Studies have revealed that patients with immune-mediated inflammatory diseases, especially those on immunomodulatory medication, have attenuated immunogenicity to COVID-19 vaccination.1, 2 These findings have informed American College of Rheumatology (ACR) and European Alliance of Associations for Rheumatology (EULAR) recommendations regarding use of immunomodulatory therapies peri-vaccination. Recent longitudinal studies in immunocompetent adults have found waning humoral immunity by 6-months post-vaccination.3, 4 However, despite an already diminished initial response to immunisation in patients with immune-mediated inflammatory diseases, there are scarce data regarding their longer-term humoral response.
We hypothesised that patients with immune-mediated inflammatory diseases who are treated chronically with certain disease-modifying rheumatic drugs (ie, methotrexate) or anti-cytokine therapies (ie, TNF inhibitors), would have lower rates of adequate humoral response over time compared with patients without these diseases or those receiving other immunomodulatory medications. Using the New York University SAGA cohort, we obtained post-vaccination blood samples from participants with immune-mediated inflammatory diseases (n=245) and healthy controls (n=27) and analysed SARS-CoV-2-spike-specific antibody titres and neutralisation capacity at 4-week, 3-month, and 6-month timepoints after vaccination. This study was approved by the NYU institutional review board (20–01078) and all patients provided informed consent for participation. Full methods can be found in the appendix (p 2).
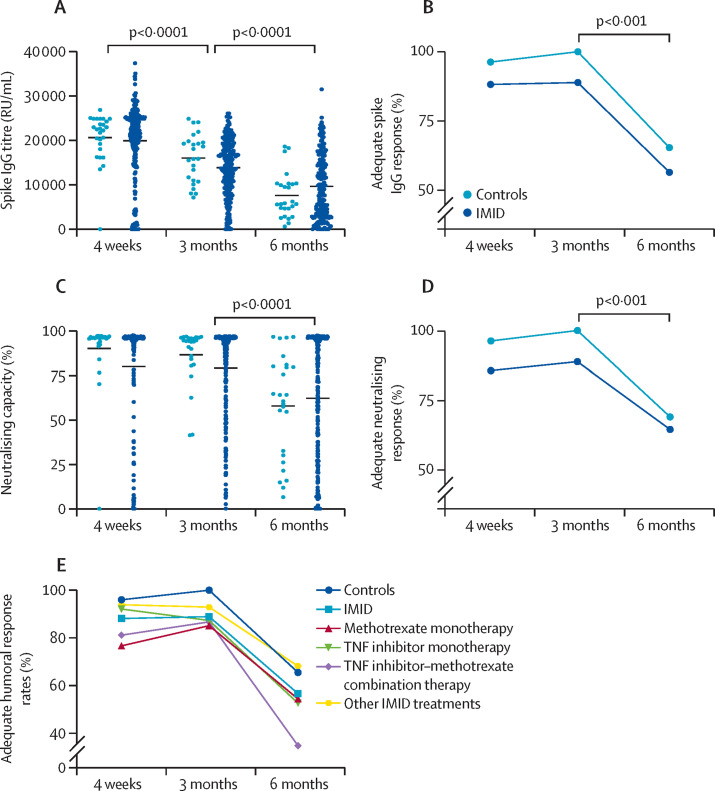
Healthy individuals and those with immune-mediated inflammatory diseases were similar in age, sex, and history of previous SARS-CoV-2 infection (appendix pp 3–4). Diagnoses included predominantly psoriatic disease, rheumatoid arthritis, and inflammatory bowel disease. The proportion of participants who achieved an adequate humoral response (ie, anti-spike IgG titre ≥5000 RU/mL) among healthy individuals and participants with immune-mediated inflammatory diseases remained stable between 4 weeks and 3 months after vaccination: 26 (96%) of 27 healthy controls were seropositive at 4 weeks versus 25 (100%) of 25 at 3 months, as were 216 (88%) of 245 participants with immune-mediated inflammatory diseases versus 193 (89%) of 217 (appendix p 5). Adequate humoral response rates decreased significantly in both groups by the 6-month timepoint (17 [65%] of 26 controls [p=0·0017] and 96 [56%] of 170 participants with IMIDs [p<0·0001]), as did IgG titres, percentage of antibodies with neutralising capacity, and rates of adequate neutralising response (ie, ≥35%) (figure ). Neutralising capacity correlated strongly with IgG antibody response (r=0·812, appendix p 14). No singular diagnosis demonstrated significant differences in adequate humoral response compared with healthy controls (appendix p 5). Previous COVID-19 infection was associated with increased antibody titres and neutralisation capacity at all timepoints and an increased adequate humoral response rate at 6 months (appendix p 6).
Figure.
Longitudinal humoral response to COVID-19 vaccination in healthy controls and patients with IMID
Humoral response represented by the spike IgG titres (A), the proportion of patients achieving an adequate spike IgG response as defined by greater than or equal to 5000 RU/mL (B), percentage neutralising capacity (C), and proportion of patients achieving an adequate neutralising capacity as defined by greater than or equal to 35% (D). (E) Proportion of patients achieving an adequate spike IgG response, by immunomodulatory use, as defined by spike IgG titres greater than or equal to 5000 RU/ml. The 4-week timepoint is defined at 4 weeks after the first dose of Janssen Ad26.COV2.S (Johnson & Johnson) and BNT162b2 (Pfizer–BioNTech) and 5 weeks after the first dose of mRNA-1273 (Moderna). IMID=immune-mediated inflammatory disease. RU=relative units.
At week 4, when compared with healthy controls, patients with immune-mediated inflammatory diseases who were not receiving methotrexate achieved a similar rate of adequate humoral response (150 [93%] of 161; appendix p 7), whereas those on methotrexate had a lower rate of adequate humoral response (66 [79%] of 84; p=0·002). Patients on methotrexate tended to be older, female, and have rheumatoid arthritis, and had a mean weekly dose of 14·5 mg. Mean IgG titres and neutralising antibodies were similarly lower in patients on methotrexate than in healthy controls and those not on methotrexate. At 3 months and 6 months after vaccination, patients on methotrexate had numerically lower rates of adequate response and titres than did healthy controls and patients not on methotrexate (66 [86%] of 77 vs 25 [100%] of 25 and 127 [91%] of 140, respectively, p=0·10 at 3 months; and 28 [46%] of 61 vs 17 [65%] of 26 and 68 [62%] of 109, respectively, p=0·08 at 6 months) with similar trends seen in antibody titre and neutralising antibodies. The overall adequate humoral response rate of patients receiving methotrexate differed significantly from healthy controls at 4 weeks (p=0·039), and was numerically lower, but did not differ significantly at 3 and 6 months (figure; appendix p 8).
The unadjusted odds ratio (OR) of achieving an adequate response to COVID-19 vaccination at week 4 for all methotrexate use (ie, alone or in combination with other medications) was 0·27 (95% CI 0·12–0·60, p=0·001), when compared with those not on methotrexate (appendix p 9). This effect remained significant when adjusting for age and sex and when restricting analysis to methotrexate monotherapy. The unadjusted OR for methotrexate use at 3 months was 0·61 (95% CI 0·26–1·45, p=0·26) and at 6 months was 0·51 (95% CI 0·27–0·97, p=0·039; appendix pp 10–11). The results at 3 and 6 months remain similar after adjusting for age and sex (appendix pp 10–11).
At 4 weeks, 37 (44%) of 84 patients on methotrexate held at least one dose immediately before or after vaccination (appendix p 7). Among patients receiving methotrexate, those who held their medication during the peri-vaccination period had an unadjusted OR of 3·50 (95% CI 1·04–11·75, p=0·043) achieving an adequate response compared with those who did not hold any doses (appendix p 9). This difference remained true at 3 months (unadjusted OR 8·33, 1·01–68·87, p=0·049) and 6 months (5·60, 1·68–18·70, p=0·005; appendix pp 10–11). There was no difference in adequate humoral response rates between the use of high-dose methotrexate (≥15 mg) and low-dose methotrexate (≤12·5 mg) in any combination of use (appendix p 12).
Patients with immune-mediated inflammatory diseases on TNF inhibitors had similar rates of adequate humoral response compared with those not on TNF inhibitors at 4 weeks (88 [88%] of 100 vs 128 [88%] of 145) and 3 months (74 [87%] of 85 vs 119 [90%] of 132), and compared with healthy controls. However, by 6 months the proportion of adequate antibody response was lower, although not statistically significant, in patients on TNF inhibitors compared with patients receiving other medications (30 [45%] of 66 vs 66 [63%] of 104, p=0·053; appendix p 13). Although adequate neutralising response was only significantly lower in the TNF group at 6 months, neutralising antibody concentration was significantly decreased at all timepoints, as were antibody titres at 3 and 6 months. Compared with those not receiving TNF inhibitors, the unadjusted OR of having an adequate humoral response following vaccination while receiving a TNF inhibitor was 0·48 (95% CI 0·26–0·90, p=0·022; appendix p 11) at 6 months post-vaccination, no such difference was seen at the 4-week and 3-month timepoints (appendix pp 9-10). The overall adequate humoral response rate for patients on TNF inhibitors declined significantly from 88 (88%) of 100 at 4 weeks and 74 (87%) of 85 at 3 months, to 30 (45%) of 66 at 6 months (p<0·0001 for both 4 weeks vs 6 months and 3 months vs 6 months; appendix pp 8–9).
Concomitant TNF inhibitor use attenuated the early suppressive effect of methotrexate (appendix p 8). However, at 6 months, combined TNF inhibitor–methotrexate use had the lowest adequate humoral response rate of any medication (9 [35%] of 26), even when compared with all methotrexate use (figure, appendix p 8). This combination had an unadjusted OR of 0·35 (95% CI 0·14–0·83, p=0·018; appendix p 11) of achieving adequate humoral response at 6 months compared with those not on this combination. This effect remained significant when adjusting for age and sex.
In the New York University SAGA cohort, participants with immune-mediated inflammatory diseases had a decline to 56% in adequate humoral response at 6 months. Importantly, healthy controls had a lower humoral response rate at 6 months (65%) than reported in previous studies (81–84%).4, 5 We continue to observe that methotrexate hampers the humoral immune response to COVID-19 vaccination. This effect is somewhat attenuated at the 3-month and 6-month timepoints, which might reflect the fact that methotrexate slows, rather than prevents, antibody production. In particular, the 3-month timepoint might reflect the peak immunogenicity of the vaccination captured in our study, thereby overcoming any observable effect on humoral response. Although dose of methotrexate did not affect humoral response; importantly, participants who held at least one dose of methotrexate during the peri-vaccination period had much higher odds of achieving an adequate humoral response than those who did not. These findings support the notion that this drug can substantially affect the biological response to vaccination and support the rationale behind current guidelines from ACR and EULAR for methotrexate use during this time.
Crucially, by 6 months, TNF inhibitors (especially in combination with methotrexate) led to further decreased rates of immunogenicity compared with earlier timepoints. Most initial studies did not demonstrate any effect of TNF inhibitors on adequate humoral response.1, 2, 6 However, Chen and colleagues7 observed reduced antibody activity against the SARS-CoV-2 delta (B.1.617.2) variant in patients receiving TNF inhibitors, especially at 3-month and 5 or 6-month timepoints. Like methotrexate,8 TNF inhibitors have previously been shown to impair the immune response to vaccines against other viral infections.9, 10 Mechanistic studies are needed to evaluate the seemingly synergistic effect of these drugs on weakening the antibody response of COVID-19 and other vaccines.
Although the relevance of our findings are constrained by the small sample size and scarcity of established correlates of levels of immunogenicity to efficacy, they show that both methotrexate and TNF inhibitors might lead to a dampened humoral response to COVID-19 vaccinations. Although TNF inhibitors do not demonstrate an initial effect on immunogenicity, persistence of adequate humoral response is significantly decreased by month 6 (appendix p 11). Taken together, these findings support the use of supplemental booster dosing in patients with immune-mediated inflammatory diseases, and specifically for those being treated with TNF inhibitors or TNF inhibitor–methotrexate combination therapy. Larger studies are needed to validate these results and to assess the effects of other immunomodulatory therapies, which will help to identify optimal timing and strategy of COVID-19 (and potentially other) vaccines.
For more on New York University SAGA see https://clinicaltrials.med.nyu.edu/clinicaltrial/1360/serologic-testing-genomic-analysis/
JUS reports consultancy fees from Janssen, Novartis, Pfizer, Sanofi, UCB, AbbVie, and Amgen, and funding for investigator-initiated studies from Pfizer, Sanofi, and Janssen. MJM reports laboratory research and clinical trials contracts with Lilly, Pfizer, and Sanofi, and personal fees for scientific advisory board service from Merck, Meissa Vaccines, and Pfizer. PMI reports consulting fees from GlaxoSmithKline and Momenta/Janssen. RHH reports consulting fees from Janssen. SA reports grant support from Johnson & Johnson. GS reports consulting fees from AbbVie. DPH reports consultancy fees from AbbVie, Bristol Myers Squibb, Janssen, Takeda, UCB, and Pfizer, and reports research support from Janssen and Pfizer. JEA reports consultancy fees from BioFire Diagnostics and Janssen; research grant support from BioFire Diagnostic; and holds US patent 2012/0052124A1. SCh reports consulting fees from AbbVie, Pfizer, and Bristol Myers Squibb. AS reports consulting fees from Lilly, GlaxoSmithKline, AstraZeneca, and Kezar. ALN reports consultancy fees from Janssen, UCB, AbbVie, and Bristol Myers Squibb, and her immediate family member owns shares of stock in Johnson & Johnson, Eli Lilly, AbbVie, and Pfizer. All other authors declare no competing interests. RHH, SU, and JEA contributed equally to this paper. DPH and JUS contributed equally to this paper. All data relevant to the study are included in the article or uploaded as supplementary information. Further de-identified data can be made available upon request via email to the corresponding author. This study was funded by US National Institutes of Health (NIH)–National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01AR074500 to Scher, T32-AR-069515 to RHH and JUS), NIH–National Institute of Diabetes and Digestive and Kidney Diseases (K23DK124570 to JEA), NIH–National Institute of Allergy and Infectious Diseases (AI082630 and AI158617 to SH), Rheumatology Research Foundation (Scientist Development Award to RHH), Bloomberg Philanthropies, Pfizer COVID-19 Competitive Grant Program, The Beatrice Snyder Foundation, The Riley Family Foundation, Crohn's and Colitis Foundation (to JEA), and the Judith and Stewart Colton Center for Autoimmunity (to JEA). No authors are employed by the NIH. We are grateful to our patients and their families for participating in this study and to our colleagues who referred patients to use. We thank Luz Alvarado, Rhina Medina, Parvathi Girija, and Jyoti Patel for coordinating and for data entry efforts.
Acknowledgments
RHH and JUS designed the study, designed the data collection tools, analysed and curated the data, and drafted and revised the paper. SU and SA designed the data collection tools, analysed and curated the data, and revised the paper. JEA and DPH designed the study, acquired data, and revised the draft. All authors approved the final version to be published and agree to be accountable for all aspects of the work. RHH and JUS verified the underlying data and had final responsibility for the decision to submit for publication. RBB, ZU, SCa, ALN, MJM, RSH, SJH, SCh, AM, GG, PMI, AS, GS, NA, JS, BDG, and PR acquired data and revised the draft.
Supplementary Material
References
- 1.Furer V, Eviatar T, Zisman D, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis. 2021;80:1330–1338. doi: 10.1136/annrheumdis-2021-220647. [DOI] [PubMed] [Google Scholar]
- 2.Haberman RH, Herati R, Simon D, et al. Methotrexate hampers immunogenicity to BNT162b2 mRNA COVID-19 vaccine in immune-mediated inflammatory disease. Ann Rheum Dis. 2021;80:1339–1344. doi: 10.1136/annrheumdis-2021-220597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levin EG, Lustig Y, Cohen C, et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N Engl J Med. 2021;385:e84. doi: 10.1056/NEJMoa2114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Israel A, Shenhar Y, Green I, et al. Large-scale study of antibody titer decay following BNT162b2 mRNA vaccine or SARS-CoV-2 infection. Vaccines (Basel) 2021;10:64. doi: 10.3390/vaccines10010064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Canaday DH, Oyebanji OA, Keresztesy D, et al. Significant reduction in humoral immunity among healthcare workers and nursing home residents 6 months after COVID-19 BNT162b2 mRNA vaccination. medRxiv. 2021 doi: 10.1101/2021.08.15.21262067. published online Aug 20. (preprint). [DOI] [Google Scholar]
- 6.Ruddy JA, Connolly CM, Boyarsky BJ, et al. High antibody response to two-dose SARS-CoV-2 messenger RNA vaccination in patients with rheumatic and musculoskeletal diseases. Ann Rheum Dis. 2021;80:1351–1352. doi: 10.1136/annrheumdis-2021-220656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen RE, Gorman MJ, Zhu DY, et al. Reduced antibody activity against SARS-CoV-2 B.1.617.2 delta virus in serum of mRNA-vaccinated patients receiving TNF-α inhibitors. Med (NY) 2021;2:1327. doi: 10.1016/j.medj.2021.11.004. 41.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hua C, Barnetche T, Combe B, Morel J. Effect of methotrexate, anti-tumor necrosis factor α, and rituximab on the immune response to influenza and pneumococcal vaccines in patients with rheumatoid arthritis: a systematic review and meta-analysis. Arthritis Care Res (Hoboken) 2014;66:1016–1026. doi: 10.1002/acr.22246. [DOI] [PubMed] [Google Scholar]
- 9.Launay O, Abitbol V, Krivine A, et al. Immunogenicity and safety of influenza vaccine in inflammatory bowel disease patients treated or not with immunomodulators and/or biologics: a two-year prospective study. J Crohn's Colitis. 2015;9:1096–1107. doi: 10.1093/ecco-jcc/jjv152. [DOI] [PubMed] [Google Scholar]
- 10.Pratt PK, Jr, David N, Weber HC, et al. Antibody response to hepatitis B virus vaccine is impaired in patients with inflammatory bowel disease on infliximab therapy. Inflamm Bowel Dis. 2018;24:380–386. doi: 10.1093/ibd/izx001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.