Significance Statement
Findings of the international prospective multicenter PEXIVAS trial challenge the role of PLEX in AAV. We conducted a retrospective study of 425 patients: 188 with AAV and renal failure treated with PLEX and 237 not treated. A score to identify patients who would benefit from PLEX was developed. With kidney biopsy data, scores more than seven achieved sensitivity and specificity of 83.1% and 96.0%, respectively, for recommending PLEX. The average effect of PLEX for those with recommended treatment corresponded to an absolute risk reduction for death or KRT at M12 of 24.6%. Patients in the PLEX-recommended group had microscopic polyangiitis, MPO-ANCA, higher serum creatinine, crescentic and sclerotic classes, and higher Brix score. These findings, which require independent validation, could provide guidance in selecting patients with AAV who will benefit from PLEX.
Keywords: plasma exchange, anti-neutrophil cytoplasmic antibody-associated vasculitis, kidney biopsy, acute kidney injury
Abstract
Background
Data from the PEXIVAS trial challenged the role of plasma exchange (PLEX) in ANCA-associated vasculitides (AAV). We aimed to describe kidney biopsy from patients with AAV treated with PLEX, evaluate whether histopathologic findings could predict kidney function, and identify which patients would most benefit from PLEX.
Methods
We performed a multicenter, retrospective study on 188 patients with AAV and AKI treated with PLEX and 237 not treated with PLEX. The primary outcome was mortality or KRT at 12 months (M12).
Results
No significant benefit of PLEX for the primary outcome was found. To identify patients benefitting from PLEX, we developed a model predicting the average treatment effect of PLEX for an individual depending on covariables. Using the prediction model, 223 patients had a better predicted outcome with PLEX than without PLEX, and 177 of them had >5% increased predicted probability with PLEX compared with without PLEX of being alive and free from KRT at M12, which defined the PLEX-recommended group. Risk difference for death or KRT at M12 was significantly lower with PLEX in the PLEX-recommended group (−15.9%; 95% CI, −29.4 to −2.5) compared with the PLEX not recommended group (−4.8%; 95% CI, 14.9 to 5.3). Microscopic polyangiitis, MPO-ANCA, higher serum creatinine, crescentic and sclerotic classes, and higher Brix score were more frequent in the PLEX-recommended group. An easy to use score identified patients who would benefit from PLEX. The average treatment effect of PLEX for those with recommended treatment corresponded to an absolute risk reduction for death or KRT at M12 of 24.6%.
Conclusions
PLEX was not associated with a better primary outcome in the whole study population, but we identified a subset of patients who could benefit from PLEX. However, these findings must be validated before utilized in clinical decision making.
Rapidly progressive GN (RPGN) is characterized by a rapid loss of kidney function, affecting both renal and overall patient survival.1 ANCA-associated vasculitides (AAV) represents one of the most frequent causes of RPGN. Up to 70% of AAV presenting with proteinase 3 (PR3)–ANCA antibodies displays kidney involvement with extensive glomerular crescent formation. Kidney disease is more frequent in myeloperoxidase (MPO)-ANCA and is found in over 80%.2,3 The major prognostic issue of RPGN is the risk for developing kidney failure (KF) or dying. Progression to KF or death can be prevented by initiating an early combination of glucocorticoids (GCs) and immunosuppressive agents, including cyclophosphamide and more recently, rituximab. In addition to GCs and immunosuppressive agents, therapeutic plasma exchanges (PLEXs) had been largely used for the treatment of severe vasculitides flares (i.e., severe kidney involvement and/or alveolar hemorrhage). More than a decade ago, the MEPEX study advocated this strategy, which was found to reduce KF by 24% at 1 year in patients receiving PLEX in comparison with those treated with intravenous pulses of methylprednisolone.4 After a median follow-up of 4 years, the long-term benefit of PLEX on a composite outcome of death or KF was not significant.5 However, the long-term follow-up study was a retrospective assessment, and half of the patients died from various causes, including infections related to the immunosuppressive regimen, explaining why it is complicated to draw any formal conclusion on the long-term prognosis of PLEX in this study.
The large, multicenter, prospective PEXIVAS trial was conducted to provide additional data in severe AAV with either alveolar hemorrhage and/or kidney disease with an eGFR<50 ml/min per 1.73 m2.5,6 This study showed that PLEX did not reduce the risk of KF or death in patients with severe AAV. However, it did not evaluate the prognostic effect of kidney histology on the kidney function and response to PLEX. More importantly, an ancillary study from the MEPEX trial showed a poor correlation between baseline kidney function and kidney function at 12 months (M12), suggesting strong heterogeneity of baseline histologic lesions in patients presenting with serum creatinine >500 µmol/L.7 Therefore, we hypothesized that the efficacy of remission-induction treatments, especially PLEX, could be affected by histopathologic findings on kidney biopsy.
In this study, we aimed to describe the effect of kidney biopsies on the treatment of patients with AAV by PLEX to understand whether histopathologic findings could predict kidney function and possibly identify patients who could most benefit from PLEX.
Methods
Eligibility Criteria
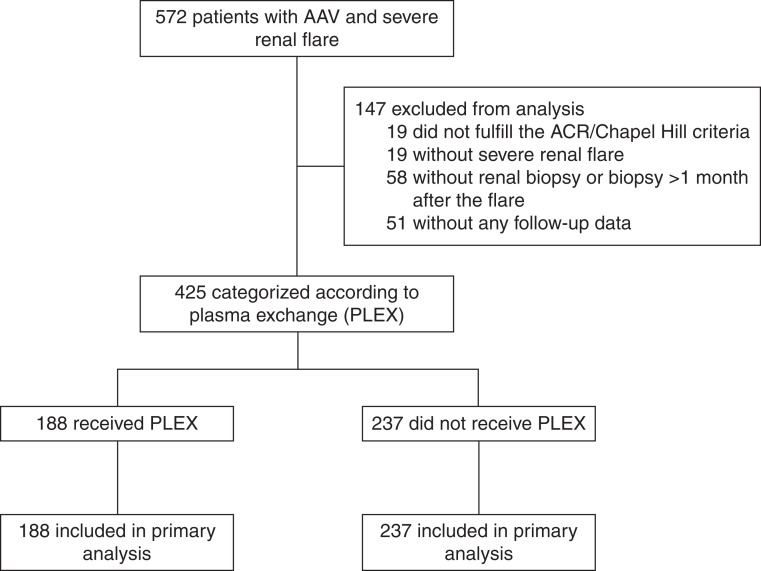
Study participants were included retrospectively and anonymously from 31 French internal medicine and nephrology departments. Patients presenting with microscopic polyangiitis, granulomatosis with polyangiitis, or renal-limited vasculitis, fulfilling American College of Rheumatology criteria8 or Chapel Hill consensus conference definitions,9 and diagnosed between May 1, 2004 and November 30, 2019 who underwent a kidney biopsy at presentation were included in the study (Figure 1). We included both patients receiving PLEX (PLEX group) and those who did not (control group). Patients on whom kidney biopsy was performed >1 month from the initiation of a remission-induction therapy were excluded. This study was conducted in compliance with the Good Clinical Practice protocol and the Declaration of Helsinki principles, and it was approved by the local ethics committee institutional review board from Cochin Hospital, Paris (Cochin-Port Royal Hospital, Paris, France; no. AAA-2020–08037).
Figure 1.
Study flow diagram. Treatment effect of PLEX according to simplified score thresholds. ACR, American College of Rheumatology.
Clinical Data
For each individual, data included age, sex, clinical manifestations, ANCA status, eGFR at baseline and during follow-up, and concomitant medications with GCs and immunosuppressive therapy. Data were collected using a standardized patient report form.
Histopathologic Classifications
Two pathology-based classifications that have previously shown a strong correlation with kidney survival were evaluated from kidney biopsy reports: the Berden classification distinguishing four categories (focal, crescentic, mixed, and sclerotic) according to the percentage of different glomerular lesions10 and the Brix score (ANCA renal risk score) distinguishing three risk groups (low, medium, or high) using the percentage of normal glomeruli and tubular atrophy/interstitial fibrosis together with baseline eGFR.11
Objectives and Outcomes
The primary outcome was mortality or KRT at M12. Secondary outcomes were the number of patients with eGFR >30 ml/min per 1.73 m2, the absolute value of changes in eGFR, and the number of patients with changes in eGFR >15 ml/min per 1.73 m2, all evaluated at M12. eGFR was estimated according to the Modification of Diet in Renal Disease.12 When dialysis was required, eGFR was considered to be 0 ml/min per 1.73 m2.
Statistical Analyses
We aimed to assess the effect of PLEX on the previously defined outcomes for the whole population (average treatment effect [ATE]). Treatment effect was expressed in terms of causal risk ratio (binary outcomes) or causal mean difference adjusted for the baseline value (changes in eGFR). We also aimed to investigate whether PLEX may benefit a subgroup of patients.
Data are described as mean (SD) or median (interquartile range [IQR]) for continuous variables and frequency (percentage) for categorical variables. The imbalance between groups at baseline was assessed by the standardized difference expressed as absolute percentage.13,14 To adjust for potential confounders, we used propensity score–based methods,15 and prognostic modeling was also used to investigate for treatment effect and additional correction for confounding. The propensity score model was estimated using logistic regression, with the treatment group as the independent variable and the aforementioned variables as covariates. For continuous variables, fractional polynomials were used to handle potentially nonlinear effects of variables.16 The primary analysis relied on the estimation of the treatment effect by using inverse probability of treatment weighting (IPTW).17,18 Stabilized IPTW weights were computed in the usual manner. Balance after weighting was assessed variable per variable and also for stabilized weights.19 Standardized mean differences of <10% are commonly considered as acceptable balance after matching or weighting.13,19 In addition, a c statistic of −0.5 was used to describe the improvement in overall balance after weighting.14 As a sensitivity analysis, we used a second version of the propensity score, and we tried to compensate for model misspecification by using a double-robust estimator of the ATE, which implies using both a model for the treatment (propensity score) and the outcome (prognostic score) and which provides a consistent estimate of the ATE.17,20 The positivity assumption, which is crucial for propensity score–based methods to be valid, was assessed by plotting the distribution of serum creatinine, eGFR, and the propensity score in both groups (Supplemental Figure 1).
Confounders were predefined and included patient characteristics (age and sex), vasculitis characteristics (AAV phenotype; i.e., granulomatosis with polyangiitis, microscopic polyangiitis, or renal-limited vasculitis; ANCA status; initial flare versus relapsing disease), serum creatinine, eGFR, histopathologic findings (Berden classification and Brix score), and remission-induction treatments (cyclophosphamide and KRT). Other treatments were too rare or too frequent to be used in the propensity score model. In a sensitivity analysis, we primarily focused on baseline variables that were also found to be prognostic because a confounder has to be associated with both treatment and outcome. Therefore, variables used were age, sex, PR3-ANCA status, serum creatinine, Berden classification, Brix score, and KRT at baseline.
Logistic regression was used to derive a multivariable prediction model for the primary outcome. Owing to the number of events, the covariates were limited to treatment group, age, sex, limited RPGN, PR3- and MPO-ANCA status, serum creatinine, Berden classification, Brix score, and cyclophosphamide at baseline. An alternative model used KRT at baseline instead of serum creatinine. eGFR was not used to limit multicollinearity. No variable selection was performed. In addition, receiver operating characteristics curves for serum creatinine and Brix score were calculated in each treatment group, and the areas under the curves were estimated.
Missing data were handled through multiple imputations by chained equations.21,22 Because approximately 10% of patients had missing potential confounders, ten independent imputed datasets were generated and analyzed separately.23 Variables used for multiple imputation were all of the potential confounders mentioned above as well as the outcomes.
Finally, to identify individuals possibly benefitting from PLEX, we used an augmented weighted approach to estimate the benefit of PLEX conditional on patients’ characteristics using both the propensity score and a prognostic model (for efficiency “augmentation” as with the double-robust estimator).24 We assumed that PLEX would be recommended as soon as the predicted probability of being alive and free from dialysis at month 12 was higher with PLEX than without and used bootstrapping with 500 replicates to correct the estimates of probability of outcome and differences within the selected subgroups.
All tests were two sided, and P values of 0.05 or less were regarded as indicating statistical significance. Analyses were performed using the R statistical software version 3.6.1.25
Results
Patients’ Characteristics
A total of 425 patients were included in the study: 188 patients receiving PLEX and 237 controls who did not (Supplemental Table 1). The average number of PLEXs in the PLEX group was 6.9±2.2. Groups were strongly imbalanced at baseline, but IPTW efficiently reduced the imbalance in baseline covariates (Supplemental Table 1). The propensity score was also well balanced after weighting (Supplemental Figure 1).
Histopathologic classifications of AAV-related RPGN were obtained for 420 of 425 patients (detailed analysis of kidney biopsy was not available infive5 patients) (Tables 1 and 2). The median number of glomeruli on each biopsy was 16 (IQR, 11–21) in the PLEX group and 16 (IQR, 12–23) in the control group. Crescentic class was more frequent in the PLEX group (39% versus 19% in the control group), whereas sclerotic and mixed classes were more frequent in the control group (33% and 27% in the control group versus 21% and 21% in the PLEX group, respectively). Median Brix score was higher in the PLEX group: seven (IQR, 3–9) versus five (IQR, 0–9) in the control group.
Table 1.
Histopathologic findings of patients treated with or without PLEX
| Variable | Number | PLEX, n=187 | Number | No PLEX, n=233 |
|---|---|---|---|---|
| No. of glomeruli, median (IQR) | 186 | 16 (11–21) | 232 | 16 (12–23) |
| Normal glomeruli, mean % (±SD) | 176 | 24.5 (±0.5) | 212 | 29.0 (±6.2) |
| Brix score, median (IQR) | 186 | 7 (3–9) | 232 | 5 (1.5–9) |
| No. of normal glomeruli, median (IQR) | 187 | 4 (0–6) | 233 | 4 (0–4) |
| Tubular atrophy/interstitial fibrosis | 182 | 233 | ||
| 0a | 107 (59) | 112 (48) | ||
| 0 or 2a | 0 (0) | 9 (4) | ||
| 2a | 75 (41) | 112 (48) | ||
| eGFR | 187 | 233 | ||
| 0b | 33 (18) | 123 (53) | ||
| 3b | 154 (82) | 110 (47) |
Only the 420 patients with available kidney biopsy data are described. Data are numbers (percentages) of patients unless otherwise stated.
0, 25% or less tubular atrophy/interstitial fibrosis; 2, >25% tubular atrophy/interstitial fibrosis. Nine patients in the non-PLEX group were classified as having zero or two because the renal pathologist could not determine whether tubular atrophy/interstitial fibrosis was ≤25% or >25%.
0, >15 ml/min per 1.73 m2; 3, ≤15 ml/min per 1.73 m2.
Table 2.
Average effect of PLEX on kidney outcomes
| Outcome | PLEX | No PLEX | Relative Risk (95% Confidence Interval) or Adjusted Mean Difference (95% Confidence Interval) |
|---|---|---|---|
| Death or dialysis at month 12 | |||
| Unweighted sample analysis | 28.0% | 18.7% | 1.50 (1.05 to 2.14) |
| IPTW analysis | 21.5% | 27.8% | 0.77 (0.50 to 1.19) |
| IPTW analysis, reduced PS model | 21.6% | 27.0% | 0.80 (0.53 to 1.21) |
| Double-robust analysis | 20.1% | 26.2% | 0.77 (0.47 to 1.25) |
| eGFR ≥30 ml/min per 1.73 m2 at month 12 | |||
| Unweighted sample analysis | 46.3% | 54.4% | 0.85 (0.70 to 1.03) |
| IPTW analysis | 49.7% | 48.0% | 1.04 (0.80 to 1.34) |
| Changes in eGFR at month 12, ml/min | |||
| Unweighted sample analysis | 19.1 (22.4) | 12.5 (18.1) | 5.7 (1.6 to 9.9) |
| IPTW analysis | 15.7 (20.5) | 12.5 (18.5) | 3.1 (−6.9 to 13.2) |
| Changes in eGFR >15 ml/min per 1.73 m2 at month 12 | |||
| Unweighted sample analysis | 52.8% | 39.2% | 1.35 (1.09 to 1.67) |
| IPTW analysis | 48.6% | 37.9% | 1.28 (0.97 to 1.70) |
All results are pooled estimations over the imputed datasets. PS, propensity score; RR, relative risk.
Kidney Outcomes
Before any statistical adjustment, the rate of patients alive and free from KRT at M12 was lower in the PLEX group than in the control group (72% versus 82%, respectively). The percentage of patients with eGFR≥30 ml/min per 1.73 m2 at M12 was also lower in the PLEX group than in the control group (46% versus 55%, respectively). However, eGFR gain was higher in the PLEX group compared with controls as well as the percentage of patients with ΔeGFR>15 ml/min per 1.73 m2 at M12 (53% versus 39%, respectively) (Supplemental Table 2).
Prediction of Kidney Outcome at 12 Months
To avoid collinearity issues, two models were fitted to predict kidney outcome at M12, one with KRT need at baseline and one with serum creatinine. Results showed that each model was strongly prognostic, especially when including the Brix score (Supplemental Table 3). PLEXs were not significatively associated with the primary outcome (death or dialysis) at month 12 both in the models using serum creatinine (odds ratio, 0.96; 95% confidence interval [95% CI], 0.52 to 1.79) and in the models using KRT (odds ratio, 0.76; 95% CI, 0.39 to 1.51).
Estimations of the Average Effect of PLEX
Unweighted analysis before any statistical adjustment revealed that PLEX was associated with a worse outcome for death or KRT at M12 (relative risk, 1.50; 95% CI, 1.05 to 2.14), whereas changes in eGFR >15 ml/min per 1.73 m2 at M12 were significatively superior in PLEX-treated patients (adjusted mean difference, 1.35; 95% CI, 1.09 to 1.67). After statistical adjustment, PLEX tended to reduce mortality or KRT at M12, but it did not achieve significant difference (Table 2). In the double-robust analysis, mortality or KRT at M12 was 20.1% in the PLEX group versus 26.2% in the control group (relative risk, 0.77; 95% CI, 0.47 to 1.25).
Identification of Patients Benefitting from PLEX
To identify patients benefitting from PLEX, we developed a model predicting the ATE of PLEX for an individual depending on covariables. Using this prediction model, 223 patients had a better predicted outcome with PLEX than without PLEX, and 177 of them (42% of the whole population) had a >5% increased predicted probability with PLEX compared with without PLEX of being alive and free from KRT at M12, the latter defining the PLEX-recommended group (Supplemental Table 4). Risk difference for death or KRT at M12 was significatively lower with PLEX in the PLEX-recommended group (−15.9%; 95% CI, −29.4 to −2.5) compared with the PLEX not recommended group (−4.8%; 95% CI, −14.9 to 5.3).
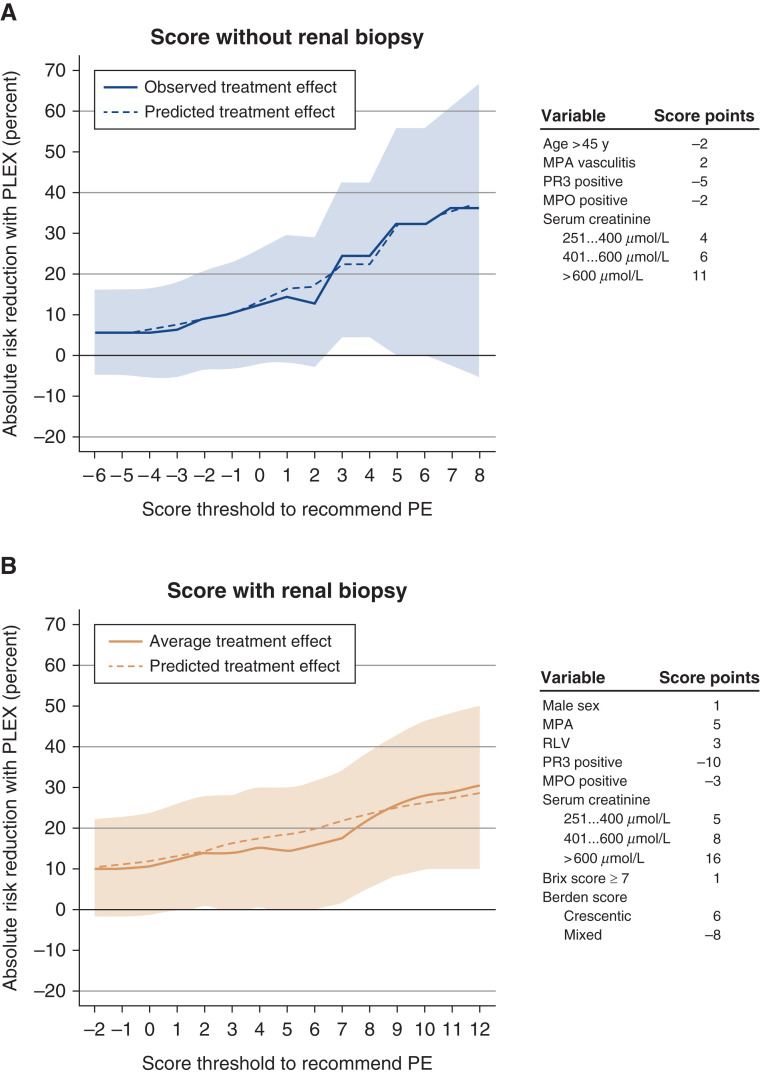
Some variables were significatively different between patients from the PLEX-recommended and PLEX not recommended groups (Table 3). Microscopic polyangiitis and positive MPO-ANCA were more frequent in the PLEX-recommended group, as well as higher serum creatinine, treatment with cyclophosphamide, need for KRT, and ventilation support. Berden classification showed more crescentic and sclerotic classes, and Brix score was significatively higher in the PLEX-recommended group (nine versus four in the PLEX not recommended group) (Supplemental Figures 2 and 3). On the basis of these findings, we established an easy to use score to identify patients who could benefit from PLEX (Figure 2). The treatment effects of different score thresholds are presented in Figure 2. Using the score without kidney biopsy data, recommending PLEX for scores more than two achieved sensitivity and specificity of 71.2% and 90.7%, respectively. Using the score with kidney biopsy findings, recommending PLEX for scores more than seven achieved sensitivity and specificity of 83.1% and 96.0%, respectively. The ATE of PLEX for those with recommended treatment reflected a reduction in the absolute risk for death or KRT dependency at M12 by 24.6%. The use of raw percentage of normal glomeruli instead of Brix score categories provided identical results (data not shown).
Table 3.
Baseline characteristics between patients in the PLEX-recommended group and the PLEX not recommended group
| Variables | PLEX Not Recommended, n=248 | PLEX Recommended, n=177 | P Value |
|---|---|---|---|
| Age, mean (SD), yr | 63.1 (13.1) | 63.6 (14.4) | 0.76 |
| Women | 112 (45) | 80 (45) | >0.99 |
| Vasculitis type | <0.001 | ||
| GPA | 116 (47) | 44 (25) | |
| MPA | 87 (35) | 107 (60) | |
| RLV | 45 (18) | 26 (15) | |
| ANCA positive | 240 (97) | 165 (93) | 0.06 |
| PR3 | 104 (42) | 34 (19) | <0.001 |
| MPO | 136 (55) | 130 (73) | <0.001 |
| Initial flare | 234 (94) | 169 (95) | 0.66 |
| Serum creatinine, median (IQR), µmol/L | 282 (187–416) | 550 (409–748) | <0.001 |
| eGFR, median (IQR), ml/min per 1.73 m2 | 18 (12–28) | 7 (3–11) | <0.001 |
| Medications | |||
| Cyclophosphamide | 172 (70) | 150 (85) | <0.001 |
| Rituximab | 70 (28) | 26 (15) | 0.001 |
| KRT | 13 (5) | 104 (59) | <0.001 |
| Ventilation support | 4 (2) | 13 (7) | 0.005 |
| Berden classification | <0.001 | ||
| Focal | 64 (26) | 21 (12) | |
| Crescentic | 33 (14) | 85 (48) | |
| Mixed | 102 (42) | 13 (7) | |
| Sclerotic | 45 (18) | 57 (32) | |
| Brix score, median (IQR) | 4 (0–7) | 9 (5–9) | <0.001 |
| Treatment received | <0.001 | ||
| PLEX | 79 (32) | 109 (62) | |
| No PLEX | 169 (68) | 68 (38) | |
Unless otherwise stated, data are presented as numbers (percentages) of patients. Because of rounding, percentages may not sum up to 100. GPA, granulomatosis with polyangiitis; MPA, microscopic polyangiitis; RLV, renal-limited vasculitis.
Figure 2.
Treatment effect of PLEX according to simplified score thresholds. In (A), the scores without results of kidney biopsy are used (recommended threshold at more than two). In (B), the scores with Brix and Berden classifications are used (recommended threshold at more than seven). The score points associated with variables in each scoring system are given on the right part of each panel. MPA, microscopic polyangiitis; PE, plasma exchanges; RLV, renal-limited vasculitis.
Discussion
RPGN is a common manifestation of AAV, and it is associated with increased morbidity and mortality. Kidney biopsy is frequently performed in the setting of AAV to confirm the diagnosis of pauci-immune crescentic GN. Even if an increasing number of nephrologists do not perform kidney biopsy when the diagnosis is established, it also provides reliable prognostic information to predict kidney outcome, as supported by the Berden classification and the Brix score. The Berden classification provides information on the glomerular lesions, whereas the Brix score integrates the number of normal glomeruli, tubular atrophy/interstitial fibrosis, and eGFR at baseline.
The rationale for using PLEX in AAV is strong, especially because it has been demonstrated that ANCA antibodies are pathogenic in animal models.26 It is thus hypothesized that removing antibodies and other proinflammatory molecules through early initiated PLEX could improve patient outcome in the period during which concomitant immunosuppressive agents are to be effective. For decades, PLEXs were largely performed on the basis of the severity of kidney involvement. Recently, the PEXIVAS study has challenged the efficacy of PLEX because PLEX was not shown to reduce the incidence of death or KF in patients with AAV. Death or KF occurred in 28.4% in the PLEX group and in 31.0% in the control group (hazard ratio with PLEX, 0.86; 95% CI, 0.65 to 1.13). When censoring the data at 1 year, PLEX tended to be associated with lower incidences of death or KF, although this was not statistically significant (hazard ratio with PLEX, 0.77; 95% CI, 0.56 to 1.06). Furthermore, there was no evidence of any benefit of PLEX in the subgroup analyses of the primary outcome, even though kidney biopsy was not featured. In an editorial of the PEXIVAS study, Derebail and Falk27 raised concerns that kidney biopsy had not been required for entry into the trial. Importantly, they reminded readers that patients with AAV frequently have a relapsing and remitting course and that diffuse tubulointerstitial and glomerular scarring can occur before initial diagnosis. Without baseline biopsy data, the proportion of patients who have kidney dysfunction caused by either active inflammation or chronic sclerosis is unknown, information that is particularly important to predict kidney function improvement during the induction therapy. In other words, a subgroup of patients with aggressive kidney disease and minimal scarring may benefit from PLEX.27 In summary, its role is now highly controversial, and no one knows if PLEX still has a role to play in selected indications.
Data presented here support a kidney pathology–based strategy to better identify patients who could benefit the most from PLEX initiation. Indeed, because of various kidney presentations at baseline and because patients with active lesions probably need a more intense immunosuppressive therapy than those with chronic lesions, irrespective of serum creatinine, such an approach seems appropriate. In our study, the two groups were strongly imbalanced at baseline because patients with more severe kidney involvement were more likely to receive PLEX, and this led us to use a propensity score to balance out the two groups. Our results show that PLEXs were not associated with a better outcome (alive and free from KRT at M12) when looking at the entire population. This is consistent with recent studies.6,28
To go further, we constructed two models that permitted the identification of 177 patients (42% of the whole cohort) who could benefit from PLEX, as defined by a gain of probability >5% to be alive and free from KRT at M12 compared with the probability in the absence of PLEX. This approach allowed us to establish a score to identify patients who would benefit the most from PLEX, with better sensitivity and specificity when including kidney pathology data. PLEXs were not associated with a better outcome in the sclerotic class, whereas in a study by Trivioli et al.,29 even if Berden classification was sclerotic in 43%, an eGFR improvement >25% was noted in 59% of treated patients.
In patients with recommended PLEX, the ATE of PLEX corresponded to an absolute risk reduction for death or KRT at M12 of 24.6%, which could represent an important benefit for patients. Importantly, only 62% of patients who were attributed to the PLEX-recommended group eventually received PLEX, and conversely, 32% of patients from the PLEX not recommended group eventually received PLEX. These findings illustrate that indications of PLEX need to be clarified. However, it is too early to use our score in clinical practice, but it would be very interesting to assess its value on other cohorts in order to select patients benefitting from PLEX.
Our study has some limitations. The study was retrospective; however, missing data were rare, and we used robust primary and secondary outcomes that remained reliable even in the absence of double-blind or prospective design. GC regimens were not analyzed, but patients were mainly treated on the basis of French guidelines from the French Vasculitis Study Group; we can assume that this does not differ between groups. Because renal biopsy was required in our study population, many patients were included from nephrology centers, which could have introduced a selection bias. Attributing an eGFR of 0 ml/min to patients requiring dialysis may overstate the gain of eGFR at M12 because of the residual kidney function (which is slightly superior to zero). However, we think that excluding them from the analysis because of the absence of this value would lead to an important loss of data that could introduce important bias. Also, a central evaluation of kidney biopsies would have been better to avoid interpretation bias, but Berden classification and Brix score were used retrospectively in order to standardize the expert nephropathologist’s interpretation. However, despite using validated classifications, the presence of acute tubular necrosis, vascular lesions (acute or chronic), or interstitial inflammation was not analyzed to assess their potential effect on treatment decision. Lastly, our score needs to be validated in forthcoming cohorts and preferentially, as part of a prospective cohort study. Finally, our study is focused on PLEX and does not address the role of future treatments. For instance, the C5a receptor inhibitor avacopan was shown to be noninferior to prednisone taper with respect to remission at week 26 in the ADVOCATE trial,30 and it will possibly affect the role of PLEX in AAV as well as the MPO inhibition strategy.31
Overall, in this large cohort of patients with AAV and kidney involvement, PLEXs were not associated with a better outcome for the whole population, as shown in the PEXIVAS trial. However, 42% of patients from this cohort could have benefitted from PLEX with a clinically relevant reduction of mortality or KRT, and such patients were efficiently identified using a score that integrates baseline characteristics and renal histopathologic findings. However, this scoring system needs to be validated in additional cohorts.
Disclosures
J. Aniort, J.-F. Augusto, P. L. Carron, C. De Moreuil, L. Guillevin, R. Mesbah, and X. Puechal participated in the PEXIVAS trial. V. Audard reports receiving honoraria from Addmedica, Alnylam, and Travere and scientific advisor or membership with Addmedica, Alnylam, and Travere. J.-F. Augusto reports honoraria from Sanofi and Therakos/Mallinckrot. S. Bally reports honoraria from Alexion Pharma France for an intervention as a speaker (about TMA and atypical HUS). C.-A. Durel reports receiving fees from Vifor Pharma and honoraria from the scientific board with Vifor Pharma France. K. El Karoui reports research funding from Amgen, Otsuka, and Sanofi and honoraria from Alexion and Otsuka. S. Faguer reports consultancy agreements with Abionyx Pharma and other interests/relationships with Vifor Pharma (symposium speaker). P. Gobert reports other interests/relationships with Société Nationale Française de Médecine Interne. S. Grange reports scientific advisor or membership with Alexion. L. Guillevin reports consultancy agreements with Behringer, Biogen, Certara, GSK, Lilly, Novartis, Novo Nordisk, Roche, Sanofi, Seattle Genetics, and UCB; research funding from Roche, which provided free rituximab for an academic study sponsored by the French Ministry of Health; honoraria for consultancies for Behringer, Biogen, Certara, GSK, Lilly, Novartis, Novo Nordisk, Roche, Sanofi, Seattle Genetics, and UCB; and other interests/relationships as a member of the board of the Haute Autorité de Santé and president of the Transparency Commission on drugs (duty finished in 2016). N. Jourde-Chiche reports research funding from Fresenius Medical Care for the CINEVAS study (NCT03635385) and honoraria from Vifor (speaking fee of 500 euros). A. Karras reports consultancy agreements with Alnylam, GSK, Novartis, and Vifor; honoraria from Abbvie, Amgen, AstraZeneca, Gilead, GSK, Pfizer, and Roche Pharmaceuticals; and scientific advisor or membership with Novartis. N. Martis reports ownership interest with Merck; honoraria from Sanofi Genzyme; and speakers bureau for Sanofi Genzyme. Z. Massy reports research funding from Amgen, Baxter, Fresenius Medical Care, Genzyme-Sanofi, GlaxoSmithKline, Lilly, Merck Sharp and Dohme-Chibret, and Otsuka; government support for the CKD REIN project and experimental projects; honoraria to charities or for travel from Baxter and Genzyme-Sanofi; and scientific advisor or membership with Journal of Nephrology, Journal of Renal Nutrition, Kidney International, Nephrology Dialysis Transplantation, and Toxins. X. Puechal reports research funding from ChemoCentryx as an investigator in studies evaluating CCX168 in ANCA-associated vasculitis, InflaRx as an investigator in studies evaluating IFX1 in ANCA-associated vasculitis, and Roche Pharma as an investigator in academic studies of ANCA-associated vasculitis for which rituximab was provided by Roche Pharma. M. Rabant reports scientific advisor or membership with Travere. C. Rafat received travel grants from Fresenius and honoraria from M3, Qualworld, and Sermo. B. Terrier received some consulting fees and/or grants from AstraZeneca, Bristol-Myers Squibb, GlaxoSmithKline, Grifols, LFB, Lilly, Roche/Chugaï, Terumo BCT, and Vifor Pharma outside of the submitted work. All remaining authors have nothing to disclose.
Funding
None.
Supplementary Material
Acknowledgments
We are indebted to the kidney pathologists for their contribution. In particular, we thank Drs. Bernadette Aymard (Metz), Nicole Pinel (Grenoble), Sophie Felix (Besançon), Anne Croue (Angers), Jean-Paul Saint-André (Angers), Jean-Paul Duong Van Huyen (Paris), Magalie Colombat (Suresnes), Jean-Louis Kemeny (Clermont-Ferrand), David Buob (Paris), Isabelle Brocheriou (Paris), Catherine Guettier (Le Kremlin Bicêtre), Sophie Ferlicot (Le Kremlin Bicêtre), Aurélie Sannier (Paris), Francine Walker (Paris), Laurent Doucet (Brest), Nathalie Rioux-Leclercq (Rennes), Damien Ambrosetti (Nice), Pascale Guerzider (Saint-Nazaire), Arnaud François (Rouen), Marion Féricot (Rouen), and Jean-Pierre Rivière (La Réunion).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Plasma Exchange in ANCA-Associated Vasculitis: For Whom (If Any)?,” on pages 465–466.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2021060771/-/DCSupplemental.
Supplemental Material. Methods.
Supplemental Figure 1. Assessment of the positivity assumption.
Supplemental Figure 2. Kidney biopsy of a patient with a predicted benefit of PLEX.
Supplemental Figure 3. Kidney biopsy of a patient without predicted benefit of PLEX.
Supplemental Table 1. Baseline characteristics of patients treated with or without PLEX.
Supplemental Table 2. Kidney function at 12 months according to PLEX or not before any statistical adjustment.
Supplemental Table 3. Baseline variables associated with death or dialysis at 12 months.
Supplemental Table 4. Treatment effect in subgroups with recommended and not recommended PLEX.
References
- 1.Berti A, Cornec-Le Gall E, Cornec D, Casal Moura M, Matteson EL, Crowson CS, et al. : Incidence, prevalence, mortality and chronic renal damage of anti-neutrophil cytoplasmic antibody-associated glomerulonephritis in a 20-year population-based cohort. Nephrol Dial Transplant 34: 1508–1517, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Millet A, Pederzoli-Ribeil M, Guillevin L, Witko-Sarsat V, Mouthon L: Antineutrophil cytoplasmic antibody-associated vasculitides: Is it time to split up the group? Ann Rheum Dis 72: 1273–1279, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Kronbichler A, Shin JI, Lee KH, Nakagomi D, Quintana LF, Busch M, et al. : Clinical associations of renal involvement in ANCA-associated vasculitis. Autoimmun Rev 19: 102495, 2020 [DOI] [PubMed] [Google Scholar]
- 4.Jayne DR, Gaskin G, Rasmussen N, Abramowicz D, Ferrario F, Guillevin L, et al. ; European Vasculitis Study Group : Randomized trial of plasma exchange or high-dosage methylprednisolone as adjunctive therapy for severe renal vasculitis. J Am Soc Nephrol 18: 2180–2188, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Walsh M, Casian A, Flossmann O, Westman K, Höglund P, Pusey C, et al. ; European Vasculitis Study Group (EUVAS) : Long-term follow-up of patients with severe ANCA-associated vasculitis comparing plasma exchange to intravenous methylprednisolone treatment is unclear. Kidney Int 84: 397–402, 2013 [DOI] [PubMed] [Google Scholar]
- 6.Walsh M, Merkel PA, Peh CA, Szpirt WM, Puéchal X, Fujimoto S, et al. ; PEXIVAS Investigators : Plasma exchange and glucocorticoids in severe ANCA-associated vasculitis. N Engl J Med 382: 622–631, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Lind van Wijngaarden RA, Hauer HA, Wolterbeek R, Jayne DR, Gaskin G, Rasmussen N, et al. : Clinical and histologic determinants of renal outcome in ANCA-associated vasculitis: A prospective analysis of 100 patients with severe renal involvement. J Am Soc Nephrol 17: 2264–2274, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Fries JF, Hunder GG, Bloch DA, Michel BA, Arend WP, Calabrese LH, et al. : The American College of Rheumatology 1990 criteria for the classification of vasculitis. Summary. Arthritis Rheum 33: 1135–1136, 1990 [DOI] [PubMed] [Google Scholar]
- 9.Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al. : 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum 65: 1–11, 2013 [DOI] [PubMed] [Google Scholar]
- 10.Berden AE, Ferrario F, Hagen EC, Jayne DR, Jennette JC, Joh K, et al. : Histopathologic classification of ANCA-associated glomerulonephritis. J Am Soc Nephrol 21: 1628–1636, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Brix SR, Noriega M, Tennstedt P, Vettorazzi E, Busch M, Nitschke M, et al. : Development and validation of a renal risk score in ANCA-associated glomerulonephritis. Kidney Int 94: 1177–1188, 2018 [DOI] [PubMed] [Google Scholar]
- 12.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. ; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. [published correction appears in Ann Intern Med 155: 408, 2011] Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Austin PC: Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 28: 3083–3107, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franklin JM, Rassen JA, Ackermann D, Bartels DB, Schneeweiss S: Metrics for covariate balance in cohort studies of causal effects. Stat Med 33: 1685–1699, 2014 [DOI] [PubMed] [Google Scholar]
- 15.Rosenbaum PR, Rubin DB: The central role of the propensity score in observational studies for causal effects. Biometrika 70: 41–55, 1983 [Google Scholar]
- 16.Royston P: A strategy for modelling the effect of a continuous covariate in medicine and epidemiology. Stat Med 19: 1831–1847, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Lunceford JK, Davidian M: Stratification and weighting via the propensity score in estimation of causal treatment effects: A comparative study. Stat Med 23: 2937–2960, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Austin PC, Stuart EA: Estimating the effect of treatment on binary outcomes using full matching on the propensity score. Stat Methods Med Res 26: 2505–2525, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Austin PC, Stuart EA: Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med 34: 3661–3679, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tchetgen EJ, Robins JM, Rotnitzky A: On doubly robust estimation in a semiparametric odds ratio model. Biometrika 97: 171–180, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rubin DB, Schenker N: Multiple imputation in health-care databases: An overview and some applications. Stat Med 10: 585–598, 1991 [DOI] [PubMed] [Google Scholar]
- 22.White IR, Royston P: Imputing missing covariate values for the Cox model. Stat Med 28: 1982–1998, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.White IR, Royston P, Wood AM: Multiple imputation using chained equations: Issues and guidance for practice. Stat Med 30: 377–399, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Chen S, Tian L, Cai T, Yu M: A general statistical framework for subgroup identification and comparative treatment scoring. Biometrics 73: 1199–1209, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.R Core Team : R: A Language and Environment for Statistical Computing, Vienna, Austria, R Foundation for Statistical Computing, 2013 [Google Scholar]
- 26.Xiao H, Heeringa P, Hu P, Liu Z, Zhao M, Aratani Y, et al. : Antineutrophil cytoplasmic autoantibodies specific for myeloperoxidase cause glomerulonephritis and vasculitis in mice. J Clin Invest 110: 955–963, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Derebail VK, Falk RJ: ANCA-associated vasculitis—refining therapy with plasma exchange and glucocorticoids. N Engl J Med 382: 671–673, 2020 [DOI] [PubMed] [Google Scholar]
- 28.Casal Moura M, Irazabal MV, Eirin A, Zand L, Sethi S, Borah BJ, et al. : Efficacy of rituximab and plasma exchange in antineutrophil cytoplasmic antibody-associated vasculitis with severe kidney disease. J Am Soc Nephrol 31: 2688–2704, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trivioli G, Gopaluni S, Urban ML, Gianfreda D, Cassia MA, Vercelloni PG, et al. : Slowly progressive anti-neutrophil cytoplasmic antibody-associated renal vasculitis: Clinico-pathological characterization and outcome. Clin Kidney J 14: 332–340, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jayne DRW, Bruchfeld AN, Harper L, Schaier M, Venning MC, Hamilton P, et al. ; CLEAR Study Group : Randomized trial of C5a receptor inhibitor avacopan in ANCA-associated vasculitis. J Am Soc Nephrol 28: 2756–2767, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Antonelou M, Michaëlsson E, Evans RDR, Wang CJ, Henderson SR, Walker LSK, et al. ; RAVE-ITN Investigators : Therapeutic myeloperoxidase inhibition attenuates neutrophil activation, ANCA-mediated endothelial damage, and crescentic GN. J Am Soc Nephrol 31: 350–364, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.