Abstract
Background:
This study aimed to investigate characteristics of severe hepatitis (SH), acute liver injury (ALI), and acute liver failure (ALF) in patients with Mushroom-induced hepatotoxicity.
Methods:
Data of patients were retrospectively reviewed between 2010-2019. Twenty-four patients withmushroominduced hepatotoxicity were included and divided into three groups: SH, ALI, and ALF. SH was defined as transaminase level≥10XULN, INR≤1.5, and the absence of hepatic encephalopathy (HE). ALI was defined as INR>1.5, presumed acute illness onset, and the absence of HE. ALF was diagnosed based on the presence of HE of any degree, with INR>1.5, presumed acute illness onset, and the absence of cirrhosis.
Results:
The mean age was 51.6 years; 13 (54.2%) were female. At admission, 18 patients (75%) had SH, 5 (21%) had ALI, 1 (4.1%) had ALF. During follow-up, 6 of 18 SH (33%) progressed to ALI, 2 of 5 ALI (40%) progressed to ALF. No progression to ALI or ALF was observed in the eight SH cases with a baseline MELD score of <15. One patient with grade 4 HE died (4.1%), none underwent liver transplantation.
Conclusion:
The survival was 100% in ALI and SH groups. MELD score of <15 at admission may be used as a predictor of no progression to ALI or ALF in patients with SH. However, since 40% of ALI cases may progress to ALF, these cases should be followed up in a tertiary care center that is equipped to perform liver transplantation and advanced therapies.
Keywords: Mushroom-induced poisoning, mushroom-induced hepatotoxicity, acute liver injury, acute liver failure
Introduction
The consumption of edible mushrooms or their isolated bioactive constituents has nutraceutical health benefits such as antioxidant, immunomodulatory, anti-inflammatory, antimicrobial, and even hepatoprotective effects. Nonetheless, certain types of mushrooms are toxic, and their ingestion could lead to acute hepatic necrosis and fulminant hepatic failure, to the extent of requiring liver transplantation. Mushroom-induced hepatotoxicity is a condition with high morbidity and mortality.1 Despite regional variations, there are 2000 to 1.5 million species of mushrooms, of which only 100 species are toxic.2 Two groups of mushroom toxins have been identified to have fatal courses of intoxication: A) The amatoxin-containing Amanita group is responsible for 90% of the cases of mushroom-induced poisoning that develop severe liver and kidney damage, possibly leading to death.3 B) Phallotoxin is believed to be responsible for gastrointestinal symptoms, ascribed to cell membrane disruption of enterocytes.4 Amatoxins selectively and irreversibly inhibit the RNA polymerase enzyme, which is critical for the synthesis of messenger RNAs and microRNAs.5 As a result of this inhibition, the protein-dependent regenerative capacity of the liver is impaired, thereby interrupting its responsiveness to repair the ongoing loss of hepatic mass and the associated intravascular hemolysis, induced by the toxic mushroom ingredient(s). The compromised liver function, in turn, leads to the development of centrilobular and periportal hemorrhagic hepatic necrosis. Likewise, the hepatocyte injury caused by the irreversible inhibition of mRNA synthesis leads to the lack of production of hepatic clotting factors and the subsequent development of coagulopathy.6 By 6-24 hours after ingestion of mushroom containing amatoxins, gastrointestinal symptoms such as nausea, vomiting, abdominal pain, and severe watery diarrhea are noticeable, followed by the deterioration of liver function and coagulopathy within 24-48 hours.7 Following ingestion of amatoxin-containing mushroom, cases might have a wide range of clinical presentations, from moderately elevated transaminase levels and symptoms of mild to severe hepatitis (SH), to acute liver injury (ALI), acute liver failure (ALF), and sometimes, death. In these patients, early diagnosis and treatment could be life-saving.3 However, there is no specific antidote treatment for mushroom intoxication. Detoxification procedures such as the use of active charcoal, hemoperfusion, extracorporeal liver support and plasmapheresis, N-acetylcysteine (NAC), penicillin, and silymarin could be used as supportive therapy in amatoxin poisoning, while liver transplantation is indicated in severe cases. In patients with amatoxin poisoning, prognosis is difficult, and thus, the mortality rate reported is high, varying between 4.8% and 34.5%.8,9,10,11
This study aimed to investigate the clinical, laboratory, and survival characteristics of patients who developed SH, ALI, and ALF due to mushroom-induced hepatotoxicity, and to determine factors that predict the progression of the disease severity.
Materials and Methods
Patients’ Data
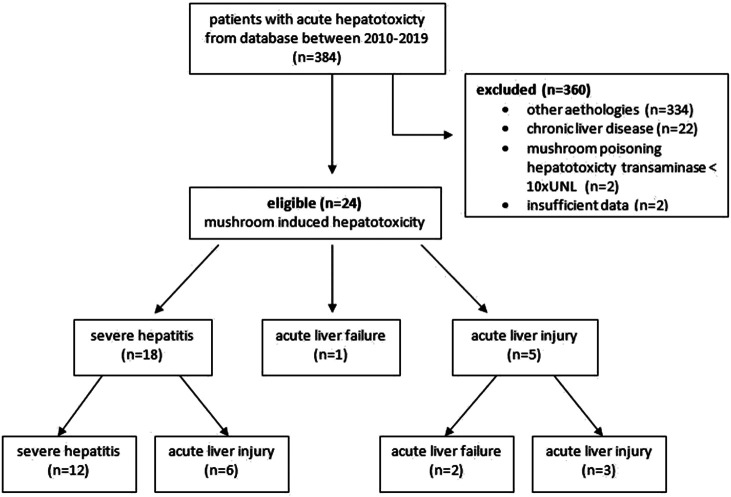
We retrieved data from electronic records of patients hospitalized due to mushroom-induced hepatotoxicity in the Gastroenterology Clinic of the Ege University Hospital, Izmir, between January 2010 and January 2019, and reviewed them retrospectively. The electronic database was searched using the keywords “acute hepatitis,” “hepatotoxicity,” “toxic hepatitis,” and “mushroom-induced hepatotoxicity.” We accessed a total of 384 patient records from the electronic files and excluded 360 cases. Our exclusion criteria were as follows: (1) the etiology of a patient’s hepatic disease not associated with mushroom-induced hepatotoxicity (e.g., drug use or autoimmune, viral, ischemic, and acute or chronic liver disease); (2) patients who had transaminase level <10 times the upper limit normal (ULN) of reference range after mushroom ingestion; and (3) patients who had incomplete data and were diagnosed with chronic liver parenchymal disease. Thus, we enrolled a total of 24 patients, based on the following inclusion criteria: (1) patients’ hepatic diseases were due to mushroom-induced hepatotoxicity, diagnosed as SH, ALI, or ALF; and (2) transaminase level after mushroom ingestion should be >10 times ULN. The consort flow diagram showing the progress of the study is shown in Figure 1.
Figure 1.
The consort flow diagram.
Patients’ Demographic and Clinical Data
The patients’ demographic data and clinical characteristics such as complaint at presentation, baseline laboratory parameters [white blood cell, hemoglobin, platelet, urea, creatinine, sodium, international normalized ratio (INR), total bilirubin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), gamma-glutamyl-transpeptidase (GGT)], and hepatic encephalopathy (HE) were recorded. The Model for End-stage Liver Disease (MELD) scores and prothrombin index were calculated. In addition, the peak laboratory values of the indicated measured parameters during follow-up were obtained. The time elapsed from mushroom ingestion to the peak of these indicated values was noted. In addition, HE development, total hospitalization time, treatment modalities, and survival data were recorded. The treatment modalities were grouped as NAC, silymarin, penicillin G (Pen G), plasmapheresis, and liver transplantation. According to the institutional protocol, NAC was administered as 300 mg/h for 24 hours, followed by 150 mg/h until the liver function test results were normalized. Silymarin was given as 5 mg/kg intravenous loading and 20 mg/kg/day as total maintenance dose divided into 4 intravenous injections.7 Pen G was administered at a dosage of 1 million U/kg/day.12 HE was graded according to the West Haven classification.13 The indications for liver transplantation were determined, according to the King’s College Criteria.14 The requirements of renal injury and renal replacement therapy were identified using the 2012 Kidney Disease: Improving Global Outcomes (KDIGO) guidelines.15
Mushroom-induced hepatotoxicity was diagnosed in cases of SH, ALI, or ALF after ingestion of mushrooms, for which other causes of hepatotoxicity were eliminated. We used the EASL guideline criteria for ALI and ALF diagnosis. However, we described another group of patients with high transaminase levels as SH. SH was confirmed if the transaminase level was ≥10 times ULN, the INR value was ≤1.5, and HE was not detected. According to the EASL manual, ALI was defined as elevated serum transaminases, the evidence of moderately severe coagulopathy (INR > 1.5), presumed acute illness onset, the absence of cirrhosis and HE; ALF was diagnosed based on the presence of HE of any degree, evidence of moderately severe coagulopathy (INR > 1.5), presumed acute illness onset, and the absence of cirrhosis.16
Statistical Analysis
The Statistical Package for the Social Sciences (SPSS) (IBM Corp.; Armonk, NY, USA) 20.0 package program was used for statistical analysis. The chi-square test (or Fisher’s exact test) was used to examine the relationship between the categorical variables, and the Mann–Whitney U-test was employed to compare the continuous variables. Spearman’s test was conducted to determine the correlations between the parameters. A P-value of <.05 was considered statistically significant.
Results
In the study, 13 of the 24 patients (54.2%) were female, and 11 (45.8%) were males. The mean age was 51.6 (range: 22-73) years. At admission, SH was detected in 18 patients (75%), ALI in 5 (21%), and ALF in 1 patient (4%). During the follow-up, 2 of the 5 ALI cases (40%) progressed to ALF, and 6 of the 18 SH cases (33%) advanced to ALI. Thus, in the follow-up period, 12 patients (50%) were evaluated as SH and 12 patients (50%) as ALI or ALF. Exactly 23 patients (95.9%), followed up for mushroom-induced hepatotoxicity, were discharged as cured. One patient (4.1%) was admitted to the hospital with grade 4 HE and died within 24 hours due to ALF. The remaining 2 patients that developed ALF during their stay in hospital were observed to have had grade 2 HE, and survived. Table 1 shows the laboratory parameters of the patients. The median time from mushroom ingestion to hospital admission was 3 days (range: 1-7). At the time of presentation, 23 patients (95.8%) had nausea and vomiting, 15 had diarrhea (62.5%), and 12 (50%) had abdominal pain (Table 1). The follow-up peak laboratory values and the time it took to reach these values are shown in Table 2. The MELD score was <15 in 8 patients (33%) and ≥15 in 16 patients (67%) at admission (11 ALI, 1 ALF, 4 SH). No progression to ALI or ALF was detected in any of the 8 SH patients with a baseline MELD score of <15. During the follow-up, ALF was detected in 2 of the 5 patients with ALI on admission. Among those 11 cases (5 on admission, 6 progressed from SH to ALI) with ALI, 8 cases with an INR between 1.5 and 2.7 did not develop HE or progress to death.
Table 1.
Clinical Characteristics and Treatments of Patients
| Variables | At Admission Median (min-max) |
|---|---|
| WBC (103/µL) | 9.14 (3.27-32.63) |
| Hemoglobin (g/dL) | 14 (8.3-1.6.2) |
| Platelet (103/µL) | 234 (99-464) |
| Urea (mg/dL) | 53.5 (20-115) |
| Creatinine (mg/dL) | 0.84 (0.54-3.1) |
| Na (mEq/L) | 138 (132-143) |
| Phosphorus (mg/dL) | 2.9 (1.1-5.4) |
| INR | 1.55 (0.9-4.5) |
| Total bilirubin (mg/dL) | 1.62 (0.1-7.86) |
| AST (U/L) | 1409 (51-5869) |
| ALT (U/L) | 1596.5 (48-8450) |
| ALP (U/L) | 95 (52-409) |
| GGT (U/L) | 37.5 (9-347) |
| MELD Score | 18.5 (6-39) |
| Symptoms | n (%) |
| Nausea-vomiting | 23 (95.8) |
| Diarrhea | 15 (62.5) |
| Abdominal pain | 12 (50) |
| Treatments | n (%) |
| NAC | 4 (16.7) |
| NAC + Pen G | 6 (25) |
| NAC + Silymarin | 2 (8.3) |
| NAC + Pen G + Silymarin | 5 (20.8) |
| NAC + Plasmapheresis | 1 (4.2) |
| NAC + Pen G + Plasmapheresis | 2 (8.3) |
| NAC + Silymarin + Plasmapheresis | 2 (8.3) |
| NAC + Pen G + Silymarin + Plasmapheresis | 2 (8.3) |
WBC, white blood cell; INR, international normalized ratio; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; GGT, gamma-glutamyl transferase; MELD, Model for End-stage Liver Disease; NAC, n-acetylcysteine, Pen G; penicillin G.
Table 2.
Peak Values and Peak Times of Biochemical Parameters of the Patients After Mushroom Ingestion
| Variables | Peak Values Median (min-max) | Peak Time (days) Median (min-max) |
|---|---|---|
| Creatinine (mg/dL) | 0.9 (0.54-3.1) | 4 (3-17) |
| INR | 2.1 (1-5.7) | 4 (3-9) |
| Total bilirubin (mg/dL) | 1.9 (0.68-13.44) | 6,5 (3-9) |
| AST (U/L) | 1409 (309-7.330) | 4 (3-8) |
| ALT (U/L) | 2929 (655-8.450) | 5 (3-8) |
| ALP (U/L) | 104 (52-409) | 5.5 (3-23) |
| GGT (U/L) | 110 (25-364) | 7 (3-14) |
INR, international normalized ratio; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; GGT, gamma-glutamyl transferase.
The prothrombin indexes were found to be >10% in our study groups. The minimum value of the prothrombin index was 13.1. No baseline factors were found related to AST, ALT, total bilirubin, INR, or creatinine peak values (P > .05). The median duration of hospitalization was 8.50 (range: 1-25) days. NAC was administered to all patients (100%), while plasmapheresis and other medical treatment combinations were employed in 7 cases (29%). Other patients received combinations of silymarin and/or penicillin G (Pen G). The treatments given are shown in Table 1. The mean duration of hospitalization was 10.8 days in the plasmapheresis group and 8.7 days in the non-plasmapheresis group, and the difference was not statistically significant (P = .3). Erythrocyte suspension, fresh frozen plasma (FFP), and/or platelet suspension replacement were not applied to any patient except for those who underwent plasmapheresis. In cases where plasmapheresis was performed, FFP was used only during the procedure. The patient that presented with grade 4 HE died before a suitable donor was found for transplantation, and the remaining patients did not meet King’s College Criteria; thus, none of them qualified for organ transplantation. During the follow-up period, 3 patients (12.5%) underwent hemodialysis for renal failure.
Discussion
The results of this study showed that out of the 24 hospitalized patients suffering from mushroom-induced hepatotoxicity, 18 were SH, 5 were ALI, and 1 was ALF at admission. During the follow-up period, 6 patients from the SH group developed ALI based on clinical findings, and 2 patients from the ALI group progressed to ALF. One patient (4.1%) with grade 4 HE died within 24 hours of admission due to ALF, and 23 patients (95.9%) were discharged as cured. Thus, the total mortality rate was 4.1%. Although the incidence of mushroom-induced poisoning is not well-known, the mortality due to this cause has been reported to vary between 4.8% and 34.5%.8,9,10,11,17,18,19,20 In a study by Karvellas et al., the survival rate in the ALI patients was reported as 100% consistent with our study.12
The clinical picture due to mushroom-induced poisoning could have a vast spectrum, ranging from very mild symptoms to liver failure, and even death. In a retrospective study including 87 patients, the clinical symptoms of mushroom-induced poisoning at the emergency service presentation were reported as nausea and vomiting in 82% of the cases, diarrhea in 68%, syncope in 10%, abdominal pain in 8%, and hallucinations in 7%.21 In our current study, the typical symptoms were nausea and vomiting (95.8%), diarrhea (62.5%), and abdominal pain (50%). The abdominal pain developed by half of the patients might have an association with liver congestion, capsule stretching, and/or gastroenteritis. The clinical course might vary depending on the time from mushroom ingestion to the onset of symptoms. While early symptomatic patients could be managed by symptomatic treatment, the risks of hepatic or renal failure, intravascular hemolysis, and electrolyte imbalance are increased in late symptomatic patients; however, this timing is not a specific predictor.22,23,24 The second important factor is the presence of gastroenteritis due to the phalloides toxin, that could facilitate early diagnosis and early treatment. The induced gastroenteritis might also reduce the absorption of hepatotoxic agents due to the shedding of the intestinal epithelium.
In our study, during the follow-up period, ALF was detected in 2 of the 5 patients that were initially admitted with ALI. Interestingly, we observed that the 3 remaining cases of ALI and the 12 remaining cases of SH did not progress to ALF in the course of the follow-up, and maintained an INR of <2.7; they neither developed HE nor progressed to death. This finding highlights the importance of coagulopathy on admission. Prothrombin time (PT) and activated partial thromboplastin time (aPTT) levels were reported to be significant predictors of mortality,3,8,18 which could be attributable to the mechanism of mushroom-induced hepatotoxicity. Further clinical studies with a larger patient series are needed to identify a threshold level for INR as a prognostic indicator.
Another intriguing finding from our study was the ability of the MELD score to predict disease prognosis in some patients. We observed no progression to ALI and/or ALF in 8 of our SH cases with a baseline MELD score of <15, which indicated that this scoring method could be used as a predictor of no ALI and/or ALF development in SH cases at the time of presentation. All of the ALI and ALF patients had initial MELD scores ≥15, which might suggest that patients with <15 MELD score on admission might have better clinical course.
Kim et al.17 found that an increased total bilirubin concentration, indirect/direct bilirubin ratio, and prolonged aPTT were significantly associated with patient mortality.17 In this study, it was shown that on the third day of hospitalization, the patients with a total bilirubin of >5 mg/dl or an aPTT of >50 s died due to ALF. In another study, the urea, AST, ALT, LDH, total bilirubin, PT, and aPTT values were found to be significantly higher in the mortality group.8 Similarly, the cases with ALT or AST >2000 IU/mL or PT >50 s were reported to be at severe risk of death.18 Ganzert et al. reported that a short interval between mushroom ingestion and the onset of diarrhea (<8 hours), severe coagulopathy, severe hyper-bilirubinemia, elevated creatinine, and a rapid increase in the prothrombin time were associated with high mortality risk.3 In our study, we were not able to analyze mortality predictors statistically due to our low patient mortality. Likewise, it has been reported that in amatoxin poisoning, a prothrombin index <10% is indicative of transplantation and often occurs before encephalopathy. However, our study found that all of the prothrombin indexes of patients were >10%.25
There is no standardized protocol for the treatment of mushroom-induced hepatotoxicity. In global literature, and NAC therapy is acknowledged as the treatment method for the reduction of mortality in all ALF patients. İn addition to NAC, silymarin, Pen G, plasmapheresis, and liver dialysis therapies such as the molecular adsorbent recirculating system (MARS) and the fractionated plasma separation and adsorption system (FPSA-Prometheus) could be used in this patient group.26,27 Whether combining NAC and other agents provides additional benefits remains controversial. In a study by Karvellas et al., NAC was administered to all patients, but the optimal treatment could not be determined definitively.12 In our clinic, we started NAC treatment for all patients of mushroom poisoning. However, we supplemented the NAC treatment with Pen G, silymarin, and plasmapheresis, depending on their availability at the time. Thus, the non-standard nature of our treatment protocol could be considered as a limiting factor, preventing us from commenting on the effectiveness of treatment in this study.
In recent years, the use of plasmapheresis as treatment has been expanding in cases with ALF. Theoretically, it could be more effective in patients of mushroom-induced poisoning than in other ALF cases with different etiologies. Considering that mushroom-induced poisoning primarily presents with clinical findings such as coagulopathy due to the termination of protein synthesis, providing protein replacement through the fresh frozen plasma via plasmapheresis would undoubtedly offer benefits. In our study, plasmapheresis was performed in 7 patients (29%), although we could not relate the effect directly to the prevention of mortality or to patients’ survival. We also found that this treatment did not shorten the hospitalization period. By considering the cost of treatment, plasmapheresis in the early-stage cases is controversial. One patient admitted to our hospital with grade 4 HE died of ALF within 24 hours, before we were able to perform plasmapheresis. Liver transplantation was also not possible due to the absence of a suitable donor. Of the 2 patients that initially presented with ALI and developed ALF in the follow-up period, 1 received a combined treatment of NAC + Silymarin + plasmapheresis, and the other patient was given NAC + Pen G treatment. Both of these cases were discharged as cured. However, due to the limited number of patients, these findings are not sufficient to enable us to comment on the effect of these treatments on saving the patients in the ALF group from mortality.
The retrospective design of the study, as well as the small size of the study group, are both limitations in all discussion. Another limitation of our study was the inability to identify the mushroom species. Amatoxins impair the regenerative capacity of the liver, which is dependent on protein synthesis. As a result, the ongoing lysis of the liver is irreparable and centrilobular, and periportal hemorrhagic hepatic necrosis develops. While coagulopathy develops due to a lack of hepatic clotting factors in the early period, it leads to secondary effects of hepatic necrosis in the later periods; hence, profound and prolonged adverse effects of coagulopathy become apparent.6 Since this usually occurs before the development of HE, compared with other etiologies, it might be necessary to create a different definition of ALF in mushroom-induced hepatotoxicity.
In conclusion, patients with mushroom-induced poisoning presented to our clinic with a broad spectrum of findings. Mushroom toxins block hepatocyte protein synthesis, thereby stopping the production of coagulation factors, which leads to coagulopathy, but this condition might not be severe enough to cause hepatocyte damage and progress to the development of HE or liver failure. Although administration of NAC appeared to be an effective treatment, the efficacy of other treatment modalities in combination with NAC remains unclear. In our study, a MELD score of <15 in patients with SH and an INR of <2.7 in patients with ALI showed good prognosis, suggesting that this group of patients could continue to be followed up. However, the follow-up of patients with higher risk should be undertaken at tertiary hospitals equipped to perform liver transplantation and advanced treatments due to the higher risk of progression.
Funding Statement
The author declared that this study has received no financial support.
Footnotes
Ethics Committee Approval: Ethics committee approval was obtained Institution name: Ege University Local Ethics Committe Approval number:19-10.1T/36 Approval date: October 16, 2019. An ethical approval for the study was obtained from the Ege University Hospital Ethics Committee (Approval 19-9.1T/35). Due to the retrospective nature of our study, the committee waived patients’ written informed consent. Our research was conducted following the ethical principles established by the Declaration of Helsinki (2008 update).
Informed Consent: Verbal or written consent was obtained.
Peer Review: Externally peer-reviewed.
Author Contributions: Concept – F.Ç., Z.K.; Design – N.G.Ü., F.Ç.; Supervision – Ö.Ö., Z.K., F.G.; Resources – F.Ç., N.G.Ü., A.Ş., S.A., İ.T.; Materials – F.Ç., N.G.Ü., A.Ş., S.A.; Data Collection and/or Processing – F.Ç., Ali Şenkaya; Analysis and/or Interpretation – S.A., İ.T.; Literature Search – F.Ç., M.B.; Manuscript Writing – F.Ç., N.G.Ü.; Critical Review – Z.K., Ö.Ö.
Acknowledgments: Authors are thankful for the medical and non- medical staff of the Ege University, Faculty of Medicine Hospital, Izmir, Turkey.
Conflict of Interest: The authors have no conflict of interest to declare.
References
- 1. . Garcia J, Costa VM, Carvalho A, et al. Amanita phalloides poisoning: mechanisms of toxicity and treatment. Food Chem Toxicol. 2015;86:41–55.. 10.1016/j.fct.2015.09.008) [DOI] [PubMed] [Google Scholar]
- 2. . Schenk-Jaeger KM, Rauber-Lüthy C, Bodmer M, et al. Mushroom poisoning: a study on circumstances of exposure and patterns of toxicity. Eur J Intern Med. 2012;23:e85–e91.. 10.1016/j.ejim.2012.03.014) [DOI] [PubMed] [Google Scholar]
- 3. . Ganzert M, Felgenhauer N, Zilker T. Indication of liver transplantation following amatoxin intoxication. J Hepatol. 2005;42:202–209.. 10.1016/j.jhep.2004.10.023) [DOI] [PubMed] [Google Scholar]
- 4. . O’Brien BL, Khuu. A fatal Sunday brunch: amanita mushroom poisoning in a Gulf Coast family. Am J Gastroenterol. 1996;91:581–583.. [PubMed] [Google Scholar]
- 5. . Wieland T, Faulstich H. Fifty years of amanitin. Experientia. 1991;47:1186–1193.. 10.1007/BF01918382) [DOI] [PubMed] [Google Scholar]
- 6. . Diaz JH. Amatoxin-containing mushroom poisonings: species, toxidromes, treatments, and outcomes. Wilderness Environ Med. 2018;29:111–118.. 10.1016/j.wem.2017.10.002) [DOI] [PubMed] [Google Scholar]
- 7. . Mengs U, Pohl RT, Mitchell T. Legalon® SIL: the antidote of choice in patients with acute hepatotoxicity from amatoxin poisoning. Curr Pharm Biotechnol. 2012;13:1964–1970.. 10.2174/138920112802273353) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. . Trabulus S, Altiparmak MR. Clinical features and outcome of patients with amatoxin-containing mushroom poisoning. Clin Toxicol (Phila). 2011;49:303–310.. 10.3109/15563650.2011.565772) [DOI] [PubMed] [Google Scholar]
- 9. . Jander S, Bischoff J, Woodcock BG. Plasmapheresis in the treatment of amanita phalloides poisoning: II. A review and recommendations. Ther Apher. 2000;4:308–312.. 10.1046/j.1526-0968.2000.004004308.x) [DOI] [PubMed] [Google Scholar]
- 10. . Bergis D, Friedrich-Rust M, Zeuzem S, et al. Treatment of amanita phalloides intoxication by fractionated plasma separation and adsorption (Prometheus®). J Gastrointestin Liver Dis. 2012;21:171–176.. [PubMed] [Google Scholar]
- 11. . Roberts DM, Hall MJ, Falkland MM, Strasser SI, Buckley NA. Amanita phalloides poisoning and treatment with silibinin in the Australian Capital Territory and New South Wales. Med J Aust. 2013;198:43–47.. 10.5694/mja12.11180) [DOI] [PubMed] [Google Scholar]
- 12. . Karvellas CJ, Tillman H, Leung AA, et al. Acute liver injury and acute liver failure from mushroom poisoning in North America. Liver Int. 2016;36:1043–1050.. 10.1111/liv.13080) [DOI] [PubMed] [Google Scholar]
- 13. . Ferenci P, Lockwood A, Mullen K, et al. Hepatic encephalopathy--definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congress of Gastroenterology, Vienna, 1998. Hepatology. 2002;35:716–721.. 10.1053/jhep.2002.31250) [DOI] [PubMed] [Google Scholar]
- 14. . Ding GK, Buckley NA. Evidence and consequences of spectrum bias in studies of criteria for liver transplant in paracetamol hepatotoxicity. Qjm. 2008;101:723–729.. 10.1093/qjmed/hcn077) [DOI] [PubMed] [Google Scholar]
- 15. . Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120:c179–c184.. 10.1159/000339789) [DOI] [PubMed] [Google Scholar]
- 16. .European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu, Clinical practice guidelines panel, Wendon, J. EASL Clinical Practical guidelines on the management of acute (fulminant) liver failure. J Hepatol. 2017;66:1047–1081.. 10.1016/j.jhep.2016.12.003) [DOI] [PubMed] [Google Scholar]
- 17. . Kim T, Lee D, Lee JH, et al. Predictors of poor outcomes in patients with wild mushroom-induced acute liver injury. World J Gastroenterol. 2017;23:1262–1267.. 10.3748/wjg.v23.i7.1262) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. . Fantozzi R, Ledda F, Caramelli L, et al. Clinical findings and follow-up evaluation of an outbreak of mushroom poisoning—survey of amanita phalloides poisoning. Klin Wochenschr. 1986;64:38–43.. 10.1007/BF01721579) [DOI] [PubMed] [Google Scholar]
- 19. . Brandão JL, Pinheiro J, Pinho D, et al. [Mushroom poisoning in Portugal]. Acta Med Port. 2011;24:269–278.. [PubMed] [Google Scholar]
- 20. . Jaeger A, Jehl F, Flesch F, Sauder P, Kopferschmitt J. Kinetics of amatoxins in human poisoning: therapeutic implications. J Toxicol Clin Toxicol. 1993;31:63–80.. 10.3109/15563659309000374) [DOI] [PubMed] [Google Scholar]
- 21. . Schmutz M, Carron PN, Yersin B, Trueb L. Mushroom poisoning: a retrospective study concerning 11-years of admissions in a swiss emergency department. Intern Emerg Med. 2018;13:59–67.. 10.1007/s11739-016-1585-5) [DOI] [PubMed] [Google Scholar]
- 22. . Diaz JH. Syndromic diagnosis and management of confirmed mushroom poisonings. Crit Care Med. 2005;33:427–436.. 10.1097/01.ccm.0000153531.69448.49) [DOI] [PubMed] [Google Scholar]
- 23. . Erden A, Esmeray K, Karagöz H, et al. Acute liver failure caused by mushroom poisoning: a case report and review of the literature. Int Med Case Rep J. 2013;6:85–90.. 10.2147/IMCRJ.S53773) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. . Ward J, Kapadia K, Brush E, Salhanick SD. Amatoxin poisoning: case reports and review of current therapies. J Emerg Med. 2013;44:116–121.. 10.1016/j.jemermed.2012.02.020) [DOI] [PubMed] [Google Scholar]
- 25. . Escudié L, Francoz C, Vinel JP, et al. Amanita phalloides poisoning: reassessment of prognostic factors and indications for emergency liver transplantation. J Hepatol. 2007;46:466–473.. 10.1016/j.jhep.2006.10.013) [DOI] [PubMed] [Google Scholar]
- 26. . Vardar R, Gunsar F, Ersoz G, Akarca US, Karasu Z. Efficacy of fractionated plasma separation and adsorption system (Prometheus) for treatment of liver failure due to mushroom poisoning. Hepatogastroenterology. 2010;57:573–577.. [PubMed] [Google Scholar]
- 27. . Pőcze B, Fazakas J, Zádori G, et al. MARS therapy, the bridging to liver retransplantation - three cases from the Hungarian liver transplant program. Interv Med Appl Sci. 2013;5:70–75.. 10.1556/IMAS.5.2013.2.3) [DOI] [PMC free article] [PubMed] [Google Scholar]