Abstract
Understanding nephron loss is a primary strategy for preventing CKD progression. Death of renal tubular cells may occur by apoptosis during developmental and regenerative processes. However, during AKI, the transition of AKI to CKD, sepsis-associated AKI, and kidney transplantation ferroptosis and necroptosis, two pathways associated with the loss of plasma membrane integrity, kill renal cells. This necrotic type of cell death is associated with an inflammatory response, which is referred to as necroinflammation. Importantly, the necroinflammatory response to cells that die by necroptosis may be fundamentally different from the tissue response to ferroptosis. Although mechanisms of ferroptosis and necroptosis have recently been investigated in detail, the cell death propagation during tubular necrosis, although described morphologically, remains incompletely understood. Here, we argue that a molecular switch downstream of tubular necrosis determines nephron regeneration versus nephron loss. Unraveling the details of this “switch” must include the inflammatory response to tubular necrosis and regenerative signals potentially controlled by inflammatory cells, including the stimulation of myofibroblasts as the origin of fibrosis. Understanding in detail the molecular switch and the inflammatory responses to tubular necrosis can inform the discussion of therapeutic options.
Keywords: acute kidney injury, ferroptosis, necroptosis, cell death, necroinflammation, acute tubular necrosis, nephron loss
Kidney disease progression as defined by a decline in GFR is associated with tubular necrosis and nephron loss.1–3 Derangements in podocytes and glomerular injury are involved in nephron loss according to some models,4 however, the massive changes in morphology require the death of tubular cells. Whereas apoptosis, the default pathway of the immunologically silent removal of cells, appears to be of limited relevance during nephron loss, the pathways of ferroptosis and necroptosis have recently attracted increasing attention. Our understanding is that no other cell death pathway explains tubular necrosis and the appearance of muddy brown casts in urine sediments better than ferroptosis.5–12 The complete loss of a functional unit, such as an entire segment of the nephron, therefore, must be considered a possible mechanism underlying nephron loss. However, it is known from clinical observations that even significant tubular injuries can regenerate and recover. Inhibitors of necroptosis and ferroptosis are being investigated in clinical trials and might hold promise for the treatment of AKI, AKI to CKD transition, and the preservation of transplant kidney quality during transport on machine perfusion.
An Introduction to Regulated Cell Death
Apoptosis: Well Defined and Well Tolerated by the Immune System
Apoptosis is a complex form of cell death associated with exposure of high levels of phosphatidylserine (PtdSer) at the outer leaflet of the plasma membrane, serving as an eat-me signal for macrophages.13 Under nonapoptotic circumstances, distribution of PtdSer within the plasma membrane is regulated by flippases,14 such as ATP11A and ATP11C,15 that constantly keep PtdSer levels high in the inner leaflet.14 On caspase activation during apoptosis, the flippases are proteolytically processes and inactivated. At the same time, caspases activate the scramblase XKR8,16 rapidly increasing the PtdSer exposure on the cell surface where macrophages sense and engulf the apoptotic cells. Importantly, during apoptosis, the plasma membrane does not lose its integrity. Therefore, immunologic consequences of apoptosis are limited to the macrophage response and to the release of tissue messengers.17 Importantly, apoptotic cells do not release damage-associated molecular patterns (DAMPs), rendering apoptosis an immunologically silent death.13,18 Apoptosis is therefore different from the other pathways described here, which are terminally executed by plasma membrane rupture, and therefore—in contrast with apoptosis—are necrotic in nature.
PtdSer is sensed by macrophages by PtdSer receptors such as the tissue injury molecule 1 (TIM-1),19,20 also referred to as kidney injury molecule 1 (KIM-1).21 TIM-1 binding will start the process of engulfment of the bound membrane that may be an entire apoptotic cell, or a fragment of a necrotic cell that exposes high levels of PtdSer on the inner leaflet of the plasma membrane after the necrotic burst. In addition, KIM-1 was demonstrated to induce fatty-acid uptake in tubular epithelial cells.22 TIM-1/KIM-1 have been demonstrated to become highly upregulated and function as a biomarker during AKI21 associated with tubular necrosis and nephron loss.23
It has been suggested that inhibition of apoptosis is protective in mouse models of AKI.24 However, clear evidence for caspase activation in the tubular cell compartment outside the embryologic development is lacking, and caspase inhibitors have failed to protect mice from ischemia-reperfusion injury (IRI).25 Along similar lines, deletion of the apoptosis-associated proteins caspase-8 or Fas-associated protein with death domain from renal tubules have not provided any protection from AKI.6 On the basis of these data and the plausibility of necrosis of renal tubules that induces KIM-1 expression, most authors agree the contribution of apoptosis to AKI in most animal models is neglectable (Box 1).
Box 1: Apoptosis in Sensu Strictu.
Outside the research field of cell death, the term “apoptosis” was commonly used as “cell death.” It has been referred to as a morphologic diagnosis by pathologists.26 As originally introduced, however, apoptosis, like necrosis, reflects a morphologic pattern of fatal cell injury that has been expanded to include biochemical assays designed to improve its detection and quantification. Within this review, we refer to apoptosis as a caspase-dependent, noninflammatory cell death program with clearly defined features: apoptosis in sensu strictu.
Caspase-3 activation drives the apoptosis program rapidly27 after proteolytic cleavage by caspase-828,29 (extrinsic apoptosis) or caspase-930–32 (intrinsic apoptosis). Cleaved caspase-3 is easily detectable in tissue slices by immunohistochemistry33 or immunofluorescence.34 In addition, cleaved caspase-3 can be detected by Western blotting and activity of caspase-3 by enzymatic assays.35 Importantly, cleaved and active caspase-3 can also be found during forms of pyroptosis,36 so the detection of cleaved caspase-3 is not sufficient to conclude on apoptosis. Likewise, the use of caspase-inhibitors (such as zVAD-fmk, qVD, or emricasan) will prevent apoptosis, but might also interfere with caspase-1 and/or caspase-11 during pyroptosis.
Another feature required for apoptosis is phospholipid flippase/scramblase activity. Anoctamin 6 (TMEM16F) is a phospholipid scramblase and a Ca-activated chloride channel involved in this process. Once PtdSer, which is expressed only on the inner leaflet of the plasma membrane in healthy cells, is exposed to the outer leaflet, it serves as an eat-me signal for macrophages that rapidly remove these cells in an immunologically silent manner.13
Another process that is inevitably associated with apoptosis is the shrinking of the cells, followed by the process of membrane blebbing, best detected in time-lapse imaging. However, ferroptotic cells may also show features of membrane blebbing, which exemplifies the need for independent assay to conclude on apoptosis.13
Apoptosis is further characterized by nuclear chromatin condensation and a phenomenon referred to as DNA laddering. It should not be concluded on apoptosis if the features mentioned here have not been fully investigated.
Finally, all known regulated cell death pathways result in the positivity of terminal deoxynucleotidyl transferase dUTP nick end labeling staining, which simply detects double-strand breaks in the DNA, and therefore is nonspecific.
Ferroptosis: Iron-dependent Necrosis by Lipid Peroxidation
A growing list of manuscripts suggest ferroptosis critically contributes to AKI (Table 1). Ferroptosis is initiated by the failure to control lipid peroxidation. In physiologic circumstances, several molecular surveilling systems prevent ferroptosis. The best studied system centrally depends on the function of the glutathione peroxidase 4 (GPX4), a glutathione (GSH) metabolizing selenoenzyme that turns oxidized lipids to respective inactivated alcohols (Figure 1).37,38 A second layer of regulation for this system is the intracellular concentration of GSH, which is provided in different ways in a cell type–specific manner, for example, by the transmembrane glutamate/cystine antiporter system Xc-, the target of the type 1 ferroptosis inducer erastin, in combination with the GSH synthase.39–41 Other pathways that regulate the GSH pool include the trans-sulfuration pathway42 and the dipeptidase-1.43
Table 1.
Evidence for the role of ferroptosis in AKI
| Number | Date of publication | Authors | Journal | Title | Type of Evidence— AKI Model | Reference— PMID |
|---|---|---|---|---|---|---|
| 1 | 2014, November | Linkermann et al. | PNAS | Synchronized renal tubular cell death involves ferroptosis | Pharmacologic (IRI) | 25385600 |
| 2 | 2014, December | Friedman-Angeli et al. | Nature Cell Biology | Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice | Genetic (spontaneous) | 25402683 |
| 3 | 2017, January | Martin-Sanchez et al. | JASN | Ferroptosis, but not necroptosis, is important in nephrotoxic folic acid-induced AKI | Pharmacologic (FA-AKI) | 27352622 |
| 4 | 2017, June | Martens et al. | Cell Death and Disease | Sorafenib tosylate inhibits directly necrosome complex formation and protects in mouse models of inflammation and tissue injury | Pharmacologic (IRI) | 28661484 |
| 5 | 2017, October | Müller et al. | Cell Mol Life Sci | Necroptosis and ferroptosis are alternative cell death pathways that operate in acute kidney failure | Histologic (ACSL4-staining) in IRI | 28551825 |
| 6 | 2018, May | Adedoyin et al. | AJP-renal | Heme oxygenase-1 mitigates ferroptosis in renal proximal tubule cells | Pharmacologic (PTCs) | 28515173 |
| 7 | 2018, May | Stoppe et al. | Science Translational Medicine | The protective role of macrophage migration inhibitory factor in acute kidney injury after cardiac surgery | Pharmacologic (rhabdomyolysis-induced AKI) | 29769287 |
| 8 | 2019, April | Guerrero-Hue et al. | FASEB Journal | Curcumin reduces renal damage associated with rhabdomyolysis by decreasing ferroptosis-mediated cell death | Pharmacologic (rhabdomyolysis-induced AKI) | 31034781 |
| 9 | 2019, June | Mishima et al. | JASN | Drugs repurposed as antiferroptosis agents suppress organ damage, including AKI, by functioning as lipid peroxyl radical scavengers | Pharmacologic (CP-AKI) | 31767624 |
| 10 | 2019, June | Huang et al. | J Cell Mol Med | Augmenter of liver regeneration protects the kidney from ischemia-reperfusion injury in ferroptosis | Pharmacologic (IRI) | 30993878 |
| 11 | 2019, November | Deng et al. | JCI | Myo-inositol oxygenase expression profile modulates pathogenic ferroptosis in the renal proximal tubule | Pharmacologic (CP-AKI) | 31437128 |
| 12 | 2019, December | Su et al. | JBC | Pannexin 1 mediates ferroptosis that contributes to renal ischemia/reperfusion injury | IRI | 31694915 |
| 13 | 2020, January | Hu et al. | Cell Death and Disease | VDR activation attenuate cisplatin induced AKI by inhibiting ferroptosis | Pharmacologic (CP-AKI) | 31996668 |
| 14 | 2020, February | Córdoba-David et al. | Scientific Reports | Effective nephroprotection against acute kidney injury with a star-shaped polyglutamate-curcuminoid conjugate | Pharmacologic (tubular cells) | 32029842 |
| 15 | 2020, February | Lee et al. | Nature Cell Biology | Energy-stress-mediated AMPK activation inhibits ferroptosis | IRI | 32029897 |
| 16 | 2020, July | Wang et al. | Journal of Advanced Research | Quercetin alleviates acute kidney injury by inhibiting ferroptosis | Pharmacologic (IRI, FI-AKI) | 33364059 |
| 17 | 2020, August | Zhao et al. | Cell Death and Disease | XJB-5–131 inhibited ferroptosis in tubular epithelial cells after ischemia-reperfusion injury | Pharmacologic (IRI, FI-AKI) | 32796819 |
| 18 | 2020, October | Ding et al. | Cell Death and Disease | miR-182–5p and miR-378a-3p regulate ferroptosis in I/R-induced renal injury | Pharmacologic (IRI) | 33116120 |
| 19 | 2020, November | Li et al. | Free Radic Biol Med | Inhibition of ferroptosis by up-regulating Nrf2 delayed the progression of diabetic nephropathy | Pharmacologic (diabetic nephropathy) | 33152439 |
| 20 | 2020, November | Yant et al. | Life Sci | Tocilizumab mimotope alleviates kidney injury and fibrosis by inhibiting IL-6 signaling and ferroptosis in UUO model | Pharmacologic (UUO) | 32979361 |
| 21 | 2020, December | Wang et al. | Eur J Pharmacology | Ferroptosis involves in renal tubular cell death in diabetic nephropathy | Pharmacologic (diabetic nephropathy) | 32976829 |
| 22 | 2021, January | Chen et al. | Cell Death and Disease | Legumain promotes tubular ferroptosis by facilitating chaperone-mediated autophagy of GPX4 in AKI | Pharmacologic (IRI, FA-AKI) | 33431801 |
| 23 | 2021, January | Guo et al. | Br J Pharmacology | Targeted inhibition of Rev-erb-α/β limits ferroptosis to ameliorate folic acid-induced acute kidney injury | Pharmacologic (FA-AKI) | 33068011 |
| 24 | 2021, January | Jiang et al. | Mol Med Rep | Effects and molecular mechanism of pachymic acid on ferroptosis in renal ischemia reperfusion injury | Pharmacologic (IRI) | 33215224 |
| 25 | 2021, January | Deng et al. | Front Pharmacol | Mitochondrial iron overload-mediated inhibition of Nrf2-HO-1/GPX4 assisted ALI-induced nephrotoxicity | Pharmacologic (AAN) | 33584308 |
| 26 | 2021, February | Zhang et al. | J Cell Physiol | Involvement of GPX4 in irisin's protection against ischemia reperfusion-induced acute kidney injury | Pharmacologic (IRI) | 32583428 |
| 27 | 2021, February | Ma et al. | Dig Dis Sci | Inhibition of ferroptosis attenuates acute kidney injury in rats with severe acute pancreatitis | Pharmacologic (pancreatitis-induced AKI) | 32219613 |
| 28 | 2021, February | Kim et al. | Cell Death and Disease | Characterization of ferroptosis in kidney tubular cell death under diabetic conditions | Pharmacologic (diabetic nephropathy) | 33558472 |
| 29 | 2021, February | Feng et al. | Front Endocrinol | Ferroptosis enhanced diabetic renal tubular injury via HIF-1α/HO-1 pathway in db/db mice | Pharmacologic (diabetic nephropathy) | 33679620 |
| 30 | 2021, March | Li et al. | Br J Pharmacology | Nuciferine protects against folic acid-induced acute kidney injury by inhibiting ferroptosis | Pharmacologic (FA-AKI) | 33450067 |
| 31 | 2021, April | Yang et al. | Clin Transl Med | Dimethyl fumarate prevents ferroptosis to attenuate acute kidney injury by acting on NRF2 | Pharmacologic (FA-AKI, CP-AKI) | 33931960 |
| 32 | 2021, July | Tonnus et al. | Nature Communications | Dysfunction of the key ferroptosis-surveilling systems hypersensitizes mice to massive tubular necrosis during acute kidney injury | Genetic (GPX4-inactivation, FSP1-ko) and Pharmacologic | 34285234 |
| 33 | 2021, September | Zhao et al. | Free Radic Biol Med | Iron deficiency exacerbates cisplatin- or rhabdomyolysis-induced acute kidney injury through promoting iron-catalyzed oxidative damage | Pharmacologic (CP-AKI)—patient data | 34298093 |
| 34 | 2021, November | Zhao et al. | Free Radic Biol Med | Endoplasmic reticulum stress-mediated autophagy activation is involved in cadmium-induced ferroptosis of renal tubular epithelial cells | Pharmacologic (cadmium-induced AKI) | 34520822 |
| 35 | 2021, September | Lin et al. | Biomedicines | Nephroprotective role of chrysophanol in hypoxia/reoxygenation-induced renal cell damage via apoptosis, ER stress, and ferroptosis | Hypoxia/reoxygenation of tubular epithelial cells | 34572468 |
FA, folic acid; PTC, proximal tubular cells; CP, cisplatin; VDR, Vitamin D receptor; FA, foliac acid; UUO, unilateral ureteral obstruction; AAN, aristolochic acid nephropathy; IRI, ischemia-reperfusion injury.
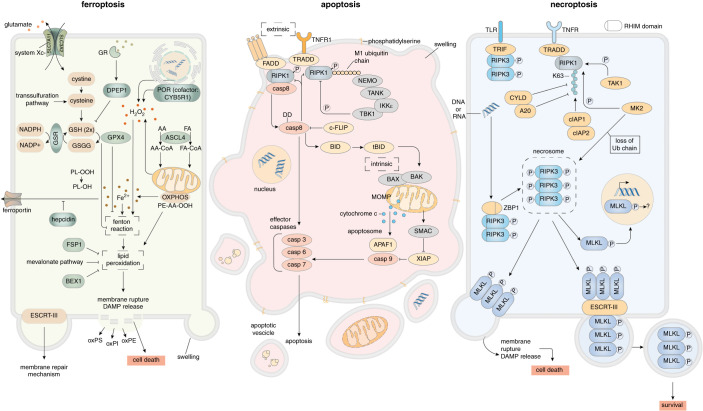
Figure 1.
Pathways of renal cell death. Ferroptosis (left) is a failsafe rather than a typical cell death pathway. In cellular homeostasis, H2O2 concentrations and iron-catalyzed Fenton reactions are limited by diverse cellular antiredox systems. The best studied system relies on GSH, which is generated intracellularly and depends on supply via system Xc-, a cys/glu antiporter in the plasma membrane, or products of the trans-sulfuration pathway. On sufficient GSH concentrations, GPX4 prevents lipid peroxidation that otherwise leads to plasma membrane rupture by unknown mechanisms. In contrast, the oxidoreductase FSP1 (also known as AIFM2) prevents lipid peroxidation on myristoylation-dependent recruitment to the plasma membrane in a GSH-independent manner. Ferroptosis occurs as a noncell-autonomous pathway in a process referred to as synchronized regulated necrosis (SRN). Apoptosis (center) represents a noninflammatory pathway that is mediated by caspases. Two distinct signaling pathways of apoptosis, extrinsic and intrinsic apoptosis, have been characterized. In extrinsic apoptosis, death receptors such as TNFR1, CD95 (Fas), and TRAIL-R, through the engagement of various intracellular adaptor proteins (Fas-associated protein with death domain, TRADD) lead to the activation of the master regulator caspase-8. On homodimerization, caspase-8 cleaves the effector caspases-3/-6/-7 to propagate the apoptosis program. On loss of mitochondrial outer membrane potential (MOMP) and the BAX-BAK–mediated release of cytochrome c from the mitochondria into the cytosol, the intrinsic apoptotic pathway is triggered. Within the cytosol, cytochrome c, APAF1 and caspase-9 form the apoptosome, which activates the effector caspases-3/-6/-7. The typical morphology includes nuclear condensation, early loss of cellular volume (shrinking), subsequent membrane blebbing, and exposure of PtdSer. Importantly, PtdSer exposure represents an eat-me signal to macrophages that eliminate apoptotic cells. Importantly, the plasma membrane does not lose its integrity during this process. Whereas apoptosis depends on the activation of caspases, necroptosis (right) is mediated by kinases. Depending on its RHIM domain, RIPK3 forms an amyloid-like structure referred to as the necrosome, the central signaling platform of necroptosis. Therein, RIPK3 phosphorylates the pseudokinase MLKL. By unknown mechanisms, pMLKL triggers a plasma membrane rupture, a process that was demonstrated to be counteracted by the membrane repair ESCRT-III complex. The necrosome can be engaged by death receptor signaling in the condition in which caspases are absent or inhibited (e.g., by viral proteins), and RIPK1 no longer intercalates with RIPK3. Other ways of engaging the necrosome are through TLRs via the RHIM-containing adaptor molecule TRIF, or by activation of the protein ZBP1/DAI in response to sensing intracellular oligonucleotides.
A second GSH-independent system centrally involves the ferroptosis suppressor protein 1 (FSP1).44–47 As GPX4, this oxidoreductase system is also important in the kidney because FSP1-deficient mice are sensitive to IRI.12 Alongside GPX4 and FSP1, the regulation of the intracellular iron pool, for example, by hepcidin48 or the heme oxidase 1 and H-ferritin,49,50 the machinery involved in H2O2 control (e.g., p450 oxidoreductase51), and lipid synthesis enzymes such as ACSL452–54 are critically involved in ferroptosis regulation. Considering the importance of ferroptosis and the rapid movement of the field,55–60 it should be stated the kidney was the first organ shown to undergo ferroptosis, and has evolved as a model system for ferroptosis researchers. However, the detailed emerging mechanism of ferroptosis is beyond the scope of this review.46,61–69 It is important to understand our novel concept of the limited immunogenicity of ferroptosis to consider that in contrast to apoptosis, necroptosis, and pyroptosis, ferroptotic cells release high amounts of oxidized phospholipids, such as oxidized phosphatidylethanolamine, oxidized PtdSer, and oxidized phosphatidylinositol.70
Necroptosis: Mixed-lineage Kinase Domain Like-mediated Necrosis To Defend Viruses
Necroptosis (Figure 1) is mediated by RIPK3-dependent phosphorylation71–73 of the pseudokinase mixed-lineage kinase domain like (MLKL) and subsequent plasma membrane rupture.74,75 RIPK3 can be activated by at least three systems. First, death receptors of the TNF superfamily, such as TNFR1, form a TNF-receptor signaling complex to unleash the pronecroptotic function of the kinase RIPK1.76,77 Human mutations in RIPK1 have been described to lead to an autoinflammatory disease.78 The only known target of RIPK1 is RIPK1 itself, but the phosphorylation at the p166 residue regulates an autoinhibitory function77,79–82 that prevents RIPK3 oligomerization via an RIP homotypic interacting motif (RHIM)-domain in both RIPK1 and RIPK3.83–87 Phosphorylation of RIPK1, therefore, drives the necroptosis machinery. Second, all toll-like receptors that engage the intracellular adapter protein TRIF can stimulate RIPK3 oligomerization and phosphorylation in an RIPK1-independent manner.88–92 Finally, intracellular nucleotides (such as misfolded zRNA) sensed by the protein ZBP1 results in RIPK3-dependent necroptosis as well.93–96 It is important for the purpose of this review that necroptosis potently simulates dendritic cell (DC)–dependent crosspriming of effector T cells.97 In addition, necroptosis is associated with the maturation and release of cytokines.78,98
The necroptosis system is of importance for AKI,99–101 kidney transplantation,102–104 and potentially for nephron loss (see below). MLKL is among the most significantly upregulated genes in a number of models of AKI,105 and RIPK3-100 and MLKL101-deficient mice are protected from IRI in the clamp ischemia model. In human ANCA-vasculitis samples106 and kidney transplant biopsies,101 individual tubular cells stain positive for a highly specific antibody against phosphorylated MLKL (pMLKL). In addition, pMLKL positivity was recognized particularly in peritubular capillaries after transplantation.107 Future studies will investigate if the expression of RIPK3 and/or MLKL in kidney biopsies is helpful for risk stratification. As for the ferrostatins, small molecule inhibitors of necroptosis (necrostatins) are readily available for clinical trials.
DAMPs: Cell-death–driven Consequences for the Immune System
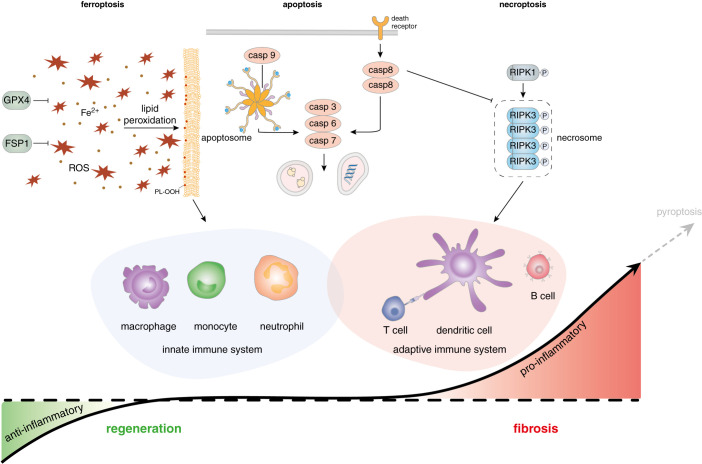
Necroinflammation is a process in which necrotic cells release DAMPs and thereby shape the immune response to the necrotic debris.108–110 Exposing necroptotic cell antigens to conventional DCs (cDC1) stimulates crosspresentation to CD8-positive cytotoxic T cells.111 Crosspresentation of necrotic antigens, such as actin,112 is regulated in a complex manner. In the case of actin, the cDC1 receptor DNGR1 is required for crosspresentation.113,114 At least in the microenvironment of cancers, however, soluble factors, such as gelsolin, are known to interfere with actin binding to DNGR1 to prevent antitumor immunity.111 Similar mechanisms are likely to contribute to necroinflammation in the kidney, but have not been investigated so far. In summary, the necrotic death of kidney cells not only results a loss of function, but leaves the remaining tissue with potentially immunogenic necrotic debris. We are only just beginning to understand how distinct necrotic cell death pathways shape the immune system in different ways.57,97 There is, however, reason to believe cDC-mediated crosspriming of T cells after necroptosis is associated with significant DC-to-myofibroblast crosstalk and potentially myofibroblast proliferation.115 Taken together, as depicted in Figure 2, necroptosis drives the adaptive immune system, which explains the proliferation of fibroblasts to cause fibrosis and irreversible nephron loss (see below).
Figure 2.
A hypothetical model of the interplay between the immune system and regulated cell death pathways. Although under fundamental debate, ferroptosis may create an anti-inflammatory environment, at least for the adaptive immune system. Although T cell activation, T cell crosspriming, and B cell activation may be inhibited by ferroptosis, cells of the innate immune system such as macrophages and neutrophils may better resist the environmental scenario of high lipid peroxidation and redox imbalance to remove necrotic debris, a feature that potentially allows necrotic kidney tubules to regenerate. In such a model, profibrotic conditions would be antagonized by the innate immune system through the removal of necrotic debris. In contrast with all other pathways of regulated cell death, classic apoptosis does not result in plasma membrane rupture and therefore does not expose intracellular epitopes to the immune system. PtdSer exposure on the surface of apoptotic cells functions as an eat-me signal and the immunologically silent removal of PtdSer-exposing cells. Necroptosis is interpreted as a defense mechanism against virus-carrying cells. During necroptosis (and most likely pyroptosis), the initiation of DC activation and T cell crosspriming alongside with the maturation of cytokines establishes a solid adaptive immune response and a vaccination against viral epitopes. Maladaptive repair and the activation of myofibroblasts in the kidney are likely a consequence of adaptive immune cell activation and nonferroptotic, nonapoptotic regulated cell death. We clearly point out that the presented model is speculative and serves as a working model that needs to be tested in the future.
In contrast with necroptotic death of renal tubular epithelial cells, ferroptosis may not trigger the adaptive immune response despite the release of DAMPs. In contrast with previous assumptions that were on the basis of the DAMP release as the exclusive immune-regulatory factor in ferroptosis,116–118 including our own previously published model,109 convincing accumulating data suggest anti-immunogenic factors are key in ferroptosis. Indeed, it was recently demonstrated that T cells are paralyzed by myeloid cells in high reactive oxygen species conditions.119 This important effect is mechanistically mediated as a direct cell-to-cell contact between myeloid cells and T cells.119 This mechanism may explain why, despite massive amounts of tubular necrosis, hardly any adaptive inflammatory response is seen in histologic samples of IRI-induced acute tubular necrosis.12,101 It is important to consider that in this scenario, the innate immune system and myeloid-derived suppressor cells are active, explaining the infiltration of macrophages and neutrophils (Figure 2). It is a new hypothesis that a microenvironment in which adaptive immunity is actively blocked may allow tubular regeneration. Of course, this model is hypothetical and future work should allow the testing of this hypothesis. However, these considerations are in line with the absence of an adaptive immune cell infiltration in GPX4-dysfunctional mice.5
Kidney Tubular Necrosis: Cell Death Propagation in the Kidney
Primary Tubular Injury
Necroptosis and ferroptosis have been convincingly demonstrated by different laboratories to contribute to tubular necrosis (see above). In most models of AKI, however, the relative contribution of these regulated necrotic cell death pathways clearly favors a major role for ferroptosis.120,121 Indeed, entire segments of proximal tubules and thick ascending limbs undergo ferroptosis.6,12 During this process, lipid peroxidation dominates the injured microenvironment between the basal laminas of the tubules and may paralyze the adaptive immune system, thereby preventing the proliferation of myofibroblasts.119
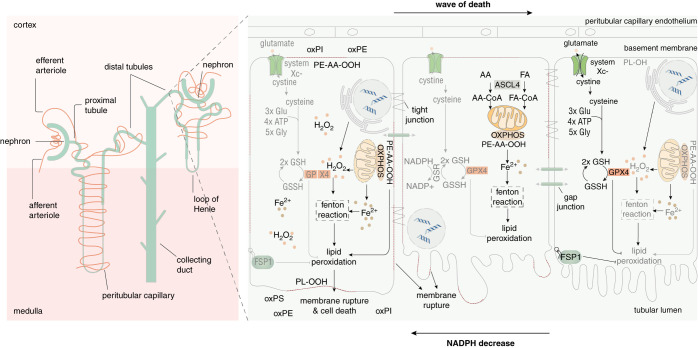
In isolated renal tubules, spontaneous release of lactate dehydrogenase was demonstrated two decades ago.122–124 A significant portion of this spontaneous lactate dehydrogenase release is accounted for by ferroptosis.12 As briefly mentioned above, ferroptosis is a cell death modality that typically involves noncell autonomous cell death propagation.5,6,125,126 Similar effects have been observed in the fins of zebrafish.127,128 This novel and expanding of field noncell autonomous cell death propagation is testing several hypotheses, some of which are depicted in Figure 3. One important aspect is the shared cytosol between connected tubular compartments, especially in its redox capacity. NADP(H) concentrations may vary along the renal tubule and become insufficient in preventing lipid peroxidation, a hallmark of ferroptosis.129 In addition, an accompanying wave of calcium was described in the cell culture, and might contribute to the cell death propagation in the renal tubules. Such factors are included in Figure 3 and account for regulatory mechanisms of the pace of cell death propagation. Future work will be required to understand these intercellular signals in more detail, to identify potential novel therapeutic targets.
Figure 3.
SRN: cell-death propagation during renal tubule ferroptosis. In most cases, it is unclear how a necrotic zone of dead cells propagates during an event of sepsis or ischemia. On the basis of time-lapse videos obtained from isolated perfused renal tubules that underwent ferroptosis, the concept of SRN emerged.6 Although this phenomenon has not been observed in humans, necrotic tubules in the urine of patients and experimental intravital microscopy videos suggest cell death occurs in a “wave of death.”6,126,128 Given the connections between cells in a functional syncytium such as a renal tubule, the adrenal gland, or the myocardium, it is tempting to speculate that the intracellular redox capacity (NADPH concentration) diffuses through intercellular junctions. If the redox capacity decreases in a dying cell, an NADPH gradient forms, which renders the closest neighbor at high risk of undergoing ferroptosis. It is now clear the bulk necrotic area that occurs during myocardial infarction or tubular necrosis originates from cells that underwent ferroptosis. We speculate that similar mechanisms might occur in other organs, such as the brain upon stroke, or the adrenal glands during sepsis or shock.
In contrast with ferroptosis, death of a tubular cell by necroptosis or apoptosis is much less common. Apoptosis of tubular cells was convincingly described during embryonic development,130 but genetically deleting key apoptosis proteins, such as caspase-8 or Fas-associated protein with death domain, in adult mice does not affect tubular necrosis patterns in AKI models or isolated kidney tubules.6 As described for several cancers, apoptotic programs in tubular cells may be reactivated during tubular regeneration. This would explain the detection of cleaved-caspase 3 signals in immunofluorescence or immunohistochemistry in some models of AKI. However, despite intensive unpublished investigations, in our own laboratory we failed to detect a single cleaved caspase-3 positive tubular cell in adult mice or human tissue in two decades of cell death research. In contrast, in addition to ferroptosis, pMLKL signals in renal tubular cells indeed indicate necroptosis, and understanding the appearance of those particularly immunogenic cells will be key to unraveling the mechanisms of nephron loss.
Tubular Necrosis Secondary to Peritubular Capillary Dysfunction
In contrast with tubular cells that predominantly die by ferroptosis, peritubular capillaries appear to follow a very different set of cell death modalities. The apoptosis/necroptosis system is known to occur in endothelial cells131 and peritubular capillaries,104 and drive capillary rarefaction.104 In those IRI studies, a nonspecific RIPK3 signal by immunohistochemistry still demonstrated tubules die in a typical synchronized manner, indicating secondary ferroptosis after peritubular capillary dysfunction.104 More experiments directed to cell death of peritubular capillaries are required to shed light on the detailed mechanism of secondary tubular necrosis. However, such experiments are tremendously important for understanding nephron loss. Some recent evidence from single-cell RNA sequencing analyses has been published and may provide the first insights into endothelial regulation.132,133 Such approaches, despite being entirely descriptive in nature and mechanistically insufficient to make conclusions about the cell death pathways involved, may indicate which mRNAs associated with transcription to the relevant cell death proteins are involved. Importantly, again, ferroptosis appears as a key mechanism in one of those studies.132
Nephron Loss
A Model of Factors that Regulate Nephron Loss
Nephron loss has been broadly discussed over the past few decades.134 However, only in selected animal models, such as of GMB nephritis, necroptotic cell death of neutrophils and the release of neutrophil extracellular traps cause the glomerular filter to collapse.106 Other models discuss protein leakage into the primary urine, but how excessive reabsorption of proteins may cause tubular necrosis remains obscure, although reactive oxygen species may be involved. On an extensive literature search, “abnormal filtrate spreading” or “obstruction by overgrowth” fail to provide clear mechanistic insights into how tubular cells could die.134 More generally, the concept of the loss of nephrons as a consequence of glomerular injury fails to explain AKI to CKD progression,3 as recently reviewed.135 The oversimplified view in which a tubule dies on glomerular dysfunction, therefore, appears unsustainable. In contrast, genetic destruction of renal tubules, for example, by deletion of GPX4, results in nephron loss by ferroptosis5 without the glomeruli being affected in any way. It is, however, entirely unclear how necrotic casts would form after tubular necrosis and how changes in tubular flow affect this process.
All data discussed above suggest acute tubular necrosis is reversible in some patients, but not in others. Therefore, the molecular switch, in other words, the decision of recovery or maladaptive repair and subsequent fibrosis, is downstream of tubular necrosis. Although to the best of our knowledge, nothing has been convincingly demonstrated on the nature of this decision process, in this study we propose a novel working hypothesis. On the basis of the effects on crosspriming and potentially the stimulation of myofibroblast proliferation, necroptosis may be upstream of maladaptive repair, fibrosis, and the clinical picture of interstitial fibrosis and tubular atrophy. In contrast, ferroptosis-mediated inhibition of the adaptive immune system might allow for recovery of the injured tissue. This hypothesis, however, cannot be interpreted as more than a working model, but we consider it conclusive that the cell death signal and the respective necroinflammatory response contribute to the divergent outcome. Considering these thoughts on a more general scale, ferroptosis may represent a means to allow regeneration of renal tubules. In models of hypoperfusion, such as IRI or septic AKI, tubular ferroptosis dominates. Similarly, a growing body of literature supports that toxic AKI is mediated by ferroptosis. In conclusion, in most cases, nephron loss is more likely to be explained by primarily tubular injury, or as a result of hypoperfusion in peritubular capillaries, secondary tubular injury. Given the insufficient quality of preclinical models of AKI-induced CKD progression, reliable data on the basis of animal models of nephron loss are sparse. Until today, no intravital microscopy-based detection of acute tubular necrosis of more than one cell136 has been reported. Along similar lines, nephron loss is a morphologic explanation for AKI to CKD progression. The number of lost nephrons may correlate with GFR decline. Even the widely accepted thoughts on age-related nephron loss methodologically relies on autopsy kidneys or enhanced computed tomography and renal biopsy analysis.137,138 The latter method may be of clinical importance to identify individuals at risk for CKD progression on the basis of low nephron endowment.139
Specific Considerations regarding Nephron Loss in Kidney Transplants
Beyond general regulators of nephron loss, kidney allografts are at risk for nephron loss as a result of additional specific factors. These include HLA incompatibility–driven inflammation, such as in acute and chronic antibody- or T cell–mediated rejection, but also nonimmunologic components. Allograft artery stenosis or thrombosis and peritransplant injuries, recipient arterial hypertension, and hyperlipidemia and general cardiovascular risk factors commonly contribute to hypoperfusion and to an insufficient partial oxygen pressure. Specific necrotic signals may result from toxicities, for example, side effects of calcineurin inhibitors (CNIs), or from the defense against virally infected tubular cells. CNIs have been demonstrated to cause characteristic tubular morphologic changes in response to necroptosis-regulating signaling, for example, through FN14, a TNFR-superfamily member.140 In contrast with CNI-induced tubular damage, cytomegalovirus infections are known to be cleared by necroptotic signaling.141,142 Along similar lines, BK virus infections are known to be accompanied by necrotic tubular damage143–145 that might be caused by necroptosis. It is possible that necroptosis of virally infected cells, according to the model presented in Figure 2, drives myofibroblast proliferation and fibrosis. Finally, after a long cold ischemia time before anastomosis, it is possible nephron loss occurs within the first hours of the reperfusion phase. In such a scenario, addition of necrostatins and/or ferrostatins to a kidney transplant machine perfusate may prevent nephron loss and associated necroinflammation.
Therapeutic Strategies
Food and Drug Administration–approved Drugs that target Ferroptosis and Necroptosis
Several Food and Drug Administration–approved drugs function as cell death inhibitors. In the case of ferroptosis, Mishima et al. published a comprehensive analysis on promethazine, omeprazole, carvedilol, estradiol, rifampicin, indole-3-carbinol, propranolol, and thyroid hormones to function as peroxyl radical scavengers.10 Although the antiferroptotic potency of these drugs is much lower compared with modern ferroptosis inhibitors, such as 3203146 or liproxstatin-1,147 it cannot be excluded that relevant antiferroptotic effects result from their application. Interestingly, IL-4–induced-1 (IL-4i1), an amino acid oxidase secreted from immune cells with high homology to snake venoms (L-amino acid oxidases), exerts antiferroptotic functions by generation of indole-3-pyruvate from tryptophan.148 These novel mechanisms have not been therapeutically targeted until today.
As with repurposed drugs that inhibit ferroptosis, the anticonvulsant phenytoin functions as an antinecroptotic compound,101 although much less potent compared with specific RIPK1 inhibitors. Along similar lines, the anticancer therapeutics ponatinib149,150and pazopanib149 were also demonstrated to function as inhibitors of RIP kinases.151 Most intriguingly, however, the original necrostatin (necrostatin-1, Nec-1152,153) contains a thiohydantoin motif base that inhibits ferroptosis by approximately 20 µM.5 Therefore, it is possible to generate combined small molecule inhibitors of necroptosis and ferroptosis as recently demonstrated by the generation of Nec-1f (where “f” means inhibition of ferroptosis).12
Potential Side Effects of Cell Death Inhibitors
It may not matter to a single cell how it dies, but it affects the surrounding tissue and the immune system. Inhibiting one cell death pathway, such as apoptosis, may allow a more immunogenic cell death modality to pursue, for example, necroptosis.154,155 This has been most clearly demonstrated for the proapoptotic caspase-8, which cleaves caspase-3 to execute apoptosis and at the same time, through a caspase cleavage site in RIPK1, inhibits necroptosis.85 Although similar connections between regulated cell death pathways have been demonstrated for an apoptosis-to-pyroptosis crosstalk,85,156–158 nothing is known in respect to the interconnectivity of any cell death pathway to ferroptosis. Importantly, however, crossregulation of ferroptosis and other pathways cannot be excluded. In conclusion, therapeutically interfering with the web of cell death pathways may favor other cell death pathways that could potentially be even more immunogenic. In cancer immunotherapy, this might more effectively treat the cancer, yet would be potentially dangerous in the case of solid organ transplantation.
As mentioned above, necroptosis is a means to defend viruses, and cytomegalovirus has already found a way to protect itself not only against apoptosis, but also against necroptosis through the expression of the viral protein M45.141,159,160 Other viruses may copy such strategies. That said, simultaneous inhibition of the critical pathways may be required for the best outcomes and potentially disease-specific and individualized cell death therapies. We consider it likely that solid organ transplant ex-vivo perfusion systems will be safe to test ferrostatins for clinical purposes.
The model on nephron loss (Figure 2) is speculative. However, as all scientists should, we worked out a hypothesis using the best of our knowledge. Every nephrologist who once saw the massive T cell infiltration into a graft on acute T cell–mediated rejection realizes the inflammatory capacity of our immune system. Despite massive tubular necrosis, nothing comparable happens to kidneys that underwent IRI. This simple consideration, to us, rules out that ferroptosis is a highly immunogenic cell death pathway. Future work should aim at investigating this hypothesis, for the sake of progression of knowledge on nephron loss.
Disclosures
A. Linkermann issued a patent for Nec-1f, a combined inhibitor of necroptosis and ferroptosis (#20160943.5); reports having consultancy agreements with Alexion, Genentech, and HBM; reports receiving research funding from Apogenix, Fresenius, Novartis, and Pfizer; reports receiving honoraria from Alexion, Genentech, and Novartis; reports being a scientific advisor or member of AJP renal, the American Journal of Transplantation, Cell Death and Differentiation Guest Editor, Cell Death and Disease, Cellular and Molecular Life Sciences Guest Editor, Clinical Kidney Journal, JASN, Molecular and Cellular Oncology, Oncotarget, the Russian Journal of Internal Medicine, and Seminars in Nephrology Guest Editor. All remaining authors have nothing to disclose. We acknowledge funding to our laboratory by the Medical Clinic 3, University Hospital Carl Gustav Carus Dresden, Germany, by the German research foundation (DFG) SFB-TRR 205, SFB-TRR 127, the international research training group 2251, the priority program on ferroptosis (SPP 3206) and by the Wilhelm-Sander-Foundation (2020.043.1)
Funding
This work was supported by the Heisenberg Professorship to A. Linkermann (project number 324141047).
Acknowledgments
We apologize for the work that could not be discussed due to special limitations. We cordially thank Dr. Peter Vandenabeele, Dr. Tom Vanden Berghe, and all researchers in the Linkermann Lab, especially Alexia Belavgeni, for their continuous helpful discussions regarding the topic reviewed here.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Romagnani P, Remuzzi G, Glassock R, Levin A, Jager KJ, Tonelli M, et al. : Chronic kidney disease. Nat Rev Dis Primers 3: 17,088, 2017 [DOI] [PubMed] [Google Scholar]
- 2.Ruiz-Ortega M, Rayego-Mateos S, Lamas S, Ortiz A, Rodrigues-Diez RR: Targeting the progression of chronic kidney disease. Nat Rev Nephrol 16: 269–288, 2020 [DOI] [PubMed] [Google Scholar]
- 3.Venkatachalam MA, Weinberg JM, Kriz W, Bidani AK: Failed tubule recovery, AKI-CKD transition, and kidney disease progression. J Am Soc Nephrol 26: 1765–1776, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glassock RJ, Rule AD: Aging and the kidneys: Anatomy, physiology and consequences for defining chronic kidney disease. Nephron 134: 25–29, 2016 [DOI] [PubMed] [Google Scholar]
- 5.Friedmann Angeli JP, Schneider M, Proneth B, Tyurina YY, Tyurin VA, Hammond VJ, et al. : Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol 16: 1180–1191, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Linkermann A, Skouta R, Himmerkus N, Mulay SR, Dewitz C, De Zen F, et al. : Synchronized renal tubular cell death involves ferroptosis. Proc Natl Acad Sci USA 111: 16836–16841, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao JY, Dixon SJ: Mechanisms of ferroptosis. Cell Mol Life Sci 73: 2195–2209, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hofmans S, Vanden Berghe T, Devisscher L, Hassannia B, Lyssens S, Joossens J, et al. : Novel ferroptosis inhibitors with improved potency and ADME properties. J Med Chem 59: 2041–2053, 2016 [DOI] [PubMed] [Google Scholar]
- 9.Lever JM, Boddu R, George JF, Agarwal A: Heme oxygenase-1 in kidney health and disease. Antioxid Redox Signal 25: 165–183, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mishima E, Sato E, Ito J, Yamada KI, Suzuki C, Oikawa Y, et al. : Drugs repurposed as antiferroptosis agents suppress organ damage, including AKI, by functioning as lipid peroxyl radical scavengers. J Am Soc Nephrol 31: 280–296, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y, Bi R, Quan F, Cao Q, Lin Y, Yue C, et al. : Ferroptosis involves in renal tubular cell death in diabetic nephropathy. Eur J Pharmacol 888: 173,574, 2020 [DOI] [PubMed] [Google Scholar]
- 12.Tonnus W, Meyer C, Steinebach C, Belavgeni A, von Mässenhausen A, Gonzalez NZ, et al. : Dysfunction of the key ferroptosis-surveilling systems hypersensitizes mice to tubular necrosis during acute kidney injury. Nat Commun 12: 4402, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagata S, Segawa K: Sensing and clearance of apoptotic cells. Curr Opin Immunol 68: 1–8, 2021 [DOI] [PubMed] [Google Scholar]
- 14.Segawa K, Kurata S, Yanagihashi Y, Brummelkamp TR, Matsuda F, Nagata S: Caspase-mediated cleavage of phospholipid flippase for apoptotic phosphatidylserine exposure. Science 344: 1164–1168, 2014 [DOI] [PubMed] [Google Scholar]
- 15.Segawa K, Yanagihashi Y, Yamada K, Suzuki C, Uchiyama Y, Nagata S: Phospholipid flippases enable precursor B cells to flee engulfment by macrophages. Proc Natl Acad Sci USA 115: 12212–12217, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suzuki J, Denning DP, Imanishi E, Horvitz HR, Nagata S: Xk-related protein 8 and CED-8 promote phosphatidylserine exposure in apoptotic cells. Science 341: 403–406, 2013 [DOI] [PubMed] [Google Scholar]
- 17.Medina CB, Mehrotra P, Arandjelovic S, Perry JSA, Guo Y, Morioka S, et al. : Metabolites released from apoptotic cells act as tissue messengers. Nature 580: 130–135, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perry JSA, Morioka S, Medina CB, Iker Etchegaray J, Barron B, Raymond MH, et al. : Interpreting an apoptotic corpse as anti-inflammatory involves a chloride sensing pathway. Nat Cell Biol 21: 1532–1543, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meyers JH, Chakravarti S, Schlesinger D, Illes Z, Waldner H, Umetsu SE, et al. : TIM-4 is the ligand for TIM-1, and the TIM-1-TIM-4 interaction regulates T cell proliferation. Nat Immunol 6: 455–464, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Umetsu SE, Lee WL, McIntire JJ, Downey L, Sanjanwala B, Akbari O, et al. : TIM-1 induces T cell activation and inhibits the development of peripheral tolerance. Nat Immunol 6: 447–454, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Ichimura T, Bonventre JV, Bailly V, Wei H, Hession CA, Cate RL, et al. : Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J Biol Chem 273: 4135–4142, 1998 [DOI] [PubMed] [Google Scholar]
- 22.Mori Y, Ajay AK, Chang JH, Mou S, Zhao H, Kishi S, et al. : KIM-1 mediates fatty acid uptake by renal tubular cells to promote progressive diabetic kidney disease. Cell Metab 33: 1042–1061.e1047, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malhotra R, Katz R, Jotwani V, Ambrosius WT, Raphael KL, Haley W, et al. : Urine markers of kidney tubule cell injury and kidney function decline in SPRINT trial participants with CKD. Clin J Am Soc Nephrol 15: 349–358, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daemen MA, van tV, Denecker G, Heemskerk VH, Wolfs TG, Clauss M, et al. : Inhibition of apoptosis induced by ischemia-reperfusion prevents inflammation. J Clin Invest 104: 541–549, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Linkermann A, Brasen JH, Himmerkus N, Liu S, Huber TB, Kunzendorf U, et al. : Rip1 (receptor-interacting protein kinase 1) mediates necroptosis and contributes to renal ischemia/reperfusion injury. Kidney Int 81: 751–761, 2012 [DOI] [PubMed] [Google Scholar]
- 26.Schumer M, Colombel MC, Sawczuk IS, Gobé G, Connor J, O’Toole KM, et al. : Morphologic, biochemical, and molecular evidence of apoptosis during the reperfusion phase after brief periods of renal ischemia. Am J Pathol 140: 831–838, 1992 [PMC free article] [PubMed] [Google Scholar]
- 27.Rehm M, Dussmann H, Janicke RU, Tavare JM, Kogel D, Prehn JH: Single-cell fluorescence resonance energy transfer analysis demonstrates that caspase activation during apoptosis is a rapid process. Role of caspase-3. J Biol Chem 277: 24506–24514, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Boldin MP, Goncharov TM, Goltsev YV, Wallach D: Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/APO-1- and TNF receptor-induced cell death. Cell 85: 803–815, 1996 [DOI] [PubMed] [Google Scholar]
- 29.Muzio M, Chinnaiyan AM, Kischkel FC, O’Rourke K, Shevchenko A, Ni J, et al. : FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death–inducing signaling complex. Cell 85: 817–827, 1996 [DOI] [PubMed] [Google Scholar]
- 30.Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, et al. : Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91: 479–489, 1997 [DOI] [PubMed] [Google Scholar]
- 31.Srinivasula SM, Hegde R, Saleh A, Datta P, Shiozaki E, Chai J, et al. : A conserved XIAP-interaction motif in caspase-9 and Smac/DIABLO regulates caspase activity and apoptosis. Nature 410: 112–116, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Pop C, Timmer J, Sperandio S, Salvesen GS: The apoptosome activates caspase-9 by dimerization. Mol Cell 22: 269–275, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Srinivasan A, Roth KA, Sayers RO, Shindler KS, Wong AM, Fritz LC, et al. : In situ immunodetection of activated caspase-3 in apoptotic neurons in the developing nervous system. Cell Death Differ 5: 1004–1016, 1998 [DOI] [PubMed] [Google Scholar]
- 34.Kamiyama K, Matsuda N, Yamamoto S, Takano K, Takano Y, Yamazaki H, et al. : Modulation of glucocorticoid receptor expression, inflammation, and cell apoptosis in septic guinea pig lungs using methylprednisolone. Am J Physiol Lung Cell Mol Physiol 295: L998–l1006, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, et al. : Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ 25: 486–541, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Y, Gao W, Shi X, Ding J, Liu W, He H, et al. : Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a Gasdermin. Nature 547: 99–103, 2017 [DOI] [PubMed] [Google Scholar]
- 37.Seiler A, Schneider M, Forster H, Roth S, Wirth EK, Culmsee C, et al. : Glutathione peroxidase 4 senses and translates oxidative stress into 12/15-lipoxygenase dependent- and AIF-mediated cell death. Cell Metab 8: 237–248, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Ingold I, Berndt C, Schmitt S, Doll S, Poschmann G, Buday K, et al. : Selenium utilization by GPX4 is required to prevent hydroperoxide-induced ferroptosis. Cell 172: 409–1422.e21, 2017 [DOI] [PubMed] [Google Scholar]
- 39.Yagoda N, von Rechenberg R, Zaganjor E, Bauer AJ, Yang WS, Fridman DJ, et al. : RAS-RAF-MEK-dependent oxidative cell death involving voltage-dependent anion channels. Nature 447: 864–868, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang WS, Stockwell BR: Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem Biol 15: 234–245, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, et al. : Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149: 1060–1072, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cao J, Chen X, Jiang L, Lu B, Yuan M, Zhu D, et al. : DJ-1 suppresses ferroptosis through preserving the activity of S-adenosyl homocysteine hydrolase. Nat Commun 11: 1251, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guan Y, Liang X, Ma Z, Hu H, Liu H, Miao Z, et al. : A single genetic locus controls both expression of DPEP1/CHMP1A and kidney disease development via ferroptosis. Nat Commun 12: 5078, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bersuker K, Hendricks J, Li Z, Magtanong L, Ford B, Tang PH, et al. : The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature 575: 688–692, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Doll S, Freitas FP, Shah R, Aldrovandi M, da Silva MC, Ingold I, et al. : FSP1 is a glutathione-independent ferroptosis suppressor. Nature 575: 693–698, 2019 [DOI] [PubMed] [Google Scholar]
- 46.Mao C, Liu X, Zhang Y, Lei G, Yan Y, Lee H, et al. : DHODH-mediated ferroptosis defence is a targetable vulnerability in cancer. Nature 593: 586–590, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zheng J, Conrad M: The metabolic underpinnings of ferroptosis. Cell Metab 32: 920–337, 2020 [DOI] [PubMed] [Google Scholar]
- 48.Scindia Y, Dey P, Thirunagari A, Liping H, Rosin DL, Floris M, et al. : Hepcidin mitigates renal ischemia-reperfusion injury by modulating systemic iron homeostasis. J Am Soc Nephrol 26: 2800–2814, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zarjou A, Bolisetty S, Joseph R, Traylor A, Apostolov EO, Arosio P, et al. : Proximal tubule H-ferritin mediates iron trafficking in acute kidney injury. J Clin Invest 123: 4423–4434, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fang X, Cai Z, Wang H, Han D, Cheng Q, Zhang P, et al. : Loss of cardiac ferritin H facilitates cardiomyopathy via Slc7a11-mediated ferroptosis. Circ Res 127: 486–501, 2020 [DOI] [PubMed] [Google Scholar]
- 51.Yan B, Ai Y, Sun Q, Ma Y, Cao Y, Wang J, et al. : Membrane damage during ferroptosis is caused by oxidation of phospholipids catalyzed by the oxidoreductases POR and CYB5R1. Mol Cell 81: 355–369.e310, 2021 [DOI] [PubMed] [Google Scholar]
- 52.Dixon SJ, Winter GE, Musavi LS, Lee ED, Snijder B, Rebsamen M, et al. : Human haploid cell genetics reveals roles for lipid metabolism genes in nonapoptotic cell death. ACS Chem Biol 10: 1604–1609, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Doll S, Proneth B, Tyurina YY, Panzilius E, Kobayashi S, Ingold I, et al. : ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol 13: 91–98, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kagan VE, Mao G, Qu F, Angeli JP, Doll S, Croix CS, et al. : Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol 13: 81–90, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jiang Z, Lim SO, Yan M, Hsu JL, Yao J, Wei Y, et al. : TYRO3 induces anti-PD-1/PD-L1 therapy resistance by limiting innate immunity and tumoral ferroptosis. J Clin Invest 131: e139434, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jourdain AA, Begg BE, Mick E, Shah H, Calvo SE, Skinner OS, et al. : Loss of LUC7L2 and U1 snRNP subunits shifts energy metabolism from glycolysis to OXPHOS. Molecular cell 81: 1905–1919.e1912, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ma X, Xiao L, Liu L, Ye L, Su P, Bi E, et al. : CD36-mediated ferroptosis dampens intratumoral CD8(+) T cell effector function and impairs their antitumor ability. Cell Metab 33: 1001–1012.e1005, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schmitt A, Xu W, Bucher P, Grimm M, Konantz M, Horn H, et al. : Dimethyl fumarate induces ferroptosis and impairs NF-κB/STAT3 signaling in DLBCL. Blood 138: 871–884, 2021 [DOI] [PubMed] [Google Scholar]
- 59.Singhal R, Mitta SR, Das NK, Kerk SA, Sajjakulnukit P, Solanki S, et al. : HIF-2α activation potentiates oxidative cell death in colorectal cancers by increasing cellular iron. J Clin Invest 131: e143691, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tan SK, Mahmud I, Fontanesi F, Puchowicz M, Neumann CKA, Griswold AJ, et al. : Obesity-dependent adipokine chemerin suppresses fatty acid oxidation to confer ferroptosis resistance. Cancer Discov 11: 2072–2093, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Beatty A, Singh T, Tyurina YY, Tyurin VA, Samovich S, Nicolas E, et al. : Ferroptotic cell death triggered by conjugated linolenic acids is mediated by ACSL1. Nat Commun 12: 2244, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Conlon M, Poltorack CD, Forcina GC, Armenta DA, Mallais M, Perez MA, et al. : A compendium of kinetic modulatory profiles identifies ferroptosis regulators. Nat Chem Biol 17: 665–674, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Garcia-Bermudez J, Birsoy K: A mitochondrial gatekeeper that helps cells escape death by ferroptosis. Nature 593: 514–515, 2021 [DOI] [PubMed] [Google Scholar]
- 64.Lee H, Zandkarimi F, Zhang Y, Meena JK, Kim J, Zhuang L, et al. : Energy-stress-mediated AMPK activation inhibits ferroptosis. Nat Cell Biol 22: 225–234, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Luo X, Gong HB, Gao HY, Wu YP, Sun WY, Li ZQ, et al. : Oxygenated phosphatidylethanolamine navigates phagocytosis of ferroptotic cells by interacting with TLR2. Cell Death Differ 28: 1971–1989, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stockwell BR: A powerful cell-protection system prevents cell death by ferroptosis. Nature 575: 597–598, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ubellacker JM, Tasdogan A, Ramesh V, Shen B, Mitchell EC, Martin-Sandoval MS, et al. : Lymph protects metastasizing melanoma cells from ferroptosis. Nature 585: 113–118, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yee PP, Wei Y, Kim SY, Lu T, Chih SY, Lawson C, et al. : Neutrophil-induced ferroptosis promotes tumor necrosis in glioblastoma progression. Nat Commun 11: 5424, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zou Y, Henry WS, Ricq EL, Graham ET, Phadnis VV, Maretich P, et al. : Plasticity of ether lipids promotes ferroptosis susceptibility and evasion. Nature 585: 603–608, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wiernicki B, Dubois H, Tyurina YY, Hassannia B, Bayir H, Kagan VE, et al. : Excessive phospholipid peroxidation distinguishes ferroptosis from other cell death modes including pyroptosis. Cell Death Dis 11: 922, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M, et al. : Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell 137: 1112–1123, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.He S, Wang L, Miao L, Wang T, Du F, Zhao L, et al. : Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell 137: 1100–1111, 2009 [DOI] [PubMed] [Google Scholar]
- 73.Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, Lin SC, et al. : RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science 325: 332–336, 2009 [DOI] [PubMed] [Google Scholar]
- 74.Sun L, Wang H, Wang Z, He S, Chen S, Liao D, et al. : Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 148: 213–227, 2012 [DOI] [PubMed] [Google Scholar]
- 75.Zhao J, Jitkaew S, Cai Z, Choksi S, Li Q, Luo J, et al. : Mixed lineage kinase domain-like is a key receptor interacting protein 3 downstream component of TNF-induced necrosis. Proc Natl Acad Sci U S A 109: 5322–5327, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dondelinger Y, Darding M, Bertrand MJ, Walczak H: Poly-ubiquitination in TNFR1-mediated necroptosis. Cell Mol Life Sci 73: 2165–2176, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lafont E, Draber P, Rieser E, Reichert M, Kupka S, de Miguel D, et al. : TBK1 and IKKε prevent TNF-induced cell death by RIPK1 phosphorylation. Nat Cell Biol 20: 1389–1399, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tao P, Sun J, Wu Z, Wang S, Wang J, Li W, et al. : A dominant autoinflammatory disease caused by non-cleavable variants of RIPK1. Nature 577: 109–114, 2020 [DOI] [PubMed] [Google Scholar]
- 79.Orozco S, Yatim N, Werner MR, Tran H, Gunja SY, Tait SW, et al. : RIPK1 both positively and negatively regulates RIPK3 oligomerization and necroptosis. Cell Death Differ 21: 1511–1521, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Geng J, Ito Y, Shi L, Amin P, Chu J, Ouchida AT, et al. : Regulation of RIPK1 activation by TAK1-mediated phosphorylation dictates apoptosis and necroptosis. Nat Commun 8: 359, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liccardi G, Ramos Garcia L, Tenev T, Annibaldi A, Legrand AJ, Robertson D, et al. : RIPK1 and caspase-8 ensure chromosome stability independently of their role in cell death and inflammation. Mol Cell 73: 413–428.e7, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Laurien L, Nagata M, Schünke H, Delanghe T, Wiederstein JL, Kumari S, et al. : Autophosphorylation at serine 166 regulates RIP kinase 1-mediated cell death and inflammation. Nat Commun 11: 1747, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dondelinger Y, Delanghe T, Priem D, Wynosky-Dolfi MA, Sorobetea D, Rojas-Rivera D, et al. : Serine 25 phosphorylation inhibits RIPK1 kinase-dependent cell death in models of infection and inflammation. Nat Commun 10: 1729, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Newton K, Wickliffe KE, Dugger DL, Maltzman A, Roose-Girma M, Dohse M, et al. : Cleavage of RIPK1 by caspase-8 is crucial for limiting apoptosis and necroptosis. Nature 574: 428–431, 2019 [DOI] [PubMed] [Google Scholar]
- 85.Newton K, Wickliffe KE, Maltzman A, Dugger DL, Reja R, Zhang Y, et al. : Activity of caspase-8 determines plasticity between cell death pathways. Nature 575: 679–682, 2019 [DOI] [PubMed] [Google Scholar]
- 86.Snyder AG, Hubbard NW, Messmer MN, Kofman SB, Hagan CE, Orozco SL, et al. : Intratumoral activation of the necroptotic pathway components RIPK1 and RIPK3 potentiates antitumor immunity. Science Immunology 4: eaaw2004, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tang Y, Tu H, Zhang J, Zhao X, Wang Y, Qin J, et al. : K63-linked ubiquitination regulates RIPK1 kinase activity to prevent cell death during embryogenesis and inflammation. Nat Commun 10: 4157, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kaiser WJ, Sridharan H, Huang C, Mandal P, Upton JW, Gough PJ, et al. : Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL. J Biol Chem 288: 31,268–31,279, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dillon CP, Weinlich R, Rodriguez DA, Cripps JG, Quarato G, Gurung P, et al. : RIPK1 blocks early postnatal lethality mediated by caspase-8 and RIPK3. Cell 157: 1189–1202, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lawlor KE, Khan N, Mildenhall A, Gerlic M, Croker BA, D’Cruz AA, et al. : RIPK3 promotes cell death and NLRP3 inflammasome activation in the absence of MLKL. Nat Commun 6: 6282, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Takemura R, Takaki H, Okada S, Shime H, Akazawa T, Oshiumi H, et al. : PolyI:C-induced, TLR3/RIP3-dependent necroptosis backs up immune effector-mediated tumor elimination in vivo. Cancer Immunol Res 3: 902–914, 2015 [DOI] [PubMed] [Google Scholar]
- 92.Nogusa S, Thapa RJ, Dillon CP, Liedmann S, Oguin TH III, Ingram JP, et al. : RIPK3 activates parallel pathways of MLKL-driven necroptosis and FADD-mediated apoptosis to protect against influenza a virus. Cell Host Microbe 20: 13–24, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Upton JW, Kaiser WJ, Mocarski ES: DAI/ZBP1/DLM-1 complexes with RIP3 to mediate virus-induced programmed necrosis that is targeted by murine cytomegalovirus vIRA. Cell Host Microbe 11: 290–297, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ingram JP, Thapa RJ, Fisher A, Tummers B, Zhang T, Yin C, et al. : ZBP1/DAI drives RIPK3-mediated cell death induced by IFNs in the absence of RIPK1. J Immunol 203: 1348–1355, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jiao H, Wachsmuth L, Kumari S, Schwarzer R, Lin J, Eren RO, et al. : Z-nucleic-acid sensing triggers ZBP1-dependent necroptosis and inflammation. Nature 580: 391–395, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang T, Yin C, Boyd DF, Quarato G, Ingram JP, Shubina M, et al. : Influenza virus Z-RNAs induce ZBP1-mediated necroptosis. Cell 180: 1115–1129.e1113, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yatim N, Jusforgues-Saklani H, Orozco S, Schulz O, Barreira da Silva R, Reis e Sousa C, et al. : RIPK1 and NF-kappaB signaling in dying cells determines cross-priming of CD8(+) T cells. Science 350: 328–334, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rickard JA, O’Donnell JA, Evans JM, Lalaoui N, Poh AR, Rogers T, et al. : RIPK1 regulates RIPK3-MLKL-driven systemic inflammation and emergency hematopoiesis. Cell 157: 1175–1188, 2014 [DOI] [PubMed] [Google Scholar]
- 99.Linkermann A, De Zen F, Weinberg J, Kunzendorf U, Krautwald S: Programmed necrosis in acute kidney injury. Nephrol Dial Transplant 27: 3412–3419, 2012 [DOI] [PubMed] [Google Scholar]
- 100.Linkermann A, Brasen JH, Darding M, Jin MK, Sanz AB, Heller JO, et al. : Two independent pathways of regulated necrosis mediate ischemia-reperfusion injury. Proc Natl Acad Sci USA 110: 12,024–12,029, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.von Mässenhausen A, Tonnus W, Himmerkus N, Parmentier S, Saleh D, Rodriguez D, et al. : Phenytoin inhibits necroptosis. Cell Death Dis 9: 359, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bonilla WV, Frohlich A, Senn K, Kallert S, Fernandez M, Johnson S, et al. : The alarmin interleukin-33 drives protective antiviral CD8(+) T cell responses. Science 335: 984–989, 2012 [DOI] [PubMed] [Google Scholar]
- 103.Lau A, Wang S, Jiang J, Haig A, Pavlosky A, Linkermann A, et al. : RIPK3-mediated necroptosis promotes donor kidney inflammatory injury and reduces allograft survival. Am J Transplant 13: 2805–2818, 2013 [DOI] [PubMed] [Google Scholar]
- 104.Yang B, Lan S, Dieude M, Sabo-Vatasescu JP, Karakeussian-Rimbaud A, Turgeon J, et al. : Caspase-3 is a pivotal regulator of microvascular rarefaction and renal fibrosis after ischemia-reperfusion injury. J Am Soc Nephrol 29: 1900–1916, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liu J, Krautzberger AM, Sui SH, Hofmann OM, Chen Y, Baetscher M, et al. : Cell-specific translational profiling in acute kidney injury. J Clin Invest 124: 1242–1254, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schreiber A, Rousselle A, Becker JU, von Mässenhausen A, Linkermann A, Kettritz R: Necroptosis controls NET generation and mediates complement activation, endothelial damage, and autoimmune vasculitis. Proceedings of the National Academy of Sciences, 2017 [DOI] [PMC free article] [PubMed]
- 107.Gong Y-N, Guy C, Olauson H, Becker JU, Yang M, Fitzgerald P, et al. : ESCRT-III acts downstream of MLKL to regulate necroptotic cell death and its consequences. Cell 169: 286–300.e216, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mulay SR, Linkermann A, Anders HJ: Necroinflammation in kidney disease. J Am Soc Nephrol 27: 27–39, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sarhan M, Land WG, Tonnus W, Hugo CP, Linkermann A: Origin and consequences of necroinflammation. Physiol Rev 98: 727–780, 2018 [DOI] [PubMed] [Google Scholar]
- 110.Tonnus W, Gembardt F, Latk M, Parmentier S, Hugo C, Bornstein SR, et al. : The clinical relevance of necroinflammation-highlighting the importance of acute kidney injury and the adrenal glands. Cell Death Differ 26: 68–82, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Giampazolias E, Schulz O, Lim KHJ, Rogers NC, Chakravarty P, Srinivasan N, et al. : Secreted gelsolin inhibits DNGR-1-dependent cross-presentation and cancer immunity. Cell 184: 4016–4031, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Srinivasan N, Gordon O, Ahrens S, Franz A, Deddouche S, Chakravarty P, et al. : Actin is an evolutionarily-conserved damage-associated molecular pattern that signals tissue injury in Drosophila melanogaster. eLife 5: e19662, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hanč P, Fujii T, Iborra S, Yamada Y, Huotari J, Schulz O, et al. : Structure of the complex of F-Actin and DNGR-1, a C-type lectin receptor involved in dendritic cell cross-presentation of dead cell-associated antigens. Immunity 42: 839–849, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sancho D, Joffre OP, Keller AM, Rogers NC, Martínez D, Hernanz-Falcón P, et al. : Identification of a dendritic cell receptor that couples sensing of necrosis to immunity. Nature 458: 899–903, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kuppe C, Ibrahim MM, Kranz J, Zhang X, Ziegler S, Perales-Patón J, et al. : Decoding myofibroblast origins in human kidney fibrosis. Nature 589: 281–286, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tang D, Kepp O, Kroemer G: Ferroptosis becomes immunogenic: Implications for anticancer treatments. OncoImmunology 10: 1,862,949, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li JY, Yao YM, Tian YP: Ferroptosis: A trigger of proinflammatory state progression to immunogenicity in necroinflammatory disease. Front Immunol 12: 701,163, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Turubanova VD, Mishchenko TA, Balalaeva IV, Efimova I, Peskova NN, Klapshina LG, et al. : Novel porphyrazine-based photodynamic anti-cancer therapy induces immunogenic cell death. Sci Rep 11: 7205, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Baumann T, Dunkel A, Schmid C, Schmitt S, Hiltensperger M, Lohr K, et al. : Regulatory myeloid cells paralyze T cells through cell-cell transfer of the metabolite methylglyoxal. Nat Immunol 21: 555–566, 2020 [DOI] [PubMed] [Google Scholar]
- 120.Martin-Sanchez D, Ruiz-Andres O, Poveda J, Carrasco S, Cannata-Ortiz P, Sanchez-Nino MD, et al. : Ferroptosis, but not necroptosis, is important in nephrotoxic folic acid-induced AKI. J Am Soc Nephrol 28: 218–229, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liu W, Chen B, Wang Y, Meng C, Huang H, Huang X-R, et al. : RGMb protects against acute kidney injury by inhibiting tubular cell necroptosis via an MLKL-dependent mechanism. Proc Natl Acad Sci USA 115: E1475–E1484, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sogabe K, Roeser NF, Venkatachalam MA, Weinberg JM: Differential cytoprotection by glycine against oxidant damage to proximal tubule cells. Kidney Int 50: 845–854, 1996 [DOI] [PubMed] [Google Scholar]
- 123.Weinberg JM, Roeser NF, Davis JA, Venkatachalam MA: Glycine-protected, hypoxic, proximal tubules develop severely compromised energetic function. Kidney Int 52: 140–151, 1997 [DOI] [PubMed] [Google Scholar]
- 124.Venkatachalam MA, Weinberg JM: The tubule pathology of septic acute kidney injury: A neglected area of research comes of age. Kidney Int 81: 338–340, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kim SE, Zhang L, Ma K, Riegman M, Chen F, Ingold I, et al. : Ultrasmall nanoparticles induce ferroptosis in nutrient-deprived cancer cells and suppress tumour growth. Nat Nanotechnol 11: 977–985, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Riegman M, Sagie L, Galed C, Levin T, Steinberg N, Dixon SJ, et al. : Ferroptosis occurs through an osmotic mechanism and propagates independently of cell rupture. Nat Cell Biol 22: 1042–1048, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Riegman M, Bradbury MS, Overholtzer M: Population dynamics in cell death: Mechanisms of propagation. Trends Cancer 5: 558–568, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Katikaneni A, Jelcic M, Gerlach GF, Ma Y, Overholtzer M, Niethammer P: Lipid peroxidation regulates long-range wound detection through 5-lipoxygenase in zebrafish. Nat Cell Biol 22: 1049–1055, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Poyan Mehr A, Tran MT, Ralto KM, Leaf DE, Washco V, Messmer J, et al. : De novo NAD(+) biosynthetic impairment in acute kidney injury in humans. Nat Med 24: 1351–1359, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ising C, Koehler S, Brahler S, Merkwirth C, Hohne M, Baris OR, et al. : Inhibition of insulin/IGF-1 receptor signaling protects from mitochondria-mediated kidney failure. EMBO Mol Med 7: 275–287, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Strilic B, Yang L, Albarran-Juarez J, Wachsmuth L, Han K, Muller UC, et al. : Tumour-cell-induced endothelial cell necroptosis via death receptor 6 promotes metastasis. Nature 536: 215–218, 2016 [DOI] [PubMed] [Google Scholar]
- 132.Ide S, Kobayashi Y, Ide K, Strausser SA, Abe K, Herbek S, et al. : Ferroptotic stress promotes the accumulation of pro-inflammatory proximal tubular cells in maladaptive renal repair. eLife 10: e68603, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kirita Y, Wu H, Uchimura K, Wilson PC, Humphreys BD: Cell profiling of mouse acute kidney injury reveals conserved cellular responses to injury. Proc Natl Acad Sci USA 117: 15,874–15,883, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kriz W, LeHir M: Pathways to nephron loss starting from glomerular diseases-insights from animal models. Kidney Int 67: 404–419, 2005 [DOI] [PubMed] [Google Scholar]
- 135.Kellum JA, Romagnani P, Ashuntantang G, Ronco C, Zarbock A, Anders HJ: Acute kidney injury. Nat Rev Dis Primers 7: 52, 2021 [DOI] [PubMed] [Google Scholar]
- 136.Linkermann A, Hackl MJ, Kunzendorf U, Walczak H, Krautwald S, Jevnikar AM: Necroptosis in immunity and ischemia-reperfusion injury. Am J Transplant 13: 2797–2804, 2013 [DOI] [PubMed] [Google Scholar]
- 137.Hughson MD, Hoy WE, Bertram JF: Progressive nephron loss in aging kidneys: Clinical-structural associations investigated by two anatomical methods. Anatomical Record 303: 2526–2536, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kanzaki G, Tsuboi N, Shimizu A, Yokoo T: Human nephron number, hypertension, and renal pathology. Anatomical Record 303: 2537–2543, 2020 [DOI] [PubMed] [Google Scholar]
- 139.Whaley-Connell AT, Sowers JR, Stevens LA, McFarlane SI, Shlipak MG, Norris KC, et al. : CKD in the United States: Kidney Early Evaluation Program (KEEP) and National Health and Nutrition Examination Survey (NHANES) 1999-2004. Am J Kidney Dis 51: S13–S20, 2008 [DOI] [PubMed] [Google Scholar]
- 140.Claus M, Herro R, Wolf D, Buscher K, Rudloff S, Huynh-Do U, et al. : The TWEAK/Fn14 pathway is required for calcineurin inhibitor toxicity of the kidneys. Am J Transplant 18: 1636–1645, 2018 [DOI] [PubMed] [Google Scholar]
- 141.Guo H, Kaiser WJ, Mocarski ES: Manipulation of apoptosis and necroptosis signaling by herpesviruses. Med Microbiol Immunol (Berl) 204: 439–448, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Omoto S, Guo H, Talekar GR, Roback L, Kaiser WJ, Mocarski ES: Suppression of RIP3-dependent necroptosis by human cytomegalovirus. J Biol Chem 290: 11,635–11,648, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Drachenberg CB, Papadimitriou JC, Wali R, Cubitt CL, Ramos E: BK polyoma virus allograft nephropathy: Ultrastructural features from viral cell entry to lysis. Am J Transplant 3: 1383–1392, 2003 [DOI] [PubMed] [Google Scholar]
- 144.Nankivell BJ, Renthawa J, Sharma RN, Kable K, O’Connell PJ, Chapman JR: BK virus nephropathy: Histological evolution by sequential pathology. Am J Transplant 17: 2065–2077, 2017 [DOI] [PubMed] [Google Scholar]
- 145.Nickeleit V, Hirsch HH, Zeiler M, Gudat F, Prince O, Thiel G, et al. : BK-virus nephropathy in renal transplants-tubular necrosis, MHC-class II expression and rejection in a puzzling game. Nephrol Dial Transplant 15: 324–332, 2000 [DOI] [PubMed] [Google Scholar]
- 146.Devisscher L, Van Coillie S, Hofmans S, Van Rompaey D, Goossens K, Meul E, et al. : Discovery of novel, drug-like ferroptosis inhibitors with in vivo efficacy. J Med Chem 61: 10,126–10,140, 2018 [DOI] [PubMed] [Google Scholar]
- 147.Zilka O, Shah R, Li B, Friedmann Angeli JP, Griesser M, Conrad M, et al. : On the Mechanism of cytoprotection by ferrostatin-1 and liproxstatin-1 and the role of lipid peroxidation in ferroptotic cell death. ACS Cent Sci 3: 232–243, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zeitler L, Fiore A, Meyer C, Russier M, Zanella G, Suppmann S, et al. : Anti-ferroptotic mechanism of IL4i1-mediated amino acid metabolism. eLife 10: e64806, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Fauster A, Rebsamen M, Huber KV, Bigenzahn JW, Stukalov A, Lardeau CH, et al. : A cellular screen identifies ponatinib and pazopanib as inhibitors of necroptosis. Cell Death Dis 6: e1767, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Najjar M, Suebsuwong C, Ray SS, Thapa RJ, Maki JL, Nogusa S, et al. : Structure guided design of potent and selective ponatinib-based hybrid Inhibitors for RIPK1. Cell Rep 10: 1850–1860, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Degterev A, Linkermann A: Generation of small molecules to interfere with regulated necrosis. Cell Mol Life Sci 73: 2251–2267, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, et al. : Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol 1: 112–119, 2005 [DOI] [PubMed] [Google Scholar]
- 153.Degterev A, Hitomi J, Germscheid M, Ch’en IL, Korkina O, Teng X, et al. : Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol 4: 313–321, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kaiser WJ, Upton JW, Long AB, Livingston-Rosanoff D, Daley-Bauer LP, Hakem R, et al. : RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature 471: 368–372, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Oberst A, Dillon CP, Weinlich R, McCormick LL, Fitzgerald P, Pop C, et al. : Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature 471: 363–367, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Orning P, Weng D, Starheim K, Ratner D, Best Z, Lee B, et al. : Pathogen blockade of TAK1 triggers caspase-8–dependent cleavage of gasdermin D and cell death. Science 362: 1064–1069, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sarhan J, Liu BC, Muendlein HI, Li P, Nilson R, Tang AY, et al. : Caspase-8 induces cleavage of gasdermin D to elicit pyroptosis during Yersinia infection. Proceedings of the National Academy of Sciences, 2018 [DOI] [PMC free article] [PubMed]
- 158.Demarco B, Grayczyk JP, Bjanes E, Le Roy D, Tonnus W, Assenmacher CA, et al. : Caspase-8-dependent gasdermin D cleavage promotes antimicrobial defense but confers susceptibility to TNF-induced lethality. Sci Adv 6: eabc3465, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Daley-Bauer LP, Roback L, Crosby LN, McCormick AL, Feng Y, Kaiser WJ, et al. : Mouse cytomegalovirus M36 and M45 death suppressors cooperate to prevent inflammation resulting from antiviral programmed cell death pathways. Proceedings of the National Academy of Sciences of the United States of America, 2017 [DOI] [PMC free article] [PubMed]
- 160.Lane RK, Guo H, Fisher AD, Diep J, Lai Z, Chen Y, et al. : Necroptosis-based CRISPR knockout screen reveals Neuropilin-1 as a critical host factor for early stages of murine cytomegalovirus infection. Proceedings of the National Academy of Sciences: 201921315, 2020 [DOI] [PMC free article] [PubMed]