Abstract
Atovaquone (also called Mepron, or 566C80) is a napthoquinone used for the treatment of infections caused by pathogens such as Plasmodium spp. and Pneumocystis carinii. The mechanism of action against the malarial parasite is the inhibition of dihydroorotate dehydrogenase (DHOD), a consequence of blocking electron transport by the drug. As an analog of ubiquinone (coenzyme Q [CoQ]), atovaquone irreversibly binds to the mitochondrial cytochrome bc1 complex; thus, electrons are not able to pass from dehydrogenase enzymes via CoQ to cytochrome c. Since DHOD is a critical enzyme in pyrimidine biosynthesis, and because the parasite cannot scavenge host pyrimidines, the drug is lethal to the organism. Oxygen consumption in P. carinii is inhibited by the drug; thus, electron transport has also been identified as the drug target in P. carinii. However, unlike Plasmodium DHOD, P. carinii DHOD is inhibited only at high atovaquone concentrations, suggesting that the organism may salvage host pyrimidines and that atovaquone exerts its primary effects on ATP biosynthesis. In the present study, the effect of atovaquone on ATP levels in P. carinii was measured directly from 1 to 6 h and then after 24, 48, and 72 h of exposure. The average 50% inhibitory concentration after 24 to 72 h of exposure was 1.5 μg/ml (4.2 μM). The kinetics of ATP depletion were in contrast to those of another family of naphthoquinone compounds, diospyrin and two of its derivatives. Whereas atovaquone reduced ATP levels within 1 h of exposure, the diospyrins required at least 48 h. After 72 h, the diospyrins were able to decrease ATP levels of P. carinii at nanomolar concentrations. These data indicate that although naphthoquinones inhibit the electron transport chain, the molecular targets in a given organism are likely to be distinct among members of this class of compounds.
Atovaquone is a member of the hydroxynapthoquinone family of compounds that were developed in the early 1980s for the treatment of malaria and were found to have activity against a broad spectrum of protozoal infections (19, 20, 23) (Fig. 1). Atovaquone has been used for the treatment of mild to moderate Pneumocystis carinii pneumonia in patients with AIDS (9, 20, 23–25); as a single agent or in combination with other compounds as an antimalarial therapy (1, 19, 20); and as an antitoxoplasmal (10) and for the treatment of babesiosis (17). Atovaquone has also been used for P. carinii prophylaxis in patients with AIDS who are not able to tolerate trimethoprim-sulfamethoxazole (TMP-SMX) (15). Resistance to atovaquone has been reported in malaria parasites, and mutations were grouped into four categories based on amino acid changes in a discrete region of the cytochrome b gene that may determine atovaquone binding affinity (33). Sequence polymorphisms in regions of the P. carinii cytochrome b gene implicated in ubiquinone binding (Qo) have been identified in small numbers of patients for whom atovaquone prophylaxis failed, suggesting that drug resistance may also be occurring in these organisms (36). It is then important to understand the mechanism of atovaquone inhibition in P. carinii as a prelude to the development of efficacious analogs.
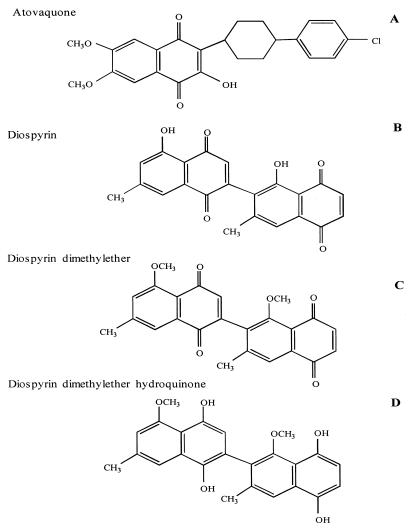
FIG. 1.
Structures of naphthoquinone drugs tested. (A) Atovaquone; (B) diospyrin; (C) diospyrin dimethylether; (D) diospyrin dimethylether hydroquinone.
Atovaquone is a lipophilic compound that has structural similarity to ubiquinone. Ubiquinone (also called coenzyme Q [CoQ]) is a mobile carrier of electrons that plays a pivotal role in cellular respiration by accepting electrons from dehydrogenase enzymes and passing them to the cytochromes of the electron transport chain (35). In addition, ubiquinone functions in ATP synthesis by translocating protons across the inner mitochondrial membrane (6, 18–20). The mechanism of its action in malarial parasites has been shown to be the inhibition of mitochondrial transport at the cytochrome bc1 complex (complex III) and subsequent breakdown of the mitochondrial membrane potential (32). This blockade does not reduce ATP pools in the malarial parasite but results in the repression of pyrimidine biosynthesis by the inhibition of dihydroorotate dehydrogenase (DHOD) (18). Several dehydrogenase enzymes, including DHOD, donate electrons either to CoQ or to the alternate oxidase system in organisms possessing this system (6). Since Plasmodium spp. cannot utilize host pyrimidines, blockage of DHOD function and de novo pyrimidine synthesis (as a consequence of respiratory chain inhibition) is lethal for this parasite (16, 19, 20). This toxicity appears to be selective for the parasite mitochondria, as the host mitochondrial function is not affected by atovaquone therapy.
Pneumocystis carinii f. sp. carinii isolated from methylprednisolone-immunosuppressed rats was shown to contain CoQ10 as the major CoQ homolog (12–14). Further, incorporation of radiolabeled precursors verified ubiquinone synthesis by P. carinii (27, 34). Previous in vitro studies utilizing short-term cultures of P. carinii sustained on cell monolayers showed a reduction in organism numbers after 72 h of treatment with clinically achievable concentrations of atovaquone (4) and a MIC of about 1 μg/ml (30). The 50% inhibitory concentration (IC50) for P. carinii O2 consumption obtained by using a [3H]p-aminobenzoate incorporation assay and a radiometric method (5) was reported as 5 × 10−8 M atovaquone (18, 19). Thus, the respiratory chain was also implicated as the site of action for atovaquone in P. carinii. It was hypothesized that, unlike that of Plasmodium, P. carinii respiration was tightly coupled to oxidative phosphorylation and hence to ATP production, and further, that the organism may salvage host pyrimidines (18, 19). Moreover, unlike Plasmodium DHOD, which is inhibited by 1 nM atovaquone (16), P. carinii DHOD activity was not inhibited by concentrations of ≤10 μM (26). Hence, it was suggested that atovaquone's lethal effect on P. carinii was the direct result of ATP depletion. Although atovaquone and other hydroxynaphthoquinone drugs are recognized as ubiquinone analogs, details on the mechanism by which these drugs inhibit electron transport in various parasites remain unclear.
In the present study, the effects of atovaquone on ATP levels in P. carinii were examined by direct quantification of ATP levels. These effects were compared with those of another group of naphthoquinoid drugs which appear promising as antiparasitic agents, the diospyrins (Fig. 1). Diospyrin, a natural product of Diospyros montana stem bark, and two of its derivatives (21, 22) exhibit activity in vitro against Plasmodium, Leishmania, and Trypanosoma spp. at micromolar concentrations (22, 37).
MATERIALS AND METHODS
P. carinii organisms.
Organisms used for the ATP assays were obtained either from (i) male Brown Norway or Long Evans rats (originally from Charles River, Madison, Wis.; transferred in 1994 to the Cincinnati Veterans Affairs Veterinary Medicine Unit) in which the infection was induced by immunosuppression with injections of methylprednisolone acetate (4 mg/week, given subcutaneously) or (ii) barrier-maintained CD rats (Charles River, Hollister, Calif.), antibody negative to common rodent viruses, which were intratracheally inoculated with form 1 of P. carinii f. sp. carinii (2, 7). Aqueous ammonium chloride was used to lyse host red blood cell contaminants; then organisms were purified using supplemented RPMI 1640 media, gravity sedimentation, filtration through 10-μm-pore-size filters, and centrifugation (3, 7). Organism preparations were immediately prepared for cryopreservation by distribution in supplemented RPMI medium (7) with a final concentration of 10% calf serum and 7.5% dimethyl sulfoxide (DMSO) and were stored in liquid nitrogen (2). Each preparation was evaluated for contaminating microorganisms prior to use by incubation at 35°C in the RPMI medium for 72 h. Preparations that were free of contaminants were used in subsequent assays by rapidly thawing the cryopreservation vials with agitation in a 37°C water bath, centrifuging the preparations to remove the cryoprotectants, and distributing the organisms in fresh supplemented medium, with and without experimental compounds. These preparations contained approximately 5% cystic forms. The ATP values represent the averages of populations of organisms comprising mainly trophic forms and reflect the effects of the compounds on these life cycle stages.
ATP assay.
Isolated organisms used for ATP analyses were suspended in a supplemented RPMI 1640 medium containing 20% calf serum (Summit Biotechnology, Fort Collins, Colo.) and other additives, pH 7.5 to 8.0, 380 mOsm, as previously described (3, 7). Drugs were added to the culture medium in DMSO (the final concentration of DMSO was <0.2%, vol/vol), and 108 organisms (as total nuclei) per ml were added to 1 to 2 ml of the culture medium in multiwell plates. For every assay, each drug concentration was assayed in triplicate. The final ATP content was expressed as the average relative light units of nine values (three readings per well). P. carinii populations were sampled at 1 to 6 h to determine the early effects of atovaquone on the ATP content. To assess the effects of extended exposure to atovaquone and to the diospyrins, the ATP contents of cultures sampled after 24, 48, and 72 h of incubation at 35°C in a 10% CO2 humidified atmosphere were measured. The media of all wells were changed on a daily basis after centrifugation of the multiwell plates at 2,400 × g and removal of the previous medium. The ATP content was determined by the luciferin-luciferase assay (Wallac, Inc., Gaithersburg, Md.) using an AutoLumat LB 953 luminometer (Wallac, Inc., Gaithersburg, Maryland) as described previously and was expressed as relative light units (3, 7). The effects of the compounds on the P. carinii ATP content were compared with the ATP contents of P. carinii populations that did not receive experimental compounds and expressed as percentages of these control values. In addition, other controls for each assay included quench controls to evaluate the effects of the highest drug concentrations used on the luciferase-luciferin reaction; vehicle controls to evaluate the effects of any solvent on the same reaction and on the organism ATP content; and pentamidine isethionate at 0.1 to 10.0 μg/ml as a comparison to experimental drug responses.
Scale of efficacy.
A comparative scale for evaluating the cytotoxic effects of compounds on the ATP levels of P. carinii f. sp. carinii was established after testing of more than 60 agents representing several different classes of compounds (7). The scale was based on the concentration of compound required to reduce the ATP levels by 50% versus levels in the untreated control (IC50) after 24 h of exposure. It was used in the present study as a means to compare the activities of the compounds tested herein with each other and with those previously evaluated. Activities were ranked as follows: very marked, <0.100 μg/ml; marked, 0.100 to 0.999 μg/ml; moderate, 1.000 to 9.999 μg/ml; and none, >10.000 μg/ml. Examples of compounds in the very marked group were potassium cyanide and camptothecin; in the marked group, TMP-SMX and pentamidine; and in the moderately effective group, sulfadoxine. Most agents tested were ineffective in reducing the ATP levels of P. carinii organisms (7).
Analysis.
Descriptive statistical analysis was performed using GraphPad InSTAT 3.0, and histograms were created with GraphPad Prism 3.0.
RESULTS
Effects of atovaquone on P. carinii ATP content over a 3-day period.
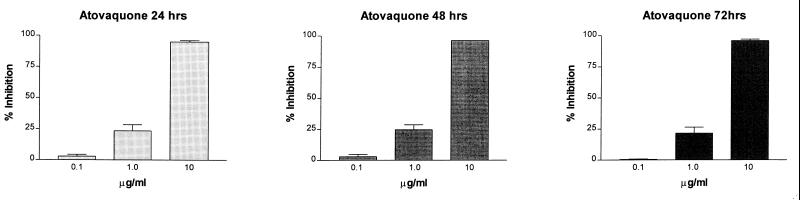
An almost-total reduction in ATP content was observed in P. carinii populations exposed to 10.0-μg/ml concentrations of atovaquone after 24 h (Fig. 2). The same effects were observed at 50 and 100 μg/ml (data not shown). The ATP levels of populations treated with 1.0 μg/ml were reduced by about 25%, whereas 0.1 μg/ml had little to no effect, even after 72 h of exposure. These results were reproducible over several experiments using different P. carinii populations. The IC50s calculated over the 3 days of atovaquone exposure were very comparable: at 24 h, 1.538 μg/ml; at 48 h, 1.393 μg/ml; and at 72 h, 1.679 μg/ml (about 4.2 μM on average) and were similar to the concentration required to reduce organism numbers by 50% in another in vitro system (29). This level of activity would be considered moderate by using the efficacy scale described in Materials and Methods (7). There was no significant increase in inhibition of ATP with any of the atovaquone concentrations after the 24-h time point (P > 0.05). These results indicated that atovaquone was effective in reducing cellular ATP levels in P. carinii populations as early as 24 h after exposure and suggested that the target of the drug was cellular respiration. Studies were then conducted to evaluate the early effects of atovaquone on P. carinii ATP levels to support this contention.
FIG. 2.
Effects of atovaquone on the cellular ATP contents of P. carinii f. sp. carinii populations in vitro, expressed as percent inhibition compared to ATP levels in untreated controls. Bars represent the averages of 12 separate experiments using P. carinii isolated from different individual rats ± standard errors of the means. The three concentrations of atovaquone tested and expressed as 0.1 to 10.0 μg are equivalent to 0.27, 2.72, and 27.2 μM, respectively.
Effects of atovaquone on P. carinii ATP content over a 6-h period.
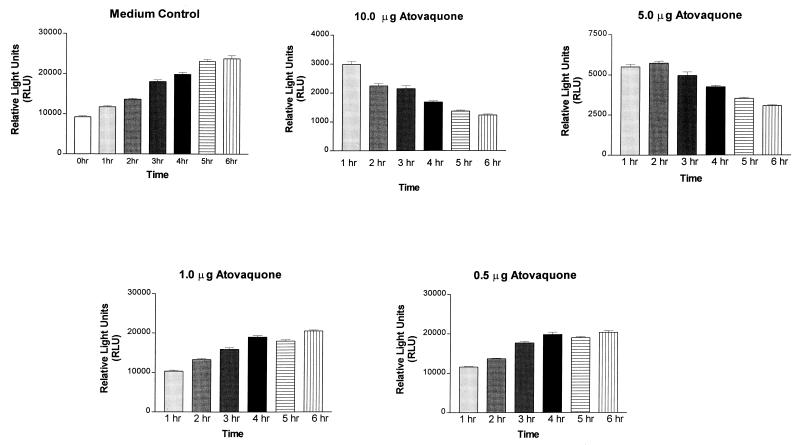
Studies to evaluate the early response of P. carinii to atovaquone were conducted by quantifying the ATP levels of the populations after 1 to 6 h of exposure to the drug (Fig. 3). These data are presented as the relative light units of organism populations, which is the direct assessment of the ATP within each population. The ATP levels of the untreated organisms approximately doubled during the 6-h observation period (Fig. 3, “Medium Control”). There was a significant increase in relative light units at each hourly increment from the time of inoculation to 5 h (P < 0.05). In contrast, ATP levels in P. carinii treated with 10 μg of atovaquone/ml had decreased by 70% after 1 h of exposure and continued to decrease throughout the 6-h period. Treatment with 5 μg/ml initially decreased the ATP levels by about 40%, with a more gradual decline over 6 h than was observed for the organisms treated with 10 μg/ml. At all time points, there were significant differences between the control group and those treated with 5 and 10 μg of atovaquone/ml (P < 0.001). Exposure to 1.0 and 0.5 μg of atovaquone/ml for 4 h caused no significant decreases in the ATP contents of the populations. At 5 and 6 h of exposure, slight but significant decreases were observed at these concentrations (P < 0.001). These studies showed a dose-dependent effect of atovaquone on the ATP pools of P. carinii and support the mechanism of action of this drug to be the organisms' electron transport chain and oxidative phosphorylation.
FIG. 3.
ATP levels of P. carinii populations exposed to varying concentrations of atovaquone over a 6-h period. “Medium control” represents the ATP levels from P. carinii organisms unexposed to experimental compounds. Data are expressed as relative light units and are averages of nine separate readings ± standard errors of the means. The atovaquone concentrations expressed as 10.0 to 0.5 μg are equivalent to 27.2, 13.6, 2.72, and 1.36 μM, respectively.
Effects of diospyrin and its derivatives on P. carinii ATP content over a 3-day period.
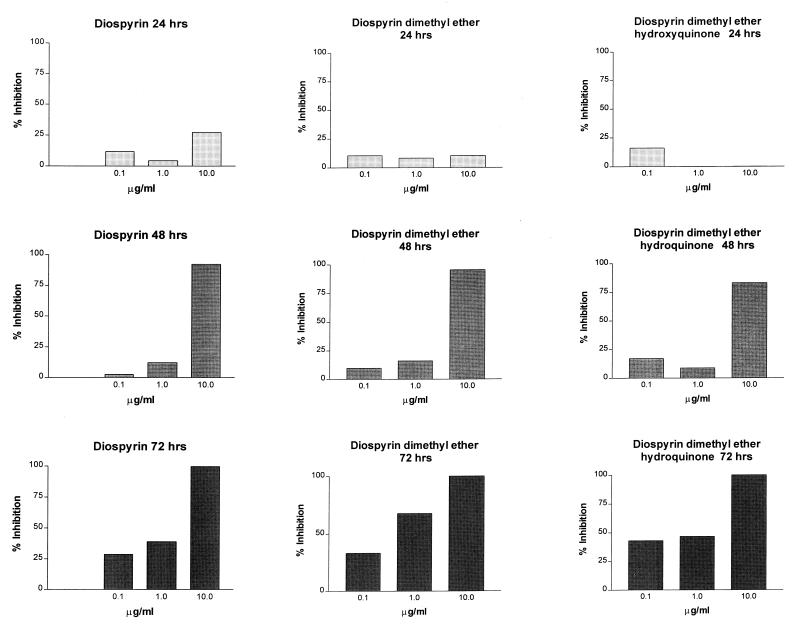
Diospyrin, diospyrin dimethylether, and diospyrin dimethylether hydroquinone were much less effective than atovaquone in reducing ATP levels in the organisms after 24 h of exposure to the drugs (Fig. 4). The degree of inhibition was less than 50% after 24 h for all three of the compounds, and the IC50 could not be calculated. However, exposure to each of the three compounds at 10 μg/ml for 48 h produced a level of ATP reduction comparable to that of atovaquone at the same concentration for the same exposure time. In contrast to the effect of atovaquone, which maintained the same level of ATP reduction over the 3 days of incubation, the inhibitory effects of the diospyrin compounds increased over time, even at the lowest concentration. The IC50s for diospyrin, diospyrin dimethylether, and diospyrin dimethylether hydroquinone at 48 h were 2.089, 1.67, and 2.59 μg/ml, respectively, which were slightly greater than that for atovaquone but would also be considered to indicate moderate activity on the efficacy scale. On a molar basis, atovaquone was more efficacious than the diospyrin compounds at this time point. However, after 72 h of exposure, the IC50s for the same three compounds were markedly reduced at 0.69, 0.31, and 0.34 μg/ml, respectively. The same trend was observed with the molar equivalents (Table 1). Such values would be ranked as having a marked effect on P. carinii ATP contents, comparable to the activity of known anti-P. carinii compounds such as pentamidine and TMP-SMX (7).
FIG. 4.
Effects of diospyrin compounds on the cellular ATP contents of P. carinii f. sp. carinii populations in vitro, expressed as percent inhibition compared to ATP levels in untreated controls. Bars represent the average results from a representative experiment performed in triplicate. The micromolar equivalents are 0.37, 3.74, and 37.4 μM for diospyrin; 0.40, 4.02, and 40.2 μM for diospyrin dimethylether; and 0.41, 4.06, and 40.6 μM for diospyrin dimethylether hydroxyquinone.
TABLE 1.
IC50s for atovaquone and the diospyrin-based drugs following different exposure times
| Drug | IC50 expressed as μg/ml (μM) at the following exposure time (h):
|
||
|---|---|---|---|
| 24 | 48 | 72 | |
| Atovaquone | 1.54 (4.10) | 1.39 (3.80) | 1.68 (4.58) |
| Diospyrin | NDa | 2.089 (7.80) | 0.69 (2.60) |
| Diospyrin dimethylether | ND | 1.67 (6.71) | 0.31 (1.25) |
| Diospyrin dimethylether hydroquinone | ND | 2.59 (10.50) | 0.34 (1.40) |
ND, not determined.
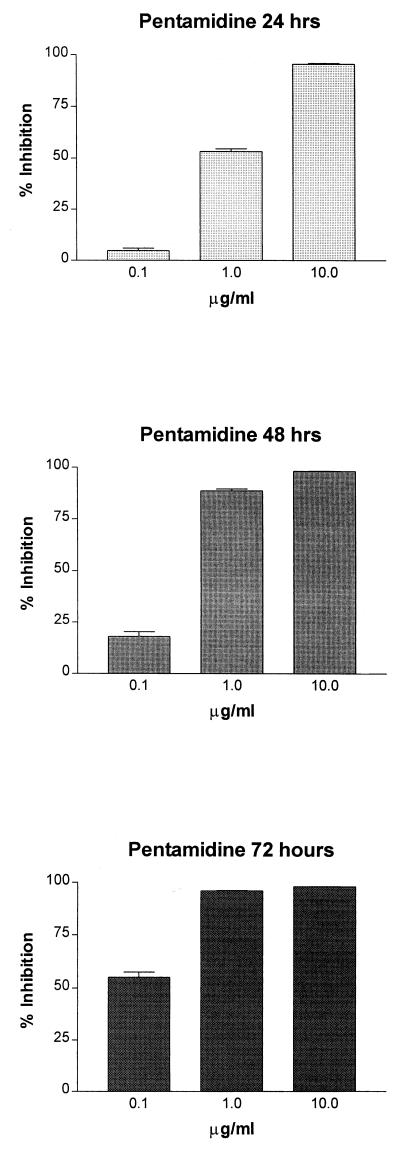
Effects of pentamidine on P. carinii ATP content over a 3-day period.
Pentamidine isethionate, a cationic diamidine, is a standard therapy for P. carinii pneumonia and was used as a positive control for evaluation of drug efficacy in all of our screening assays. We have previously shown that it exerted an inhibitory effect on P. carinii ATP levels as early as 1 h after exposure and that this effect increased over time (7). In contrast to the effects of atovaquone, pentamidine was more effective at lower concentrations, and the inhibitory effect on the ATP levels of P. carinii increased over time at 0.1 and 1.0 μg/ml (Fig. 5). In the present series of studies, the IC50s for pentamidine at the 24-, 48-, and 72-h time points were 0.957 μg/ml (1.61 μM), 0.350 μg/ml (0.59 μM), and 0.029 μg/ml (0.05 μM), respectively. These values would be considered as achieving a marked effect on the ATP levels after the first 48 h of exposure and a very marked effect after 72 h, the highest activity level for a compound according to our previously reported ranking system (7). Such inhibition was comparable to the effects of the known respiratory inhibitors, antimycin A and 2,4-dinitrophenol, on P. carinii ATP pools (7). The early effects of both atovaquone and pentamidine suggest that they are active against the respiratory chain of the mitochondrion, but the kinetics of ATP inhibition suggest that pentamidine may have additional targets that enhance the decrease in ATP levels over time.
FIG. 5.
Effects of pentamidine on the cellular ATP contents of P. carinii f. sp. carinii populations in vitro, expressed as percent inhibition compared to ATP levels in untreated controls. Bars represent the averages ± standard errors of the means of three to nine separate experiments using different P. carinii populations. The micromolar equivalents of the concentrations of pentamidine evaluated are 0.169, 1.69, and 16.87 μM.
DISCUSSION
Effect of atovaquone on P. carinii ATP levels.
Atovaquone is effective in the treatment of mild to moderate cases of P. carinii pneumonia (9, 19, 25). Gutteridge (18) predicted that oxidative phosphorylation activity is high in P. carinii and that it is tightly coupled to electron transport and ATP synthesis in the organism. Hence, unlike the situation in Plasmodium, in which disruption of electron transport results in the inhibition of de novo pyrimidine biosynthesis, the consequence of blocking electron transport in P. carinii would be expected to be the reduction of ATP synthesis and the eventual death of the organism. By performing direct measurements of the ATP content of isolated and purified organism preparations, we demonstrated that the inhibitory effects of the drug involve ATP depletion. Whether the effect on the organisms is a lethal one cannot be addressed with the current limitations of in vitro culture systems for P. carinii. Inoculation of the treated P. carinii directly into susceptible rats is also made difficult by recent evidence that fewer than 10 organisms are required to initiate infection (8).
Atovaquone exerted ATP-depleting effects as early as 1 h after exposure to intact P. carinii organism populations at 27.2 and 13.6 μM concentrations. After 5 to 6 h of exposure, lower concentrations (2.72 and 1.36 μM) reduced the ATP pools slightly but significantly. The average IC50 of atovaquone on P. carinii cellular ATP contents at 24, 48, or 72 h was approximately 1.54 μg/ml, or 4.2 μM. It was previously reported that the drug inhibited P. carinii respiration (measured polarographically) at an IC50 of 50 nM (18, 19). Since it is known that the activity of atovaquone in Plasmodium is the consequence of binding to the mitochondrial cytochrome bc1 complex (16, 18, 19), and that it apparently also binds to the P. carinii bc1 complex (19), it is not surprising that inhibition of P. carinii respiration was observed before ATP depletion was detected. Other biosynthetic cycles, such as glycolysis, also produce ATP and could contribute to intracellular pools. Reduction of P. carinii growth in primary culture was observed with atovaquone at 3 μM but not at 0.3 μM (26). The difference between the 3 μM concentration required for growth inhibition in the previous studies and the 4.2 μM concentration required for ATP depletion in the present study is likely due to differences in organism isolation, culture conditions, and methods of assessment.
Effects of diospyrin, diospyrin dimethylether, and diospyrin dimethylether hydroquinone on P. carinii.
It has been suggested that other quinoid drugs with antiparasite activity, as well as quinoid metabolites of other drugs (e.g., primaquine), may also block electron transport by functioning as analogs of ubiquinone (18). Of the napthoquinone drugs tested in this study, atovaquone was the most effective in reducing ATP levels after 24 h, but this inhibitory effect then remained stable through the duration of the 3-day study. In contrast, the diospyrin-based quinoid drugs were ineffective in reducing cellular ATP levels after 24 h of exposure. These compounds were able to reduce ATP pools in P. carinii only after 48 h of exposure, and this effect dramatically increased after 72 h of exposure. All of the diospyrin compounds had greater activity, expressed as the IC50, than atovaquone at the 72-h time point (Table 1). When considered individually, the diospyrin dimethylether analog exhibited greater activity against P. carinii than the other quinoid compounds at 48 and 72 h of exposure (Table 1). The same compounds were tested against Leishmania donovani, Trypanosoma cruzi, and Trypanosoma brucei brucei using enumeration of parasites after 5 days in culture to determine the 50% effective doses (ED50) (37). The parent compound, diospyrin, and the dimethylether were ineffective in inhibiting the replication of L. donovani but could reduce the growth of both trypanosome species at 27 to 50 μM concentrations for diospyrin and at 2 to 17 μM for the dimethyl derivative. The hydroxyquinoid derivative was effective against L. donovani at 2.2 μM and against T. brucei at 0.7 μM. These concentrations do not dramatically deviate from those required for inhibition of P. carinii ATP pools in the present study. Unlike the selective effects of the diospyrin compounds observed in the parasite study, all of these compounds were effective against P. carinii ATP pools after 48 h, suggesting that the targets may not be shared among the affected organisms. Of interest was the reported ED50 of 0.02 μM for pentamidine after 5 days of exposure in the T. brucei study (36), where it was also included as a control compound. In the present study, exposure of P. carinii populations to pentamidine for 3 days resulted in a similar IC50 of 0.05 μM. As in the parasite studies, we found pentamidine to be superior to any of the napthoquinones evaluated in the present study in depleting the ATP pools of P. carinii.
Exposure of P. carinii to pentamidine or the diospyrins caused a continual decrease in ATP levels over time. In contrast, the decrease in ATP levels at all concentrations of atovaquone remained the same from 24 to 72 h. Since all the compounds targeted the electron transport chain, as evidenced by the decrease in ATP, these differences in the kinetics likely involve effects on other cellular processes. Both pentamidine (11) and the diospyrins (31) have been reported to inhibit protistan topoisomerase activity. Topoisomerases are involved in many cellular processes and are classified into two groups by virtue of the nicks made in single-stranded (type I) or double-stranded (type II) DNA. These breaks relax the DNA strands during replication and chromosomal separation. Since P. carinii replicates very slowly in the in vitro setting, deleterious effects from the inhibition of topoisomerases may require longer exposure to manifest. Previous studies using the present system have shown P. carinii to be highly susceptible to camptothecin, another type I topoisomerase inhibitor, and it is thus possible that collateral effects of the compounds tested herein could be detected (7). Although topoisomerase activity has not been reported for atovaquone, Kaneshiro et al. (28) recently reported the effect of atovaquone on ubiquinone biosynthesis in P. carinii. At the concentrations used in the present study, atovaquone inhibited the incorporation of p-hydroxybenzoate into ubiquinone in vitro, but the diospyrin compounds did not exhibit this inhibition. Therefore, targeting of the diospyrins and pentamidine to cellular processes outside of the respiratory chain may have contributed to the cumulative ATP decreases.
We have shown that atovaquone directly decreases the ATP levels in P. carinii populations maintained in a short-term in vitro system, and this depletion occurs as early as 1 h after exposure. Diospyrin and its dimethylether and dimethylether hydroxyquinone analogs also decrease the ATP levels of P. carinii, but only after more-extended exposure. The differences observed in the kinetics of inhibition could involve the transport of the compounds and/or distinct mechanisms of action for reducing P. carinii ATP contents that might involve both primary and secondary targets. Since there are very few known targets for the design of chemotherapeutic agents against P. carinii, the use of combinations of compounds targeting the biosynthesis of ATP and other cellular processes seems a viable strategy and will be pursued in future studies.
ACKNOWLEDGMENTS
This work was supported by NIH/NIAID grants NO1 AI75319, RO1 AI38167 (MTC), RO1 AI29316, and RO1 AI38758 (E.S.K.); the Medical Research Service, Department of Veterans Affairs (M.T.C.); and the International Foundation for Science, Stockholm grant F/1836 (B.H.).
REFERENCES
- 1.Anabwani G, Canfield C J, Hutchinson D B. Combination atovaquone and proguanil hydrochloride vs. halofantrine for treatment of acute Plasmodium falciparum malaria in children. Pediatr Infect Dis J. 1999;18:456–461. doi: 10.1097/00006454-199905000-00011. [DOI] [PubMed] [Google Scholar]
- 2.Boylan C J, Current W L. Improved model of Pneumocystis carinii pneumonia: induced laboratory infection in Pneumocystis-free animals. Infect Immun. 1992;60:1589–1597. doi: 10.1128/iai.60.4.1589-1597.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen F, Cushion M T. Use of an ATP bioluminescent assay to evaluate viability of Pneumocystis carinii from rats. J Clin Microbiol. 1994;32:2791–2800. doi: 10.1128/jcm.32.11.2791-2800.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cirioni O, Giacometti A, Scalise G. In vitro activity of atovaquone, sulphamethoxazole and dapsone alone and combined with inhibitors of dihydrofolate reductase and macrolides against Pneumocystis carinii. J Antimicrob Chemother. 1997;39:45–51. doi: 10.1093/jac/39.1.45. [DOI] [PubMed] [Google Scholar]
- 5.Comley J C W, Mullin R J, Wolfe L A, Hanlon M H, Ferone R. A radiometric method for objectively screening large numbers of compounds against Pneumocystis carinii in vitro. J Protozool. 1991;38:144S–146S. [PubMed] [Google Scholar]
- 6.Crane F L. Development of concepts for the role of ubiquinones in biological membranes. In: Lenaz G, Barnabei O, Rabbi A, Battino M, editors. Highlights in ubiquinone research. London, United Kingdom: Taylor & Francis; 1990. pp. 3–17. [Google Scholar]
- 7.Cushion M T, Chen F, Kloepfer N. A cytotoxicity assay for evaluation of candidate anti-Pneumocystis agents. Antimicrob Agents Chemother. 1997;41:379–384. doi: 10.1128/aac.41.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cushion M T, Linke M J, Collins M, Keely S P, Stringer J R. The minimum number of Pneumocystis carinii f. sp. carinii organisms required to establish infections is very low. J Eukaryot Microbiol. 1999;46:111S. [PubMed] [Google Scholar]
- 9.Dohn M N, Frame P T, Baughman R P, Lafon S W, Smulian A G, Caldwell P, Rogers M D. Open-label efficacy and safety trial of 42 days of 566C80 for Pneumocystis carinii pneumonia in AIDS patients. J Eukaryot Microbiol. 1992;38:220S–221S. [PubMed] [Google Scholar]
- 10.Djurkovic-Djakovic O, Nikolic T, Robert-Gangneux F, Bobic B, Nikolic A. Synergistic effect of clindamycin and atovaquone in acute murine toxoplasmosis. Antimicrob Agents Chemother. 1999;43:2240–2244. doi: 10.1128/aac.43.9.2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dykstra C C, Tidwell R R. Inhibition of topoisomerases from Pneumocystis carinii by aromatic dicationic molecules. J Protozool. 1991;6:78S–81S. [PubMed] [Google Scholar]
- 12.Ellis J E. Coenzyme Q homologs in parasitic protozoa as targets for chemotherapeutic attack. Parasitol Today. 1994;10:296–301. doi: 10.1016/0169-4758(94)90079-5. [DOI] [PubMed] [Google Scholar]
- 13.Ellis J E, Setchell K D, Kaneshiro E S. Detection of ubiquinone in parasitic and free-living protozoa, including species devoid of mitochondria. Mol Biochem Parasitol. 1994;65:213–224. doi: 10.1016/0166-6851(94)90073-6. [DOI] [PubMed] [Google Scholar]
- 14.Ellis J E, Wyder M A, Zhou L, Gupta A, Rudney H, Kaneshiro E S. Composition of Pneumocystis carinii neutral lipids and identification of coenzyme Q10 as the major ubiquinone homolog. J Eukaryot Microbiol. 1996;43:154–170. doi: 10.1111/j.1550-7408.1996.tb01385.x. [DOI] [PubMed] [Google Scholar]
- 15.El Sadr W M, Murphy R L, Yurik T M, Luskin-Hawk R, Cheung T W, Balfour Jr H H, Eng R, Hooton T M, Kerkering T M, Schutz M, van der Horst C, Haffner R. Atovaquone compared with dapsone for the prevention of Pneumocystis carinii pneumonia in patients with HIV infection who cannot tolerate trimethoprim, sulfonamides, or both. N Engl J Med. 1998;339:1889–1895. doi: 10.1056/NEJM199812243392604. [DOI] [PubMed] [Google Scholar]
- 16.Fry M, Pudney M. Site of action of the antimalarial hydroxynaphthoquinone, 2-[trans-4-(4′-chlorophenyl) cyclohexyl]-3-hydroxy-1,4- naphthoquinone (566C80) Biochem Pharmacol. 1992;43:1545–1553. doi: 10.1016/0006-2952(92)90213-3. [DOI] [PubMed] [Google Scholar]
- 17.Gray J S, Pudney M. Activity of atovaquone against Babesia microti in the Mongolian gerbil, Meriones unguiculatus. J Parasitol. 1999;85:723–728. [PubMed] [Google Scholar]
- 18.Gutteridge W E. Pneumocystis carinii: potential targets for chemotherapeutic attack. In: Coombs G H, North M J, editors. Biochemical protozoology. London, United Kingdom: Taylor & Francis; 1991. pp. 35–51. [Google Scholar]
- 19.Gutteridge W E. 566C80, an antimalarial hydroxynaphthoquinone with broad spectrum: experimental activity against opportunistic parasitic infections of AIDS patients. J Protozool. 1991;38:141S–143S. [PubMed] [Google Scholar]
- 20.Haile L G, Flaherty J F. Atovaquone: a review. Ann Pharmacother. 1993;27:1488–1494. doi: 10.1177/106002809302701215. [DOI] [PubMed] [Google Scholar]
- 21.Hazra B, Pal S, Ghosh R, Banerjee A. Studies on changes in tumour-inhibitory activities through structural modification of a diospyrin derivative. Med Sci Res. 1994;22:621–623. [Google Scholar]
- 22.Hazra B, Ghosh R, Banerjee A, Kirby G C, Warhurst D C, Phillipson J D. In vitro antiplasmodial effects of diospyrin, a plant-derived naphthoquinoid, and a novel series of derivatives. Phytother Res. 1995;9:72–74. [Google Scholar]
- 23.Hudson A T, Dickins M, Ginger C D, Gutteridge W E, Holdich T, Hutchinson D B, Pudney M, Randall A W, Latter V S. 566C80: a potent broad spectrum anti-infective agent with activity against malaria and opportunistic infections in AIDS patients. Drugs Exp Clin Res. 1991;17:427–435. [PubMed] [Google Scholar]
- 24.Hughes W T, Gray V L, Gutteridge W E, Latter V S, Pudney M. Efficacy of a hydroxynaphthoquinone, 566C80, in experimental parasitic infections of AIDS pneumonitis. Antimicrob Agents Chemother. 1990;34:225–228. doi: 10.1128/aac.34.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hughes W T. The role of atovaquone tablets in treating Pneumocystis carinii pneumonia. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;8:247–252. doi: 10.1097/00042560-199503010-00005. [DOI] [PubMed] [Google Scholar]
- 26.Ittarat I, Asawamahasakda W, Bartlett M S, Smith J W, Meshnick S R. Effects of atovaquone and other inhibitors on Pneumocystis carinii dihydroorotate dehydrogenase. Antimicrob Agents Chemother. 1995;39:325–328. doi: 10.1128/aac.39.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaneshiro E S, Ellis J E, Zhou L H, Rudney H, Gupta A, Jayasimhulu K, Setchell K D R, Beach D H. Isoprenoid metabolism in Pneumocystis carinii. J Eukaryot Microbiol. 1994;41:93S. [PubMed] [Google Scholar]
- 28.Kaneshiro E S, Sul D, Hazra B. Effects of atovaquone and diospyrin-based drugs on ubiquinone biosynthesis in Pneumocystis carinii organisms. Antimicrob Agents Chemother. 2000;44:14–18. doi: 10.1128/aac.44.1.14-18.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mulenga M, Sukwa T Y, Canfield C J, Hutchinson D B. Atovaquone and proguanil vs. pyrimethamine/sulfadoxine for the treatment of acute falciparum malaria in Zambia. Clin Ther. 1999;21:841–852. doi: 10.1016/s0149-2918(99)80006-x. [DOI] [PubMed] [Google Scholar]
- 30.Queener S F, Bartlett M S, Richardson J D, Durkin M M, Jay M A, Smith J W. Activity of clindamycin with primaquine against Pneumocystis carinii in vitro and in vivo. Antimicrob Agents Chemother. 1988;32:807–813. doi: 10.1128/aac.32.6.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ray S, Hazra B, Mittra B, Das A, Majumder H K. Diospyrin, a bisnaphthoquinone: a novel inhibitor of type I DNA topoisomerase of Leishmania donovani. Mol Pharmacol. 1998;34:994–999. doi: 10.1124/mol.54.6.994. [DOI] [PubMed] [Google Scholar]
- 32.Srivastava I K, Rottenberg H, Vaidya A B. Atovaquone, a broad spectrum antiparasitic drug, collapses mitochondrial membrane potential in a malarial parasite. J Biol Chem. 1997;272:3961–3966. doi: 10.1074/jbc.272.7.3961. [DOI] [PubMed] [Google Scholar]
- 33.Srivastava I K, Morrisey J M, Darrouzet E, Daldal F, Vaidya A B. Resistance mutations reveal the atovaquone-binding domain of cytochrome b in malaria parasites. Mol Microbiol. 1999;33:704–711. doi: 10.1046/j.1365-2958.1999.01515.x. [DOI] [PubMed] [Google Scholar]
- 34.Sul D, Kaneshiro E S. Ubiquinone synthesis by Pneumocystis carinii: incorporation of radiolabeled polyprenyl chain and benzoquinone ring precursors. J Eukaryot Microbiol. 1997;44:60S. doi: 10.1111/j.1550-7408.1997.tb05780.x. [DOI] [PubMed] [Google Scholar]
- 35.Sun I L, Sun E E, Crane F L, Morré D J, Lindgren A, Low H. Requirement for coenzyme Q in plasma membrane electron transport. Proc Natl Acad Sci USA. 1992;89:11126–11130. doi: 10.1073/pnas.89.23.11126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walker D J, Wakefield A E, Dohn M N, Miller R F, Baughman R P, Hossler P A, Bartlett M S, Smith J W, Kazanjian P, Meshnick S R. Sequence polymorphisms in the Pneumocystis carinii cytochrome b gene and their association with atovaquone prophylaxis failure. J Infect Dis. 1998;178:1767–1775. doi: 10.1086/314509. [DOI] [PubMed] [Google Scholar]
- 37.Yardley V, Snowdon D, Croft S, Hazra B. In vitro activity of diospyrin and derivatives against Leishmania donovani, Trypanosoma cruzi and Trypanosoma brucei brucei. Phytother Res. 1996;10:559–562. [Google Scholar]