Abstract
The brain is an endocrine organ whose day-to-day function is tied to the rhythmic production of neuromodulatory hormones. Yet, traditional approaches to studying brain–hormone relationships in humans are often coarse in scope. By contrast, dense-sampling neuroimaging offers the unique ability to probe dynamic interactions between the nervous and endocrine systems. This review summarizes recent evidence of sex hormones’ influence on structural and functional properties of the human brain. In particular, findings from the ‘28andMe’ project suggest that estradiol modulates the topology of large-scale functional brain networks and progesterone rapidly shapes medial temporal lobe morphology across the menstrual cycle. This nascent body of work sets the stage for additional studies in larger cohorts. We end by discussing the potential of dense-sampling designs to further elucidate endocrine modulation of the brain, with implications for personalized medicine.
Keywords: deep imaging, sex hormones, personalized medicine, MRI
Since its inception, the field of neuroendocrinology has provided cross-species evidence for the tightly-coupled relationship between the nervous and endocrine systems [1,2]. Sex steroid hormones act as critical neuromodulators, influencing the brain from the level of microscopic intracellular and synaptic events [3,4] to macroscopic structural [5–7] and functional [8,9] brain networks. Two major sex steroid hormones, 17β-estradiol and progesterone, are critical components of cell survival and plasticity, exerting excitatory and inhibitory effects that are evident across multiple spatial and temporal scales [10].
Scientists have routinely pushed the bounds of experimental creativity to tease apart the inherently complex nature of brain–hormone interactions. Here, we address a new set of methodological innovations for probing estrogen and progesterone action in the human brain. A central feature of the mammalian endocrine system is that hormone secretion varies over time, and this rhythmicity is essential for sustaining many physiological processes. Traditional approaches to studying brain–hormone interactions rely largely on cross-sectional designs that, by nature, cannot capture fluctuations in sex hormone production. A growing trend in human neuroimaging is to flip the cross-sectional model, densely-sampling individuals over timescales of days, weeks, months, or years to provide greater insight into the dynamic properties of the human brain. Dense-sampling neuroimaging studies extend longitudinal designs by collecting multiple phenotypic measurements at a higher frequency or over a larger number of sessions (> 10 time points) in individuals [11,12].
In this review, we discuss how dense-sampling methods can be adopted within the field of human neuroendocrinology. A handful of recent human neuroimaging studies have begun leveraging dense-sampling techniques to uncover the dynamic endocrine modulation of the nervous system across novel timescales, including the ‘28andMe’ Project from the University of California, Santa Barbara. After reviewing key findings from this emerging literature, we discuss how these methods could be leveraged to improve personalized medicine for endocrine-related neurological disorders.
I. Traditional experiments reveal brain–hormone relationships
Cross-sectional investigations
In cognitive neuroscience, a classic approach for studying the brain involves collecting data from a number of individuals at a single time point and then group-averaging, thus increasing the ability to generalize findings to a broader population. Similarly, testing differences between cross-sections of the population has been valuable for brain–hormone investigations. For example, differences in brain structure and function have emerged when comparing groups of women at different stages of the menstrual cycle [13] or menopausal transition [14,15] and similar comparisons are useful for investigating the brain’s response to exogenous hormone exposure [16]. In a recent study, Zeydan and colleagues (2018) compared the effects of reproductive stage on brain structure among middle-aged women [17]. Those that had undergone surgical menopause before the age of 50 had thinner parahippocampal/entorinhal cortices, smaller amygdala volumes, and lower entorhinal white matter fractional anisotropy values compared to women who underwent spontaneous menopause later in life. One strength of this cross-sectional approach is that it allows researchers to isolate how reproductive stage, over and above chronological age, shapes the brain [18].
Large-scale ‘population neuroscience’ datasets are also being leveraged to test brain– hormone associations. Recently, de Lange and colleagues used the UK Biobank database to examine the association between a woman’s reproductive health history and brain age [19,20]. Using machine learning on data from over 12,000 middle-aged women, they found that multiparous women were classified as having ‘younger-looking’ brains relative to nulliparous women, particularly within striatal and limbic regions—an effect that held after accounting for age, ethnicity, education, body mass index, age at first birth, and number of reproductive years.
Longitudinal investigations—insights from ‘sparse-sampling’ designs
Longitudinal studies provide insight into the degree of stability or change within groups of individuals over time, a particularly powerful approach for revealing hormone-related changes in the brain. Recent longitudinal brain–hormone studies have sampled individuals before and after major neuroendocrine events such as pregnancy [21] and across discrete stages of the menopausal transition [22]. One such study examined first-time mothers prospectively, finding reductions in gray matter volumes post-pregnancy in regions largely overlapping with theory-of-mind circuitry. These changes were not observed in age-matched nulliparous women or male counterparts scanned over the same time-period [21]. Longitudinal studies like these offer valuable clues about how the brain responds to periods of significant hormonal change. A number of recent studies have also applied this approach to the menstrual cycle, sampling women several (e.g. 2–4) times in order to compare brain structure and function by cycle stage [8,23,24]. However, inconsistences emerge due to the inherent limitation of applying a static sampling rate to the study of a dynamical system. For example, Schmalenberger and colleagues (2020) argue that exploring within-person menstrual cycle effects requires, at minimum, sampling three time points across one cycle [25]. Still, three time points are insufficient to explore how the brain responds to transient changes in sex hormones, a critical feature of the endocrine system [9,26,27].
Together, findings from cross-sectional and traditional longitudinal studies support a role for ovarian hormones in shaping the brain across the life course [1–2,4,10]. However, new approaches with improved temporal resolution are needed to illuminate the time-sensitive coupling between hormone fluctuations and the functional and structural architecture of the human brain.
II. Dense-sampling as a new avenue for probing brain–hormone interactions
Neuroimaging studies that densely sample the individual connectome are beginning to transform our understanding of human brain organization over time [11,12]. This method is particularly well-suited for examining relationships between brain dynamics and physiological variables that vary over relatively short time scales, such as ovarian hormone production over the human menstrual cycle. To that end, a small but emerging body of work has begun to reveal sex hormones’ ability to drive changes in the brain’s structure and function [5,7,9,27–30].
Densely-sampling the functional connectome across the menstrual cycle
Arélin and colleagues densely-sampled a naturally-cycling female every 2–3 days over a four-month period, collecting brain imaging and blood hormone data across 32 sessions [5,29]. Using resting-state fMRI, the authors observed a positive relationship between progesterone and functional connectivity between the hippocampus, dorsolateral prefrontal cortex (PFC), and sensorimotor cortex, suggesting that changes in inter-regional connectivity are tied to hormone fluctuations across the cycle [29]. This sampling rate provided novel evidence for a relationship between functional connectivity and hormone fluctuations, while accounting for potential intra-individual hormone variation across multiple cycles. However, the study was unable to address whether changes in sex hormones drive variation in brain states, which requires a higher within-person measurement frequency (e.g. day to day assessments) and time-lagged analyses.
To that end, our group completed the ‘28andMe’ project, in which a participant underwent brain imaging and venipuncture over 30 consecutive days across a complete menstrual cycle (Study 1), followed by 30 consecutive days on an oral hormonal contraceptive regimen (0.02 mg ethinyl-estradiol, 0.1 mg levonorgestrel, Aubra, Afaxys Pharmaceuticals) (Study 2) one year later. To probe time-synchronous and time-lagged effects of ovarian hormones on functional brain networks, we sampled the participant every 24 hours. The unique strength of these studies derives from their ability to capture, with high spatial and temporal resolution, the brain’s response to a central feature of the mammalian endocrine system: hormonal rhythmicity.
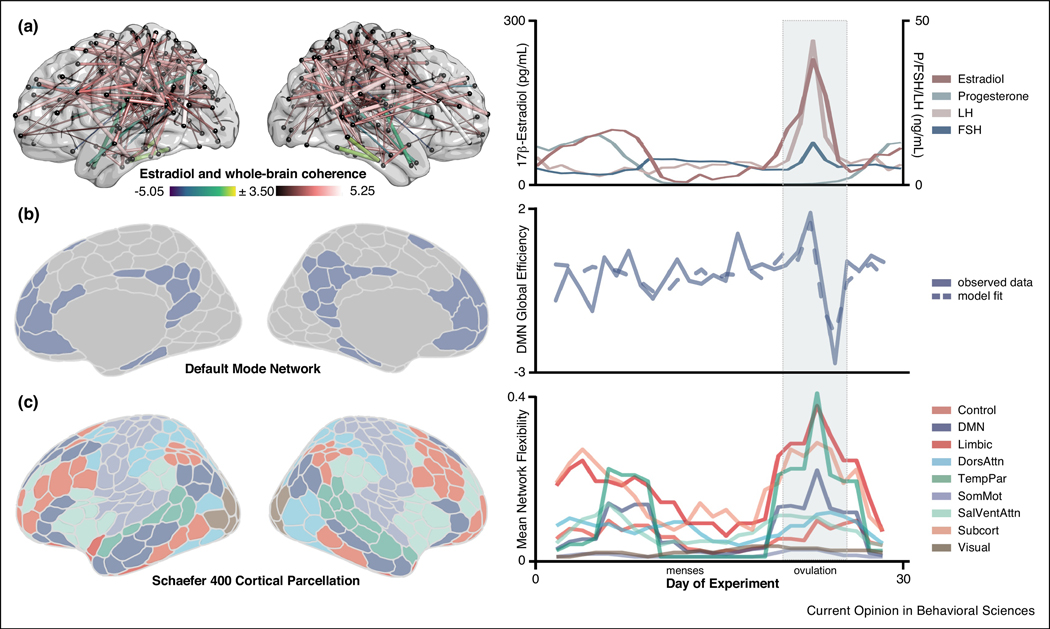
To begin, we tested the hypothesis that whole-brain, resting-state functional connectivity (rs-fc) is associated with intrinsic fluctuations in estradiol and progesterone in a time-synchronous (i.e. day-by-day) fashion. Based on enriched expression of estrogen receptors in frontal cortex [31], we predicted that the Default Mode, Frontoparietal Control, and Dorsal Attention Networks would be most sensitive to hormone fluctuations across the cycle. We observed robust increases in coherence across the brain as a function of increasing estradiol (Fig. 1A) [9]. In contrast to estradiol’s proliferative effects, progesterone was primarily associated with reduced coherence across the whole brain. Next, we used time-lagged methods from dynamical systems analysis to test the temporal directionality of these associations, finding that estradiol enhances within-network integration (i.e. global efficiency) in several large-scale brain networks, with the strongest effects in Default Mode (DMN) (Fig. 1B) and Dorsal Attention (DAN) Networks. These results replicated across Study 2 (where progesterone, but not estradiol, was selectively suppressed), further strengthening the notion that estradiol drives changes in network connectivity. In fact, across both studies, estradiol often predicted brain states better than previous states of the brain itself.
Figure 1. Estradiol has a robust impact on intrinsic brain network properties in a densely sampled female.
A) Transient changes in estradiol across the menstrual cycle were associated with whole-brain coherence at rest (left) [9]. Warmer colors indicate increased coherence with higher concentrations of estradiol; cool colors indicate the reverse. Nodes without significant edges are omitted for clarity. Ovarian steroid hormones (estradiol, progesterone) and gonadotropin (LH, FSH) concentrations are plotted across the 30-day study (right). B) Time-lagged analyses suggest that estradiol drives Default Mode Network topology (left). Observed data (solid lines) vs. Vector Autoregressive model fits (dotted lines) are depicted for within-network efficiency (right). Note that the peak/trough of DMN network efficiency coincides with estradiol’s characteristic rise and fall across the ovulatory window (gray band) [9]. C) Network flexibility (calculated over a 5-day sliding window [27]) was also noticeably higher in many regions of the Temporal Parietal, Limbic, and Default Mode Networks during the ovulatory phase of the cycle. Peaks in flexibility were coincident with the ovulatory window (days 22–25) and the secondary peak in estradiol (days 5–10) (right). These findings are based on a densely-sampled female and should be examined in a larger cohort to assess generalizability. Abbreviations: DMN, Default Mode Network; DorsAttn, Dorsal Attention Network; FSH, Follicle Stimulating Hormone; LH, Luteinizing Hormone; SalVentAttn, Salience/Ventral Attention Network; SomMot, SomatoMotor Network; TempPar, Temporal Parietal Network.
However, questions remained regarding how hormones shape large-scale functional brain network reorganization: which nodes are driving this network reorganization and how do they reorganize? To address this, we applied methods from complex systems analysis—dynamic community detection (DCD)—to identify periods of time when functionally coupled regions began to shift the network communities with which they were affiliated: so-called network ‘flexibility’ [27]. Despite a large degree of network stability over the menstrual cycle, a striking reorganization event occurred within the DMN, coincident with the peaks in serum estradiol (Fig. 1C). During the 3-day ovulatory window, the DMN core split into two smaller groups, leading to the transient formation of a new functional community. This was one of only two large-scale reorganization events—the other occurring during the luteal phase’s secondary peak in estradiol, which involved an overlapping set of nodes located predominantly within the PFC.
While DCD expanded upon our earlier finding by highlighting cycle-dependent alterations in DMN organization, unique effects emerged when comparing naturally-cycling (Study 1) and oral contraceptive (Study 2) conditions. Our original investigation [9] revealed that estradiol’s ability to drive global efficiency within the DMN was invariant to condition (i.e. evident under naturally cycling conditions and when progesterone was selectively suppressed). In contrast, a closer look with DCD revealed increased DMN network flexibility only during ovulation: networks were largely stable under the oral contraceptive regimen, with no major reorganizations despite the fact that fluctuations in estradiol were on par with those observed under naturally cycling conditions. It is possible that hormone-driven changes in sub-network organization are specific to conditions in which gonadotropins and ovarian hormones (e.g. luteinizing hormone, follicle stimulating hormone, estradiol, and progesterone) exert coordinated action in the brain, as occurs across the menstrual cycle.
Together, these dense-sampling studies provide initial evidence that transient fluctuations in ovarian hormones over the cycle drive coordinated reorganization across the functional connectome, especially within the DMN. Interestingly, a major DMN subnetwork encompasses theory-of-mind circuitry [32], a cognitive process that underlies the ability to infer another individual’s mental state [33]. Given that pregnancy leads to select structural changes in this circuit [21], there may be a specific functional significance of cycle-related changes in DMN connectivity – i.e. to support social cognitive processes. This hypothesis could be tested in future dense-sampling experiments in which theory-of-mind performance, resting-state fMRI, and endocrine evaluations are assessed in diverse cohorts of women across the menstrual cycle. Further, examining DMN properties across other major endocrine transition periods (e.g. puberty, menopause) and in response to direct pharmacological manipulation will strengthen our understanding of sex hormones’ role in this circuit.
Densely-sampling brain structure across the menstrual cycle
In addition to sex hormone–driven changes in the functional connectome, dense-sampling studies are also beginning to reveal hormone-related changes in morphology over short timescales. Animal studies provide powerful evidence that sex hormones play a critical role in the synaptic organization of the hippocampus. In rodents, peak levels of estradiol during the estrous cycle induce a 30% increase in dendritic spine density in the CA1 region of the hippocampus, while progesterone rapidly reverses this effect. On average, women experience an 8-fold increase in estradiol and an 80-fold increase in progesterone across a menstrual cycle; yet, few studies have investigated the relationship between endogenous hormone fluctuations and hippocampal subfield morphology in humans [34]. Furthermore, prior to 28andMe, none had sampled at sufficiently high temporal and spatial (submillimeter) resolutions to probe whether the pronounced effects of sex hormones observed at the microscopic scale in rodents [35] are evident at the mesoscopic scale detectable with human MRI.
Using high-resolution hippocampal subfield imaging, we discovered that endogenous hormone fluctuations and exogenous hormone manipulations alter medial temporal lobe morphology [7]. Across the menstrual cycle, intrinsic fluctuations in progesterone were associated with volumetric changes in CA2/3, entorhinal, perirhinal, and parahippocampal cortex. Chronic progesterone suppression (with 0.02 mg ethinyl-estradiol, 0.1 mg levonorgestrel, Aubra, Afaxys Pharmaceuticals) abolished these cycle-dependent effects. These results suggest progesterone has the ability to dynamically shape medial temporal lobe morphology over rapid timescales.
Considerations from 28andMe
A major limitation of the 28andMe project is that it densely tracked one individual, limiting our ability to generalize findings to a more diverse sample of women. Indeed, the pattern of results we observed may vary across women depending upon the duration of a woman’s ovulatory window, sensitivity to hormone fluctuations, or sex hormone receptor expression (to name a few). Rather, findings from the 28andMe Project should be treated as indicative of the type that might emerge when applying dense-sampling methods to reveal endocrine modulation of the human brain. We hope that this project motivates future dense-sampling studies that investigate the dynamic role endogenous/exogenous sex hormones play in shaping the brain of both sexes across the lifespan. For example, future work using dense-sampling to characterize the brain’s response to sex hormone fluctuations in men (e.g. androgens) and other steroid hormones (e.g. cortisol) will improve our understanding of the time-course through which hormones influence brain structure and function. Critically, the extent to which hormone fluctuations cause mesoscopic brain variability remains rich for exploration. Models of interactions lagged in time (such as VAR) may offer a promising first step, but they require strong ad hoc specification and make considerable assumptions about the temporal structure of brain-hormone dynamics. Ovarian hormones are more likely a nonlinear system, for which other modeling techniques are better-suited. Ultimately, although dense-sampling studies in neuroendocrinology remain rare, this approach could elucidate general principles of brain-hormone interactions in the human brain at an unprecedented temporal resolution.
III. Relevance for personalized medicine
Personalized medicine is the development of therapeutic approaches catered to the individual through phenotypic and genotypic characterization. Increasing the frequency of within-person measurements (i.e. dense-sampling) in neuroimaging provides a new opportunity to identify neural biomarkers that can lead to individualized inferences, detection, and treatment of psychiatric and neurologic conditions—personalized neuroscience. Throughout the life course, major hormonal transition periods (including puberty, pregnancy, initiation of oral contraceptive use, and perimenopause) coincide with an increased risk for major depressive disorder [36]. Modeling dynamic changes in these tightly-coupled systems (e.g. brain networks, ovarian hormones) in the healthy brain could help us better predict the likely consequences of their disruption in the disordered brain. Doing so could afford unique insight into why symptoms emerge in some individuals but not others.
Disruptions in functional brain networks are implicated in a number of neurodegenerative disorders, including Alzheimer’s Disease (AD). The DMN is particularly vulnerable to AD progression, demonstrating distinct abnormalities in functional connectivity compared to healthy controls [37] and hypoactivity due to amyloid-β aggregation [38]. Notably, DMN connectivity is also altered in MDD [39], a known risk factor for AD [40]. At the morphological level, the entorhinal cortex is one of the first cortical regions of the brain to develop neuropathology in the progression of AD, followed by surrounding medial temporal lobe regions (MTL) [41,42]. A distinct characteristic of these AD-sensitive circuits (e.g. the DMN and MTL) is that they are heavily populated by sex hormone receptors and are regulated by sex hormones [7–10,13,17,18,21,31]. In 28andMe, we discovered that estradiol is a major driver of global efficiency within DMN [9], a network that also exhibited the most striking reorganization event (transiently splitting into two distinct subnetworks) coincident with peaks in estradiol. Next, our dense-sampling study revealed that the most pronounced effects of chronic progesterone suppression occurred in entorhinal cortex [7], demonstrating strong regulatory effects of progesterone on entorhinal cortex volume, which adds to a growing body of work underscoring the need for clinical studies to investigate the neuroendocrine basis of cognitive decline and dementia risk [43–46]. Few studies of the aging brain consider the midlife period, and even fewer consider the neural effects of reproductive aging [43]. Given that women are disproportionately affected by AD [44,45], applying dense-sampling methods to the menopausal transition (a neurological transition state when ovarian hormone production declines by >90% [46]) would allow us to observe how an individual’s brain—especially DMN and MTL architecture— responds to a changing hormonal milieu decades before disease onset. Such studies may yield important clues about what constitutes normative reproductive aging versus a prodromal period of heightened disease risk.
IV. Conclusions
While human neuroimaging studies that densely sample the individual connectome have begun to transform our understanding of the dynamics of human brain organization, they routinely omit sex steroid hormones as variables of interest. This approach is uniquely well-suited, however, to test hypotheses and build models of nervous and endocrine system interactions across the lifespan, capturing details of brain–hormone coupling that may be overlooked by traditional cross-sectional or sparse-sampling approaches. Advancing our understanding of how hormones shape the brain is imperative for our basic understanding of the brain and for women’s health.
Highlights.
Sex steroid hormones influence the brain across multiple spatiotemporal scales
Dense-sampling can uncover brain–hormone interactions at higher temporal resolution
Transient increases in estradiol enhance global efficiency of several large-scale networks
Progesterone shapes medial temporal lobe volume across the menstrual cycle
Incorporating endocrine factors into neuroimaging could improve personalized medicine
Acknowledgements:
work was supported by the Brain and Behavior Research Foundation (EGJ), the National Institutes of Health AG063843 (EGJ), and the Rutherford B. Fett Fund. We would like to thank Scott Grafton, Joshua Mueller, Evan Layher, Shuying Yu, Morgan Fitzgerald, Michael Miller, and Mario Mendoza for their contributions to the 28andMe project.
Footnotes
COI
The authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].McEwen BS, 2018. Redefining neuroendocrinology: Epigenetics of brain-body communication over the life course. Frontiers in Neuroendocrinology 49, 8–30. 10.1016/j.yfrne.2017.11.001 [DOI] [PubMed] [Google Scholar]
- [2]. Beltz AM, Moser JS, 2020. Ovarian hormones: a long overlooked but critical contributor to cognitive brain structures and function. Annals of the New York Academy of Sciences 1464, 156–180. 10.1111/nyas.14255 *A historical review of sex hormones’ influence on brain structure and function in cognitive neuroscience, detailing empirical findings across hormonal transition periods such as puberty, menstrual cycles, exogenous hormone use, and menopause.
- [3].Lu Y, Sareddy GR, Wang J, Wang R, Li Y, Dong Y, Zhang Q, Liu J, O’Connor JC, Xu J, Vadlamudi RK, Brann DW, 2019. Neuron-Derived Estrogen Regulates Synaptic Plasticity and Memory. The Journal of Neuroscience 39, 2792–2809. 10.1523/JNEUROSCI.1970-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. Taxier LR, Gross KS, Frick KM, 2020. Oestradiol as a neuromodulator of learning and memory. Nature Reviews Neuroscience 21, 535–550. 10.1038/s41583-020-0362-7 **A seminal review paper that provides evidence for estradiol’s role as a neuromodulator in the central nervous system of both sexes.
- [5].Barth C, Steele CJ, Mueller K, Rekkas VP, Arélin K, Pampel A, Burmann I, Kratzsch J, Villringer A, Sacher J, 2016. In-vivo Dynamics of the Human Hippocampus across the Menstrual Cycle. Scientific Reports 6. 10.1038/srep32833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zsido RG, Heinrich M, Slavich GM, Beyer F, Kharabian Masouleh S, Kratzsch J, Raschpichler M, Mueller K, Scharrer U, Löffler M, Schroeter ML, Stumvoll M, Villringer A, Witte AV, Sacher J, 2019. Association of Estradiol and Visceral Fat With Structural Brain Networks and Memory Performance in Adults. JAMA Network Open 2, e196126. 10.1001/jamanetworkopen.2019.6126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7]. Taylor CM, Pritschet L, Olsen RK, Layher E, Santander T, Grafton ST, Jacobs EG, 2020. Progesterone shapes medial temporal lobe volume across the human menstrual cycle. NeuroImage 220, 117125. 10.1016/j.neuroimage.2020.117125 ** A dense-sampling study from the 28andMe project used high-resolution hippocampal subfield imaging to reveal that endogenous fluctuations in progesterone across the menstrual cycle alter medial temporal lobe morphology, effects that were mitigatd when progesterone concentrations were pharmacologically suppressed.
- [8].Weis S, Hodgetts S, Hausmann M, 2019. Sex differences and menstrual cycle effects in cognitive and sensory resting state networks. Brain and Cognition 131, 66–73. 10.1016/j.bandc.2017.09.003 [DOI] [PubMed] [Google Scholar]
- [9]. Pritschet L, Santander T, Taylor CM, Layher E, Yu S, Miller MB, Grafton ST, Jacobs EG, 2020. Functional reorganization of brain networks across the human menstrual cycle. NeuroImage 220, 117091. 10.1016/j.neuroimage.2020.117091 **A dense-sampling study from the 28andMe project found that transient changes in estradiol drive intrinsic brain network properties. Estradiol enhanced global efficiency in Default Mode and Dorsal Attention Networks.
- [10].Galea LAM, Frick KM, Hampson E, Sohrabji F, Choleris E, 2017. Why estrogens matter for behavior and brain health. Neuroscience & Biobehavioral Reviews 76, 363–379. 10.1016/j.neubiorev.2016.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Poldrack RA, Laumann TO, Koyejo O, Gregory B, Hover A, Chen M-Y, Gorgolewski KJ, Luci J, Joo SJ, Boyd RL, Hunicke-Smith S, Simpson ZB, Caven T, Sochat V, Shine JM, Gordon E, Snyder AZ, Adeyemo B, Petersen SE, Glahn DC, Reese Mckay D, Curran JE, Göring HHH, Carless MA, Blangero J, Dougherty R, Leemans A, Handwerker DA, Frick L, Marcotte EM, Mumford JA, 2015. Long-term neural and physiological phenotyping of a single human. Nature Communications 6. 10.1038/ncomms9885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gratton C, Laumann TO, Nielsen AN, Greene DJ, Gordon EM, Gilmore AW, Nelson SM, Coalson RS, Snyder AZ, Schlaggar BL, Dosenbach NUF, Petersen SE, 2018. Functional Brain Networks Are Dominated by Stable Group and Individual Factors, Not Cognitive or Daily Variation. Neuron 98, 439–452.e5. 10.1016/j.neuron.2018.03.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Petersen N, Kilpatrick LA, Goharzad A, Cahill L, 2014. Oral contraceptive pill use and menstrual cycle phase are associated with altered resting state functional connectivity. NeuroImage 90, 24–32. 10.1016/j.neuroimage.2013.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Jacobs EG., Weiss B., Makris N., Whitfield-Gabrieli S., Buka SL., Klibanski A., Goldstein JM., 2016. Reorganization of Functional Networks in Verbal Working Memory Circuitry in Early Midlife: The Impact of Sex and Menopausal Status. Cerebral Cortex bhw 127. 10.1093/cercor/bhw127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Jacobs EG, Weiss BK, Makris N, Whitfield-Gabrieli S, Buka SL, Klibanski A, Goldstein JM, 2016. Impact of Sex and Menopausal Status on Episodic Memory Circuitry in Early Midlife. Journal of Neuroscience 36, 10163–10173. 10.1523/JNEUROSCI.0951-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Votinov M, Wagels L, Hoffstaedter F, Kellermann T, Goerlich KS, Eickhoff SB, Habel U, 2020. Effects of exogenous testosterone application on network connectivity within emotion regulation systems. Scientific Reports 10. 10.1038/s41598-020-59329-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17]. Zeydan B, Tosakulwong N, Schwarz CG, Senjem ML, Gunter JL, Reid RI, Gazzuola Rocca L, Lesnick TG, Smith CY, Bailey KR, Lowe VJ, Roberts RO, Jack CR, Petersen RC, Miller VM, Mielke MM, Rocca WA, Kantarci K, 2019. Association of Bilateral Salpingo-Oophorectomy Before Menopause Onset With Medial Temporal Lobe Neurodegeneration. JAMA Neurology 76, 95. 10.1001/jamaneurol.2018.3057 **This case-control study compares the effects of surgical vs. spontaneous menopause on medial temporal lobe structure in the human brain. The abrupt hormonal changes associated with bilateral salpingo-oophorectomy resulted in structural abnormalities in the amygdala and parahippocampal-entorhinal cortex.
- [18].Jacobs EG, Goldstein JM, 2018. The middle-aged brain: biological sex and sex hormones shape memory circuitry. Current Opinion in Behavioral Sciences 23, 84–91. 10.1016/j.cobeha.2018.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19]. de Lange A-MG, Kaufmann T, van der Meer D, Maglanoc LA, Alnæs D, Moberget T, Douaud G, Andreassen OA, Westlye LT, 2019. Population-based neuroimaging reveals traces of childbirth in the maternal brain. Proceedings of the National Academy of Sciences 116, 22341–22346. 10.1073/pnas.1910666116 *This paper uses a ‘Big Data’ approach to explore the impact of childbirth on brain aging. Women who had a higher number of births had less apparent brain aging. This cross-sectional evidence is consistent with the theory that greater estrogen exposure in adulthood may have an enduring influence on the brain decades later in life.
- [20].de Lange AG, Barth C, Kaufmann T, Anatürk M, Suri S, Ebmeier KP, Westlye LT, 2020. The maternal brain: Region specific patterns of brain aging are traceable decades after childbirth. Human Brain Mapping 41, 4718–4729. 10.1002/hbm.25152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21]. Hoekzema E, Barba-Müller E, Pozzobon C, Picado M, Lucco F, García-García D, Soliva JC, Tobeña A, Desco M, Crone EA, Ballesteros A, Carmona S, Vilarroya O, 2017. Pregnancy leads to long-lasting changes in human brain structure. Nature Neuroscience 20, 287–296. 10.1038/nn.4458 *This prospective study examined a group of women pre- and post-pregnancy, observing reductions in gray matter volume in a network largely overlapping with theory-of-mind circuitry. Many changes persisted two years later and the degree of volumetric change (relative to nulliparous women scanned over the same interval) was correlated with measures of maternal-infant attachment following birth.
- [22].Mosconi L, Rahman A, Diaz I, Wu X, Scheyer O, Hristov HW, Vallabhajosula S, Isaacson RS, de Leon MJ, Brinton RD, 2018. Increased Alzheimer’s risk during the menopause transition: A 3-year longitudinal brain imaging study. PLOS ONE 13, e0207885. 10.1371/journal.pone.0207885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hidalgo-Lopez E, Mueller K, Harris T, Aichhorn M, Sacher J, Pletzer B, 2020. Human menstrual cycle variation in subcortical functional brain connectivity: a multimodal analysis approach. Brain Structure and Function 225, 591–605. 10.1007/s00429-01902019-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Pletzer B, Harris T-A, Scheuringer A, Hidalgo-Lopez E, 2019. The cycling brain: menstrual cycle related fluctuations in hippocampal and fronto-striatal activation and connectivity during cognitive tasks. Neuropsychopharmacology 44, 1867–1875. 10.1038/s41386-019-0435-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Schmalenberger KM, Tauseef HA, Barone JC, Owens SA, Lieberman L, Jarczok MN, Girdler SS, Kiesner J, Ditzen B, Eisenlohr-Moul TA, 2021. How to study the menstrual cycle: Practical tools and recommendations. Psychoneuroendocrinology 123, 104895. 10.1016/j.psyneuen.2020.104895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Schmidt PJ, Martinez PE, Nieman LK, Koziol DE, Thompson KD, Schenkel L, Wakim PG, Rubinow DR, 2017. Premenstrual Dysphoric Disorder Symptoms Following Ovarian Suppression: Triggered by Change in Ovarian Steroid Levels But Not Continuous Stable Levels. American Journal of Psychiatry 174, 980–989. 10.1176/appi.ajp.2017.16101113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Mueller JM, Pritschet L, Santander T, Taylor CM, Grafton ST, Jacobs EG, Carlson JM, 2020. Dynamic community detection reveals transient reorganization of functional brain networks across a female menstrual cycle. Network Neuroscience 1–28. 10.1162/netn_a_00169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Fitzgerald M, Pritschet L, Santander T, Grafton ST, Jacobs EG, 2020. Cerebellar network organization across the human menstrual cycle. Sci Rep 10, 20732. 10.1038/s41598-020-77779-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Arélin K, Mueller K, Barth C, Rekkas PV, Kratzsch J, Burmann I, Villringer A, Sacher J, 2015. Progesterone mediates brain functional connectivity changes during the menstrual cycle—a pilot resting state MRI study. Frontiers in Neuroscience 9. 10.3389/fnins.2015.00044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Karch JD, Filevich E, Wenger E, Lisofsky N, Becker M, Butler O, Mårtensson J, Lindenberger U, Brandmaier AM, Kühn S, 2019. Identifying predictors of within-person variance in MRI-based brain volume estimates. NeuroImage 200, 575–589. 10.1016/j.neuroimage.2019.05.030 [DOI] [PubMed] [Google Scholar]
- [31].Wang ACJ., Hara Y., Janssen WGM., Rapp PR., Morrison JH., 2010. Synaptic Estrogen Receptor- Levels in Prefrontal Cortex in Female Rhesus Monkeys and Their Correlation with Cognitive Performance. Journal of Neuroscience 30, 12770–12776. 10.1523/JNEUROSCI.3192-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Buckner RL, DiNicola LM, 2019. The brain’s default network: updated anatomy, physiology and evolving insights. Nat Rev Neurosci 20, 593–608. 10.1038/s41583-019-02127 [DOI] [PubMed] [Google Scholar]
- [33].Frith C. and Frith U, 2005. Theory of mind. Current biology 15, 644–645. [DOI] [PubMed] [Google Scholar]
- [34].Protopopescu X, Butler T, Pan H, Root J, Altemus M, Polanecsky M, McEwen B, Silbersweig D, Stern E, 2008. Hippocampal structural changes across the menstrual cycle. Hippocampus 18, 985–988. 10.1002/hipo.20468 [DOI] [PubMed] [Google Scholar]
- [35].Woolley CS, McEwen BS, 1993. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. The Journal of Comparative Neurology 336, 293–306. 10.1002/cne.903360210 [DOI] [PubMed] [Google Scholar]
- [36].Rubinow DR, Schmidt PJ, 2019. Sex differences and the neurobiology of affective disorders. Neuropsychopharmacology 44, 111–128. 10.1038/s41386-018-0148-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, Hedden T, Andrews-Hanna JR, Sperling RA, Johnson KA, 2009. Cortical Hubs Revealed by Intrinsic Functional Connectivity: Mapping, Assessment of Stability, and Relation to Alzheimer’s Disease. Journal of Neuroscience 29, 1860–1873. 10.1523/JNEUROSCI.5062-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Pascoal TA, Mathotaarachchi S, Kang MS, Mohaddes S, Shin M, Park AY, Parent MJ, Benedet AL, Chamoun M, Therriault J, Hwang H, Cuello AC, Misic B, Soucy JP, Aston JAD, Gauthier S, Rosa-Neto P, 2019. Aβ-induced vulnerability propagates via the brain’s default mode network. Nature Communications 10. 10.1038/s41467-01910217-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Scalabrini A, Vai B, Poletti S, Damiani S, Mucci C, Colombo C, Zanardi R, Benedetti F, Northoff G, 2020. All roads lead to the default-mode network—global source of DMN abnormalities in major depressive disorder. Neuropsychopharmacology 45, 2058–2069. 10.1038/s41386-020-0785-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Herbert J, Lucassen PJ, 2016. Depression as a risk factor for Alzheimer’s disease: Genes, steroids, cytokines and neurogenesis – What do we need to know? Frontiers in Neuroendocrinology 41, 153–171. 10.1016/j.yfrne.2015.12.001 [DOI] [PubMed] [Google Scholar]
- [41].Bobinski M, De Leon MJ, Convit A, De Santi S, Wegiel J, Tarshish CY, Saint Louis LA and Wisniewski HM, 1999. MRI of entorhinal cortex in mild Alzheimer’s disease. The Lancet 353, 38–40. 10.1016/S0140-6736(05)74869-8 [DOI] [PubMed] [Google Scholar]
- [42].Olsen RK., Yeung L-K., Noly-Gandon A., D’Angelo MC., Kacollja A., Smith VM., Ryan JD., Barense MD., 2017. Human anterolateral entorhinal cortex volumes are associated with cognitive decline in aging prior to clinical diagnosis. Neurobiology of Aging 57, 195–205. 10.1016/j.neurobiolaging.2017.04.025 [DOI] [PubMed] [Google Scholar]
- [43].Taylor CM, Pritschet L, Yu S, Jacobs EG, 2019. Applying a Women’s Health Lens to the Study of the Aging Brain. Frontiers in Human Neuroscience 13. 10.3389/fnhum.2019.00224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Mielke MM, Ferretti MT, Iulita MF, Hayden K, Khachaturian AS, 2018. Sex and gender in Alzheimer’s disease - Does it matter? Alzheimer’s & Dementia 14, 1101–1103. 10.1016/j.jalz.2018.08.003 [DOI] [PubMed] [Google Scholar]
- [45].Nebel RA, Aggarwal NT, Barnes LL, Gallagher A, Goldstein JM, Kantarci K, Mallampalli MP, Mormino EC, Scott L, Yu WH, Maki PM, Mielke MM, 2018. Understanding the impact of sex and gender in Alzheimer’s disease: A call to action. Alzheimer’s & Dementia 14, 1171–1183. 10.1016/j.jalz.2018.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Brinton RD, Yao J, Yin F, Mack WJ, Cadenas E, 2015. Perimenopause as a neurological transition state. Nature Reviews Endocrinology 11, 393–405. 10.1038/nrendo.2015.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Boker SM, Neale MC, Klump KL, 2014. In: Molenaar PC, Lerner R, Newll K. (Eds.), A Differential Equations Model for the Ovarian Hormone Cycle. Guilford Press, New York, pp. 369–391. [Google Scholar]