Since the emergence of COVID-19 more than 2 years ago, allogeneic haematopoietic stem-cell transplantation (HSCT) recipients have been considered at especially high risk of developing severe forms of the disease. The first studies focusing on COVID-19 after HSCT confirmed the poor prognosis in this population,1 highlighting the urgent need for efficient preventive and curative treatment strategies. In this regard, mRNA vaccines have emerged with the ability to confer a high protection rate, mostly against severe disease, and a good safety profile; however, in post-HSCT vaccination, humoral response might be altered due to intake of immunosuppressive drugs and delay of B-cell recovery. Although a weak immune response after two doses of mRNA vaccine against SARS-CoV-2 has been reported in around 40% of allogeneic HSCT recipients,2 a third early vaccine dose has been shown to have a positive impact on humoral response in this subpopulation of poorly responding recipients.3 We addressed whether pre-HSCT vaccination of donors has an impact on humoral response to early post-HSCT vaccination of recipients, at a time when they are still receiving immunosuppressive drugs.
As vaccination became widely applied in patients and the general population, the first HSCTs of recipients or from donors vaccinated with the BNT162b2 (mRNA) vaccine (Pfizer–BioNTech) were performed in March, 2021, in our department. We monitored all vaccinated patients and donors for anti-spike glycoprotein-specific IgG, namely IgG(S-receptor-binding domain [RBD]) and anti-nucleocapsid protein (N) IgG titres 1 month after the last vaccine dose (appendix p 1). Based on functional studies using the same IgG(S-RBD) quantification assay as the present study,3 IgG(S-RBD) titres above 1000 AU/mL characterised humoral protection in HSCT recipients.
Among 14 patients vaccinated before HSCT, 11 received their HSCT from a vaccinated donor (D+/R+) and three were transplanted from non-vaccinated donors (D–/R+). All recipients were re-vaccinated with two doses, 4 weeks apart, at 3 months after HSCT, at a time when their residual IgG(S-RBD) mean titre was 294 AU/mL (SD 407), as compared with a mean titre of 901 AU/mL (SD 2173) immediately before HSCT after two vaccine doses (p=0·21; appendix p 2). Two additional recipients received transplants from a vaccinated donor without themselves being vaccinated before HSCT, because they initially refused vaccination against COVID-19. For subsequent analyses focused on the specific role of pre-HSCT donor vaccination, these last D+/R– patients were included with 11 other D+/R+ patients into a D+/R± group.
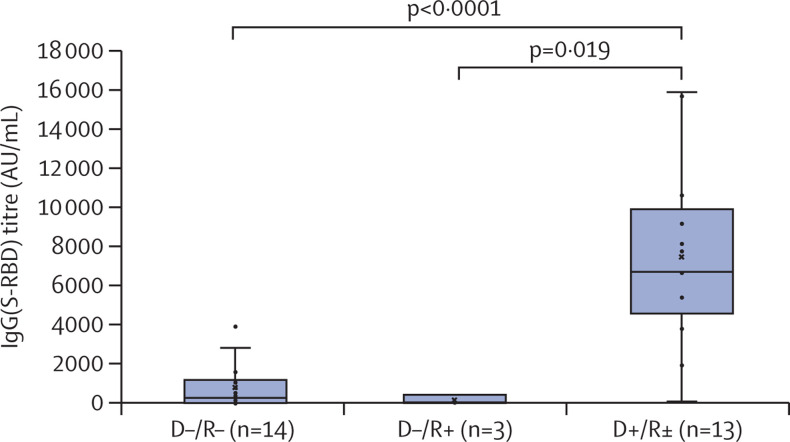
We further compared the humoral response to post-HSCT vaccination with two doses in these two groups of recipients (D+/R± and D–/R+), in parallel with 14 naive recipients transplanted from non-vaccinated donors (D–/R–) who were vaccinated within the same early period of 3–5 months after HSCT. As shown in the figure , IgG(S-RBD) titres after post-HSCT vaccination were significantly higher in D+/R± recipients (7492 AU/mL [SD 4650]) than D–/R– recipients (828 AU/mL [1205]; p<0·0001) and D–/R+ recipients (170 AU/mL [228]; p=0·019). Patient and HSCT characteristics of these three donor–recipient groups, as well as their respective recipient immune cell counts in peripheral blood at time of re-vaccination (lymphocytes as well as T cells and B cells), showed no significant differences (appendix p 3). All recipients still received systemic immunosuppressive drugs at the time of post-HSCT vaccination and their past exposure to rituximab (a major limiting factor of humoral response to vaccination) did not differ between groups. Notably, one recipient within each of the three donor–recipient groups had previous COVID-19 documented by positive PCR or positive anti-N IgG detection, or both, at any time during follow-up. Among these patients, anti-N IgG were still detected at time of response analysis to post-HSCT vaccination in the two D–/R– and D–/R+ recipients, but not in the D+/R+ recipient. In the 13 D+/R± pairs, post-HSCT sequential monitoring of chimerism in peripheral blood mononuclear cell (PBMCs), as well as in T cells and B cells, indicated their origin was mostly from the donor over the first 3 months after HSCT (appendix p 4). In this cohort, we did not observe any serious adverse event of vaccination either post-HSCT COVID-19 or cases of graft-versus-host disease induction or flare-up that could be related to the vaccination schedule.
Figure.
Impact of donor vaccination on recipient response to vaccination after HSCT
All recipients were vaccinated within the same 3–5 month period after HSCT. The D+/R± group included 11 D+/R+ and two D+/R– pairs. The only patient from this group who had an IgG(S-RBD) titre below 1000 AU/mL was D+/R+, and had received rituximab at day 6 of HSCT within a desensitisation regimen for preventing the risk of graft rejection in the setting of anti-donor HLA immunisation. Related to rituximab infusion, the patient had no detectable B cells in peripheral blood at time of re-vaccination. None of the patients in this cohort received chimeric antigen receptor T-cell therapy, blinatumomab, or inotuzumab. p values were calculated using Fisher's exact test. Error bars represent non-atypical minimal and maximal values. HSCT=haematopoietic stem-cell transplantation. D+=donor vaccinated. D–=donor unvaccinated. R+=recipient vaccinated. R–=recipient unvaccinated. R±=combined group of vaccinated and unvaccinated recipients. IgG(S-RBD)=IgG(S-receptor-binding domain).
In recipients who were vaccinated before HSCT, as well as in those who received HSCT from vaccinated donors, we observed low IgG(S-RBD) titres over the first 3 months after HSCT, indicating that pre-HSCT vaccination of recipients and donors, or both, was not sufficient to raise protective IgG(S-RBD) levels after HSCT. On this basis, all recipients—irrespective of their previous immunisation against SARS-CoV-2—were vaccinated (or re-vaccinated) at 3 months after HSCT, at a time when their residual IgG(S-RBD) titre was below 1000 AU/mL (with the exception of one recipient whose titre was >1000 AU/mL). Recipients who were transplanted from a vaccinated donor (11 D+/R+ and two D+/R–) had significantly higher IgG(S-RBD) titres after this post-HSCT re-vaccination, as compared with naive donor–recipient pairs against SARS-CoV-2 who received the same vaccination in the same early period after HSCT. The patient and HSCT characteristics of these two D+/R± and D–/R– groups were comparable, as well as their respective recipient immune cell counts in peripheral blood at time of post-HSCT vaccination. As controls, a small group of three D–/R+ pairs had no humoral response to post-HSCT vaccination in the same timeframe, confirming the role of donor rather than recipient immunity in the enhanced vaccine response observed in D+/R+ pairs. Sequential chimerism monitoring in these recipients showed the donor origin of PBMC (>99·9 %) over the first 3 months after HSCT in all D–/R+ pairs.
The present study adds to previous demonstrations of the adoptive transfer of an immune memory against an infectious agent from the donor to the recipient in the setting of HSCT. The impact of donor natural immunisation against pathogens is well demonstrated for the cytomegalovirus (CMV), with recipients transplanted from a seropositive donor (when they are seropositive themselves) having an overall survival benefit related to protection against CMV re-activation as compared with those receiving their HSCT from a naive donor against CMV.4 Thus, donor CMV serological status is a key factor in the algorithm of donor choice. Besides natural (ie, post-infectious) immunity, the transfer of donor vaccine-induced immunity has been previously suggested for some bacterial and viral pathogens such as Haemophilus influenzae type B,5, 6 tetanus,7 diphtheria,6 or hepatitis B virus.8 This was not the case for influenza9 or Streptococcus pneumoniae.5, 7 These discrepancies might, at least in part, be related to the nature and composition of the vaccine involved, as protein-conjugated polysaccharide antigens have been reported to be more immunogenic than polysaccharide antigens alone in HSCT recipients.10 In relation to their recent emergence, the impact of mRNA vaccines on post-HSCT immune response has, to our knowledge, never been studied. Our data suggest, for the first time to our knowledge, that B-cell-mediated immunity against SARS-CoV-2 can be transferred from donor to recipient in the setting of HSCT, as pre-HSCT vaccination of donors significantly boosts post-HSCT immune response to BNT162b2.
A limitation of the present study is the absence of quantitative assays for neutralising antibodies or lymphocyte function tests. However, the Abbott assay has a wide range of linear IgG(S-RBD) quantification, which consists of most neutralising antibodies generated after natural infection or vaccination. The assay has been tested and validated against WHO international standards, although analytical differences are still detected between commercially available assays. Using a fluorescence-based neutralisation assay, we also observed strong correlations between IgG(S-RBD) detected in serum and in vitro neutralising capacity for all tested variants (spike protein substitution D614G, variant B.1.351 [beta], and variant B.1.617.2 [delta]) in allogeneic HSCT recipients, as well as in immunocompetent vaccinated individuals (data not shown). The data are also limited by their single-centre, retrospective nature. The negligible impact of recipient pre-HSCT vaccination evidenced by very low IgG(S-RBD) titres observed after post-HSCT vaccination in the D–/R+ group should be considered with caution, since this result was from only three donor–recipient pairs in this group.
In conclusion, our study suggests that pre-HSCT donor vaccination has an impact on post-HSCT humoral response to early SARS-CoV-2 mRNA vaccination after HSCT. Although these results require further validation, they provide a rationale for incorporating donor serological status against SARS-CoV-2 into the algorithm of donor choice, or inciting donor vaccination before donation, if feasible. We believe that early and efficient vaccination of every HSCT recipient should be a priority, starting at 3 months post-HSCT. The number of required initial doses should be determined by careful monitoring of humoral response, with the possibility of a booster dose 6 months later. During the early months after HSCT, while awaiting an efficient anti-SARS-CoV-2 immune response to be attained, treatment options like pre-exposure and post-exposure prophylaxis with neutralising monoclonal antibodies could be considered.
All authors declare no competing interests. ML and RR contributed equally to this study. ML, RR, SF, and SM designed research. All authors performed research. ML, RR, SF, and SM analysed data. SM prepared the figures. ML and SM wrote the manuscript.
Supplementary Material
References
- 1.Ljungman P, de la Camara R, Mikulska M, et al. COVID-19 and stem cell transplantation; results from an EBMT and GETH multicenter prospective survey. Leukemia. 2021;35:2885–2894. doi: 10.1038/s41375-021-01302-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Redjoul R, Le Bouter A, Beckerich F, Fourati S, Maury S. Antibody response after second BNT162b2 dose in allogeneic HSCT recipients. Lancet. 2021;398:298–299. doi: 10.1016/S0140-6736(21)01594-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Redjoul R, Le Bouter A, Parinet V, Fourati S, Maury S. Antibody response after third BNT162b2 dose in recipients of allogeneic HSCT. Lancet Haematol. 2021;8:e681–e683. doi: 10.1016/S2352-3026(21)00274-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grob JP, Grundy JE, Prentice HG, et al. Immune donors can protect marrow-transplant recipients from severe cytomegalovirus infections. Lancet. 1987;1:774–776. doi: 10.1016/s0140-6736(87)92800-5. [DOI] [PubMed] [Google Scholar]
- 5.Molrine DC, Guinan EC, Antin JH, et al. Donor immunization with Haemophilus influenzae type b (HIB)-conjugate vaccine in allogeneic bone marrow transplantation. Blood. 1996;87:3012–3018. [PubMed] [Google Scholar]
- 6.Parkkali T, Käyhty H, Hovi T, et al. A randomized study on donor immunization with tetanus-diphtheria, Haemophilus influenzae type b and inactivated poliovirus vaccines to improve the recipient responses to the same vaccines after allogeneic bone marrow transplantation. Bone Marrow Transplant. 2007;39:179–188. doi: 10.1038/sj.bmt.1705562. [DOI] [PubMed] [Google Scholar]
- 7.Storek J, Dawson MA, Lim LC-L, et al. Efficacy of donor vaccination before hematopoietic cell transplantation and recipient vaccination both before and early after transplantation. Bone Marrow Transplant. 2004;33:337–346. doi: 10.1038/sj.bmt.1704336. [DOI] [PubMed] [Google Scholar]
- 8.Ilan Y, Nagler A, Shouval D, et al. Development of antibodies to hepatitis B virus surface antigen in bone marrow transplant recipient following treatment with peripheral blood lymphocytes from immunized donors. Clin Exp Immunol. 1994;97:299–302. doi: 10.1111/j.1365-2249.1994.tb06084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ambati A, Boas LSV, Ljungman P, et al. Evaluation of pretransplant influenza vaccination in hematopoietic SCT: a randomized prospective study. Bone Marrow Transplant. 2015;50:858–864. doi: 10.1038/bmt.2015.47. [DOI] [PubMed] [Google Scholar]
- 10.Harris AE, Styczynski J, Bodge M, Mohty M, Savani BN, Ljungman P. Pretransplant vaccinations in allogeneic stem cell transplantation donors and recipients: an often-missed opportunity for immunoprotection? Bone Marrow Transplant. 2015;50:899–903. doi: 10.1038/bmt.2015.49. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.