Abstract
Background
Post-stroke depression (PSD), a common neuropsychiatric comorbidity after stroke, has a negative impact on the functional recovery and quality of life of survivors. It lacks effective therapeutic drugs with good curative effects and few adverse reactions. Preliminary experiments have shown that the optimized acupuncture and moxibustion treatment (OAMT), including acupuncture, moxibustion, and auricular intradermal acupuncture, improved depressive symptoms and neurological deficits in patients with PSD. However, the evidence for its effectiveness is still insufficient. Hence, we designed this study to evaluate the efficacy and safety of the OAMT in the treatment of PSD and to explore its possible mechanism from the perspective of executive functions.
Methods/Design
This is a randomized controlled trial, which comprises a total of 134 patients with PSD. Participants are randomized into intervention group and control group at a 1:1 ratio. All treatments are given five times per week for 4 weeks. The primary outcome is the severity of depression, which is evaluated by the Hamilton Depression Scale-17 (HAMD-17) and the Beck Depression Rating Scale (BDI). Secondary outcomes are executive abilities, which are measured by several neuropsychological tests, including the Stroop Color and Word Test (SCWT), the Trial Making Test (TMT), the Digit Symbol Substitution Test (DSST), and the Matrix Reasoning Test (MRT). All outcomes have been evaluated at baseline and weeks 4, 8, 12, and 20. At the same time, functional MRI (fMRI) is used to measure the functional connectivity in the cognitive control network (CCN) at baseline and 4 weeks after intervention.
Discussion
This study aims to provide high-quality evidence for the efficacy and safety of the OAMT for treating PSD. In addition, this trial is the first trial to explore if the improvement condition of depression in the OAMT group is related to the improvement of executive functions and the favorable changes in the structure.
Clinical Trial Registration
Chinese Clinical Trial Registry, identifier: ChiCTR2100048431.
Keywords: post-stroke depression (PSD), acupuncture, moxibustion, auricular intradermal acupuncture, executive function, fMRI, randomized controlled trial, protocol
Introduction
Stroke is the leading cause of death and disability worldwide, contributing to a high burden of disease (1). Post-stroke depression (PSD) is a common neuropsychiatric comorbidity after stroke. It is reported that 20–65% of people suffer from PSD, and the cumulative percentage of patients with depression in the first 5 years after stroke is 39–52% (2, 3). In addition, PSD is not only related to the poor results of rehabilitation but also increases the risk of stroke recurrence and mortality. Unluckily, most guidelines for stroke do not address the best way to identify and treat depression in these patients, and existing studies on PSD are still insufficient (4–8).
A Cochrane review showed that there is limited evidence on the effectiveness of drug interventions for PSD (9). Commonly used drugs, such as serotonin reuptake inhibitors (SSRIs) and serotonin norepinephrine reuptake inhibitors (SNRIs), however, may cause a series of adverse reactions, as well as bring risks, including potential cerebral hemorrhage, myocardial infarction, and all-cause mortality (10–12). Therefore, it is of great significance to seek effective complementary and alternative therapies with a few side effects.
Acupuncture has a history of more than 2,000 years in China and is used to treat neuropsychiatric diseases such as stroke and depression. A meta-analysis shows that acupuncture significantly reduces the degree of PSD and has a better safety profile than antidepressants (13). Its antidepressant effect is reflected in the improvement of the Hamilton Depression Scale-17 (HAMD-17) score and the quality of life (14–18). Compared with the routine single acupuncture treatment, the addition of auricular intradermal acupuncture and moxibustion could consolidate and prolong the curative effect (19, 20). However, the efficacy of the optimized acupuncture and moxibustion treatment (OAMT) in the treatment of patients with PSD remains to be proven, and its mechanism has not been fully elucidated.
Depressive symptoms after stroke have been reported to be closely related to executive dysfunction (21). Pohjasvaara et al. (22) showed that executive dysfunction was detected in 40.6% (n = 104) of 256 patients 3–4 months after stroke, and this dysfunction was proven to be related to depressive symptoms. In the same vein, patients with PSD with executive dysfunction show more severe depressive symptoms and have a significantly higher incidence of cerebral infarction in the frontal lobe-subcortical circuit compared with patients with PSD without executive dysfunction (21). In neuroanatomy, links between the two have also been reported (23). Both are associated with alterations in intrinsic and extrinsic structural and functional connectivity in the convolutional neural network (CNN). Specifically, both may have structural disconnections in frontal, parietal, and subcortical areas (24–26), and these disconnections have contacts with lower intrinsic functional connectivity in the CNN (27). Cognitive control network (CCN) is the frontal parietal loop (28), which participates in top-down, attention-dependent executive functions such as decision-making and task switching (29–31). The dorsolateral prefrontal cortex (DLPFC) is an important node of this network (32), and it has been proven that the left DLPFC functional connectivity is negatively correlated with the severity of PSD (33). Several studies using DLPFC as a seed have reported the decreased functional connectivity within the CCN after depression (34–36). Alexopoulos et al. (35) pointed out that lower CCN connectivity can predict the lower recovery rate and symptom improvement of depressed individuals after taking escitalopram. Ye et al. (36) found that the node centrality of DLPFC in patients with depression is lower than that of normal people, which also reflects the weakening of the network function of CCN in patients with depression. Therefore, DLPFC is used as a seed to observe the functional connectivity in the CCN, which may be a key for exploring the neural mechanism of the OAMT in the treatment of patients with PSD. In addition, studies have shown that acupuncture is conducive to relieving executive dysfunction (37, 38), which may be resulted from taking an effect on the central nervous system through local reflex, affecting neurotransmitter levels, etc., to regulate the executive control system (39, 40). Therefore, this trial not only evaluates the degree of depression and executive functions in patients with PSD but also observes the CCN network's functional connectivity, providing stronger evidence for the efficacy and mechanism of the OAMT from multiple perspectives.
Methods and Analysis
Design and Setting
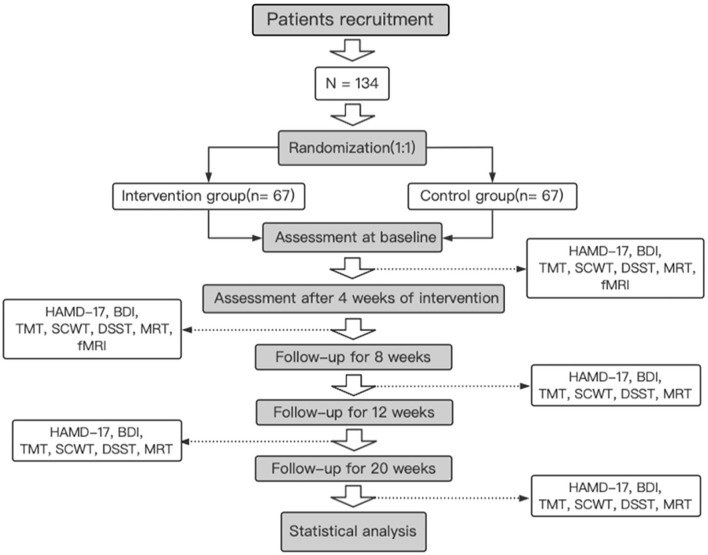
This is a prospective, randomized controlled trial conducted by the First Affiliated Hospital of Henan University of Chinese Medicine. A total of 134 patients who meet the inclusion and exclusion criteria are randomly divided into two groups. One group receives the OAMT and routine medicine and rehabilitation treatment, and another group receives routine medicine and rehabilitation treatment only. All treatments are provided five times per week for 4 weeks. The primary outcome is the severity of depression, which is evaluated by the HAMD-17 and the Beck Depression Rating Scale (BDI). Secondary outcomes are executive abilities, which are measured by several neuropsychological tests, including the Stroop Color and Word Test (SCWT), Trial Making Test (TMT), Digit Symbol Substitution Test (DSST), and Matrix Reasoning Test (MRT). All outcomes are evaluated at baseline and weeks 4, 8, 12, and 20. At the same time, functional MRI (fMRI) is used to measure the functional connectivity in the CCN at baseline and 4 weeks after intervention. The study's flow chart is shown in Figure 1, and the process chart is shown in Table 1.
Figure 1.
A flow chart of the trial.
Table 1.
A process chart of the trial.
| Study period | ||||||
|---|---|---|---|---|---|---|
| Timepoint | Enrollment | Baseline | Treatment phase | Follow-up phase | ||
| −1 week | 0 week | 4 weeks | 8 weeks | 12 weeks | 20 weeks | |
| Enrollment | ||||||
| Eligibility screen | × | |||||
| Informed consent | × | |||||
| Medical history | × | |||||
| Merger disease | × | |||||
| Randomization | × | |||||
| Interventions | ||||||
| Intervention group | × | × | ||||
| Control group | × | × | ||||
| Assessments | ||||||
| HAMD-17 | × | × | × | × | × | |
| BDI | × | × | × | × | × | |
| SCWT | × | × | × | × | × | |
| TMT | × | × | × | × | × | |
| DSST | × | × | × | × | × | |
| MRT | × | × | × | × | × | |
| fMRI | × | × | ||||
| Safety evaluation | × | × | ||||
| Adverse events | × | × | × | × | × | |
Recruitment of Participants
This trial is conducted at the First Affiliated Hospital of Henan University of Chinese Medicine, China. Patients who meet the criteria are recruited through the outpatient and inpatient systems and advertisements. The recruitment begins on January 1, 2022 and is expected to end in June 2023.
Inclusion Criteria
Participants who meet the following criteria are included
(1) Aged between 40 and 85.
(2) Diagnosed with ischemic stroke, and met the diagnostic criteria for “Depressive disorder due to another medical condition” in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V) (41).
(3) Had a first episode of stroke within 6 months.
(4) The HAMD-17 score >7 points, or <24 points.
(5) Have not taken antidepressants systematically.
(6) Patients or their immediate families sign an informed consent and voluntarily participate in this study.
Exclusion Criteria
Participants with the following conditions are excluded:
(1) The HAMD-17 score ≤ 7 points, or ≥24 points.
(2) Patients with obvious suicidal tendencies assessed by specialists.
(3) Diagnosed with depression, cognitive impairment, schizophrenia, bipolar disorder, substance abuse, or other mental disorders before stroke.
(4) Patients who are taking antidepressant drugs.
(5) Patients who have severe heart, liver, kidney, and other medical diseases or tumors.
(6) Patients who are diagnosed with bleeding disorders, coagulation dysfunction, and skin infections are not suitable for acupuncture and moxibustion treatment.
(7) Pregnant or lactating women.
(8) Contraindication for an MRI examination.
(9) Patients participating in any other clinical trials.
Randomization
The randomization sequence is generated by an independent statistician from the Henan Evidence-based Medicine Center of Traditional Chinese Medicine, using the PROCPLAN process of the SAS statistical analysis system. Then, the randomization sequence is placed in opaque sealed envelopes and assigned to the eligible patients who can be included.
Blinding
Due to the characteristics of the OAMT and the technical limitations, we are unable to conduct a double-blind study design. However, evaluators and statisticians of the outcome are blinded to the assignments. Patients are treated separately to avoid communication, and the OAMT sessions are strictly performed by licensed and experienced acupuncturists. Acupuncturists provide any information about the allocation to the patients, evaluators, or statisticians. In the process of data management and statistical analysis, a professional statistician who is not in this study is invited to undertake analysis tasks.
Interventions
Patients receive treatments in separate rooms five times a week for 4 weeks. All treatments are performed by licensed acupuncturists with more than 3 years of experience in practice. The location of the acupoints follows the WHO standards (42–44). At the same time, routine medicine and rehabilitation treatment of each patient is performed by physicians and therapists who do not know the allocation. The interventions of the two groups are as follows.
Intervention Group
Patients receive the OAMT and routine medicine and rehabilitation treatment once a day, five times a week, and for 4 weeks. The OAMT consists of acupuncture, moxibustion, and auricular intradermal acupuncture. The specific operations are as follows:
Acupuncture
After skin disinfection with 75% alcohol cotton swabs, patients receive acupuncture at Baihui (GV20), Shenting (GV24), Yintang (GV29), bilateral Hegu (LI4), Jiuwei (CV15), Zhongwan (CV12), Qihai (CV6), bilateral Sanyinjiao (SP6), and bilateral Taichong (LR3). Disposable sterile needles (0.25 ×25 mm; Huatuo, Suzhou Medical Appliance Fact. 215005 Suzhou, China) are used. Using the tube-guide method, the needles are inserted into the acupoints and operated for the sense of “De qi.” Specific acupuncture methods of each acupoint are shown in Table 2. The needles are kept in the acupoints for 30 min.
Table 2.
Specific acupuncture methods of each acupoint.
| Acupoints | Location | Insert angle | Insert depth |
|---|---|---|---|
| Baihui (GV20) | On the head, 5 cun directly above the midpoint of the anterior hairline | 15° | 0.5 cun |
| Shenting (GV24) | On the head, 0.5 cun directly above the midpoint of the anterior hairline | 15° | 0.5 cun |
| Yintang (GV29) | On the head, at the intersection of the line between the two brows and the front midline | 15° | 0.5 cun |
| Hegu (LI4) (bilateral) | On the dorsum of the hand, between the first and second metacarpal bones, approximately in the middle of the second metacarpal bone on the radial side | 90° | 0.5 cun |
| Jiuwei (CV15) | On the anterior median line of the upper abdomen, 1 cun below the Xiphisternal Synchondrosis | 45° | 0.5 cun |
| Zhongwan (CV12) | On the anterior median line of the upper abdomen, 4 cun above the navel | 90° | 1 cun |
| Qihai (CV6) | On the lower abdomen, on the front midline, 1.5 cun below the navel | 90° | 0.5 cun |
| Sanyinjiao (SP6) (bilateral) | On the medial side of the shank, 3 cun above the medial malleolus, by the posterior of the tibia | 90° | 1 cun |
| Taichong (LR3) (bilateral) | On the dorsum of the foot, in the depression proximal to the first metatarsal space | 45° | 0.5 cun |
A 1 cun (≈20 mm) is defined as the width of the interphalangeal joint of the patient's thumb.
Moxibustion
There are two acupoint selection plans used alternatively: (1) Feishu (BL13), Geshu (BL17), Danshu (BL19), and Yongquan (KI1). (2) Pohu (BL42), Geguan (BL46), Yanggang (BL48), and Yongquan (KI1). The location of the acupoints is shown in Table 3. The ignited moxa roll (herbal preparation of Artemisia vulgaris, Z32021062, Oriental Moxa Co., Suzhou, China) is applied 3 cm above the skin of acupoints, making the patient feel warm. When the skin turns red, the burning ash is moved away in time to avoid burning injury. Moxibustion for 30 min each time.
Table 3.
The location of moxibustion acupoints.
| Acupoints | Location |
|---|---|
| Feishu (BL13) | Under the spinous process of the third thoracic vertebrae, at the midpoint of the line between the medial edge of the scapula and the spine |
| Geshu (BL17) | Under the spinous process of the seventh thoracic vertebrae, at the midpoint of the line between the medial edge of the scapula and the spine |
| Danshu (BL19) | Under the spinous process of the ninth thoracic vertebrae, at the midpoint of the line between the medial edge of the scapula and the spine |
| Yongquan (KI1) | On the mid-line of the sole of the foot, 2/3 of the way forward from the back of the heel |
| Pohu (BL42) | Under the spinous process of the third thoracic vertebrae, at the medial edge of the scapula |
| Geguan (BL46) | Under the spinous process of the seventh thoracic vertebrae, at the medial edge of the scapula |
| Yanggang (BL48) | Under the spinous process of the second lumbar vertebra, 1 cun beside the spine |
A 1 cun (≈20 mm) is defined as the width of the interphalangeal joint of the patient's thumb.
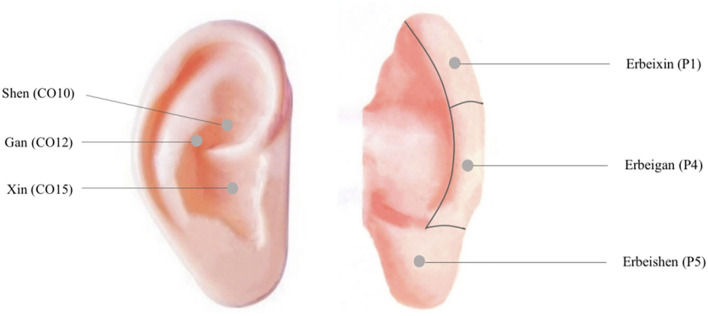
Auricular Intradermal Acupuncture
Following the acupuncture and moxibustion treatment, patients receive auricular intradermal acupuncture. There are two acupoint selection plans used alternatively: (1) Xin (CO15), Gan (CO12), and Shen (CO10) and (2) Erbeixin (P1), Erbeigan (P4), and Erbeishen (P5). The location of the acupoints is shown in Figure 2. After skin disinfection, the acupuncturist holds a sterile intradermal needle (0.22 × 1.5 mm, ZHONGYANTAIHE, AN2016, Wujiang Shenling Medical Equipment Co., Wujiang, China) to penetrate the auricle's skin. The insert angle is < 10°. Then, the needle is fixed on the acupoint with a medical tape. Intradermal needles are kept for 4 h in each session.
Figure 2.
The location of auricular intradermal acupuncture acupoints.
Control Group
Patients receive routine medicine and rehabilitation treatment for 4 weeks. For stroke and underlying diseases (such as hypertension and diabetes), we have provided symptomatic treatments, including antiplatelet aggregation, lowering lipids and stabilizing plaques, blood pressure, and blood sugar treatment, and have tried to avoid the drugs that affect mental factors, referring to the Guidelines for the Prevention and Treatment of Cerebrovascular Diseases in China (45). At the same time, routine rehabilitation treatment, such as exercise therapy and physical therapy, is provided equally.
Sample Size
The main outcome of this study is the improvement of depressive symptoms. Our team's preliminary pre-experiment (n = 30) showed that the OAMT for 4 weeks can reduce the HAMD-17 score by 14.53 ± 1.66. When α = 0.05, β = 0.1, 1 – β = 0.9, and the sample size of the intervention group and the control group are equal, the normal distribution quantile table shows that Zα/2 = 1.96, Zβ/2 = 1.282, σ = 1.66 means the SD of the intervention group and δ = 1.28 means the mean difference of the intervention group. Bring the data in the following formula:
After the calculation, the sample size is N = 60. Taking the 10% dropout rate into account, the sample size is 67 patients per group (134 in total). All 134 participants receive fMRI scans, and this sample size is much larger than the 12 cases per group required by the technical requirements of quality control and network analysis of acupuncture brain functional imaging (46), which can make the test results more reliable.
Outcome Measures
Participants are evaluated at baseline and weeks 4, 8, 12, and 20. All evaluations are conducted by researchers who are blinded to the treatment allocation.
Primary Outcomes
Primary outcomes are the HAMD-17 and the BDI.
Hamilton Depression Scale-17
The HAMD-17 is one of the most widely used scales in the evaluation of depression and is used to assess the severity of depression (47, 48). It consists of 17 items. The higher the score, the more severe the depression (49). The classification is as follows: normal (< 7), possible depression (7–17), diagnosed depression (18–24) and severe depression (> 24).
Beck Depression Rating Scale
The BDI is a commonly used self-evaluation scale to measure depression. It measures the intensity of depression by judging the main symptoms of depression syndrome (50). The advantages of the BDI lie in its international spread, high internal consistency of psychiatric and non-psychiatric samples, high content validity, high sensitivity to changes, and high convergence validity. The entire scale includes 21 groups of items, each group has four options, and each option corresponds to a certain score. A total score of <10 means no depression. The higher the score, the more severe the depression (51).
Secondary Outcomes
Secondary outcomes will be obtained using several neuropsychological tests and fMRI scanning. Neuropsychological tests include SCWT, TMT, DSST, and MRT.
Stroop Color and Word Test
The SCWT is a neuropsychological test widely used for experimental and clinical purposes. It can measure not only the ability to suppress cognitive interference but also a variety of cognitive functions (such as attention, processing speed, cognitive flexibility, and working memory) (52). The test is generally divided into three parts: (1) quickly name the color pictures; (2) quickly read the nouns that represent the name of the color; and (3) a set of cards have been presented with the nouns representing the color names written in colors different from the meaning of the words. A researcher checks the patient's ability to distinguish color names from actual colors and quickly read nouns representing color names. The degree to which the patient is affected by the color of the words is used as an index to measure his/her cognitive control ability.
Trial Making Test
The TMT is a commonly used neuropsychological test (53), and its reliability and effectiveness have been previously proven (54). TMT consists of two parts, involving visual search and scanning capabilities, processing speed, mental flexibility, and executive functions. TMT-A requires a patient to connect the circled numbers distributed on a piece of paper one by one. The requirements of TMT-B are similar to those of TMT-A, but the patient must alternate between numbers and graphics. The total score is calculated based on the task completion time and the accuracy rate.
Digit Symbol Substitution Test
The DSST was originated from the Wechsler Adult Intelligence Scale (55), which can evaluate participants' abilities related to digital decoding, memory, attention, and operating speed (56). Patients are provided with a table showing various symbols and matching numbers, and they are asked to write down matching symbols for each number in several rows of numbers. The score is the number of correctly coded numbers completed in 120 s (57).
Matrix Reasoning Test
The MRT is a sub-test of the Wechsler Intelligence Abbreviation Scale (58, 59), which measures non-verbal reasoning ability. It is composed of a sequence or a set of graphic matrixes. Patients are required to select the patterns or symbols that can be filled in the vacant part according to the pattern or symbol change rules in the incomplete sequence or graphic matrix. The score is the number of items completed correctly.
Functional MRI
An Ingenia 3.0 Tesla MRI scanner (Philips MedicalSystems, Best, Netherlands) with a head orthogonal coil is used for fMRI data acquisition. Participants are instructed to lie supine, close their eyes, and keep quiet and awake. High-resolution three-dimensional T1-weighted MRIs are collected at the beginning of the scanning session with the following parameters: TR = 1,900 ms, TE = 2.56 ms, flip angle = 9°, FOV = 250 × 250 mm, matrix size = 246 × 246 mm, and slice thickness = 1 mm. Then, the resting-state data are acquired as follows: TR = 2,000 ms, TE = 30 ms, flip angle = 90°, FOV = 240 × 240 mm, matrix size = 64 × 64 mm, slice thickness = 5 mm, and slice number = 32 slices.
Safety Evaluation and Adverse Events
Safety indicators are tested before and after the treatment, including general physical examination (blood pressure, pulse, and breathing), ECG, blood routine, urine routine, stool routine, and liver and kidney function tests. In the case report forms (CRFs), adverse events such as pain, fainting, local infection, and allergies are recorded in detail. Serious adverse events, such as death or life-threatening events, are reported to the researcher immediately and reported to the ethics committee within 24 h. In those circumstances, the research team gives the patient treatment and suggestions based on the situation, evaluates whether he/she continues to participate in the research, and compensates him/her accordingly.
Data Management
The trained evaluators use CRFs to record patients' information and data in detail and import them into an electronic database. After the study is completed, the paper CRFs are stored in a locked cabinet. Meanwhile, the electronic database has also been locked, so researchers do not able to modify the data. Participants' personal data are kept anonymously and strictly confidential. For patients who discontinue or leave the trial, we obtain their data by telephone with their consent. The Data Monitoring Committee of the Rehabilitation Center of the First Affiliated Hospital of Henan University of Chinese Medicine is established. They are independent of researchers to monitor the trial progress, regularly monitor the safety of the trial, and check the completeness and accuracy of the CRFs.
Data Analysis
Clinical Data Analysis
We invite third-party professional statisticians who do not know the trial protocol to conduct a statistical analysis and participate in the whole process comprising trial design, implementation, and data analysis. If the necessary data are available, subgroup analyses are performed by different clinicopathological features, such as gender, age, time of stroke onset, and stroke severity. A statistical analysis is performed using SPSS 22.0.
The data are statistically described by mean ± SD. Continuous variables are compared using Student t-test or Wilcoxon rank sum test, and categorical variables are compared using Pearson χ2 test or Wilcoxon rank-sum test. The comparison between the groups is carried out by an independent sample t-test or the Mann–Whitney U-rank-sum test based on whether the measurement data present a normal distribution and homogeneous variance. When doing a statistical analysis with two-tailed testing, the significance level is set at 5%. When p < 0.05, differences have been considered as statistically significant.
MRI Data Analysis
The SPM8 software platform (SPM8, Wellcome Department of Imaging Neuroscience, London, UK; http://www.fil.ion.ucl.ac.uk/spm/) is used to preprocess the MRI data. Referring to the study of DLPFC resting-state connectivity of patients with PSD (33), we select the seeds from bilateral DLPFC, draw the region of interest (ROI) in WFU Pickatlas (60) to tap into the CCN, and perform a whole-brain regression analysis. The DLPFC resting-state connectivity in the CCN is compared between the intervention group and the control group. A paired t-test is used to evaluate brain changes in each group through an intragroup analysis (before and after the treatment). We include factors such as age and gender as covariates in the data analysis. The Pearson correlation coefficient is used to analyze the relationship between the improvement value of the correlation scales and the change of fMRI image data.
Quality Control
Qualified acupuncturists, physicians, therapists, and statisticians in the First Affiliated Hospital of Henan University of Chinese Medicine monitored and revised this trial protocol. All relevant personnel are trained in accordance with the prepared standard operating procedure (SOP) to ensure consistency in the comprehension and implementation of interventions and evaluations. At the same time, a quality control team is established to conduct quality control, and the qualified clinical trial experts are invited to supervise the trial once a month.
Discussion
There is a large volume of published studies showing that acupuncture and related therapies effectively improved depression, and the combination therapy appeared to have superior efficacy (14–16, 19, 20). However, these studies used diverse acupoint selection plans and intervention methods, and lacked a unified standard acupuncture program. Moreover, they remain narrow in focus dealing only with efficacy without exploring the mechanism. The OAMT is a combination therapy based on the acupuncture theory, our previous experiments, and practical experience. It was developed according to the theory of “ShuGanTiaoShen” (smoothing the liver and regulating mental activities). Chinese doctor believes that the cause of PSD is closely related to “Liver Failing to Maintain Normal Flow of Qi” and “Disorder of Cerebral Soul” (61, 62). Referring to this, this study select acupoint plans with the effect of “ShuGanTiaoShen.”
Based on the clinical practice and previous experimental results of the First Affiliated Hospital of Henan University of Chinese Medicine, we completed the first draft of the protocol for this study. Subsequently, the ethics committee of the hospital discussed and revised the draft, and the final version of the protocol was unanimously approved by the ethics committee.
This trial is the first trial to explore the OAMT treatment of the PSD population from the executive control system. It has been proven that the reduction of functional connectivity in the CCN affects depression vulnerability and maintenance through an impact on the cognitive control of emotional information (63, 64). In other words, neurological deficits are considered as a basis for the difficulty of cognitive control mechanisms (e.g., attention control, inhibition, and reassessment) that support emotion regulation. These deficits may hinder the function of CNN. Therefore, observing neurological defects of CCN through fMRI is of certain significance to explore the mechanism of the OAMT in the treatment of PSD.
However, this study has some limitations. First, it is a single-center study in which patients from different areas cannot be recruited. Second, although this study followed the principle of separation of researchers, operators, and statisticians due to the nature of acupuncture and moxibustion treatment, we did not use blind methods in the OAMT. This may cause partial deviations in the results. Despite all of these, we strive to standardize the process of this study to provide high-quality medical evidence for the OAMT, as well as an optimized plan as a complementary therapy for PSD.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the First Affiliated Hospital of Henan University of Chinese Medicine (reference number: 2021HL-184-01). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
XF and JG designed this study. ML and ZD drafted the manuscript together. XS revised this manuscript and conducted the preliminary pre-experiment. CL, RL, KS, and XW recruited patients and provided treatment. WF and YB conducted a statistical analysis in the pre-experiment. All authors agreed to the final version of this manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (Grant Nos. 82104973 and 81574042), the Ministry of Science and Technology of the People's Republic of China (Grant No. 2018YFC1706004), the Department of Science and Technology of Henan Province (Grant No. 222102310715), and the Henan Provincial Administration of Traditional Chinese Medicine (Grant No. 2022ZY1162).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank all the staff who contributed to our study and also for their effort and support.
Glossary
Abbreviations
- PSD
post-stroke depression
- OAMT
optimized acupuncture and moxibustion treatment
- HAMD-17
Hamilton Depression Scale-17
- BDI
Beck Depression Rating Scale
- TMT
Trial Making Test
- SCWT
Stroop Color and Word Test
- DSST
Digit Symbol Substitution Test
- MRT
Matrix Reasoning Test
- CCN
cognitive control network
- SSRIs
serotonin reuptake inhibitors
- SNRIs
serotonin norepinephrine reuptake inhibitors
- DLPFC
dorsolateral prefrontal cortex
- DSM-V, Diagnostic and Statistical Manual of Mental Disorders
Fifth Edition
- SOP
standard operating procedure
- CRFs
case report forms.
References
- 1.Collaborators GBDS. Global, regional, and national burden of stroke, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. (2019) 18:439–58. 10.1016/S1474-4422(19)30034-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharma GS, Gupta A, Khanna M, Prakash NB. Post-stroke depression and its effect on functional outcomes during inpatient rehabilitation. J Neurosci Rural Pract. (2021) 12:543–9. 10.1055/s-0041-1731958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ayerbe L, Ayis S, Wolfe CD, Rudd AG. Natural history, predictors and outcomes of depression after stroke: systematic review and meta-analysis. Br J Psychiatry. (2013) 202:14–21. 10.1192/bjp.bp.111.107664 [DOI] [PubMed] [Google Scholar]
- 4.Carod-Artal FJ. Post-stroke depression (I). Epidemiology, diagnostic criteria and risk factors. Rev Neurol. (2006) 42:169–75. 10.33588/rn.4203.2005049 [DOI] [PubMed] [Google Scholar]
- 5.Dafer RM, Rao M, Shareef A, Sharma A. Poststroke depression. Top Stroke Rehabil. (2008) 15:13–21. 10.1310/tsr1501-13 [DOI] [PubMed] [Google Scholar]
- 6.Sturm JW, Donnan GA, Dewey HM, Macdonell RA, Gilligan AK, Srikanth V, et al. Quality of life after stroke: the North East Melbourne Stroke Incidence Study (NEMESIS). Stroke. (2004) 35:2340–5. 10.1161/01.STR.0000141977.18520.3b [DOI] [PubMed] [Google Scholar]
- 7.Morris PL, Robinson RG, Andrzejewski P, Samuels J, Price TR. Association of depression with 10-year poststroke mortality. Am J Psychiatry. (1993) 150:124–9. 10.1176/ajp.150.1.124 [DOI] [PubMed] [Google Scholar]
- 8.Morris PL, Robinson RG, Samuels J. Depression, introversion and mortality following stroke. Aust N Z J Psychiatry. (1993) 27:443–9. 10.3109/00048679309075801 [DOI] [PubMed] [Google Scholar]
- 9.Hackett ML, Anderson CS, House A, Xia J. Interventions for treating depression after stroke. Cochr Database Syst Rev. (2008) CD003437. 10.1002/14651858.CD003437.pub3 [DOI] [PubMed] [Google Scholar]
- 10.Coupland C, Dhiman P, Morriss R, Arthur A, Barton G, Hippisley-Cox J. Antidepressant use and risk of adverse outcomes in older people: population based cohort study. BMJ. (2011) 343:d4551. 10.1136/bmj.d4551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smoller JW, Allison M, Cochrane BB, Curb JD, Perlis RH, Robinson JG, et al. Antidepressant use and risk of incident cardiovascular morbidity and mortality among postmenopausal women in the Women's Health Initiative study. Arch Intern Med. (2009) 169:2128–39. 10.1001/archinternmed.2009.436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hackam DG, Mrkobrada M. Selective serotonin reuptake inhibitors and brain hemorrhage: a meta-analysis. Neurology. (2012) 79:1862–5. 10.1212/WNL.0b013e318271f848 [DOI] [PubMed] [Google Scholar]
- 13.Liu R, Zhang K, Tong QY, Cui GW, Ma W, Shen WD. Acupuncture for post-stroke depression: a systematic review and meta-analysis. BMC Comp Med Ther. (2021) 21:109. 10.1186/s12906-021-03277-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen SX, Liu FF. [Effect of tiaoshen kaiyu acupuncture (regulating vitality and dredging stasis) combined with psychological intervention on patients of mild depression after stroke]. Zhen Ci Yan Jiu. (2018) 43:39–43. 10.13702/j.1000-0607.170049 [DOI] [PubMed] [Google Scholar]
- 15.You Y, Zhang T, Shu S, Qian X, Zhou S, Yao F. Wrist-ankle acupuncture and Fluoxetine in the treatment of post-stroke depression: a randomized controlled clinical trial. J Tradit Chin Med. (2020) 40:455–60. 10.19852/j.cnki.jtcm.2020.03.014 [DOI] [PubMed] [Google Scholar]
- 16.Nie RR, Huang CH. [Post-stroke depression treated with acupuncture and moxibustion: an evaluation of therapeutic effect and safety]. Zhongguo Zhen Jiu. (2013) 33:490–4. [PubMed] [Google Scholar]
- 17.Zhang GJ, Shi ZY, Liu S, Gong SH, Liu JQ, Liu JS. Clinical observation on treatment of depression by electro-acupuncture combined with Paroxetine. Chin J Integr Med. (2007) 13:228–30. 10.1007/s11655-007-0228-0 [DOI] [PubMed] [Google Scholar]
- 18.Smith CA, Armour M, Lee MS, Wang LQ, Hay PJ. Acupuncture for depression. Cochrane Database Syst Rev. (2018) 3:CD004046. 10.1002/14651858.CD004046.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hung CY, Wu XY, Chung VC, Tang EC, Wu JC, Lau AY. Overview of systematic reviews with meta-analyses on acupuncture in post-stroke cognitive impairment and depression management. Integr Med Res. (2019) 8:145–59. 10.1016/j.imr.2019.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wen X, Li K, Wen H, Wang Q, Wu Z, Yao X, et al. Acupuncture-related therapies for Parkinson's disease: a meta-analysis and qualitative review. Front Aging Neurosci. (2021) 13:676827. 10.3389/fnagi.2021.676827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vataja R, Pohjasvaara T, Mantyla R, Ylikoski R, Leskela M, Kalska H, et al. Depression-executive dysfunction syndrome in stroke patients. Am J Geriatr Psychiatry. (2005) 13:99–107. 10.1097/00019442-200502000-00003 [DOI] [PubMed] [Google Scholar]
- 22.Pohjasvaara T, Leskela M, Vataja R, Kalska H, Ylikoski R, Hietanen M, et al. Post-stroke depression, executive dysfunction and functional outcome. Eur J Neurol. (2002) 9:269–75. 10.1046/j.1468-1331.2002.00396.x [DOI] [PubMed] [Google Scholar]
- 23.Jaywant A, DelPonte L, Kanellopoulos D, O'Dell MW, Gunning FM. The structural and functional neuroanatomy of post-stroke depression and executive dysfunction: a review of neuroimaging findings and implications for treatment. J Geriatr Psychiatry Neurol. (2022) 35:3–11. 10.1177/0891988720968270 [DOI] [PubMed] [Google Scholar]
- 24.Respino M, Hoptman MJ, Victoria LW, Alexopoulos GS, Solomonov N, Stein AT, et al. Cognitive control network homogeneity and executive functions in late-life depression. Biol Psychiatry Cogn Neurosci Neuroimaging. (2020) 5:213–21. 10.1016/j.bpsc.2019.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roussel M, Dujardin K, Henon H, Godefroy O. Is the frontal dysexecutive syndrome due to a working memory deficit? Evidence from patients with stroke. Brain. (2012) 135 (Pt 7):2192–201. 10.1093/brain/aws132 [DOI] [PubMed] [Google Scholar]
- 26.Kuceyeski A, Navi BB, Kamel H, Relkin N, Villanueva M, Raj A, et al. Exploring the brain's structural connectome: A quantitative stroke lesion-dysfunction mapping study. Hum Brain Mapp. (2015) 36:2147–60. 10.1002/hbm.22761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Foulon C, Cerliani L, Kinkingnehun S, Levy R, Rosso C, Urbanski M, et al. Advanced lesion symptom mapping analyses and implementation as BCBtoolkit. Gigascience. (2018) 7:1–17. 10.1093/gigascience/giy004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron. (2009) 62:42–52. 10.1016/j.neuron.2009.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Y, Hu G, Yu Y, Jiang Z, Yang K, Hu X, et al. Structural and functional reorganization within cognitive control network associated with protection of executive function in patients with unilateral frontal gliomas. Front Oncol. (2020) 10:794. 10.3389/fonc.2020.00794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. (2007) 27:2349–56. 10.1523/JNEUROSCI.5587-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sakai K, Passingham RE. Prefrontal set activity predicts rule-specific neural processing during subsequent cognitive performance. J Neurosci. (2006) 26:1211–8. 10.1523/JNEUROSCI.3887-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tops M, Boksem MA. A potential role of the inferior frontal gyrus and anterior insula in cognitive control, brain rhythms, and event-related potentials. Front Psychol. (2011) 2:330. 10.3389/fpsyg.2011.00330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Egorova N, Cumming T, Shirbin C, Veldsman M, Werden E, Brodtmann A. Lower cognitive control network connectivity in stroke participants with depressive features. Transl Psychiatry. (2018) 7:4. 10.1038/s41398-017-0038-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liston C, Chen AC, Zebley BD, Drysdale AT, Gordon R, Leuchter B, et al. Default mode network mechanisms of transcranial magnetic stimulation in depression. Biol Psychiatry. (2014) 76:517–26. 10.1016/j.biopsych.2014.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alexopoulos GS, Hoptman MJ, Kanellopoulos D, Murphy CF, Lim KO, Gunning FM. Functional connectivity in the cognitive control network and the default mode network in late-life depression. J Affect Disord. (2012) 139:56–65. 10.1016/j.jad.2011.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ye M, Yang T, Qing P, Lei X, Qiu J, Liu G. Changes of functional brain networks in major depressive disorder: a graph theoretical analysis of resting-state fMRI. PLoS ONE. (2015) 10:e0133775. 10.1371/journal.pone.0133775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tan TT, Wang D, Huang JK, Zhou XM, Yuan X, Liang JP, et al. Modulatory effects of acupuncture on brain networks in mild cognitive impairment patients. Neural Regen Res. (2017) 12:250–8. 10.4103/1673-5374.200808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiong J, Zhang Z, Ma Y, Li Z, Zhou F, Qiao N, et al. The effect of combined scalp acupuncture and cognitive training in patients with stroke on cognitive and motor functions. NeuroRehabilitation. (2020) 46:75–82. 10.3233/NRE-192942 [DOI] [PubMed] [Google Scholar]
- 39.Hori E, Takamoto K, Urakawa S, Ono T, Nishijo H. Effects of acupuncture on the brain hemodynamics. Auton Neurosci. (2010) 157:74–80. 10.1016/j.autneu.2010.06.007 [DOI] [PubMed] [Google Scholar]
- 40.Liu G, Ma HJ, Hu PP, Tian YH, Hu S, Fan J, et al. Effects of painful stimulation and acupuncture on attention networks in healthy subjects. Behav Brain Funct. (2013) 9:23. 10.1186/1744-9081-9-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.American Psychiatric Association. DSM-5. 5th ed. Arlington, VA: American Psychiatric Association; (2013). [Google Scholar]
- 42.World Health Organization . A Proposed Standard International Acupuncture Nomenclature: Report of a WHO Scientific Group. Geneva: (1991). Available online at: https://apps.who.int/iris/bitstream/handle/10665/40001/9241544171_eng.pdf?sequence=1 [Google Scholar]
- 43.Wang D. The Third WHO regional workshop on the standardization of acupuncture nomenclature. J Trad Chin Med. (1988) 8:221. [PubMed] [Google Scholar]
- 44.World Health Organization . WHO Standard Acupuncture Point Locations in the Western Pacific Region. Geneva: World Health Organization; (2008). [Google Scholar]
- 45.Liu L, Chen W, Zhou H, Duan W, Li S, Huo X, et al. Chinese Stroke Association guidelines for clinical management of cerebrovascular disorders: executive summary and 2019 update of clinical management of ischaemic cerebrovascular diseases. Stroke Vasc Neurol. (2020) 5:159–76. 10.1136/svn-2020-000378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qiu K, Jing M, Sun R, Yang J, Liu X, He Z, et al. The status of the quality control in acupuncture-neuroimaging studies. Evid Based Comp Altern Med. (2016) 2016:3685785. 10.1155/2016/3685785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. (1960) 23:56–62. 10.1136/jnnp.23.1.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. (1967) 6:278–96. 10.1111/j.2044-8260.1967.tb00530.x [DOI] [PubMed] [Google Scholar]
- 49.Leucht S, Fennema H, Engel R, Kaspers-Janssen M, Lepping P, Szegedi A. What does the HAMD mean? J Affect Disord. (2013) 148:243–8. 10.1016/j.jad.2012.12.001 [DOI] [PubMed] [Google Scholar]
- 50.Hautzinger M. [The Beck Depression Inventory in clinical practice]. Nervenarzt. (1991) 62:689–96. [PubMed] [Google Scholar]
- 51.Richter P, Werner J, Heerlein A, Kraus A, Sauer H. On the validity of the beck depression inventory. A review. Psychopathology. (1998) 31:160–8. 10.1159/000066239 [DOI] [PubMed] [Google Scholar]
- 52.Scarpina F, Tagini S. The stroop color and word test. Front Psychol. (2017) 8:557. 10.3389/fpsyg.2017.00557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Giovagnoli AR, Del Pesce M, Mascheroni S, Simoncelli M, Laiacona M, Capitani E. Trail making test: normative values from 287 normal adult controls. Ital J Neurol Sci. (1996) 17:305–9. 10.1007/BF01997792 [DOI] [PubMed] [Google Scholar]
- 54.Sanchez-Cubillo I, Perianez JA, Adrover-Roig D, Rodriguez-Sanchez JM, Rios-Lago M, Tirapu J, et al. Construct validity of the Trail Making Test: role of task-switching, working memory, inhibition/interference control, and visuomotor abilities. J Int Neuropsychol Soc. (2009) 15:438–50. 10.1017/S1355617709090626 [DOI] [PubMed] [Google Scholar]
- 55.Wechsler D. Wechsler Adult Intelligence Scale. 3rd ed. San Antonio, TX: The Psychological Corporation; (1997). 10.1007/978-1-4419-1698-3_101553 [DOI] [Google Scholar]
- 56.Bright P, Hale E, Gooch VJ, Myhill T, van der Linde I. The National Adult Reading Test: restandardisation against the Wechsler Adult Intelligence Scale-Fourth edition. Neuropsychol Rehabil. (2018) 28:1019–27. 10.1080/09602011.2016.1231121 [DOI] [PubMed] [Google Scholar]
- 57.Zhang N, Du S, Tang Z, Zheng M, Ma G. Effect of water supplementation on cognitive performances and mood among male college students in Cangzhou, China: study protocol of a randomized controlled trial. Int J Environ Res Public Health. (2017) 14:966. 10.3390/ijerph14090966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chiaravalloti ND, Weber E, Wylie G, Dyson-Hudson T, Wecht JM. Patterns of cognitive deficits in persons with spinal cord injury as compared with both age-matched and older individuals without spinal cord injury. J Spinal Cord Med. (2020) 43:88–97. 10.1080/10790268.2018.1543103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wechsler D. Wechsler Abbreviated Scale of Intelligence Manual (WASI). San Antonio, TX: Harcourt Assessment; (1999). [Google Scholar]
- 60.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. (2003) 19:1233–9. 10.1016/S1053-8119(03)00169-1 [DOI] [PubMed] [Google Scholar]
- 61.Zhang JS. [Effect of shugan jiannao tiaoyu tablets (SJTT) on hypothalamic corticotrophin releasing hormone gene expression in model rat of post-stroke depression]. Zhongguo Zhong Yao Za Zhi. (2008) 33:2037–40. [PubMed] [Google Scholar]
- 62.Sun PY, Li PF, Wang T, Wu J, Li N, Liu H, et al. [Effect of Tongdu Tiaoshen acupuncture on PI3K/Akt/mTOR signaling pathway and autophagy-related proteins of hippocampus in rats with post-stroke depression]. Zhongguo Zhen Jiu. (2020) 40:1205–10. 10.13703/j.0255-2930.20200522-k0006 [DOI] [PubMed] [Google Scholar]
- 63.De Raedt R, Koster EH. Understanding vulnerability for depression from a cognitive neuroscience perspective: a reappraisal of attentional factors and a new conceptual framework. Cogn Affect Behav Neurosci. (2010) 10:50–70. 10.3758/CABN.10.1.50 [DOI] [PubMed] [Google Scholar]
- 64.Disner SG, Beevers CG, Haigh EA, Beck AT. Neural mechanisms of the cognitive model of depression. Nat Rev Neurosci. (2011) 12:467–77. 10.1038/nrn3027 [DOI] [PubMed] [Google Scholar]