Abstract
To describe the frequency and nature of premedication practices for neonatal tracheal intubation (TI) in 2011; to identify independent risk factors for the absence of premedication; to compare data with those from 2005 and to confront observed practices with current recommendations. Data concerning TI performed in neonates during the first 14 days of their admission to participating neonatal/pediatric intensive care units were prospectively collected at the bedside. This study was part of the Epidemiology of Procedural Pain in Neonates study (EPIPPAIN 2) conducted in 16 tertiary care units in the region of Paris, France, in 2011. Multivariate analysis was used to identify factors associated with premedication use and multilevel analysis to identify center effect. Results were compared with those of the EPIPPAIN 1 study, conducted in 2005 with a similar design, and to a current guidance for the clinician for this procedure. One hundred and twenty‐one intubations carried out in 121 patients were analyzed. The specific premedication rate was 47% and drugs used included mainly propofol (26%), sufentanil (24%), and ketamine (12%). Three factors were associated with the use of a specific premedication: nonemergent TI (Odds ratio (OR) [95% CI]: 5.3 [1.49‐20.80]), existence of a specific written protocol in the ward (OR [95% CI]:4.80 [2.12‐11.57]), and the absence of a nonspecific concurrent analgesia infusion before TI (OR [95% CI]: 3.41 [1.46‐8.45]). No center effect was observed. The specific premedication rate was lower than the 56% rate observed in 2005. The drugs used were more homogenous and consistent with the current recommendations than in 2005, especially in centers with a specific written protocol. Premedication use prior to neonatal TI was low, even for nonemergent procedures. Scientific consensus, implementation of international or national recommendations, and local written protocols are urgently needed to improve premedication practices for neonatal intubation.
Keywords: evidence‐based practice, neonate, pain, premedication, tracheal intubation
1. INTRODUCTION
Tracheal intubation (TI) is an essential but potentially hazardous procedure for neonates in life‐threatening situations. This painful and stressful invasive procedure is often associated with immediate adverse effects such as laryngospasm, hemodynamic changes, and increased risk of intracranial hemorrhage. 1 , 2 , 3 , 4 A specific premedication, defined by the use of analgesic and/or sedative drug(s) regardless of the use of a vagolytic agent, can blunt the patient's physiologic responses to TI and has been recommended for nonemergent neonatal TI since 2001, with an update in 2010 by the American Academy of Pediatrics (AAP). 5 , 6 , 7 Despite this guidance for the clinicians, the staff awareness of pain and its consequences in the neonatal period, and the publication in the last years of several studies on the possible drug combinations to use for this procedure, many neonatal and pediatric intensive care units (NICU/PICU) or individuals caregivers have not incorporated routine use of neonatal premedication into their practices. 8 , 9 , 10
In 2005, a large regional French longitudinal study (Epidemiology of Procedural Pain in Neonates (EPIPPAIN 1)) was conducted in 13 tertiary care centers in the region of Paris to collect epidemiological data on neonatal pain. 11 More than 60 000 painful or stressful procedures were collected in 430 neonates during the 2 months of study period with a total of 101 TI in 91 patients. The specific premedication rate was 56% and included mostly opioids (67%) and midazolam (53%). 12 In univariate analysis, infants without a specific premedication compared with others were younger at the time of intubation (median age: 0.7 vs 2.0 days), displayed significantly more frequent spontaneous breathing at the time of intubation (31% vs 12%), and a higher percentage of analgesia for all other painful procedures (median values: 16% vs 6%). 12 In multivariate analysis, no patient or center‐related independent risk factor for the absence of premedication was identified in this study. 12 In 2011, the EPIPPAIN 2 study took place in the same 13 centers plus three additional centers in the Paris region. The aim of our study was to analyze the practices of premedication before TI in intensive care units (ICU) in 2011 and to compare them with the results from 2005.
2. OBJECTIVES AND HYPOTHESIS
The objectives of the study were as follows:
To describe the frequency and the nature of premedication used prior to neonatal TI in NICUs and PICUs;
To describe the centers’ characteristics and practices;
To identify risk factors for the absence of premedication;
To confront the observed practices with the current recommendations 7 and compare them with the practices in 2005 (EPIPPAIN 1). 12
Our hypotheses were that:
The premedication rate had increased since 2005 and would be over 60%, and the drugs used would be more consistent with the current recommendations. 7 , 9 , 10
The premedication rate would be lower for the most premature neonates and for the youngest babies (in hours of life), 13 , 14 in case of emergent TI 7 , 10 and during the night. 15
Some centers would promote more than others a specific premedication. 10
3. PATIENTS AND METHODS
3.1. Study design
From the EPIPPAIN (Epidemiology of Procedural Pain in Neonates) 2 study (Trial registration: ClinicalTrials.gov identifier: NCT01346813), we extracted all intubations performed in participating NICUs/PICUs. The EPIPPAIN 2 study was a prospective observational study that collected data at bedside on all painful and stressful procedures performed in neonates as well as pain management (pharmacological and nonpharmacological) for these procedures. It was conducted in all 16 tertiary care centers including 13 neonatal intensive care units (NICUs) and three pediatric intensive care units (PICUs) in the biggest region of France, the Paris Region (Ile de France). The three participating PICUs had a NICU area; medical and nursing staff was rotating personnel common to NICU and PICU areas. The inclusion period in each unit lasted six weeks, from June 2, 2011 to July 12, 2011. Included neonates were followed from their admission to the 14th day of their ICU stay, discharge, or death, whichever occurred first. Only newly admitted neonates were included; those already hospitalized at the start of the study were not included. The study included all preterm neonates younger than 45 postconceptional weeks and term neonates younger than 28 days on the day of ICU admission. Providers were aware of the ongoing study and its objectives. In each unit, medical and nurse coordinators were designated and were shown how to complete the study forms. All participating units had their own protocols. No instructions or interventions were given to modify the standard of care for pain management in neonates.
Practices for TI were specifically assessed in the EPIPPAIN 2 study. The present study focused on neonates who had at least one TI during their first 14 days of hospitalization, whatever the reason. TI performed outside the ICU (in the delivery room or by medical transport team for outborn neonates) were not included. In case of several TI for the same infant, data for the first IT only were analyzed. The emergency degree of the procedure was assessed by the operator who performed the tracheal intubation using the following definitions: emergent TI (to be performed within 10 minutes after the decision), semi‐emergent TI (to be performed between 10 and 30 minutes after the decision), and nonemergent TI (can be performed more than 30 minutes after the decision).
We conducted a retrospective analysis of the prospectively collected data to describe the frequency and type of premedication used prior to TI and the centers’ characteristics and practices, to compare the premedication practices to current recommendations in 2011, and to identify factors associated with the absence of premedication. We then compared the practices between 2005 and 2011 to assess the evolution in a 6 years period.
3.2. Studied variables
Clinical variables: age, sex, gestational age (GA) at birth, birth weight, Clinical Risk Index for Babies (CRIB) score, 16 Apgar score, main indication for TI, respiratory mode at time of TI, in‐ or outborn status, previous TI before admission in ICU, availability of an IV access at time of TI.
-
Intubation data:
○Total number of intubations.
○Postnatal age (hours) at first intubation in the ward.
○Description of each intubation: main operator, number of attempts, emergency degree, time of the day. We divided 24 hours in 2 periods: “day” (7:00‐18:59) and “night” (19:00‐6:59). These timings were chosen because in France, most of the day and night nurse shifts start at 7:00 and 19:00, respectively.
-
Drugs used:
○Specific premedication was defined by the use of an anesthetic and/or a short acting analgesic through IV or intranasal routes. The use of atropine, alone or in association, was not assessed in this study as it acts only as a vagolytic. Oral or intra‐rectal routes were not considered as specific analgesia because the onset delay related to these routes was considered too long.
Two groups were defined: the group of intubations performed with a specific premedication (Premed group) and the group of intubations performed without any specific premedication (No premed group).
○Nonspecific concurrent sedation‐analgesia: if a patient was receiving continuous IV sedation or analgesia at the time of TI without loading dose, bolus or additional treatment immediately prior to the procedure, that was not considered as a specific premedication. The reasons, exact start time, and thus delay before IT of concurrent sedation‐analgesia were not collected. For some patients, this treatment could have been started just before TI, in order to provide continuous sedation or analgesia for the subsequent invasive ventilation period.
○Nonpharmacological treatment use such as sweet‐solutions, pacifier, facilitating tucking, parental presence, or other comfort measures was not considered as a specific premedication.
Centers’ characteristics: ICU type (NICU or PICU), university hospital or not and existence of a specific written protocol for TI.
Comparison to EPIPPAIN 1 study: main clinical data (age, sex, GA, birth weight, CRIB score, respiratory mode at time of TI, availability of an IV access at time of procedure), intubation's data except emergency degree of the procedure which was not collected for EPIPPAIN 1, and centers’ characteristics were compared for the two studies. The drugs used in EPIPPAIN 2 were collected and classified as for EPIPPAIN 1 in order to compare practices. For both studies, we used the following guidance as reference.
3.3. Guidance used as reference
The AAP guidance for the clinicians was the reference to classify premedication regimen as “preferred,” “acceptable,” “not recommended,” or “not described” in the EPIPPAIN 1 study. 11 , 12 Although this guidance applies only to nonemergent intubation and both studies included all intubations, we considered that most intubations carried out with a specific premedication (Premed group) were semi‐emergent or nonemergent intubations. This guidance was published after EPIPPAIN 1 but before EPIPPAIN 2 study, which allowed us to compare the two periods and to analyze the change and the adequacy to this guidance. It includes several drugs, except ketamine and sufentanil, and was the first to classify premedication regimens.
3.4. Statistical analysis
Chi‐squared or Fisher's exact tests were used to compare categorical variables and distribution of premedication among centers. For continuous variables, data were analyzed with t tests for normally distributed variables and Wilcoxon tests for nonnormally distributed variables. The infants and centers characteristics of the Premed and No premed groups were compared in univariate analysis. A P value < 0.05 was considered significant. Multivariate analysis to identify factors associated with the absence of premedication was performed by creating a binary logistic regression model including variables clinically relevant and variables identified with a P value < 0.20 from univariate analysis. Considering that infants’ management in each center could be influenced by local policies, a multilevel model with center as random effect and the variables included in the logistic regression as a fixed effect was created. All analyses were performed with R software, version 3.6.1.
3.5. Ethics
The local committee for the protection of human subjects reviewed the study protocol. Because this was an observational study with no changes in the standard of care, the human subjects committee established that further approvals or parental consent were not required according to French law. The computerized data collection was approved by the French Data Protection Authority (Commission Nationale de l’Informatique et des Libertés).
4. RESULTS
Among the 589 patients included in the EPIPPAIN 2 study, a total of 134 intubations were performed in 121 patients (11 patients were intubated twice and one patient three times) (Figure 1). One hundred patients (82.6%) were admitted to NICUs and 21 (17.4%) to PICUs. Mean (SD) GA at birth was 32.2 (4.6) weeks, and 73 (60%) of neonates were born before 33 weeks GA. Mean (SD) birth weight was 1783 (919) g. Median [25th‐75th] 5‐minute Apgar score and median [25th‐75th] CRIB score were 9 [8‐10] and 1 [0‐3], respectively. Before their admission in ICU, 41 (34%) patients had already been intubated once (33 (80%) in the delivery room). Fifty‐one patients were still hospitalized after 14 days. Patients’ characteristics and intubations data in the Premed and No premed groups are presented in Table 1.
FIGURE 1.
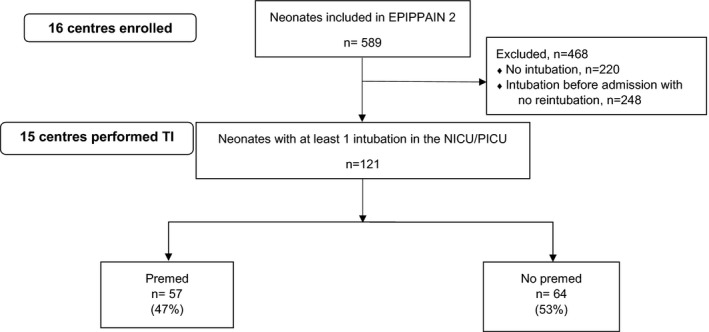
Population flow chart. Abbreviations: EPIPPAIN, Epidemiology of Procedural Pain in Neonates; NICU/PICU, Neonatal Intensive Care Unit/Pediatric Intensive Care Unit; Premed/No premed: Premedication/No premedication groups; TI, Tracheal Intubation
TABLE 1.
Patients’ and intubations’ characteristics in the Premed and No premed groups
| No premed n = 64 | Premed n = 57 | P value | |
|---|---|---|---|
| Mean GA at birth, weeks (SD) | 32.03 (4.56) | 32.37 (4.63) | 0.68 |
| GA categories, n (%) | |||
| <33 GW | 40 (62.5) | 33 (57.9) | 0.74 |
| ≥33 GW | 24 (37.5) | 24 (42 0.1) | |
| Male sex, n (%)) | 31 (48.4) | 32 (56.1) | 0.50 |
| Mean weight at birth, g (SD) | 1771 (971) | 1797 (866) | 0.87 |
| Mean CRIB score (SD) | 2.3 (2.7) | 2.1 (2.4) | 0.58 |
| ICU type: NICU, n (%) | 56 (86.1) | 44 (78.5) | 0.39 |
| Outborn status, n (%) | 21 (32.8) | 17 (29.8) | 0.87 |
| Still at hospital at D14, n (%) | 25 (39.1) | 26 (45.6) | 0.58 |
| Respiratory mode before TI, n (%) | |||
| Tracheal ventilation | 11 (17.2) | 6 (10.5) | 0.61 |
| Noninvasive ventilation | 43 (67.2) | 42 (73.7) | |
| Spontaneous ventilation | 10 (15.6) | 9 (15.8) | |
| Main reason for TI, n (%) | |||
| Respiratory distress | 37 (57.8) | 33 (57.9) | 0.88 |
| Apnea | 14 (21.9) | 13 (22.8) | |
| Endotracheal tube replacement | 6 (9.3) | 4 (7.0) | |
| Others | 7 (11) | 7 (12.3) | |
| Available intravenous access, n (%)) | 56/59 a (94.9) | 57 (100) | 0.25 |
| Procedure performed during daytime | |||
| [7:00‐18:59], n (%) | 30 (46.9) | 31 (53.4) | 0.52 |
| Median postnatal age at TI, h [25th‐75th] | 24.15 [3.1‐105.3] | 31 [6.02‐170] | 0.12 |
| Postnatal age categories at TI, n (%) | |||
| ≤H24 | 31 (48.4) | 26 (45.6) | 0.89 |
| >H24 | 33 (51.6) | 31 (54.4) | |
| Emergency degree of the procedure, n (%) | |||
| Emergent TI | 25 (39.1) | 13 (22.8) | 0.095 |
| Semi‐Emergent TI | 32 (50.0) | 32 (56.1) | |
| Nonemergent TI | 7 (10.9) | 12 (21.1) | |
| Emergency degree of the procedure, n (%) | |||
| Emergent TI | 25 (39.1) | 13 (22.8) | 0.054 |
| Nonurgent TI (semi‐emergent, nonemergent) | 39 (60.9) | 44 (77.2) | |
| Median number of attempts, n [25th‐75th] | 1 [1‐2] | 1 [1‐2] | 0.70 |
| Number of attempts, n (%) | |||
| 1 | 45 (70.3) | 37 (64.9) | 0.42 |
| 2 | 7 (10.9) | 11 (19.3) | |
| >2 | 12 (18.8) | 9 (15.8) | |
| Continuous sedation‐analgesia, n (%)) | 34 (53.1) | 19 (33.3) | 0.045 |
| Specific written protocol for TI, n (%) | 20 (31.2) | 36 (63.2) | 0.001 |
Abbreviations: CRIB, Clinical Risk Index for Babies; D14: 14th day; g, grams; GA, gestational age; GW, gestational weeks; h, hours; ICU, intensive care unit; NICU, neonatal intensive care unit; TI, tracheal intubation.
Data were available for n = 59 patients in the No premed group.
4.1. Specific analgesia for the procedure
Of the 121 intubations, 57 (47.1%) were performed with a specific premedication. We found 15 different premedication regimens, including mainly propofol and sufentanil, alone or in combination (Table 2). The drugs used were different according to the existence of a specific written protocol or not (Figure 2). The doses used were not collected in this study.
TABLE 2.
Specific drugs used before tracheal intubation
| Drugs used | n (%) |
|---|---|
| Propofol | 15 (26.3) |
| Sufentanil | 14 (24.5) |
| IV ketamine | 7 (12.3) |
| Fentanyl | 4 (7.0) |
| Morphine | 5 (8.8) |
| IV midazolam | 3 (5.3) |
| Nalbuphine | 1 (1.7) |
| Nasal midazolam | 1 (1.7) |
| IV ketamine + propofol | 1 (1.7) |
| Propofol + muscle relaxant | 1 (1.7) |
| Propofol + fentanyl | 1 (1.7) |
| Fentanyl + IV midazolam | 1 (1.7) |
| Sufentanil + muscle relaxant | 1 (1.7) |
| Morphine + sufentanil | 1 (1.7) |
| IV nalbuphine + nasal midazolam | 1 (1.7) |
| Total | 57 |
Abbreviation: IV, intravenous.
FIGURE 2.
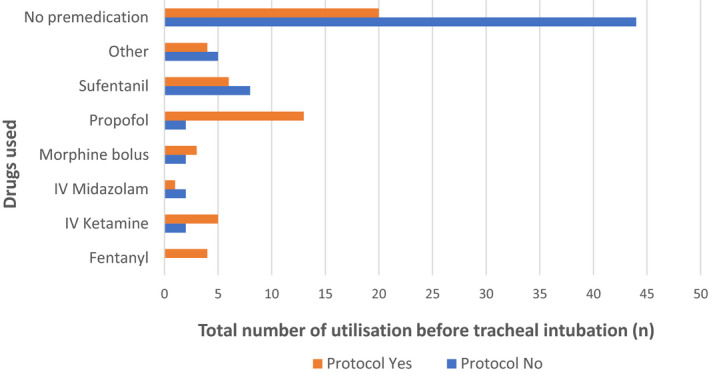
Drugs used according to the existence of a specific written protocol for premedication
4.2. Nonspecific analgesia
Of the 121 intubations, 91 (75.2%) were performed with a form of pharmacological sedation and/or analgesia. Thus, a quarter of the neonates in this study underwent this painful procedure without any form of analgesia. Before intubation, 53 (43.8%) patients were already receiving an analgesic or a sedative infusion. Among them, 19 (35.8%) received a specific premedication before the procedure. Ten on 19 (52%) and 31/85 (36%) neonates on spontaneous ventilation or under noninvasive ventilation, respectively, were receiving a continuous analgesic and/or a sedative at time of the procedure. Respectively, 4 and 11 of them also received a specific premedication for TI, with a bolus of an ongoing drug or with a different drug. The mode of ventilation before the procedure, type of continuous analgesia/sedation, and premedications are shown in Table 3.
TABLE 3.
Mode of ventilation prior to tracheal intubation, type of continuous sedation‐analgesia and associated premedication
|
Spontaneous ventilation (n = 19) |
Non‐invasive ventilation (n = 85) |
Tracheal ventilation (n = 17) |
|||
|---|---|---|---|---|---|
|
Continuous sedation‐analgesia (n = 10) |
Premedication |
Continuous sedation‐analgesia (n = 31) |
Premedication |
Continuous sedation‐analgesia (n = 12) |
Premedication |
| Sufentanil/Midazolam (n = 4) |
Propofol (n = 1) Midazolam (n = 1) None (n = 2) |
Sufentanil/Midazolam (n = 3) | None (n = 3) | Sufentanil/Midazolam (n = 1) | None |
| Sufentanil (n = 3) |
Propofol (n = 1) None (n = 2) |
Sufentanil (n = 11) |
Propofol (n = 2) Fentanyl (n = 1) None (n = 8) |
Sufentanil (n = 3) |
Propofol (n = 1) None (n = 2) |
| Morphine/Midazolam (n = 1) | Propofol (n = 1) | Morphine/Midazolam (n = 8) |
Morphine bolus (n = 2) IV ketamine (n = 2) None (n = 4) |
Morphine/Midazolam (n = 1) | None |
| Morphine (n = 1) | None | Morphine (n = 3) |
Morphine bolus (n = 1) Fentanyl (n = 1) None (n = 1) |
Morphine (n = 4) |
Sufentanil (n = 1) Morphine bolus (n = 1) None (n = 2) |
| Nalbuphine (n = 1) | None | Nalbuphine (n = 1) | Nalbuphine (n = 1) | ||
| Fentanyl (n = 1) | None | ||||
| Midazolam (n = 2) | None (n = 2) | Midazolam (n = 1) | None | ||
| Midazolam/Fentanyl (n = 1) | None | Midazolam/Fentanyl (n = 2) |
Fentanyl/Propofol (n = 1) None (n = 1) |
||
| Sufentanil/Propofol (n = 1) | Propofol (n = 1) | ||||
|
No continuous sedation‐analgesia (n = 9) |
Kétamine/Propofol (n = 1) Morphine (n = 1) Sufentanil/muscle relaxant (n = 1) IN midazolam (n = 1) Nalbuphine/IN midazolam (n = 1) None (n = 4) |
No continuous sedation‐analgesia (n = 54) |
Sufentanil (n = 12) Propofol (n = 7) IV ketamine (n = 5) IV midazolam (n = 2) Fentanyl (n = 2) Fentanyl/midazolam (n = 1) Morphine/Sufentanil (n = 1) Propofol/curare (n = 1) None (n = 43) |
No continuous sedation‐analgesia (n = 5) |
Sufentanil (n = 1) Propofol (n = 1) None (n = 3) |
Abbreviations: IN, Intra Nasal; IV, Intravenous.
4.3. Nonpharmacological analgesia
Comfort measures were applied in 22 cases. In 10 cases, there were associated with a specific analgesia (Premed group) (9 swaddling, 1 swaddling associated with sweet solution and pacifier). In 12 cases (11 swaddling and one sweet solution without pacifier), these measures were the only “analgesic” procedures (No Premed group).
4.4. Parental presence
A parent was present in 4 of these 121 intubations (2 in each group).
4.5. Characteristics of the procedure
During the study period, intubations were equally performed during day or night‐time. Eighty‐three out of 121 (68.6%) TI were considered nonemergent or semi‐emergent and were performed mainly for a respiratory cause (including surfactant therapy), as 86% of patients were on spontaneous ventilation or noninvasive ventilation before the procedure (Table 1). Ten replacement of endotracheal tube were done, and only one of them was considered as emergent TI. The procedure was most often successful after one attempt, but 2 or more attempts were necessary in one out of three cases (Table 1). The main operator was always a medical doctor, assisted by a nurse. The medical degree (junior or senior doctor) was not specified. In the univariate analysis, a trend for a less frequent use of a specific premedication was observed in case of emergent TI (P =0.054), but there were no differences between groups (Premed/No premed) for the number of attempts (Table 1).
4.6. Intravenous access
An intravenous access (umbilical catheter, percutaneous catheter, or peripheral catheter) was available for 113/116 (97.4%, five patients with missing data) neonates.
4.7. Centers
Most of the patients were managed in a NICU (13/16 centers). The 121 TI were performed in 15 centers. Among them, 10 were university hospitals. A specific written premedication protocol was available in 7 (46.7%) centers, including five university hospitals. Premedication rates were low in each center as the highest rate was 45.8% and the lowest was 12.5%. The overall rates of premedication did not seem different among all centers (P = 0.77) (Figure 3) despite a probable variety of practices, or between university or nonuniversity hospitals (P = 0.13) but they were significantly different between those with a specific written protocol and those without (63% vs 37%, respectively, P = 4.10‐4). The emergency degree of the procedure was not statistically different between centers with and without specific written protocol (P = 0.46).
FIGURE 3.
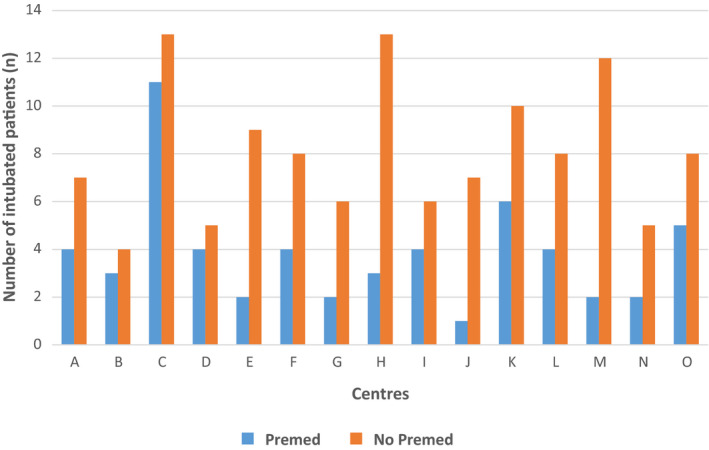
Premedication use before tracheal intubation within centers. Centers A‐C‐D‐F‐K‐N‐O had a specific written protocol
4.8. Factors associated with premedication
After multivariate analysis, nonemergent TI (OR [95% CI]: 5.30 [1.49‐20.80]), the existence of a specific written protocol (OR [95% CI]: 4.80 [2.12‐11.57]), and the absence of a continuous sedation/analgesia infusion before TI (OR [95% CI]: 3.41 [1.46‐8.45]) were significantly associated with a premedication before the procedure (Table 4). When considering the center with a multilevel analysis, we did not observe any center effect with similar odds ratio for the 3 previously identified risk factors: nonemergent TI (OR [95% CI]: 5.29 [1.36‐20.57]), existence of a specific written protocol (OR [95% CI]: 4.48 [1.69‐11.92]), and absence of continuous sedation/analgesia infusion before TI (OR [95% CI]: 3.64 [1.46‐9.06]).
TABLE 4.
Factors associated with a premedication before tracheal intubation in multivariate analysis
| Variable | Crude OR [95% CI] | P value | Adjusted OR [95% CI] | P value |
|---|---|---|---|---|
| GA (weeks) | ||||
| <33 WG | Reference | Reference | ||
| ≥33 WG | 1.001 [0.99‐1.003] | 0.60 | 1.59 [0.70‐3.72] | 0.27 |
| Postnatal age at TI (hours) | ||||
| ≤24 h | Reference | Reference | ||
| >24 h | 1.12 [0.55‐2.30] | 0.75 | 2.05 [0.88‐5.02] | 0.10 |
| Emergency degree of the procedure | ||||
| Emergent TI | Reference | Reference | ||
| Semi‐Emergent TI | 1.92 [0.84‐4.50] | 0.12 | 1.97 [0.79‐5.08] | 0.15 |
| Nonemergent TI | 3.30 [1.07‐10.86] | 0.04 | 5.30 [1.49‐20.8] | 0.012 |
| Continuous sedation‐analgesia | ||||
| Yes | Reference | Reference | ||
| No | 2.26 [1.09‐4.80] | <0.001 | 3.41 [1.46‐8.45] | 0.006 |
| Specific written protocol for TI | ||||
| No | Reference | Reference | ||
| Yes | 2.26 [1.09‐4.80] | 0.001 | 4.80 [2.12‐11.57] | < 0.001 |
Abbreviations: GA, gestational age; GW, gestational weeks; h, hours; TI, tracheal intubation.
4.9. Comparison between EPIPPAIN 1 and EPIPPAIN 2
The population characteristics were similar between the two periods (Table 5 and Ref. 11). A specific premedication was carried out more often in 2005 than in 2011, although this difference was not statistically significant (56% vs 47%, respectively, P = 0.19), but a continuous sedation/analgesia was significantly more frequently infused at the time of TI in 2011 (27/91 (30%) vs 53/121 (44%), respectively, P = 0.035). 12 In both studies, the rate of TI with no form of sedation or analgesia was high: 29% (26/91) in 2005 and 25% (30/121) in 2011. No independent factor was associated with a premedication use in the EPIPPAIN 1 study. 12 At that time, the emergency degree of the procedure was not collected.
TABLE 5.
Comparison of populations and premedication practices between EPIPPAIN 1 and EPIPPAIN 2
| Characteristics | EPIPPAIN 1 | EPIPPAIN 2 | ||
|---|---|---|---|---|
| Year | 2005 | 2011 | ||
| Number of included patients | 430 | 589 | ||
| Number of TI analysed | 91 | 121 |
|
No premed n = 40 (44%) |
Premed n = 51 (56%) |
No premed n = 64 (53%) |
Premed n = 57 (47%) |
|
|---|---|---|---|---|
| Mean GA at birth, weeks (SD) | 32.7 (5) | 32.3 (4.4) | 32.0 (4.5) | 32.37 (4.6) |
| Mean weight at birth, g (SD) | 1927 (1013) | 1795 (875) | 1771 (971) | 1797 (866) |
| Sex (Male, n (%)) | 21 (52 0.5) | 21 (41.2) | 31 (48.4) | 32 (56.1) |
| Procedure performed during daytime [7:00‐18:59], n (%) | 19 (48) | 34 (67) | 30 (48) | 31 (54) |
| Median postnatal age at intubation, days [25th‐75th] | 0.7 [0.5‐4.9] | 2.0 [0.2‐13.1] | 1.0 [0.13‐4.4] | 1.29 [0.25‐7.1] |
| Median number of attempts, n [25th‐75th] | 1 [1‐2] | 1 [1‐2] | 1 [1‐2] | 1 [1‐1] |
| Number of attempts, n (%) | ||||
| 1 | 29 (73) | 34 (67) | 45 (70) | 37 (65) |
| 2 | 6 (15) | 12 (23) | 7 (11) | 11 (19) |
| >2 | 5 (12) | 5 (10) | 12 (19) | 9 (16) |
| Continuous sedation/analgesia, n (%) | 14 (35) | 13 (25) | 34 (53) | 19 (33) |
| Available intravenous access, n (%) | 34 (85) | 51 (100) | 56 (94.9) | 57 (100) |
| Specific written protocol for TI, n (%) | 7 (18) | 10 (20) | 20 (31) | 36 (63) |
| ICU type (NICU/PICU) | 26/14 | 35/16 | 55/9 | 45/12 |
Abbreviations: g, grams; GA, gestational age; ICU (NICU/PICU): intensive care unit (Neonatal/Pediatric); TI, tracheal intubation.
The drugs regimens used changed between the two periods (Figure 4). The use of midazolam, alone or in association, was predominant in the first study followed by fentanyl, sufentanil, and morphine, while in EPIPPAIN 2, propofol, fentanyl, sufentanil, and ketamine were the most used drugs, with a different distribution according to the presence of a protocol or not (Figure 2 and Ref. 12).
FIGURE 4.
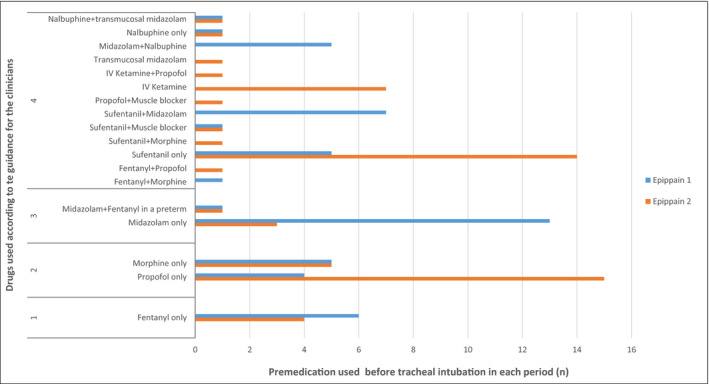
Classification of drugs used for premedication before neonatal TI in EPIPPAIN 1 and EPIPPAIN 2 according to the American Academy of Paediatrics guidance for the clinicians. Legend: 1, Preferred drug; 2, Acceptable drugs; 3, Not recommended drugs; 4, Not described drugs
As compared to EPIPPAIN 1, more units had a written premedication protocol for TI in EPIPPAIN 2 (4/12 (33%) vs 7/15(47%), respectively), but this was not statistically significant (P = 0.92). In 2005, a local written protocol was not associated with a higher premedication rate 12 whereas it was the case in 2011 (see above).
5. DISCUSSION
Despite the 20‐year‐old international consensus statement to use premedication before TI in neonates 6 and unlike what we expected, our results indicate no improvement of specific premedication rates before neonatal TI in the participating ICUs. Instead specific premedication rates decreased from 56% to 47% between 2005 and 2011. 7 , 12 In this study, 25% of TI were still performed without any sedation or analgesia, even though the majority (70%) were non‐ or semi‐urgent and an intravenous access was available for most patients (95% in the non‐Premed group). There were no differences according to the patients’ GA or postnatal age, nor the time shift of the day. Nonetheless, as compared to 2005, the drugs used in 2011 were more consistent with the AAPs guidance for the clinicians, 7 especially in units with protocols which were more numerous in 2011 than in 2005. A specific protocol, a nonemergent TI, and the absence of continuous sedation‐analgesia were the 3 independent factors associated with a higher specific premedication administration in this study, with no identified center effect.
These results are surprising and highlight the difficulties to standardize practices among centers and caregivers for premedication before TI in the neonate. As shown by several declarative or observational studies, 17 , 18 , 19 , 20 the premedication rates for this procedure vary and increase slowly according to the countries and as years go by. 10
Three reasons may explain the difficulties to apply evidence‐based medicine for this procedure, if nonurgent: the absence of a specific protocol, the difficulty to choose the most appropriate drug(s) for a given neonate, and the apparent uselessness and loss of time to apply a premedication for a trained practitioner. In our study, the presence of a protocol was an independent factor for the use of premedication before TI (OR [95% CI] = 4.80 [2.11‐11.57]). The implementation of a premedication algorithm or guideline, even more so if computerized, increases the premedication rate, improves the self‐confidence of the practitioners with the medication regimens used, reduces the team stress, and standardizes the practices in a same unit. 2 , 8 , 21 , 22 To help for the premedication fulfillment and to gain time for the preparation of the drugs, ready‐to‐use kits, as well anticipated prescriptions for the most at risk children could be done in each ICU. 8 As TI is less performed nowadays thanks to noninvasive ventilation, training sessions and standard processes to prepare and administer premedication should be in place in each unit. 8 , 23 Yet, in our study, even in the centers with a specific protocol, the premedication rates were low and did not exceed 45%. This underlines that implementation of a protocol alone may not lead to sustained quality improvement without routine monitoring and ongoing education to ensure effectiveness. 24
This low specific premedication rate could also be explained by an important use of a continuous sedation‐analgesia which increased from 30% to 44% between 2005 and 2011. 12 This rate was high among infants not receiving invasive ventilation and higher than observed elsewhere in Europe. 25 We can speculate that, at least in some case, continuous sedation/analgesia was started just before TI in order to improve the comfort or ease subsequent invasive ventilation. However, such practice without a loading dose or a bolus should not be considered as specific premedication since intubation itself causes acute pain, which should be treated. 26
Despite several guidelines on the drugs to use, there is still no consensus worldwide, and almost no systematic use, for nonemergent TI premedication. 7 , 27 Maybe there cannot be a single universal regimen because of countries’ different habits and availability of drugs, but at least a defined list of drugs to use alone or in association could be established in each country. Medication with rapid onset and short duration of action are preferable, with an association of analgesic and/or hypnotic drugs at anesthetic dose as well as a rapid onset muscle relaxant. 7 , 27 Fentanyl and remifentanil are preferred or acceptable analgesics, even if there are safety considerations for remifentanil. 7 , 27 In our study, fentanyl and sufentanil were the most frequently used opioids, with a decreased use of fentanyl in 2011 as compared to 2005, and a larger use of sufentanil in centers without protocol in 2011. In France, sufentanil is frequently used in neonates but only 2 studies assessed sufentanil for nonemergent TI in neonates and more data are needed to validate its efficacy and safety. 28 , 29 Synthetic opioids can induce chest wall rigidity, which can be prevented by muscle relaxants that have been shown to reduce the risk of adverse effects, decrease the number of attempts and total procedure time. 1 , 7 , 29 , 30 , 31 , 32 Midazolam, which was widely used in EPIPPAIN 1, has been replaced by propofol, especially in centers with a protocol in EPIPPAIN 2. Midazolam alone should not be used for TI, because of its long delay of action and its absence of analgesic effects. Propofol is an acceptable hypnotic agent for TI and has been shown to be a suitable sedative with good tolerance for nonemergent TI. 28 , 33 Its use is spreading among French NICUs/PICUs. In the EPIPPAIN 2 study, ketamine, alone or in association, was more frequently used than in the EPIPPAIN 1 study. Ketamine was not in the 2010 AAPs guidance for the clinicians. 7 At its publication date, there were scarce data on its use in the neonatal population and concerns about its cerebral toxicity, as for other drugs. 34 , 35 , 36 Since then, ketamine has been shown effective in reducing pain and stress due to TI in neonates, mainly in the delivery room. 27 , 37 , 38 Thus, updated guidance might include this drug.
We consider that the often‐used arguments that premedication is not necessary for the practitioner and is a loss of time for performing the procedure must be discarded. Several studies showed that premedication, even more so if associated with a muscle relaxant, decreases the number of attempts and time to achieve the procedure, and subsequently its adverse effects, including among extremely low birth weight infants. 1 , 2 , 4 , 39 , 40 Furthermore, it helps to decrease team stress, self‐confidence for pediatric residents and fellows who perform this procedure less often than before. 8 , 9 , 10 , 21 , 41
Several limitations of this study should be considered. First, the data are 10‐year‐old and since 2011 practices have changed. Second, although no instructions were given to the caregivers to modify their standard of care for pain management, it is possible that they slightly modified their practices. However, the study's duration of six weeks should have limited this bias, and the premedication rate is low. The third limitation is that data were collected in real time for 24 hour a day. Thus, it was impossible for the authors to verify at the point of care the accuracy of all the data entered by caregivers for each procedure. Nonetheless, all the procedures listed in the patient's hospital file were verified and double‐checked with the data forms. Even if specific information on TI was collected, we did not collect the experience of the operator which could be considered as potential risk factors for the absence of premedication, 20 , 39 , 42 neither the time to get first successful attempt nor tolerance or tracheal intubation adverse effects (TIAEs). TIAEs seem to be better indicators of the quality of premedication rather than the number of attempts 1 , 9 , 30 , 43 and further studies on premedication for TI should focus on them.
6. CONCLUSION
Despite international guidance for the clinician and the existing evidence of adverse effects of awake TI and immediate and long‐term effects on pain in the developing brain, a significant percentage of French practitioners do not use premedication prior to this procedure. These results will be presented to each center, as well as in national medical congresses to help neonatologists to become more aware of the state of this subject and the urgent necessity to improve our practices. A qualitative study to try to understand individual and institutional attitudes could also be undertaken. National and international neonatal and pain societies must establish straight guidelines to improve premedication practices before neonatal intubation.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
ETHICAL APPROVAL
This study was approved by the local committee for the protection of human subjects (CPP) and the French Data Protection Authority (CNIL). This study was registered at ClinicalTrials.gov (NCT01346813).
ACKNOWLEDGMENTS
We gratefully acknowledge the contributions of the physicians, nurses, and other healthcare providers at the participating institutions.
We thank the nurse coordinators: Céline Butel, Valérie Dubuche, Anne‐Marie Ferreira, Marie‐France Goiset, Mélanie Goussot, Céline Guiot, Etienne Huraux, Valérie Jolly, Sylvie Lacoste, Audrey Lagarde, Gladys Lajoie, Valérie Maillard, Marie‐Christine Nanquette, Claire Orfèvre, Sandrine Séjourné, and Betty Sgaggero.
We thank Colin Gentile, Juliana Guilheri, Alicia Marzouk, Astrid Polaert, Jessica Rousseau, Dalila Selmane, Dienaba Sylla, Tony Toulorge, and Solange Yuego for assistance with data collection.
We also thank Pierre‐Yves Ancel, PhD, and the Inserm UMR 1153 Obstetrical, Perinatal and Pediatric Epidemiology Research Team (Epopé) Center for Epidemiology and Statistics Sorbonne Paris Cité DHU Risks in pregnancy Paris Descartes University, for counseling and analysis strategy.
Walter‐Nicolet E, Marchand‐Martin L, Guellec I, et al. Premedication practices for neonatal tracheal intubation: Results from the EPIPPAIN 2 prospective cohort study and comparison with EPIPPAIN 1. Paediatr Neonatal Pain. 2021;3:46–58. 10.1002/pne2.12048
Funding information
This study was supported by a grant from the CNP Foundation (Caisse Nationale de Prevoyance‐Assurances, Paris, France) and a grant from the Programme Hospitalier de Recherche Clinique Regional (PHRC regional) 2012 with the file number 136.
The main author received a financial contribution from the Apicil Foundation (Fondation Apicil, Lyon, France) to carry out a master's degree
REFERENCES
- 1. Foglia EE, Ades A, Napolitano N, Leffelman J, Nadkarni V, Nishisaki A. Factors associated with adverse events during tracheal intubation in the NICU. Neonatology. 2015;108(1):23‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dupree Hatch L, Grubb PH, Lea AS, et al. Interventions to improve patient safety during intubation in the neonatal intensive care unit. Pediatrics. 2016;138(4):e20160069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sauer CW, Kong JY, Vaucher YE, et al. Intubation attempts increase the risk for severe intraventricular hemorrhage in preterm infants—a retrospective cohort study. J Pediatr. 2016;177:108‐113. [DOI] [PubMed] [Google Scholar]
- 4. Wallenstein MB, Birnie KL, Arain YH, et al. Failed endotracheal intubation and adverse outcomes among extremely low birth weight infants. J Perinatol. 2016;36(2):112‐115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McAllister JD, Gnauck KA. Rapid sequence intubation of the pediatric patient. Fundamentals of practice. Pediatr Clin North Am. 1999;46(6):1249‐1284. [DOI] [PubMed] [Google Scholar]
- 6. Anand KJS, Aynsley‐Green A, Bancalari E, et al. Consensus statement for the prevention and management of pain in the newborn. Arch Pediatr Adolesc Med. 2001;155(2):173‐180. [DOI] [PubMed] [Google Scholar]
- 7. Kumar P, Denson SE, Mancuso TJ. Premedication for nonemergency endotracheal intubation in the neonate. Pediatrics. 2010;125(3):608‐615. [DOI] [PubMed] [Google Scholar]
- 8. Johnston L, Kwon SH. Moving from controversy to consensus: Premedication for neonatal intubation. J Perinatol. 2018;38(6):611‐613. [DOI] [PubMed] [Google Scholar]
- 9. Foglia EE, Ades A, Sawyer T, et al. Neonatal intubation practice and outcomes: An international registry study. Pediatrics. 2019;143(1):e20180902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mari J, Franczia P, Margas W, Rutkowski J. International consensus is needed on premedication for non‐emergency neonatal intubation after survey found wide‐ranging policies and practices in 70 countries. Acta Paediatr. 2020;109(7):1369‐1375. [DOI] [PubMed] [Google Scholar]
- 11. Carbajal R, Rousset A, Danan C, et al. Epidemiology and treatment of painful procedures in neonates in intensive care units. JAMA. 2008;300(1):60‐70. [DOI] [PubMed] [Google Scholar]
- 12. Durrmeyer X, Daoud P, Decobert F, et al. Premedication for neonatal endotracheal intubation: results from the epidemiology of procedural pain in neonates study. Pediatr Crit Care Med. 2013;14(4):e169‐e175. [DOI] [PubMed] [Google Scholar]
- 13. Caldwell CD, Watterberg KL. Effect of premedication regimen on infant pain and stress response to endotracheal intubation. J Perinatol. 2015;35:415‐418. [DOI] [PubMed] [Google Scholar]
- 14. Sawyer T, Foglia EE, Ades A, et al. Incidence, impact and indicators of difficult intubations in the neonatal intensive care unit: A report from the National Emergency Airway Registry for Neonates. Arch Dis Child Fetal Neonatal Ed. 2019;104(5):F461‐466. [DOI] [PubMed] [Google Scholar]
- 15. Guedj R, Danan C, Daoud P, et al. Does neonatal pain management in intensive care units differ between night and day? An observational study. BMJ Open. 2014;4(2):e004086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. The International Neonatal Network . The CRIB (clinical risk index for babies) score: a tool for assessing initial neonatal risk and comparing performance of neonatal intensive care units. Lancet 1993;342, 8(8883):1365. [PubMed] [Google Scholar]
- 17. Whyte S, Birrell G, Wyllie J. Premedication before intubation in UK neonatal units. Arch Dis Child Fetal Neonatal Ed. 2000;82(1):F38‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chaudhary R, Chonat S, Gowda H, Clarke P, Curley A. Use of premedication for intubation in tertiary neonatal units in the United Kingdom. Paediatr Anaesth. 2009;19(7):653‐658. [DOI] [PubMed] [Google Scholar]
- 19. Sarkar S, Schumacher RE, Baumgart S, Donn SM. Are newborns receiving premedication before elective intubation? J Perinatol. 2006;26(5):286‐289. [DOI] [PubMed] [Google Scholar]
- 20. Muniraman HK, Yaari J, Hand I. Premedication Use before Nonemergent Intubation in the Newborn Infant. Am J Perinatol. 2015;32(9):821‐824. [DOI] [PubMed] [Google Scholar]
- 21. Umoren RA, Sawyer TL, Ades A, et al. Team stress and adverse events during neonatal tracheal intubations: a report from NEAR4NEOS. Am J Perinatol. 2020;37(14):1417‐1424. [DOI] [PubMed] [Google Scholar]
- 22. Fleishman R, Mossabeb R, Menkiti O, Young M, Bains V, Cooperberg D. Transition to routine premedication for nonemergent intubations in a level IV neonatal intensive care unit. Am J Perinatol. 2018;35(4):336‐344. [DOI] [PubMed] [Google Scholar]
- 23. Glenn T, Grathwol M, McClary J, et al. Decreasing time from decision to intubation in premedicated neonates: a quality improvement initiative. Pediatr Qual Saf. 2019;4(6):e234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yaghmai BF, Di Gennaro JL, Irby GA, Deeter KH, Zimmerman JJ. A pediatric sedation protocol for mechanically ventilated patients requires sustenance beyond implementation. Pediatr Crit Care Med. 2016;17(8):721‐726. [DOI] [PubMed] [Google Scholar]
- 25. Carbajal R, Eriksson M, Courtois E, et al. Sedation and analgesia practices in neonatal intensive care units (EUROPAIN): results from a prospective cohort study. Lancet Respir Med. 2015;3(10):796‐812. [DOI] [PubMed] [Google Scholar]
- 26. Cara DM, Norris AM, Neale LJ. Pain during awake nasal intubation after topical cocaine or phenylephrine/lidocaine spray. Anaesthesia. 2003;58(8):777‐780. [DOI] [PubMed] [Google Scholar]
- 27. Ancora G, Lago P, Garetti E, et al. Evidence‐based clinical guidelines on analgesia and sedation in newborn infants undergoing assisted ventilation and endotracheal intubation. Acta Paediatr. 2019;108(2):208‐217. [DOI] [PubMed] [Google Scholar]
- 28. Durrmeyer X, Breinig S, Claris O, et al. Effect of atropine with Propofol vs Atropine with atracurium and sufentanil on oxygen desaturation in neonates requiring nonemergency intubation a randomized clinical trial. JAMA. 2018;319(17):1790‐1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Durrmeyer X, Dahan S, Delorme P, et al. Assessment of atropine‐sufentanil‐atracurium anaesthesia for endotracheal intubation: an observational study in very premature infants. BMC Pediatr. 2014;14:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ozawa Y, Ades A, Foglia EE, et al. Premedication with neuromuscular blockade and sedation during neonatal intubation is associated with fewer adverse events. J Perinatol. 2019;39(6):848‐856. [DOI] [PubMed] [Google Scholar]
- 31. Barrington KJ, Finer NN, Etches PC. Succinylcholine and atropine for premedication of the newborn infant before nasotracheal intubation: a randomized, controlled trial. Crit Care Med. 1989;17(12):1293‐1296. [DOI] [PubMed] [Google Scholar]
- 32. Dempsey EM, Al Hazzani F, Faucher D, Barrington KJ. Facilitation of neonatal endotracheal intubation with mivacurium and fentanyl in the neonatal intensive care unit. Arch Dis Child Fetal Neonatal Ed. 2006;91(4):F279‐F282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ghanta S, Abdel‐Latif ME, Lui K, Ravindranathan H, Awad J, Oei J. Propofol compared with the morphine, atropine, and suxamethonium regimen as induction agents for neonatal endotracheal intubation: a randomized, controlled trial. Pediatrics. 2007;119(6):e1248‐e1255. [DOI] [PubMed] [Google Scholar]
- 34. Bhutta AT. Ketamine: a controversial drug for neonates. Semin Perinatol. 2007;31(5):303‐308. [DOI] [PubMed] [Google Scholar]
- 35. Young C, Jevtovic‐Todorovic V, Qin YQ, et al. Potential of ketamine and midazolam, individually or in combination, to induce apoptotic neurodegeneration in the infant mouse brain. Br J Pharmacol. 2005;146(2):189‐197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Anand KJ, Soriano SG. Anesthetic agents and the immature brain: are these toxic or therapeutic? Anesthesiology. 2004;101(2):527‐530. [DOI] [PubMed] [Google Scholar]
- 37. Barois J, Tourneux P. Ketamine and atropine decrease pain for preterm newborn tracheal intubation in the delivery room: an observational pilot study. Acta Paediatr. 2013;102(12):e534‐e538. [DOI] [PubMed] [Google Scholar]
- 38. Milési C, Baleine J, Mura T, et al. Nasal midazolam vs ketamine for neonatal intubation in the delivery room: a randomised trial. Arch Dis Child Fetal Neonatal Ed. 2018;103(3):F221‐226. [DOI] [PubMed] [Google Scholar]
- 39. Le CN, Garey DM, Leone TA, Goodmar JK, Rich W, Finer NN. Impact of premedication on neonatal intubations by pediatric and neonatal trainees. J Perinatol. 2014;34(6):458‐460. [DOI] [PubMed] [Google Scholar]
- 40. Krick J, Gray M, Umoren R, Lee G, Sawyer T. Premedication with paralysis improves intubation success and decreases adverse events in very low birth weight infants: a prospective cohort study. J Perinatol. 2018;38(6):681‐686. [DOI] [PubMed] [Google Scholar]
- 41. Brady J, Kovatis K, Oaposdea CL, Gray MAA. What Do NICU fellows identify as important for achieving competency in neonatal intubation? Neonatology. 2019;116(1):10‐16. [DOI] [PubMed] [Google Scholar]
- 42. Haubner LY, Barry JS, Johnston LC, et al. Neonatal intubation performance: Room for improvement in tertiary neonatal intensive care units. Resuscitation. 2013;84(10):1359‐1364. [DOI] [PubMed] [Google Scholar]
- 43. Hatch LD, Grubb PH, Lea AS, et al. Endotracheal intubation in neonates: a prospective study of adverse safety events in 162 infants. J Pediatr. 2016;168:62‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]


