Abstract
The advent of increasingly sophisticated medical technology, surgical interventions, and supportive healthcare measures is raising survival probabilities for babies born premature and/or with life‐threatening health conditions. In the United States, this trend is associated with greater numbers of neonatal surgeries and higher admission rates into neonatal intensive care units (NICU) for newborns at all birth weights. Following surgery, current pain management in NICU relies primarily on narcotics (opioids) such as morphine and fentanyl (about 100 times more potent than morphine) that lead to a number of complications, including prolonged stays in NICU for opioid withdrawal. In this paper, we review current practices and challenges for pain assessment and treatment in NICU and outline ongoing efforts using Artificial Intelligence (AI) to support pain‐ and opioid‐sparing approaches for newborns in the future. A major focus for these next‐generation approaches to NICU‐based pain management is proactive pain mitigation (avoidance) aimed at preventing harm to neonates from both postsurgical pain and opioid withdrawal. AI‐based frameworks can use single or multiple combinations of continuous objective variables, that is, facial and body movements, crying frequencies, and physiological data (vital signs), to make high‐confidence predictions about time‐to‐pain onset following postsurgical sedation. Such predictions would create a therapeutic window prior to pain onset for mitigation with non‐narcotic pharmaceutical and nonpharmaceutical interventions. These emerging AI‐based strategies have the potential to minimize or avoid damage to the neonate's body and psyche from postsurgical pain and opioid withdrawal.
Keywords: neonatal intensive care unit, neonatal pain assessment, neonatal pain prediction, newborn pain management, opioid‐based pain management
1. INTRODUCTION
For newborns of all birth weights in the United States, there is a trend toward the increased likelihood of admission to the neonatal intensive care unit (NICU). 1 , 2 The availability of highly specialized care for treatment of various healthcare emergencies raises the probability that premature and sick infants will survive. However, hospitalization of newborns for life‐threatening illnesses requires repeated episodes of acute and/or prolonged pain from surgery and other types of tissue trauma. 3 A major challenge for the scientific community is to mitigate the adverse effects of postsurgical pain on newborns, given their inability to verbally express pain, the vulnerability of their developing nervous system, and the effects of pain and pain management on the structural and functional changes that take place during the early neonatal period.
Before 1980, pain in newborns was for the most part discounted, ignored, or under‐treated 4 due to several factors, including the lack of a working definition for pain in newborns; and, self‐reporting as the gold standard for pain assessment, which is clearly not possible for newborns in NICU where only indirect pain assessment is possible. 5 , 6 In contrast to assumptions that the undeveloped neural pathways of newborns do not support the perception or recall of perinatal pain, substantial basic and clinical research confirms that pain triggers a range of chemical, behavioral, and morphological changes in the neonate's developing nervous system. 7 , 8 , 9 Recent studies using functional magnetic resonance imaging (fMRI) confirm that pro‐pain brain regions undergo similar activation patterns in infants as in adults. 10 , 11 Consistent with these findings is the evolutionary notion that pain responses reflect the newborn's ability to adapt, as needed, to ensure the organism's survival.
The categorical imperative for minimizing pain and its associated toxic stress in neonates is now self‐ evident. 7 , 12 , 13 , 14 , 15 , 16 By 2010, the most common drugs used to treat postsurgical pain and anxiety in the NICU were the highly addictive narcotics (opioids) that require prolonged withdrawal prior to discharge and a range of non‐narcotic benzodiazepines, barbiturates, ketamine, propofol, acetaminophen, and local and topical anesthetics. 17 Today, the opioids morphine and fentanyl, a fast‐acting narcotic that are 20‐40× and 100× more potent than heroin and morphine, respectively, remain the cornerstone drugs for the therapeutic management of postsurgical pain in NICUs worldwide. 18
Preterm births account for about 15 million newborns worldwide, or about 10% of all births 19 annually. In the United States, a similar fraction of about 380 000 out of 3.8 million live births are assessed early in terms of gestational age (<37 weeks) or low birth weight (<2500 g). 15 , 20 Preterm and term babies born with mild, moderate, and serious healthcare issues typically spend their first weeks after birth in the NICU. To some extent, remarkable though rare technological achievements of the past four decades confer realistic possibilities for survival even for preterm babies born as early as 23 weeks gestational age. 15 Equally remarkable are the high costs for this high survival rate in terms of days in the NICU, which average about five days but in cases with severe complications can climb to 80 days and beyond. 3 , 21 In the United States, the financial costs for NICU care of preterm births average about $78 000 per birth, or an annual total cost of about $14 billion nationally. 22
Figure 1 shows the number of babies (per 1000 births) in specific regions of North America (United States, Canada) born from 2000 to 2014 with Neonatal Opioid Withdrawal Syndrome (NOWS). These data collected from different sources 23 , 24 , 25 , 26 show clear evidence of increasing opioid addiction in mothers and cases of NOWS in their babies. Though based on fewer and less comprehensive studies (data not shown), the numbers of newborns with NOWS stayed stable or diminished in England and Australia, respectively, during roughly the same years (for more details, see 23 , 24 , 25 , 26 ). Given the evidence showing the harmful effects of opioid withdrawal to the developing brain, as discussed in the following section, these trends emphasize the importance to explore novel opioid‐sparing strategies for the therapeutic management of neonatal pain in NICU, especially those in North America.
FIGURE 1.
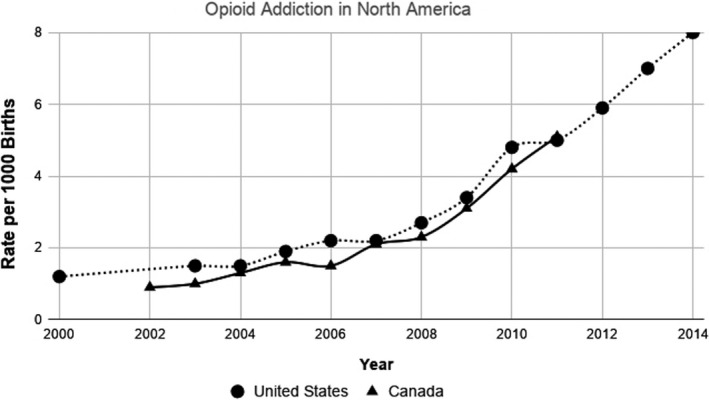
Comparison of NOWS incidents per 1000 births in North America
During the past four decades, animal and human studies have documented the toxic stress of opioid exposure and withdrawal in neonates. These studies indicate that opioids, which act by binding (stimulating) the endogenous opioid receptors in the neonate's nervous system, cause a long‐term and likely permanent disruption of developing newborn's brain structure and function, including reduced brain volume; decreased neuronal packing density, and impaired dendritic growth 27 ; impaired learning ability 28 ; and, reductions in locomotor activity. 29 As expected, opioid antagonists such as naltrexone that block the mu receptor exert opposite effects—increases in brain size, dendritic arborization, and numbers of dendritic spines, 30 indicating that stimulation of opioid receptors by endogenous opioids (enkephalins and endorphins) plays a critical role in neuronal differentiation, synaptic pruning, and dendritic growth during early neurodevelopment. 31 Further studies in animals support the view that overuse of opioids has long‐term consequences on newborns 32 in terms of a heightened risk for neurodevelopmental abnormalities 33 , 34 , 35 including potential sex differences with preterm males more vulnerable to aberrant neurodevelopment trajectories 36 and preterm females more likely to experience slowed brain development. 31 , 37 Notably, these adverse neurodevelopmental effects add to other well‐known nonanalgesic complications of opioid treatment on postsurgical neonates, that is, reduced time to feeding, constipation, sedation, respiratory depression, and nausea, as well delays in wound healing. 38 , 39 , 40 , 41 , 42 , 43 For instance, Rook et al 39 found that the topical application of morphine gel resulted in delayed wound closure in animals in a dose‐dependent manner.
Substantial evidence indicates that opioid‐based treatments of postsurgical pain in human newborns alter brain morphology and disrupt at least some cognitive and sensorimotor domains in later life. Ferguson et al 35 reported differences at term‐equivalent ages and during childhood in head circumference for 14 morphine‐treated and five placebo‐treated children born at 23‐32 weeks of gestation. By 5‐7 years, short‐term memory was significantly worse in children treated with morphine compared with those treated with placebo. 35 A recent neuroimaging study 44 in preterm infants (24‐32 weeks) found that greater neonatal exposure to morphine was associated with smaller cerebellar volume than at term‐equivalent ages and that greater morphine exposure led to poorer cognitive and motor outcomes in the same children at 18 months. Another 2018 study 45 showed strong negative correlations between neonatal morphine exposure and volumes of pain‐related brain regions, total gray volume, and cerebral white matter at school age in children born preterm at 26‐36 weeks of gestation. In addition to morphine, fentanyl also has disruptive effects on the brains of developing newborns. In preterm infants (23‐30 weeks), a higher cumulative fentanyl dose was associated with a higher incidence of cerebellar hemorrhage, as well as lower cerebellar diameter. 46 However, this study and another 47 found no relation between cumulative fentanyl dose and developmental outcome assessed at two years of age. This finding may be due to the fact that only general mental functioning can be assessed at this young age. The various higher‐order cognitive functions of the cerebellum such as visuospatial processing, attention, and executive functioning 48 typically do not manifest before school age. 49 Longer‐term neuropsychological follow‐up of low and very low birth weight children is critical for improving our understanding of the neurodevelopmental outcomes of early morphine and fentanyl exposure on brain structure and function at later ages. In sum, these findings support the adage, “the way it wires is the way it fires,” since both pain and opioid exposure alter the development of neuronal circuits in the developing neonate's brain, leading to measurable changes in cognitive, sensorimotor, and addictive behaviors in later life.
This review outlines the current approaches for pain assessment of newborns in NICU, highlights the particular challenges of pain management following life‐saving surgery in newborns, and explores the potential uses of artificial intelligence (AI)‐based frameworks for understanding, assessing, treating, and averting postsurgical pain and opioid withdrawal. Like AI‐based machine learning models in current use for predicting weather and climate events, 50 AI frameworks can use single or multiple combinations of continuous variables, for example, facial and body movements, crying frequencies, and physiological data (vital signs) for reliable time‐to‐pain onset, thereby creating a therapeutic window for interventions with non‐narcotic pharmaceutical and nonpharmaceutical methods. For instance, acetaminophen (paracetamol) achieves analgesia in neonates within minutes of administration by the IV (intravenous) route. 51 Recent prospective, randomized controlled trials report that neonates and infants receiving intermittent IV acetaminophen required significantly less IV morphine following major cardiac 52 and noncardiac 53 surgeries. With a 30‐minute window warning of ensuing pain, clinical staff could alert family as part of a “constellation” of nonpharmacological interventions using individualized strategies where possible. With the goal of staying ahead of the infants’ pain, this constellation of interventions can include, for instance, sweet solutions (sucrose); music; holding; reassuring vocalizations; acupressure; skin‐to‐skin contact (kangaroo care), and scent therapy. 54 , 55 , 56 , 57 When effective either alone or in conjunction with nonopioid medications (IV acetaminophen or NSAIDs), such interventions have the potential to mitigate damage to the neonate's developing central and peripheral nervous systems caused by both pain and withdrawal from opioid‐based medications. In this way, early pain detection (EPD) using AI‐based frameworks in combination with a priori opioid‐sparing pain avoidance strategies could minimize or avert damage caused by postsurgical pain and opioid withdrawal to newborns in NICU.
Among the various options for addressing this need are continuous monitoring and assessment of neonatal pain in NICU, 58 novel strategies for the therapeutic management of prolonged pain and artificial intelligence (AI)‐based predictive approaches for pain avoidance. This review outlines the current pain management strategies in NICU, highlights the challenges associated with pain mitigation of newborns, and explores the potential of AI using computer vision and machine learning to improve our understanding, assessment, treatment, and avoidance of neonatal pain in the future.
2. PAIN TYPE AND INDICATORS
Routine care in the NICU requires newborns to undergo various injections and blood draws, 3 , 14 , 59 the majority of which occur in the absence of analgesia medication. 60 The majority of newborns in NICU undergo multiple painful procedures 3 , 14 , 59 , 61 , 62 for a range of purposes: diagnostic (heel stick, venipuncture); therapeutic (bladder catheterization); and surgical, including minor operations (circumcision) and major repairs (intestinal atresia, ventricular‐septal defect). This number rises precipitously for babies born with potential health emergencies and leads to the requirement of prolonged stays in the NICU.
Pain in newborns was initially categorized into three types: acute procedural, acute prolonged, and chronic pain. 63 , 64 Acute procedural pain refers to short‐term pain caused by a painful stimulus that quickly dissipates (eg, immunization). Acute prolonged pain follows a major surgery, such as omphalocele repair, and continues for a longer period. Finally, chronic pain is recurrent pain, not associated with a single event, which continues for a longer duration than prolonged acute pain.
Several researchers have argued that this pain classification fails to characterize the full spectrum of neonatal pain. 65 Anand et al 65 proposed a more complete pain classification based on the temporal features (onset and duration); subjective features (burning, piercing, and shooting); secondary effects (hyperalgesia, allodynia); and response pattern (behavioral and physiological). This revised scheme includes five categories of neonatal pain: acute episodic, acute recurrent, prolonged, persistent, and chronic. An immediate benefit of this classification is the ability for bedside clinicians to understand the pain level and expected duration, thus allowing for personalized therapeutic pain management in individual newborns, rather than general treatment strategies across broad pain categories.
Pain indicators include both behavioral and physiological signs. 14 , 65 The major behavioral signs are facial expression, crying patterns, sleeping patterns, body movements, and communication patterns. Physiological signs refer to various information from bedside telemetry such as blood pressure, heart rate, respiration rate, oxygen saturation, variations in skin color, and conductance‐based on signals of brain activity such as electroencephalography (EEG). Among all the pain signs, facial expression is the most prominent, 66 , 67 , 68 but also the most difficult to translate into clinical practice, especially for example in postoperative (acute prolonged) cases with facial occlusions in place such as oxygen masks or surgical tapes. 69 , 70 On the other hand, vital signs are the least specific because they can be associated with other conditions such as inter‐ and intra‐individual variations of heart rate, noise, underlying disease, hunger, or anger. 68 Hence, a multimodal approach for pain assessment takes better account of pain as a multimodal event. 71 , 72 Further, this approach allows pain assessment during circumstances in which not all pain indicators are available such as occlusions (prone position and swaddling), clinical condition (eg, Bell's palsy), level of activity (eg, physical exertion), and sedation.
3. PAIN MEASUREMENT
At the bedside, pain in neonates is typically assessed by caregivers using subjective and nonlinear scales (+1, +2, +3…). However, numerous difficulties arise in the interpretation of these semi‐quantitative data due to the lack of standardization and variable training 65 and numerous confounds such as premature birth. 59 The next section outlines the range of subjective pain scales in current use and introduces some more objective tools under development for assessing and rating pain in newborns. We also discuss more objective and advanced technologies 70 , 72 , 73 using computer vision and machine learning recently proposed to help standardize pain scales using one or more modalities (face and body movements, crying frequency, vital signs, EEG signals, etc).
3.1. Subjective pain rating
At least 30 pain scales 74 , 75 have been proposed for assessing newborn pain by bedside caregivers, including general pain scales for all newborns and others specifically designed for subgroups such as preterm and term newborns. Broadly speaking, these pain scales fall into either unimodal (one‐dimensional) or multimodal (multidimensional) groups. 74 For instance, unimodal pain scales reflect a single dimension such as facial expressions, crying patterns, or body movements. Examples of unimodal pain scales are the Behavioral Indicators of Infant Pain (BIIP) 76 ; Liverpool Infant Distress Score (LIDS) 77 for preterm infants; Neonatal Pain Analyzer—ABC analyzer 78 for term infants; ABC Pain Scale 79 ; Neonatal Facial Coding System (NFCS) 80 for both preterm and term newborns; Children's and Infant's Postoperative Pain Scale (CHIPPS) 81 ; and Faces Legs Activity Cry Consolability Pain Scale (FLACC) 82 for both infants and children. As the name implies, multimodal pain scales combine multiple indicators such as facial expression, physiological signs, and crying sounds simultaneously. Examples of these multimodal scales include Pain Assessment Scale for Preterm Infants (PASPI) 83 for preterm infants; Neonatal Infant Pain Scale (NIPS) 84 ; Neonatal‐Pain, Agitation and Sedation Scale (N‐PASS) 85 ; Crying, Requires oxygen, Increased vital signs, Expressions and Sleepless (CRIES) 86 ; Premature Infant Pain Profile (PIPP) 87 for both preterm and term infants; and, COMFORT 88 for newborns, infants and children. All these unimodal and multimodal scales are valid for one or more types of pain (ie, acute pain, prolonged pain, or both).
Considerable difficulties arise with the validation of these subjective scales due to poor inter‐rater agreement, especially for premature babies born at <29 weeks of gestation. 59 A “faceless” acute neonatal pain scale (FANS) 89 has been proposed for premature babies which does not depend on facial expression, though more work is needed to confirm the reliability of this scale. 59
All subjective pain rating scales must address the difficulty in generalizing their assessments across specific pain types and age groups of newborns. Interactions arise, for instance, based on the patient's age, type of painful stimuli, environment, and clinical condition, which can confound efforts to generalize a specific pain rating scale across multiple patients. 74 Again, the inherent subjectivity of these scales often leads to the disagreement between two or more raters on the pain score for specific newborns. Finally, caregivers (raters) tend to gain experience in one or two specific pain scales, which increases time and expense and reduces motivation for raters to gain proficiency in novel scales.
3.2. Objective pain rating
A desirable alternative to semi‐quantitative subjective scales would be to standardize pain assessment in newborns around objective data from one or more modalities. Several groups have begun exploring this approach using a range of automated tools for rating neonatal pain with computer vision, signal processing, machine learning, and deep learning. These works can be divided according to pain indication (modality), pain type, and goals as discussed below.
3.2.1. Based on modality
Both newborns and adults manifest pain using a range of different modalities that can be expressed individually (unimodal) or across domains (multimodal). For newborns, one proposal for objective unimodal pain assessment based on facial expression is the COPE project. 90 This approach uses a total of 204 static images collected from 26 subjects (age range: 18 hours—3 days) grouped into pain, crying, resting, and response to stimulation with friction or air. Features were extracted from facial image and PCA (principal component analysis) and LDA (linear discriminant analysis) feature reduction approaches followed by Support Vector Machine (SVM) classifier. More recently, this project has expanded to iCOPE 91 where facial data were collected from video rather than a single static image. Other unimodal approaches based on crying sounds include a range of hand‐crafted features Harmonic Product Spectrum (HPS), 92 Mel Frequency Cepstral Coefficients (MFCC), 92 , 93 , 94 , 95 Short‐time Frame Energies (STE), 96 Local Binary Pattern (LBP), 97 Fuzzy Support Vector Machine (FSVM) 93 and deep learning‐based features such as spectrogram analysis 73 followed by VGG16, RestNet50 to separate infant's pain from discomfort. Recently, research work 98 supports using only brain data such as Near‐Infrared Spectroscopy (NIRS) to identify the impact of brain activity in case of premature babies. Since newborns cannot convey subjective assessments of their own pain, an objective approach is needed for automatic pain assessment based on behavioral and physiological signs. Multimodal approaches 99 use objective data for indicators such as facial expression, body movement, crying sounds, as well as physiological and electrophysiological signals to assess and predict the onset, quality, and duration of neonatal pain. The use of a standardized pain scale such as NIPS for training the deep learning model facilitates, at least in theory, the translation of this approach into the clinical environment. For instance, recently research work 71 reported a multimodal approach for neonatal pain assessment based on facial expression, body movement, and vital signs. Pain labeling was done by NICU clinicians using the NIPS pain scale, then an extended version of the same multimodal data set, together with data on crying frequency, was used to train traditional classifier model 72 and deep learning model. 73 A comprehensive survey of existing automated methods (unimodal and multimodal) for neonatal pain assessment can be found in. 100
Another potential direction of neonatal pain identification would be the use of neurophysiological data (eg, fMRI, EEG). 101 Recently, machine learning research using this data has shown success for adult pain. 102 However, these procedures have been less thoroughly explored for neonates. One of the main barriers is that regular neonatal pain scales do not include the fMRI or EEG as a separate pain modality, though recent research suggests this direction for future AI. 103
3.2.2. Based on pain type
As mentioned earlier, one variable refers to pain types, which can be divided into acute procedural, acute prolonged, and chronic pain. 63 , 64 Most research to date has been done on acute pain, which is easier to collect during a short procedure (eg, heel stick) than prolonged pain following a more complex surgical procedure. However, there is a significant need to include assessment and predication of postoperative pain due to its greater clinical value. As reported in, 70 , 100 , 104 automated detection of facial and/or body features can be more challenging for postoperative pain, as compared to acute procedural pain, because of the reduced intensity for postoperative pain; faces blocked by masks, tape, and facial position; and, body motions obscured by a blanket. 69 , 70 Similar differences exist for acute versus postoperative pain with regard to the time to onset and duration of pain, which in turn depend on age and other idiosyncratic factors. Thus, more clear definitions of neonatal pain and associated variables provide the foundation for developing more objective and therefore more clinically valuable approaches to neonatal pain assessment, prediction, and management in NICU.
3.2.3. Based on goal
The primary goals of neonatal pain assessment are pain identification and estimation of pain intensity. Pain identification includes the precise differentiation of pain from other classes such as hunger and need for diaper change that trigger behavioral and physiological signs, for example, crying, facial, and body movements. The majority of the current automatic assessment tools for this purpose 69 , 71 , 72 , 104 , 105 focus on identifying pain class while others focus on differentiating pain from other classes such as rest, hunger, discomfort, joy, sleep, and unhappy. 106 In the clinical environment, the more fruitful approach is to monitor the neonates continuously, 58 thereby generating an unbroken set of pain score signals. In this case, estimation of pain intensity is the desired goal for both automatic and objective approaches as it is for subjective pain scales. Some recent works have focused on objective estimation of pain intensity following a unimodal approach using only facial expression. 70
4. COLLECTION OF NEONATAL PAIN DATA
Research involving neonatal pain differs in several important ways from that involving older cohorts, especially with regard to the paucity of publicly available datasets. 100 , 107 The primary reasons for this critical shortage are outlined below.
There are some unique challenges and barriers of access to the NICU environment. Most NICUs require special permission for external persons to enter the environment.
Parents, staff, and administrators are understandably sensitive to research that in any way delays, distracts, or could endanger neonates due to under‐ or over‐treatment.
As humans in an early, preverbal stage of brain development, neonates cannot understand or consent to any type of data collection. In the event that the baby is under critical care, parents are unlikely to provide permission for any but the most basic procedures.
While parents may agree to the concept of research in general, the use of device for recording data of their baby in pain is sufficient grounds for many parents to deny consent.
In most developed countries, data collection for human subjects requires researchers to establish and follow approved Institutional Review Board (IRB) protocols. Many IRB protocols specifically disallow sharing data with others without data de‐identification, which is currently difficult for modern machine learning algorithms to accomplish.
A critical component of all types of Artificial Intelligence (AI) techniques, including computer vision, machine learning, and deep learning, is the proper labeling of ground truth. An important complication in the case of pain in preverbal neonates is that bedside caregivers must make subjective decisions based on a combination of qualitative data (facial expression and body movements) and quantitative data (vital sign telemetry). Even when these subjective determinations are used in concert with standardized pain rating scales for neonates, there is frequent disagreement between caregivers based on difference in available time and bias due to human factors, for example, training, experience, and fatigue.
Data collection in the NICU is highly dependent on pain type, variable procedures, the availability of trained caregivers, and other uncontrolled variables. The time available for data collection from newborns in acute pain (heel stick) is drastically less, typically up to 10 minutes, compared with hours or days for prolonged and chronic pain, respectively. Hence, in the case of data collection for postoperative pain, caregivers are typically only able to perform routine checks.
Trained caregivers in the NICU are subject to changing shifts and interruptions due to changing demands for their attention and skillsets.
The NICU environment lacks controlled conditions for the collection of experimental data. For instance, poor lighting, background noise, covered faces, and instrument sensors can confound efforts to standardize data collection.
Table 1 summarizes the recent neonatal pain datasets that are publicly available for research use.
TABLE 1.
List of publicly available neonatal pain datasets for research
| Dataset | Age range | Pain | Modalities | Subjects | Pain scale | ||||
|---|---|---|---|---|---|---|---|---|---|
| Face | Body | Sound | VS/PS | NIRS | |||||
| COPE 90 | 18 h‐3 d | A | ✓ | ✗ | ✗ | ✗ | ✗ | 26 | — |
| YouTube Infant 118 | 0‐12 m | A | ✓ | ✓ | ✓ | ✗ | ✗ | 142 | FLACC 82 |
| Human Neonates 103 | GA 29‐47 w, PA 0.5‐96 d | A | ✗ | ✗ | ✗ | ✓ | ✗ | 112 | PIPP 87 |
| APN‐db a , 110 | GA 28‐41 w, 0‐26 w | A | ✓ | ✗ | ✗ | ✗ | ✗ | 101, 112 | NFLAP 110 |
| FENP a , 105 | 2 d‐4 w | A | ✓ | ✗ | ✗ | ✗ | ✗ | 106 | NFCS 119 |
| USF‐MNPAD‐I 120 | GA 27‐41 w | A, P | ✓ | ✓ | ✓ | ✓ | ✓ | 58 | NIPS, 84 N‐PASS 85 |
Abbreviations: A, acute pain (procedural pain); d, day; GA, gestational age; h, hour; m, month; NIRS, near‐infrared spectroscopy; P, postoperative pain; PA, postnatal age; PS, physiological signals; VS, vital signs; w, week.
Declared to be public dataset, but was not publicly available while writing this article.
5. EARLY PAIN DETECTION
5.1. Future strategies for neonatal pain management
In order to identify areas in greatest need for novel technology in the field of neonatal pain management, in Fall 2019 our group carried out in‐person interviews.1 We interviewed over three dozen clinical staffs affiliated with three NICUs at local hospitals in the Tampa Bay region (Tampa General Hospital, St. Joseph's Hospital, Johns Hopkins All Children's Hospital). From these interviews, we learned that current pain management of newborns in NICUs can be generally characterized as manual, subjective, and discontinuous with NICU nurses treating neonates emerging from postsurgical sedation with pain management plans based on intermittent, subjective ratings with poor inter‐rater agreement. In the vast majority of cases, newborns undergo pain mitigation with Schedule II narcotics (morphine, fentanyl) that require at least 4‐5 extra days for withdrawal due to opioid dependence. 32 The vast majority (>90%) of clinical staff interviewed favored the development of novel approaches for objective Early Pain Detection (EPD) combined with effective nonpharmacologic and nonopioid medications over the current approach of subjective pain assessment followed by opioid treatment for NICU‐based management of prolonged postsurgical pain in neonates. The major reasons given for positive impacts of EPD on short‐ and long‐term health outcomes in this vulnerable population are outlined below:
Objective pain detection: EPD based on an AI framework could relieve the current burden on NICU clinical staff who must rely on subjective qualitative and semi‐quantitative pain assessment scales as the basis for pain management in newborns.
Newborns with NAS/NOWS: According to 2015 United States data 25 from the National Institute for Drug Abuse, 32 000 babies per year, or 1 baby every 15 minutes, are born either (a) addicted to opioids with Neonatal Abstinence Syndrome (NAS); or, (b) in opioid withdrawal with Neonate Opioid Withdrawal Syndrome (NOWS). These newborns require on average 23 days in NICU at a cost of $322K per newborn ($14K/day 23 days). In financial terms, AI‐based frameworks for objective early pain monitoring, prediction, and management have the potential to reduce the length of stay in NICU for NAS/NOW babies by a least 5 days at a cost savings of over $70K per newborn in state Medicare funds.
Reduced stays in NICU: The goal of postoperative care of neonates should be minimize opioids with effective pain avoidance and control. EPD in concert with reduced reliance on opioids for pain mitigation could reduce postsurgical stays in NICU by 4‐5 days, that is, to 8‐9 days from the current average of 12‐13 days per neonate. At about $14K per day for stays in NICU, opioid‐sparing pain management could save on average about $50K to $60K per neonate stay in NICU. However, time to discharge can be completely unrelated to surgery and therefore may miss the gains from decreasing the use of opioids.
Assessing effects of opioid‐sparing treatments: Outcomes for interventions in babies can be difficult to quantify, though those that have been used successfully during postoperative period are related to returning the baby to preoperative state. These outcome measures include time to enteral feeding; time to discontinuation of parenteral feeding 108 ; average opioid consumption up to 3 postoperative days; pain intensity (N‐PASS scores); reduced level of ventilator support and feeding; and, parent and nurse satisfaction. 109
Reduced health risk: A further goal of reducing the length of stay in NICU for preterm and term births and babies born with NAS and NOW is to minimize the opportunity for nosocomial infections to bring further harm and costs to hospitalized neonates.
Our conclusions from these interviews 1 support the use of strategies centered around EPD to reduce unmanaged and undermanaged pain in the NICU. Furthermore, we learned that these approaches, if effective, would likely receive favorable acceptance in the clinical setting.
5.2. Benefits of automatic EPD
Efforts are currently underway to develop AI‐based approaches for automatic Early Pain Detection (EPD) for enhancing the assessment, management, and prediction of postsurgical pain in neonates. Recent research also supports the view that AI systems can estimate pain intensity 70 as distinct from pain assessment. By standardizing pain management in the NICU, computer vision and deep learning technologies could support an intelligent AI platform for continuous monitoring of behavioral and physiological variables and assessment of postoperative pain in neonates. 69 , 70 , 71 , 72 Analogous to predicting the time to onset of adverse weather events, for example, thunderstorms, based on deep learning models trained by a range of weather indicators, for example, wind speed, temperature, and barometric pressure, 50 a similar approach could use a set of behavioral and physiological signs such as facial expression, 69 , 90 , 91 , 110 , 111 crying sounds (frequency), 92 , 96 body movements, 71 , 95 as well as multimodal combinations of these inputs 69 , 71 , 92 , 95 to estimate time to onset of postoperative pain in neonates.
Figure 2 represents a typical example of pain scale rating of a NICU patient. The dashed line indicates the pain threshold required for a pain signal, and the solid line shows the opioid threshold for application of narcotics for pain control. EPD technology involves training machine learning models (algorithms) using real pain data from patients, rather than data entered by programmers. In conjunction with real‐time patient data, these models allow for early pain predictions, that is, windows of time‐to‐pain onset. Highly probable predictions about time‐to‐pain onset, for example, “>90% probability of pain in 30 minutes,” allow caregivers to initiate opioid‐sparing medications and nonpharmacological interventions with the goal of preventing newborn trauma from both pain and opioid withdrawal.
FIGURE 2.
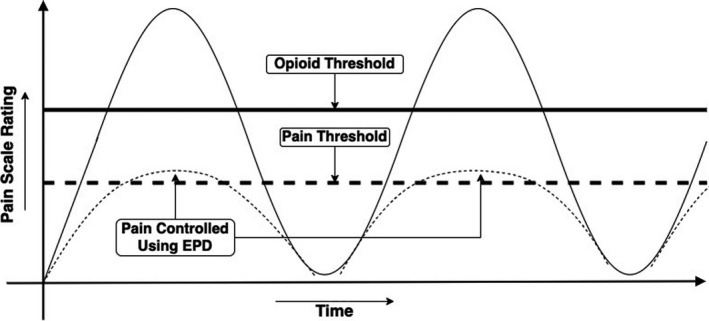
Potential benefits of Early Pain Detection (EPD) in neonates. This schematic shows how pain prediction prior to pain onset could create a time window (30‐40 min) for controlling pain using fast‐acting, nonopioid pain medications, for example, intravenous acetaminophen or ibuprofen. The goal of EPD is to “flatten the curve” for the recurring cycle of intermittent postsurgical pain, narcotic treatment, and opioid withdrawal (as shown by larger peaks and valleys), leading to less toxic stress (smaller peaks and valleys) on babies in NICU
The goal of an EPD system is to support continuous and objective monitoring of neonatal pain that will allow a minimum of 30 minutes prior to pain onset for pain mitigation using non‐narcotic drugs, including but not limited to acetaminophen and nonsteroidal anti‐inflammatory drugs (NSAIDs), rather than opioid medications such as fentanyl and morphine. If EPD can reduce or avoid the need for severe pain and opioid medications in the majority of cases, the EPD could substantially reduce the consequences of long‐lasting toxic stress trauma including behavioral impairments, epigenetic modifications, and increased complications caused by extreme pain and opioid addiction on neonates in NICU. Finally, it is expected that EPD will achieve these treatment goals while decreasing the economic burden on patients, private hospitals, and government agencies by reducing the length of stay and complications associated with the treatment of opioid withdrawal. In this way, AI‐based EPD technology could protect the neonatal brain from the damage wrought by both untreated pain onset and withdrawal from opioid addiction. 32
5.3. Pain prediction by artificial intelligence
Machine learning techniques have already shown substantial progress in neonatal pain assessment (Section III‐B). Recent deep learning approaches 69 , 72 have advanced this field by providing high accuracy in case of pain classification, 72 , 104 estimation of pain intensity, 70 and the ability to capture temporal pain dynamics. 69 , 70 , 72 Based on this evidence, 69 , 70 , 73 it can be estimated that AI can also be used to predict the pain earlier in the future. Similar to weather analyses 50 , 112 , 113 that gathers multimodal variables for making predictions, machine learning‐based methods can utilize the neonate's facial expressions, body movements, crying frequency, and vital sign data (eg, heart rate, blood pressure, and oxygen saturation level) to assign a probability of experiencing pain. Deep features using CNNs (Convolutional Neural Network) 114 , 115 can be extracted from different modality and temporal pain dynamics learned by Recurrent Neural Network (for example: LSTM 116 ) or Reinforcement Learning. 117 Moreover, based on the patient's history (ie, previous medical condition, family history, medication, and genetics) the AI model can boost up its learning performance, leading to more efficient and accurate predictions of future pain.
A potential system for providing EPD in neonates requires minimal hardware components that can include a data reading device (eg, A/V recorder such as a camera and/or microphone, vital signs reader) for visualizing and recording the neonate's facial expressions, voice, vital signs, and body movement including arms/legs (Figure 3). A facial expression classifier can be used for evaluating the pain via the facial expressions, where the facial expression classifier produces a facial expression score; a voice classifier for evaluating the pain via the infant's crying, where the voice classifier can produce a voice score based on the frequency and pitch of those inarticulate sounds (eg, using speech signal analysis). A vital signs classifier can be used to evaluate the neonate's pain according to its physical condition (eg, heart rate, breathing rate, oxygen saturation, changes in cerebral deoxyhemoglobin concentration, etc) and produce a vital sign score. The system software can include a processor that runs a machine learning algorithm (eg, parametric, nonparametric, optical flow, facial strain, local binary patterns, linear predictive coding, linear regression, and neural network) for processing images, videos, signals, and/or a combination thereof. The facial expression score, voice classifier score, body motions score, and vital signs score can be combined/weighed to produce a total score for pain assessment. The system can also include an output device, for example, meter, LED indicator, for outputting the total score to NICU personnel for pain monitoring.
FIGURE 3.
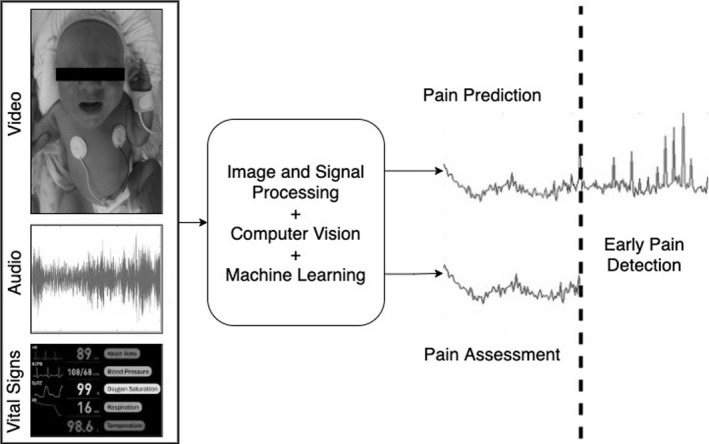
AI system for early pain detection of neonates. The future multimodal AI system can observe several modalities such as face, body, crying sound, physiological signals, and assess the current pain as well as predict the pain beforehand
Figure 3 shows a potential approach of future EPD technology. Multimodal data from NICU patients such as facial expression, body movement, crying sound, and physiological signals can be used by AI algorithms (ie, computer vision, signal processing, and machine learning altogether) to simultaneously assess pain and predict it before it occurs.
In future work, we plan to explore partnerships with industry‐based pharmacologists and medicinal chemists to “close the loop” by expanding our approach to include not only pain data from neonates but also the pharmacological actions and effects, as well as undesired effects of various non‐narcotic pain relievers in newborns. The novel availability of an AI‐based non‐narcotic pain avoidance could stimulate new funding, research, and incentives to better understand the timelines of absorption, bioavailability, distribution, metabolism, and excretion of various non‐narcotic pain relievers, which has received less attention in newborns due to an overreliance on morphine, fentanyl, and other narcotics. The goals of such a closed‐loop system would be in line with our AI‐based EPD method—more effective and safer mitigation of postsurgical pain and neonatal abstinence syndrome in this highly vulnerable population.
6. CONCLUSION
There is a large and growing need for the development of novel strategies for the therapeutic management of pain in the NICU. Current approaches for post hoc pain mitigation using narcotics fail to account for the detrimental and potentially permanent damage to the neonate from both pain and opioid withdrawal during the postoperative period. AI‐based frameworks using continuous monitoring of multiple modalities provide the necessary tools for creating a critical time window to pain onset. Such a time window could support proactive use of safer, that is, non‐narcotics pharmaceutical and nonpharmaceutical, interventions aimed at avoiding or minimizing damage to the neonate from both postsurgical pain and withdrawal due to opioid dependence.
CONFLICT OF INTEREST
All authors declare that this work is presented in the absence of any real or perceived conflict of interest. Authors PRM, SLE, MSS, DG, YS, TH, and GZ are named inventors on US patent application, “System and method for multimodal spatio‐temporal pain assessment.”
ACKNOWLEDGMENTS
We are grateful to all of our interviewees who expressed their feedback during the National Science Foundation (NSF) USF I‐Corps program. This research is partially supported by University of South Florida Nexus Initiative (UNI) Grant and National Institutes of Health Grant (NIH R21NR018756).
Salekin MS, Mouton PR, Zamzmi G, et al. Future roles of artificial intelligence in early pain management of newborns. Paediatr Neonatal Pain. 2021;3:134–145. 10.1002/pne2.12060
Footnotes
National Science Foundation (NSF) Innovation Corps (I‐Corps) program at the University of South Florida, Fall 2019.
REFERENCES
- 1. Mahendra M, Steurer‐Muller M, Hohmann SF, Keller RL, Aswani A, Dudley RA. Predicting NICU admissions in near‐term and term infants with low illness acuity. J Perinatol. 2021;41(3):478‐485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schulman J, Braun D, Lee HC, et al. Association between neonatal intensive care unit admission rates and illness acuity. JAMA Pediatr. 2018;172(1):17‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Williams MD, Lascelles BDX. Early neonatal pain—a review of clinical and experimental implications on painful conditions later in life. Front Pediatr. 2020;8:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Anand K, Hall RW. Controversies in neonatal pain: an introduction. Semin Perinatol. 2007;5:273‐274. [DOI] [PubMed] [Google Scholar]
- 5. DiLorenzo M, Pillai Riddell R, Holsti L. Beyond acute pain: understanding chronic pain in infancy. Children. 2016;3(4):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Johnston C. Neonatal pain: a journey spanning three decades. Paediatr Neonatal Pain. 2020;2(2):33‐39. 10.1002/pne2.12020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Anand KJ, Hickey PR, et al. Pain and its effects in the human neonate and fetus. N Engl J Med. 1987;317(21):1321‐1329. [DOI] [PubMed] [Google Scholar]
- 8. McIntosh N. Pain in the newborn, a possible new starting point. Eur J Pediatr. 1997;156(3):173‐177. [DOI] [PubMed] [Google Scholar]
- 9. Marchant A. ‘Neonates do not feel pain': a critical review of the evidence. Biosci Horiz. 2014;7:hzu006. [Google Scholar]
- 10. Goksan S, Hartley C, Emery F, et al. fMRI reveals neural activity overlap between adult and infant pain. Elife. 2015;4:e06356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Duff EP, Moultrie F & van der Vaart M et al. Inferring pain experience in infants using quantitative whole‐brain functional MRI signatures: a cross‐sectional, observational study. Lancet Digit Health. 2020;2(9). 10.1016/S2589-7500(20)30168-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Coughlin ME. Trauma‐Informed Care in the NICU: Evidenced‐Based Practice Guidelines for Neonatal Clinicians. United States: Springer Publishing Company; 2016. [Google Scholar]
- 13. Hall RW, Anand KJ. Pain management in newborns. Clin Perinatol. 2014;41(4):895‐924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Eriksson M, Campbell‐Yeo M. Assessment of pain in newborn infants. Semin Fetal Neonat Med. 2019;24(4):101003 [DOI] [PubMed] [Google Scholar]
- 15. Altimier L, Phillips R. The neonatal integrative developmental care model: Advanced clinical applications of the seven core measures for neuroprotective family‐centered developmental care. Newborn Infant Nurs Rev. 2016;16(4):230‐244. 10.1053/j.nainr.2016.09.030 [DOI] [Google Scholar]
- 16. Tompkins AD, Greg HJ, Compton P. Providing chronic pain management in the Fifth Vital Sign Era: historical and treatment perspectives on a modern‐day medical dilemma. Drug Alcohol Depend. 2017;173(1):S11‐S21. 10.1016/j.drugalcdep.2016.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hall RW, Shbarou RM. Drugs of choice for sedation and analgesia in the NICU. Clin Perinatol. 2009;36(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Anand K, Hall R. Pharmacological therapy for analgesia and sedation in the newborn. Arch Dis Child Fetal Neonatal Ed. 2006;91(6):F448‐F453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. March of Dimes, PMNCH, Save the Children, WHO . In: Howson CP, Kinney MV, Lawn JE, eds. Born Too Soon: The Global Action Report on Preterm Birth. Geneva: World Health Organization, 2012. [Google Scholar]
- 20. Martin JA, Hamilton BE, Ventura SJ, Osterman MJ, Wilson EC, Mathews T. Births: final data for 2010; 2012. [PubMed]
- 21. Lee HC, Bennett MV, Schulman J, Gould JB. Accounting for variation in length of NICU stay for extremely low birth weight infants. J Perinatol. 2013;33(11):872‐876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Grosse SD, Waitzman NJ, Yang N, Abe K, Barfield WD. Employer‐sponsored plan expenditures for infants born preterm. Pediatrics. 2017;140(4):e20171078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Davies H, Gilbert R, Johnson K, et al. Neonatal drug withdrawal syndrome: cross‐country comparison using hospital administrative data in England, the USA, Western Australia and Ontario, Canada. Arch Dis Child Fetal Neonatal Ed. 2016;101(1):26‐30. [DOI] [PubMed] [Google Scholar]
- 24. Pryor JR, Maalouf FI, Krans EE, Schumacher RE, Cooper WO, Patrick SW. The opioid epidemic and neonatal abstinence syndrome in the USA: a review of the continuum of care. Arch Dis Child Fetal Neonatal Ed. 2017;102(2):F183‐F187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Drug Abuse NI . Dramatic Increases in Maternal Opioid Use and Neonatal Abstinence Syndrome. NIDA, USDHHS Bethesda; 2015. [Google Scholar]
- 26. Winkelman TN, Villapiano N, Kozhimannil KB, Davis MM, Patrick SW. Incidence and costs of neonatal abstinence syndrome among infants with Medicaid: 2004–2014. Pediatrics. 2018;141(4):e20173520. 10.1542/peds.2017-3520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Seatriz JV, Hammer RP Jr. Effects of opiates on neuronal development in the rat cerebral cortex. Brain Res Bull. 1993;30(5‐6):523‐527. [DOI] [PubMed] [Google Scholar]
- 28. Zagon IS, Mclaughlin PJ, Thompson CI. Development of motor activity in young rats following perinatal methadone exposure. Pharmacol Biochem Behav. 1979;10(5):743‐749. [DOI] [PubMed] [Google Scholar]
- 29. Zagon IS, McLaughlin PJ, Thompson CI. Learning ability in adult female rats perinatally exposed to methadone. Pharmacol Biochem Behav. 1979;10(6):889‐894. [DOI] [PubMed] [Google Scholar]
- 30. Zagon IS, McLaughlin PJ. Increased brain size and cellular content in infant rats treated with an opiate antagonist. Science. 1983;221(4616):1179‐1180. [DOI] [PubMed] [Google Scholar]
- 31. Hauser KF, McLaughlin PJ, Zagon IS. Endogenous opioids regulate dendritic growth and spine formation in developing rat brain. Brain Res. 1987;416(1):157‐161. [DOI] [PubMed] [Google Scholar]
- 32. Squillaro A, Mahdi EM, Tran N, Lakshmanan A, Kim E, Kelley‐Quon LI. Managing procedural pain in the neonate using an opioid‐sparing approach. Clin Ther. 2019;41(9):1701‐1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cheong JL, Burnett AC, Treyvaud K, Spittle AJ. Early environment and long‐term outcomes of preterm infants. J Neural Transm. 2020;127(1):1‐8. [DOI] [PubMed] [Google Scholar]
- 34. Lien R. Neurocritical care of premature infants. Biomed J. 2020;43(3):259‐267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ferguson SA, Ward WL, Paule MG, Hall RW, Anand KJS. A pilot study of preemptive morphine analgesia in preterm neonates: effects on head circumference, social behavior, and response latencies in early childhood. Neurotoxicol Teratol. 2012;34:47‐55. [DOI] [PubMed] [Google Scholar]
- 36. Skiöld B, Alexandrou G, Padilla N, Blennow M, Vollmer B, Ådén U. Sex differences in outcome and associations with neonatal brain morphology in extremely preterm children. J Pediatr. 2014;164(5):1012‐1018. [DOI] [PubMed] [Google Scholar]
- 37. Schneider J, Duerden EG, Guo T, et al. Procedural pain and oral glucose in preterm neonates: brain development and sex‐specific effects. Pain. 2018;159(3):515‐525. [DOI] [PubMed] [Google Scholar]
- 38. Rook JM, McCarson KE. Delay of cutaneous wound closure by morphine via local blockade of peripheral tachykinin release. Biochem Pharmacol. 2007;74(5):752‐757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rook JM, Hasan W, McCarson KE. Temporal effects of topical morphine application on cutaneous wound healing. Anesthesiology. 2008;109(1):130‐136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rook JM, Hasan W, McCarson KE. Morphine‐induced early delays in wound closure: involvement of sensory neuropeptides and modification of neurokinin receptor expression. Biochem Pharmacol. 2009;77(11):1747‐1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sassani JW, Zagon IS, McLaughlin PJ. Opioid growth factor modulation of corneal epithelium: uppers and downers. Curr Eye Res. 2003;26(5):249‐262. [DOI] [PubMed] [Google Scholar]
- 42. Küchler S, Wolf NB, Heilmann S, et al. 3D‐wound healing model: influence of morphine and solid lipid nanoparticles. J Biotechnol. 2010;148(1):24‐30. [DOI] [PubMed] [Google Scholar]
- 43. Stein C, Kuchler S. Non‐analgesic effects of opioids: peripheral opioid effects on inflammation and wound healing. Curr Pharm Des. 2012;18(37):6053‐6069. [DOI] [PubMed] [Google Scholar]
- 44. Zwicker JG, Miller SP, Grunau RE, et al. Smaller cerebellar growth and poorer neurodevelopmental outcomes in very preterm infants exposed to neonatal morphine. J Pediatr. 2016;172:81‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schiller R, Allegaert K, Hunfeld M, van den Bosch G, van den Anker J, Tibboel D. Analgesics and sedatives in critically ill newborns and infants: the impact on long‐term neurodevelopment. J Clin Pharmacol. 2018;58:S140‐S150. [DOI] [PubMed] [Google Scholar]
- 46. McPherson C, Haslam M, Pineda R, Rogers C, Neil JJ, Inder TE. Brain injury and development in preterm infants exposed to fentanyl. Ann Pharmacother. 2015;49(12):1291‐1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lammers EM, Johnson PN, Ernst KD, et al. Association of fentanyl with neurodevelopmental outcomes in very‐low‐birth‐weight infants. Ann Pharmacother. 2014;48(3):335‐342. [DOI] [PubMed] [Google Scholar]
- 48. O’Halloran CJ, Kinsella GJ, Storey E. The cerebellum and neuropsychological functioning: a critical review. J Clin Exp Neuropsychol. 2012;34(1):35‐56. [DOI] [PubMed] [Google Scholar]
- 49. Anderson PJ. Neuropsychological outcomes of children born very preterm. Semin Fetal Neonatal Med. 2014;19(2):90‐96. [DOI] [PubMed] [Google Scholar]
- 50. Grover A, Kapoor A, Horvitz E. A deep hybrid model for weather forecasting. In: Proceedings of the 21th ACM SIGKDD International Conference on Knowledge Discovery and Data Mining; 2015:379‐386. 10.1145/2783258.2783275 [DOI] [Google Scholar]
- 51. Pacifici GM, Allegaert K. Clinical pharmacology of paracetamol in neonates: a review. Curr Ther Res. 2015;77:24‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zeilmaker‐Roest GA, van Rosmalen J, van Dijk M, et al. Intravenous morphine versus intravenous paracetamol after cardiac surgery in neonates and infants: a study protocol for a randomized controlled trial. Trials. 2018;19(1):318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ceelie I, de Wildt SN, van Dijk M, et al. Effect of intravenous paracetamol on postoperative morphine requirements in neonates and infants undergoing major noncardiac surgery: a randomized controlled trial. JAMA. 2013;309(2):149‐154. [DOI] [PubMed] [Google Scholar]
- 54. Golianu B, Krane E, Seybold J, Almgren C, Anand K. Non‐pharmacological techniques for pain management in neonates. Semin Perinatol. 2007;31(5):318‐322. [DOI] [PubMed] [Google Scholar]
- 55. Cignacco E, Hamers JPH, Stoffel L, et al. The efficacy of non‐pharmacological interventions in the management of procedural pain in preterm and term neonates: a systematic literature review. Eur J Pain. 2007;11(2):139‐152. 10.1016/j.ejpain.2006.02.010 [DOI] [PubMed] [Google Scholar]
- 56. Lago P, Garetti E, Pirelli A, et al. Non‐pharmacological intervention for neonatal pain control. Ital J Pediatr. 2014;40:A52. [Google Scholar]
- 57. Bucsea O, Riddell RP. Non‐pharmacological pain management in the neonatal intensive care unit: Managing neonatal pain without drugs. Semin Fetal Neonatal Med. 2019;24(4):101017. [DOI] [PubMed] [Google Scholar]
- 58. Anand KJ, Eriksson M, Boyle EM, et al. Assessment of continuous pain in newborns admitted to NICU s in 18 E uropean countries. Acta Paediatr. 2017;106(8):1248‐1259. [DOI] [PubMed] [Google Scholar]
- 59. Meesters NJ, Simons SH, van Rosmalen J, Holsti L, Reiss IK, Van Dijk M. Acute pain assessment in prematurely born infants below 29 weeks: a long way to go. Clin J Pain. 2019;35(12):975‐982. [DOI] [PubMed] [Google Scholar]
- 60. Carbajal R, Rousset A, Danan C, et al. Epidemiology and treatment of painful procedures in neonates in intensive care units. JAMA. 2008;300(1):60‐70. [DOI] [PubMed] [Google Scholar]
- 61. Cruz M, Fernandes A, Oliveira C. Epidemiology of painful procedures performed in neonates: a systematic review of observational studies. Eur J Pain. 2016;20(4):489‐498. [DOI] [PubMed] [Google Scholar]
- 62. Laudiano‐Dray MP, Pillai Riddell R, Jones L, et al. Quantification of neonatal procedural pain severity: a platform for estimating total pain burden in individual infants. Pain. 2020;161(6):1270‐1277. [DOI] [PubMed] [Google Scholar]
- 63. Stevens BJ, Pillai Riddell R, Oberlander TE, Gibbins S. Assessment of pain in neonates and infants. Pain Neonates Infants. 2007;3:67‐90. [Google Scholar]
- 64. Anand KJ, Stevens BJ, McGrath PJ, et al. Pain in Neonates and Infants: Pain Research and Clinical Management Series, Vol. 10. Netherlands: Elsevier Health Sciences; 2007. [Google Scholar]
- 65. Anand KJ. Defining pain in newborns: need for a uniform taxonomy? Acta Pædiatr. 2017;106(9):1438‐1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Grunau RV, Johnston CC, Craig KD. Neonatal facial and cry responses to invasive and non‐invasive procedures. Pain. 1990;42(3):295‐305. [DOI] [PubMed] [Google Scholar]
- 67. Lindh V, Wiklund U, Sandman P‐O, Håkansson S. Assessment of acute pain in preterm infants by evaluation of facial expression and frequency domain analysis of heart rate variability. Early Human Dev. 1997;48(1‐2):131‐142. [DOI] [PubMed] [Google Scholar]
- 68. Bellieni CV. Pain assessment in human fetus and infants. AAPS J. 2012;14(3):456‐461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Salekin MS, Zamzmi G, Goldgof D, Kasturi R, Ho T, Sun Y. Multi‐channel neural network for assessing neonatal pain from videos. In: 2019 IEEE International Conference on Systems, Man and Cybernetics (SMC). IEEE; 2019:1551‐1556. 10.1109/SMC.2019.8914537 [DOI] [Google Scholar]
- 70. Salekin MS, Zamzmi G, Goldgof D, Kasturi R, Ho T, Sun Y. First investigation into the use of deep learning for continuous assessment of neonatal postoperative pain. In: 2020 15th IEEE International Conference on Automatic Face and Gesture Recognition (FG). IEEE; 2020:415‐419. 10.1109/FG47880.2020.00082 [DOI] [Google Scholar]
- 71. Zamzmi G, Pai CY, Goldgof D, Kasturi R, Ashmeade T, Sun Y. An approach for automated multimodal analysis of infants’ pain. In: 2016 23rd International Conference on Pattern Recognition (ICPR). IEEE; 2016:4148‐4153. 10.1109/ICPR.2016.7900284 [DOI] [Google Scholar]
- 72. Zamzmi G, Pai CY, Goldgof D, Kasturi R, Ashmeade T, Sun Y. A comprehensive and context‐sensitive neonatal pain assessment using computer vision. IEEE Trans Affect Comput. 2019:1. 10.1109/TAFFC.2019.2926710 [DOI] [Google Scholar]
- 73. Salekin MS, Zamzmi G, Paul R, et al. Harnessing the power of deep learning methods in healthcare: neonatal pain assessment from crying sound. In: 2019 IEEE Healthcare Innovations and Point of Care Technologies, (HI‐POCT). IEEE; 2019:127‐130. 10.1109/HI-POCT45284.2019.8962827 [DOI] [Google Scholar]
- 74. Melo GMD, Lélis ALPdA, Moura AFD, Cardoso MVLML, Silva VMD. Pain assessment scales in newborns: integrative review. Rev Paul Pediatr. 2014;32(4):395‐402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Giordano V, Edobor J, Deindl P, et al. Pain and sedation scales for neonatal and pediatric patients in a preverbal stage of development: a systematic review. JAMA Pediatr. 2019;173(12):1186‐1197. [DOI] [PubMed] [Google Scholar]
- 76. Holsti L, Grunau RE. Initial validation of the behavioral indicators of infant pain (BIIP). Pain. 2007;132(3):264‐272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Morgan M, Choonara I, Al‐Waidh M, Sambrooks J. Measuring pain in neonates: an objective score. Paediatr Nurs. 1996;8(10):24‐27. [PubMed] [Google Scholar]
- 78. Sisto R, Bellieni CV, Perrone S, Buonocore G. Neonatal pain analyzer: development and validation. Med Biol Eng Comput. 2006;44(10):841. [DOI] [PubMed] [Google Scholar]
- 79. Bellieni CV, Bagnoli F, Sisto R, Neri L, Cordelli D, Buonocore G. Development and validation of the ABC pain scale for healthy full‐term babies. Acta Paediatr. 2005;94(10):1432‐1436. [DOI] [PubMed] [Google Scholar]
- 80. Peters JWB, Koot HM, Grunau RE, et al. Neonatal facial coding system for assessing postoperative pain in infants: item reduction is valid and feasible. Clin J Pain. 2003;19(6):353‐363. [DOI] [PubMed] [Google Scholar]
- 81. Büttner W, Finke W. Analysis of behavioural and physiological parameters for the assessment of postoperative analgesic demand in newborns, infants and young children: a comprehensive report on seven consecutive studies. Paediatr Anaesth. 2000;10(3):303‐318. 10.1046/j.1460-9592.2000.00530.x [DOI] [PubMed] [Google Scholar]
- 82. Voepel‐Lewis T, Shayevitz JR, Malviya S. The FLACC: a behavioral scale for scoring postoperative pain in young children. Pediatr Nurs. 1997;23(3):293‐297. [PubMed] [Google Scholar]
- 83. Liaw JJ, Yang L, Chou HL, Yin T, Chao SC, Lee TY. Psychometric analysis of a Taiwan‐version pain assessment scale for preterm infants. J Clin Nurs. 2012;21(1‐2):89‐100. [DOI] [PubMed] [Google Scholar]
- 84. Hudson‐Barr D, Capper‐Michel B, Lambert S, Palermo TM, Morbeto K, Lombardo S. Validation of the pain assessment in neonates (PAIN) scale with the neonatal infant pain scale (NIPS). Neonatal Netw. 2002;21(6):15‐22. [DOI] [PubMed] [Google Scholar]
- 85. Hummel P, Puchalski M, Creech S, Weiss M. Clinical reliability and validity of the N‐PASS: neonatal pain, agitation and sedation scale with prolonged pain. J Perinatol. 2008;28(1):55. [DOI] [PubMed] [Google Scholar]
- 86. Krechel SW, Bildner J. CRIES: a new neonatal postoperative pain measurement score. Initial testing of validity and reliability. Paediatr Anaesth. 1995;5(1):53‐61. [DOI] [PubMed] [Google Scholar]
- 87. Stevens BJ, Gibbins S, Yamada J, et al. The premature infant pain profile‐revised (PIPP‐R): initial validation and feasibility. Clin J Pain. 2014;30(3):238‐243. [DOI] [PubMed] [Google Scholar]
- 88. van Dijk M, de Boer JB, Koot HM, Tibboel D, Passchier J, Duivenvoorden HJ. The reliability and validity of the COMFORT scale as a postoperative pain instrument in 0 to 3‐year‐old infants. Pain. 2000;84(2‐3):367‐377. [DOI] [PubMed] [Google Scholar]
- 89. Milesi C, Cambonie G, Jacquot A, et al. Validation of a neonatal pain scale adapted to the new practices in caring for preterm newborns. Arch Dis Child Fetal Neonatal Ed. 2010;95(4):F263‐F266. [DOI] [PubMed] [Google Scholar]
- 90. Brahnam S, Nanni L, Sexton R. Introduction to neonatal facial pain detection using common and advanced face classification techniques. In: Advanced Computational Intelligence Paradigms in Healthcare‐1. Berlin, Heidelberg: Springer; 2007:225‐253. 10.1007/978-3-540-47527-9_9 [DOI] [Google Scholar]
- 91. Brahnam S, Nanni L, McMurtrey S, et al. Neonatal pain detection in videos using the iCOPEvid dataset and an ensemble of descriptors extracted from Gaussian of Local Descriptors. Appl Comput Inform. 2020. 10.1016/j.aci.2019.05.003 [DOI] [Google Scholar]
- 92. Pal P, Iyer AN, Yantorno RE. Emotion detection from infant facial expressions and cries. In: 2006 IEEE International Conference on Acoustics Speech and Signal Processing Proceedings, Vol. 2. IEEE; 2006:II‐II. 10.1109/ICASSP.2006.1660444 [DOI] [Google Scholar]
- 93. Barajas‐Montiel SE, Reyes‐Garcia CA. Fuzzy support vector machines for automatic infant cry recognition. In: Intelligent Computing in Signal Processing and Pattern Recognition. Springer; 2006:876‐881. 10.1007/978-3-540-37258-5_107 [DOI] [Google Scholar]
- 94. Patil HA. “Cry baby”: using spectrographic analysis to assess neonatal health status from an infant’s cry. In: Advances in Speech Recognition. Springer; 2010:323‐348. 10.1007/978-1-4419-5951-5_14 [DOI] [Google Scholar]
- 95. Zamzmi G, Pai CY, Goldgof D, Kasturi R, Sun Y, Ashmeade T. Automated pain assessment in neonates. In: Scandinavian Conference on Image Analysis. Springer; 2017:350‐361. 10.1007/978-3-319-59129-2_30 [DOI] [Google Scholar]
- 96. Vempada RR, Kumar BSA, Rao KS. Characterization of infant cries using spectral and prosodic features. In: 2012 National Conference on Communications (NCC). IEEE; 2012:1‐5. 10.1109/NCC.2012.6176851 [DOI] [Google Scholar]
- 97. Felipe GZ, Aguiat RL, Costa YM, et al. Identification of infants’ cry motivation using spectrograms. In: 2019 International Conference on Systems, Signals and Image Processing (IWSSIP). IEEE; 2019:181‐186. 10.1109/IWSSIP.2019.8787318 [DOI] [Google Scholar]
- 98. Compton M, Zamzmi G & Mhaskar R et al. Pain analysis, in premature infants, using near infrared spectroscopy (NIRS); 2019. arXiv preprint arXiv:190810240.
- 99. van der Vaart M, Duff E, Raafat N, Rogers R, Hartley C, Slater R. Multimodal pain assessment improves discrimination between noxious and non‐noxious stimuli in infants. Paediatr Neonatal Pain. 2019;1(1):21‐30. 10.1002/pne2.12007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Zamzmi G, Kasturi R, Goldgof D, Zhi R, Ashmeade T, Sun Y. A review of automated pain assessment in infants: features, classification tasks, and databases. IEEE Rev Biomed Eng. 2018;11:77‐96. [DOI] [PubMed] [Google Scholar]
- 101. Hartley C, Duff EP, Green G, et al. Nociceptive brain activity as a measure of analgesic efficacy in infants. Sci Transl Med. 2017;9(388):eaah6122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Vijayakumar V, Case M, Shirinpour S, He B. Quantifying and characterizing tonic thermal pain across subjects from EEG data using random forest models. IEEE Trans Biomed Eng. 2017;64(12):2988‐2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Jones L, Laudiano‐Dray MP, Whitehead K, et al. EEG, behavioural and physiological recordings following a painful procedure in human neonates. Sci Data. 2018;5(1):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Salekin MS, Zamzmi G, Goldgof D, Kasturi R, Ho T, Sun Y. Multimodal spatio‐temporal deep learning approach for neonatal postoperative pain assessment. Comput Biol Med. 2021;129: 104150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Yan J, Lu G, Li X, et al. FENP: a database of neonatal facial expression for pain analysis. IEEE Trans Affect Comput. 2020. 10.1109/TAFFC.2020.3030296 [DOI] [Google Scholar]
- 106. Li C, Pourtaherian A, van Onzenoort L, Tjon A Ten WE, de With PHN. Infant facial expression analysis: towards a real‐time video monitoring system using R‐CNN and HMM. IEEE J Biomed Health Inform. 2021;25(5):1429‐1440. [DOI] [PubMed] [Google Scholar]
- 107. Werner P, Lopez‐Martinez D, Walter S, Al‐Hamadi A, Gruss S, Picard R. Automatic recognition methods supporting pain assessment: a survey. IEEE Trans Affect Comput. 2019. 10.1109/TAFFC.2019.2946774 [DOI] [Google Scholar]
- 108. Fraga MV, Laje P, Peranteau WH, et al. The influence of gestational age, mode of delivery and abdominal wall closure method on the surgical outcome of neonates with uncomplicated gastroschisis. Pediatr Surg Int. 2018;34(4):415‐419. [DOI] [PubMed] [Google Scholar]
- 109. Czarnecki ML, Hainsworth K, Simpson PM, et al. A pilot randomized controlled trial of outcomes associated with parent‐nurse controlled analgesia vs. continuous opioid infusion in the neonatal intensive care unit. Pain Manag Nurs. 2020;21(1):72‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Egede J, Valstar M, Torres MT, Sharkey D. Automatic neonatal pain estimation: An acute pain in neonates database. In: 2019 8th International Conference on Affective Computing and Intelligent Interaction (ACII). IEEE; 2019:1‐7. 10.1109/ACII.2019.8925480 [DOI] [Google Scholar]
- 111. Zamzmi G, Paul R, Salekin MS, et al. Convolutional neural networks for neonatal pain assessment. IEEE Trans Biometr Behav Identity Sci. 2019;1(3):192‐200. 10.1109/TBIOM.2019.2918619 [DOI] [Google Scholar]
- 112. Qing X, Niu Y. Hourly day‐ahead solar irradiance prediction using weather forecasts by LSTM. Energy. 2018;148:461‐468. [Google Scholar]
- 113. Xingjian S, Chen Z, Wang H, Yeung DY, Wong WK, Wc W. Convolutional LSTM network: a machine learning approach for precipitation nowcasting. In: Advances in Neural Information Processing Systems; 2015:802‐810. https://dl.acm.org/doi/10.5555/2969239.2969329 [Google Scholar]
- 114. Simonyan K, Zisserman A. Very deep convolutional networks for large‐scale image recognition; 2014. arXiv preprint arXiv:14091556.
- 115. He K, Zhang X, Ren S, Sun J. Deep residual learning for image recognition. In: Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition; 2016:770‐778. 10.1109/CVPR.2016.90 [DOI] [Google Scholar]
- 116. Hochreiter S, Schmidhuber J. Long short‐term memory. Neural Comput. 1997;9(8):1735‐1780. [DOI] [PubMed] [Google Scholar]
- 117. Sutton RS, Barto AG. Reinforcement Learning: An Introduction. MIT Press; 2018. [Google Scholar]
- 118. Harrison D, Sampson M, Reszel J, et al. Too many crying babies: a systematic review of pain management practices during immunizations on YouTube. BMC Pediatr. 2014;14(1):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Grunau RE, Oberlander T, Holsti L, Whitfield MF. Bedside application of the neonatal facial coding system in pain assessment of premature infants. Pain. 1998;76(3):277‐286. [DOI] [PubMed] [Google Scholar]
- 120. Salekin MS, Zamzmi G, Hausmann J, et al. Multimodal neonatal procedural and postoperative pain assessment dataset. Data Brief. 2021;35:106796. [DOI] [PMC free article] [PubMed] [Google Scholar]


