Abstract
Background:
To investigate the association between interleukin-21 (IL-21) expression level and virological relapse (VR) of HBeAg positive chronic hepatitis B (CHB) after discontinuance of entecavir (ETV).
Methods:
The serum IL-21 level of 112 CHB patients was measured at 0, 12, 24, 52, and 104 weeks after ETV discontinuance. ELISA was used for the measurement of serum IL-21 level. VR was defined as two continuous examinations with an interval of 1 month with both showing HBV DNA >10 000 copies/mL after drug discontinuance.
Results:
The serum IL-21 levels at 0, 12, 24, 52, and 104 weeks after discontinuance of ETV were significantly higher in the durable virological remission (DVR) group than in the VR group (all P < .01). The area under the ROC curve (AUC) was 0.728 (95% CI: 0.630-0.827, P < .001), while the best cut-off value was 49.8 pg/mL. Multivariate Cox model showed that the factors affecting the relapse included age, followed by HBsAg level at the serological conversion of HBeAg and serum IL-21 level (all P < .05).
Conclusion:
Serum IL-21 level at ETV discontinuance is an independent risk factor for CHB relapse. IL-21 acts as an immunomodulatory factor in maintaining DVR in HBeAg positive CHB patients after ETV discontinuance.
Keywords: Cytokine, hepatitis B, chronic, entecavir, discontinuance, relapse
INTRODUCTION
Hepatitis B, a major global health problem, is a life-threatening liver infection caused by the hepatitis B virus (HBV). It has been estimated that globally 2 billion people are infected with HBV, among which 257 million carry a chronic HBV infection. According to the statistics, an approximate 0.65 million people die because of HBV infection-induced liver failure, liver cirrhosis, and hepatocellular carcinoma every year.1,2 Currently, an anti-viral therapy has shown to be effective for patients with chronic hepatitis B (CHB); the therapy suppresses the replication of HBV, inhibits the progression of liver disease, and reduces the incidence of decompensated liver cirrhosis, liver failure, hepatocellular carcinoma, and other complications.3 Entecavir (ETV) is a novel deoxyguanosine analog that has been recommended for the treatment of CHB.4 The advantages of ETV compared to other drugs are the following: high virological response rate, high genetic barrier effects, and low side-effects. Nevertheless, the treatment of ETV is expensive. Currently, only some Chinese patients with CHB can afford this kind of therapy, and the relatively long treatment duration often leads to heavy mental stress and economic pressure to the patients. Consequently, compliance to drug therapy is relatively poor and voluntary or non-standard drug discontinuance occasionally occurs.5 Achieving safe ETV discontinuance has become one of the hotspots, as well as a challenge, to numerous researchers.
In 2012, the Asia-Pacific Consensus for the Diagnosis and Treatment of Hepatitis B recommended that when using nucleoside analogs for patients with CHB, drug discontinuance should be implemented after 12 months of consolidation therapy following HBeAg serological conversion and attainment of HBV DNA values below the lower limit of detection.3 However, relapse could still occur in some patients even if drug discontinuance is conducted according to the recommendations.6 The virological relapse (VR) or hepatitis relapse could abrogate treatment efficacy and lead to acute aggravation of the disease, and, in some severe cases, even death.7 Unfortunately, the understanding of the factors affecting the relapse after ETV discontinuance is limited. The immune state could be closely associated with the VR after ETV discontinuance; yet, so far, only few studies have investigated the association between immune response and VR.8,9
Interleukin 21 (IL-21) is a multifunctional cytokine produced by the activated CD4+ T cells, Thl7 cells, and follicular helper T cells (TFH).10-13 Previous studies have suggested that the relatively high serum IL-21 levels might predict the serological conversion of HBeAg approximately 12 weeks after treatment with telbivudine. Compared with CHB patients, the patients in the immunocontrol phase have significantly higher serum IL-21 level. The count of CD4+IL-21+T cells is higher in patients with serological conversion of HBeAg than in the ones without serological conversion of HBeAg at 24 weeks of telbivudine treatment, suggesting that IL-21 has an important role in controlling HBV replication.14 Therefore, we speculated that serum IL-21 levels could be associated with DVR after ETV discontinuance. Thus, the aim of this study was to investigate the dynamic changes of IL-21 expression after ETV discontinuance in HBeAg positive CHB patients, as well as the value of serum IL-21 in predicting the relapse of CHB.
MATERIALS AND METHODS
Patients
A total of 112 HBeAg positive CHB patients (including 74 males and 38 females) who were hospitalized or admitted to the outpatient departments of the Wuxi Fifth Affiliated Hospital of Jiangnan University, the First Affiliated Hospital of Soochow University, and the Second Affiliated Hospital of Nanchang University between January 2009 and December 2011 were included in the study. The mean age of the patients was 40.1 ± 11.2 years. The mean duration of antiviral treatment with ETV was 30.7 ± 3.5 months (26-40 months). All the patients were diagnosed based on the criteria for drug discontinuance recommended by the Asia-Pacific Consensus for the Diagnosis and Treatment of Hepatitis B: HBV DNA in the HBeAg positive patients was <100 copies/mL, ALT levels were normal, and the indicators were stable for at least 1 year after serological conversion of HBeAg.
The patients with one or more of the following characteristics were excluded: (1) patients who had confirmed liver cirrhosis by imaging, and/or Ishak fibrosis score of liver biopsy >4-point before ETV treatment; (2) those with history of resistance to any kind of nucleoside analogs; (3) patients with history of relapse after drug discontinuance; (4) if the disease was combined with hepatitis C, autoimmune liver disease, cholestatic liver disease, malignant tumor, or severe systemic disease; (5) pregnant women; (6) those who were prescribed immunomodulators over the last 6 months.
All the patients signed the informed consent from the Ethics Committees of the corresponding hospital (WXFAH-No. 1125). The medical records of all the patients were complete, and all the patients volunteered to participate in the follow up.
Measurements and Data Collection
Liver functions, HBV DNA, serological markers of HBV, and serum IL-21 levels were measured at 0, 12, 24, 52, and 104 weeks after ETV discontinuance. Automatic biochemical analyzer (7600, Hitachi) and the corresponding reagents were used to analyze liver functions. PCR-fluorescent probe method was used for the measurement of HBV DNA. Serological markers of HBV were analyzed with 1235 time-resolved fluorescence immunoanalyzer. Chemiluminescence immune detection system of i2000 (Abbott, USA) and reagents from Abbott were used for the quantification of HBsAg, with a diagnostic range from 0.05 IU/mL to 250 IU/mL. When the HBsAg level was >250 IU/mL, a special diluent was used to dilute the sample 100–1000 times, and precise quantitative tests were conducted. The Architect platform (Abbott) was also used to quantify qHBeAg, with a diagnostic range from 0.13 PEIU/mL to 100 PEIU/mL. ELISA was used for the measurement of serum IL-21 level (eBioscience, USA). Other cytokines of Th1 (IL-2, IFN-γ, TNF-α), Th2 (IL-4, IL-6, IL-10), and Th17 (IL-17A) were examined using cytometric bead array (CBA) (Cat. # 560484 BD Pharmingen).
Virological response referred to serum HBV DNA <100 copies/mL. Durable virological remission (DVR) was defined as maintenance of HBV DNA level <10 000 copies/mL for more than 12 months after treatment discontinuation. VR was defined as two continuous examinations with an interval of 1 month, with both showing HBV DNA >10 000 copies/mL after drug discontinuance in patients with maintained virologic response.3
Statistical Analysis
SPSS 16.0 software (SPSS Inc., Chicago, IL, USA) was used for the statistical analysis. Quantitative data were described with means ± standard divisions, and compared with t-test. χ2 was used for the comparisons of rates between the two groups. For the patients with HBV DNA lower than the detection limit, 2.0 lg IU/mL was used for the analysis. For the patients with relapse, the results measured at each follow up were used for the analysis. Cox model was adopted to investigate the associations between the factors and relapse. Receiver operating characteristic (ROC) curve was used to assess the value of IL-21 in predicting relapse after discontinuance of ETV. All the statistical analyses were two-sided, and P < .05 was considered statistically significant.
RESULTS
General Clinical Data
In this study, 112 non-cirrhotic patients were included by imaging and/or Ishak fibrosis score <4-point before ETV treatment or at ETV cessation. The course of ETV treatment in these patients lasted for 26-36 months (average, 30.7 ± 3.5 months).15 The observation period after ETV cessation was 104 weeks. The mean age of VR and sustained virological response (SVR) was 46.5 ± 10.6 years and 34.1 ± 9.3 years, respectively; the difference in age was statistically significant (P < .001). The HBV relapse rates at ≤12 weeks, 13-24 weeks, 25-36 weeks, 37-52 weeks, and 52-104 weeks were 7.1% (8/112), 24.1% (27/112), 11.7% (13/112), 5.4% (6/112), and 9.9% (10/112), respectively. The cumulative rate of VR rate was 48.2% (54/112) and 57.1% (64/112) at 52 weeks and 104 weeks after ETV discontinuance, respectively. While HBsAg disappearance was found in one patient, for the patients with relapse, ETV treatment was conducted again, and the follow up was stopped. However, all patients were followed up during regular anti-viral therapy. The baseline age and HBV DNA were significantly different between the VR and SVR groups (both P < .05, Table 1). In contrast, sex, ALT, genotype, time of virological response, and treatment duration were not significantly different between the two groups (all P > .05, Table 1). The baseline characteristics of the relapsers VR and SVR are shown in Table 1.
Table 1.
Comparison of Baseline Characteristics Between VR and DVR
| Variables | Total (n = 112) | VR (n = 64) | SVR (n = 48) | P |
|---|---|---|---|---|
| Age (years), mean (SD) | 40.1 ± 11.2 | 46.5 ± 10.6 | 34.1 ± 9.3 | <.001 |
| Sex, male/female, n | 74/38 | 44/20 | 30/18 | .689 |
| ALT at ETV commencement (IU/L), mean (SD) | 195.6 ± 90.1 | 185.3 ± 92.6 | 213.1 ± 98.6 | .079 |
| Total bilirubin (mg/dL), mean (SD) | 2.2 ± 0.5 | 2.3 ± 0.6 | 2.1 ± 0.5 | .174 |
| HBV DNA at ETV commencement (log10 copies/mL), mean (SD) | 6.5 ± 1.0 | 6.9 ± 1.6 | 6.2 ± 0.9 | .012 |
| HBV genotype (B/C) | 66/46 | 34/30 | 32/16 | .177 |
| Baseline HBsAg at ETV commencement (log10 IU/mL), mean (SD) | 3.8 ± 0.7 | 3.9 ± 0.8 | 3.6 ± 0.6 | .314 |
| Time of virological response (weeks) | 20.6 ± 9.4 | 22.9 ± 10.4 | 18.8 ± 8.8 | .059 |
| Course of treatment (months) | 30.7 ± 3.5 | 30.1 ± 4.1 | 31.8 ± 3.9 | .108 |
VR, virologic relapse; DVR, durable virologic remission; SD, standard deviation; ALT, alanine aminotransferase; ETV, entecavir; HBV, hepatitis B virus; HBsAg, hepatitis B surface antigen.
Serum IL-21 Level at ETV Discontinuance Was Associated With Relapse
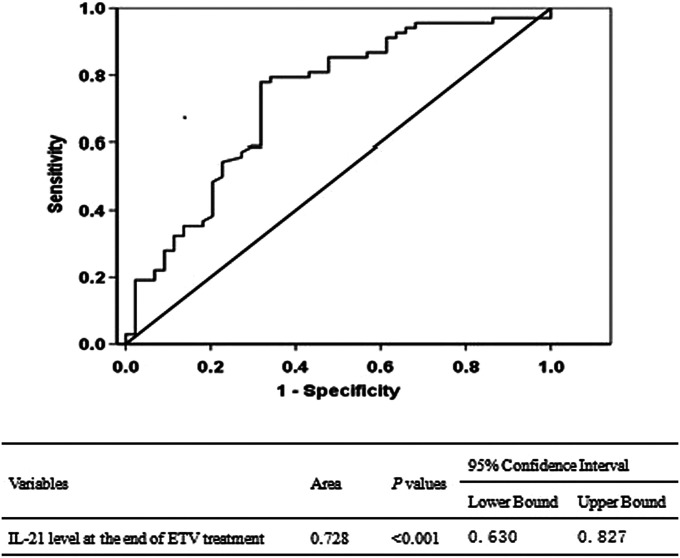
As shown in Figure 1, at ETV discontinuance, higher serum IL-21 levels were observed in the DVR group compared to the VR group (DVR: 61.8 ± 12.8 pg/mL; VR: 46.1.2 ± 10.8 pg/mL, P < .001). The ROC curve was plotted using the serum IL-21 level of 112 patients at ETV discontinuance, and the area under the ROC curve (AUC) was 0.728 (95% CI: 0.630-0.827, P < .001, Figure 2). The best cut-off value was 49.8 pg/mL, which resulted in a sensitivity of 77.9% and specificity of 68.2%. Moreover, the rate of VR was 30.2% (19/63) and 91.8% (45/49) for the patients with IL-21 ≥49.8 pg/mL and <49.8 pg/mL at drug discontinuance, respectively, and the difference was statistically significant (χ2 = 11.548, P = .001).
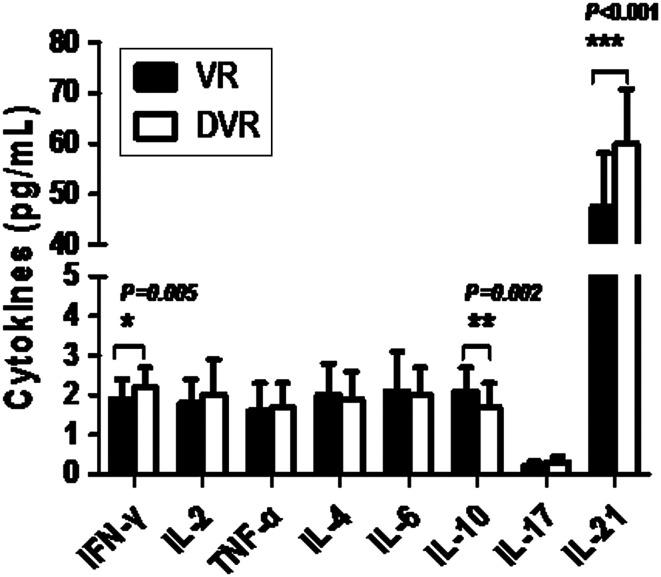
Figure 1.
The cytokine levels of Th1 (IL-2, IFN-γ, TNF-α)/Th2 (IL-4, IL-6, IL-10)/Th17 (IL-17A) and level of IL-21 at the end of ETV treatment. ETV, entecavir.
Figure 2.
Area under the receiver operating characteristic curve of IL-21 level at the end of ETV treatment. IL-21, interleukin-21; ETV, entecavir.
Our previous studies have shown that age is an independent predictive factor for relapse after drug discontinuance.15 In this study, the patients were divided into 3 subgroups according to age and IL-21 levels that were measured after drug discontinuance: group A, age <50 years and IL-21 ≥49.8 pg/mL; group B, age <50 years and IL-21 <49.8 pg/mL; and group C, age ≥50 years, and IL-21 ≥49.8 pg/mL or <49.8 pg/mL. The rate of VR in these three subgroups was 17.1% (6/35), 68.6% (35/51), and 88.5% (23/26), respectively; and the difference was statistically significant (P < .001). Further analysis showed that the VR rate in group A was significantly different from groups B and C (P < .001), while the VR rates in groups C and B were not significantly different (P = .092).
The Cytokines Level of Th1/Th2/Th17 at the End of ETV Treatment
As shown in Figure 1, at ETV discontinuance, the serum levels of IFN-γ (Th1) in SVR patients were significantly higher than those in VR patients (P < .05, Figure 1). The serum levels of IL-10 (Th2) in SVR patients were significantly lower than that in VR patients (P < .05, Figure 1). The comparison of serum levels of IL-17A (Th17) between the two groups was without significance (P > .005, Figure 1).
Multivariate Cox Model Analysis
First, single-variate analysis was conducted for age, sex, baseline ALT, baseline HBV DNA, HBV genotype, time of virologic response, HBsAg level at the serological conversion of HBeAg, and HBsAg level at ETV discontinuance. The variables with statistical significances, namely age, baseline HBV DNA, HBsAg level at the serological conversion of HBeAg, HBsAg level at ETV discontinuance, and serum IL-21 level at ETV discontinuance, and IFN-γ and IL-10 level at ETV discontinuance were included in the multivariate Cox model for further analyses. The results showed that the factors affecting the relapse after ETV discontinuance included HBsAg level at serological conversion of HBeAg (P = .001), followed by age (P = .008), HBsAg level at ETV cessation (P = .032), and serum IL-21 levels (P = .036) (Table 2).
Table 2.
Factors Predictive of Post-Treatment HBV Relapse for 112 Patients
| Factors | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age (years) | 1.051 | 1.030-1.072 | <.001 | 1.031 | 1.008-1.055 | .008 |
| HBV DNA at ETV commencement (log10 copies/mL) | 1.339 | 1.069-1.677 | .011 | 1.133 | 0.909-1.414 | .266 |
| HBsAg level at e antigen seroconversion (log IU/mL) | 2.801 | 1.892-4.148 | <.001 | 2.034 | 1.315-3.174 | .001 |
| HBsAg level at ETV cessation (log IU/mL) | 2.342 | 1.548-3.542 | <.001 | 1.692 | 1.046-2.737 | .032 |
| IFN-γ level at ETV cessation (pg/mL) | 0.471 | 0.272-0.811 | .008 | 0.799 | 0.481-1.326 | .384 |
| IL-10 level at ETV cessation (pg/mL) | 1.835 | 1.196-2.823 | .006 | 1.197 | 0.791-1.811 | .396 |
| IL-21 level at ETV cessation (pg/mL) | 0.946 | 0.924-0.969 | <.001 | 0.974 | 0.951-0.998 | .036 |
HBV, hepatitis B virus; HR, hazard ratio; CI, confidence interval; ALT, alanine aminotransferase; ETV, entecavir; HBsAg, hepatitis B surface antigen; HBeAg, hepatitis B e antigen; IL-21, interleukin-21.
Changes of ALT, HBV DNA, HBsAg, and HBeAg Levels After ETV Discontinuance
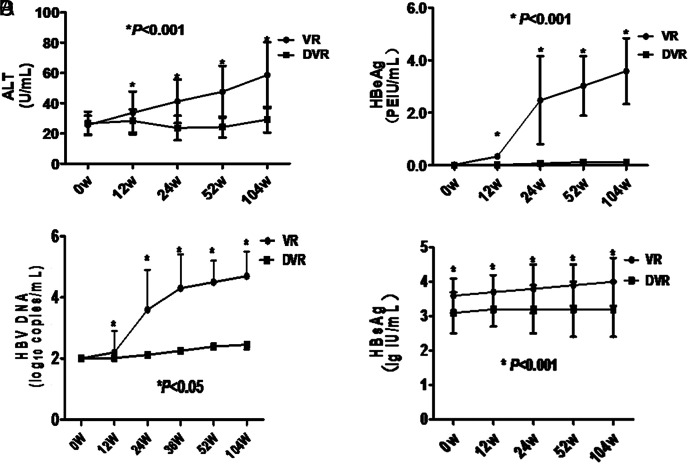
The ALT, HBV DNA, and HBeAg level at 12, 24, 52, and 104 weeks after ETV discontinuance in the VR group all increased significantly, compared with the levels before ETV discontinuance (0 week) (all P < .001, Figure 3A–C). The HBsAg levels after ETV discontinuance also showed an increasing tendency, and the level at 104 weeks after ETV discontinuance was also significantly higher than 0 week (P < .001, Figure 3D). Moreover, the ALT, HBV DNA, HBsAg, and HBeAg levels at 12, 24, 52, and 104 weeks after ETV discontinuance in the DVR group were relatively stable and were not significantly different compared with the levels at 0 week (all P > .05, Figure 3A–C). The ALT level, HBV DNA level, and HBeAg level at 12, 24, 52, and 104 weeks after ETV discontinuance were all different between the DVR and VR groups (all P < .001, Figure 3A–C). In addition, the HBsAg levels at 0, 12, 24, 52, and 104 weeks after ETV discontinuance were also significantly different between the DVR group and VR group (all P < .01, Figure 3D).
Figure 3.
ALT, HBeAg, HBV DNA, and HBsAg levels at different timepoints after ETV cessation (weeks). VR, virological relapse; DVR, durable virologic remission; ALT, alanine aminotransferase; HBsAg, hepatitis B surface antigen; HBeAg, hepatitis B e antigen; ETV, entecavir.
Patients with DVR After ETV Discontinuance Had Higher Serum IL-21 Levels
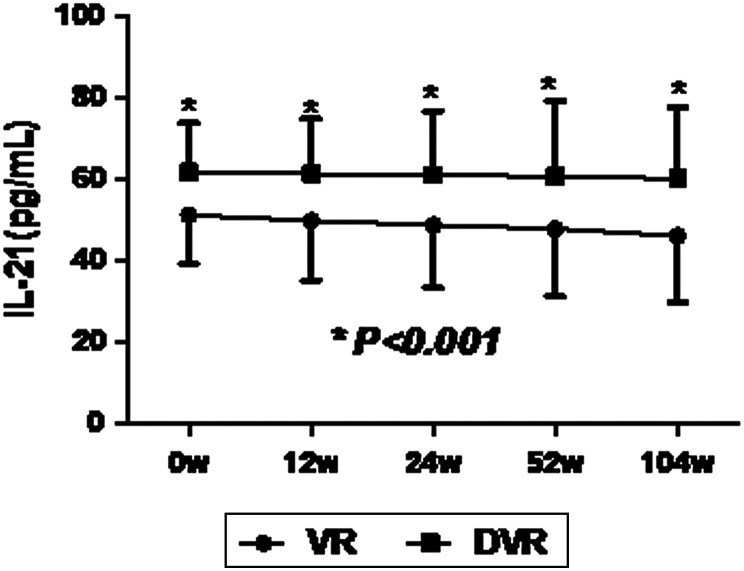
At 0, 12, 24, 52, and 104 weeks after ETV discontinuance, significantly higher levels of serum IL-21 were found in the DVR group compared to the VR group; nevertheless, the serum IL-21 levels at different timepoints of follow up were not significantly different from the baseline level (all P > .05, Figure 4).
Figure 4.
The level of serum IL-21 at timepoints after ETV cessation (weeks). *Comparison of IL-21 level between the DVR group and VR group at 0, 12, 24, 52 and 104 week after ETV cessation. ETV, entecavir.
Association Between Serum IL-21 and HBsAg Levels After ETV Discontinuance
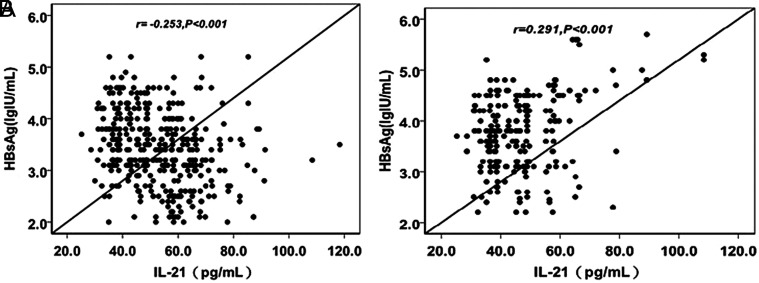
The total serum IL-21 level was not significantly associated with the titer of serum HBsAg after EVT discontinuance (r = −0.051, P = .356). However, for the patients in the DVR group, serum IL-21 remained at relatively high levels, while serum HBsAg titer remained at relatively low levels during the follow up. The serum IL-21 level was negatively associated with the titer of serum HBsAg in the DVR group (r = −0.253, P < .001; Figure 5A). In contrast, positive association between these two factors was found in the VR group (r = 0.291, P < .001; Figure 5B).
Figure 5.
The correlation between serum IL-21 and HBsAg after ETV cessation. (A) The correlation between serum IL-21 and HBsAg after ETV cessation in the DVR group. (B) The correlation between serum IL-21 and HBsAg after ETV cessation in the VR group. DVR, durable virologic remission; HBsAg, hepatitis B surface antigen; ETV, entecavir.
DISCUSSION
At present, cessation of nucleoside (nucleotide) analog attracts people’s attention. When medication is discontinued according to the recommendation of APASL,3 relapse would occur in some patients clinically. In the studies of Song et al., the maintenance rate of virological response after the discontinuation of ETV and clevudine therapy in HBeAg-positive patients reaches 41.6%,16, and Ridruejo et al. reported a 26% VR rate in HBeAg-positive patients at a median of 48 weeks after the cessation of ETV treatment.17 Our study showed that relapse rate in HBeAg-positive patients was 48.2% (54/112) and 57.1% (64/112) at 52 weeks and 104 weeks after ETV discontinuance, respectively. Relapse rate of 62.5% (40/54) occurred 12-36 weeks after ETV cessation. Therefore, we hold that it is necessary to monitor HBV DNA levels closely (within 36 weeks in particular) after ETV cessation to predict and detect VR in a timely manner.15
Previous studies have demonstrated that IL-21 is a type I cytokine with potential immunomodulatory effects. The specific binding of IL-21 to the receptors could mediate various biological immune responses, and in turn, affect the B and T cells at different phases via different effector cells in the immune network.18-20 In vitro studies have shown that IL-21 could directly inhibit HBV replication, as well as indirectly inhibit HBV replication via the suppression of IL-10.19,21 In the peripheral blood of the patients with acute HBV infection, the level of IL-21 increases. This increase has shown to be closely associated with the negative conversion of HBsAg, which further suggests that IL-21 participates in the eradication of HBV.22 In addition, the high expression of IL-21 before anti-viral treatment predicts virological responses at an earlier time in CHB patients. Furthermore, in patients with acute attack of chronic HBV infection, the IL-21 levels in peripheral blood also increase, which further promotes the seroconversion of HBeAg, and in turn the virological control.23 Recent studies have shown that the IL-21 produced by CD4+ T lymphocytes has important effects on maintaining the functions of CD8+ T lymphocytes. IL-21 could enhance the responses of CD8+ T lymphocytes during the processes of chronic viral infection and thus is essential in the chronicity of the infection.24,25 In the present study, we found that the serum IL-21 levels at ETV discontinuance were significantly higher in DVR patients than in the VR group. In addition, the serum IL-21 levels at ETV discontinuance were negatively associated with HBsAg levels. Moreover, both single and multivariate Cox regression showed that IL-21 levels at ETV discontinuance were associated with VR after ETV discontinuance, suggesting that IL-21 level was an independent predictive factor for relapse after ETV discontinuance. We found that the AUC under ROC was 0.728. In addition, we found that the rate of VR in patients with the IL-21 at ETV discontinuance ≥49.8 pg/mL was 30.2% (19/63), while it was only 17.1% (6/35) in patients with the IL-21 at ETV discontinuance ≥49.8 pg/mL and age <50 years. In contrast, the rate of VR was 88.5% (23/26) in patients over ≥50 years old, regardless of the IL-21 levels at ETV discontinuance. The rate of VR was also very high (68.6% (35/51)) in patients with IL-21 at ETV discontinuance <49.8 pg/mL and age <50 years. To sum up, our data suggest that antiviral treatments should be continued for such patients. However, there are many factors that affect the recurrence of ETV. IL-21 is only one of the effective factors to predict recurrence. The sensitivity, specificity, and the AUC of IL-21 as a single factor to predict recurrence are not high. Whether combining hepatitis B core-associated antigen (HBcAg), HBV RNA, and other factors can improve the predictive value remains to be further studied.
After HBV infection, IL-21 has various roles in the different phases of disease progression.22,25,26 The specific cytotoxic CD8+ T lymphocytes could be effectively restored after anti-viral treatment or in the immune control phase. The stable and high expression of IL-21 could promote the secretion of effector molecules of CD8+ T lymphocytes, thus enhancing the responses and maintaining the killing functions of CD8+ T lymphocytes during HBV infection, which are important for maintaining the immunocontrol and eradication of the virus.22,25 Our data showed that serum IL-21 levels after ETV discontinuance tended to increase in the DVR group, while they slightly decreased in the VR group. In addition, the serum IL-21 levels in the DVR group were all significantly higher than in the VR group at all the follow ups after ETV discontinuance. These findings showed that after ETV discontinuance, IL-21 may promote the immune control of HBV, thus maintaining stable and high levels of serum IL-21, which is important for maintaining the virological response after ETV discontinuance. In addition, the IL-21 levels were negatively associated with HBsAg levels in the DVR group, but positively associated with HBsAg levels in the VR group. The associations between IL-21 levels and HBsAg levels were different in the two groups, and its specific mechanism was not clear ,which needs to be confirmed by further research.27
This study has a few limitations. First, ours is a retrospectively observational study and our findings come from a small number of 112 patients; therefore, any new finding such as the association of the best cut-off value of IL-21 with the probability of VR after discontinuance of ETV needs to be confirmed in additional larger studies. Second, this study did not determine whether a different duration of prolonged consolidation therapy would influence the relapse rate. Third, the follow-up time after ETV discontinuance was relatively short, and thus more studies with longer follow-up time are needed to further verify the association between IL-21 levels and disease relapse after ETV discontinuance.
In summary, the IL-21 levels at ETV discontinuance are associated with disease relapse in HBeAg positive CHB patients after ETV discontinuance. In patients with DVR, the serum IL-21 is maintained at high levels and is positively associated with the serum HBsAg levels, which suggests that it has an important role in the immune control of HBeAg positive CHB patients after ETV discontinuance.
Funding Statement
This study was supported by the Chinese Foundation for Hepatitis Prevention and Control—TianQing Liver Disease Research (grant no.: TQGB2011004), Nanjing Medical University of Science and Technology (grant no.: 2012NJMU072), and Wuxi Technology Development Fund (grant no.: 2013CSE31N1318).
Footnotes
Ethics Committee Approval: The project was approved by the Ethics Committee of the Wuxi Fifth Affiliated Hospital of Jiangnan University on June 10, 2011, and approval number is WXFAH-No. 1125.
Informed Consent: All the patients signed the informed consents from the Ethics Committees of the Wuxi Fifth Affiliated Hospital of Jiangnan University.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – Y.W.Q., L.H.H.; Design - Y.W.Q., J.H.G.; Supervision - Y.W.Q., L.H.H., J.H.G; Resource - L.Z., W.L.Y., Z.W., T.T.S.; Materials - L.Z., Y.P.D., W.L.Y.; Data Collection and/or Processing - L.Z., W.L.Y., Z.W., T.T.S.; Analysis and/or Interpretation - Y.W.Q.; Literature Search - Y.W.Q.; Writing - Y.W.Q.; Critical Reviews - Y.W.Q., L.H.H.
Acknowledgments: The authors thank Professor Jiming Zhang (Department of Infections, Huashan Hospital, affiliated to Fudan University) for his helpful advice and for his help on this research.
Conflict of Interest: The authors have no conflict of interest to declare.
References
- 1. . Seto WK, Lo YR, Pawlotsky JM, Yuen MF. Chronic hepatitis B virus infection. Lancet. 2018;392(10161):2313–2324. 10.1016/S0140-6736(18)31865-8) [DOI] [PubMed] [Google Scholar]
- 2. . Wang FS, Fan JG, Zhang Z, Gao B, Wang HY. The global burden of liver disease: the major impact of China. Hepatology. 2014;60(6):2099–2 108. 10.1002/hep.27406) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. . Liaw YF, Kao JH, Piratvisuth T.et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2012 update. Hepatol Int. 2012;6(3):531–561. 10.1007/s12072-012-9365-4) [DOI] [PubMed] [Google Scholar]
- 4. .European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67(2):370–398. 10.1016/j.jhep.2017.03.021) [DOI] [PubMed] [Google Scholar]
- 5. . Kamezaki H, Kanda T, Arai M.et al. Adherence to medication is a more important contributor to viral breakthrough in chronic hepatitis B patients treated with entecavir than in those with lamivudine. Int J Med Sci. 2013;10(5):567–5 74. 10.7150/ijms.5795) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. . Shim JJ. Long-term suppression of viral replication in chronic hepatitis B: outcomes and future directions. Gut Liver. 2015;9(3):265–26 6. 10.5009/gnl15105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. . Reijnders JG, Perquin MJ, Zhang N, Hansen BE, Janssen HL. Nucleos(t)ide analogues only induce temporary hepatitis B e antigen seroconversion in most patients with chronic hepatitis B. Gastroenterology. 2010;139(2):491–49 8. 10.1053/j.gastro.2010.03.059) [DOI] [PubMed] [Google Scholar]
- 8. . Aljumah AA, Bin Selayem NA, Al-Howti SY.et al. Clinical and virological outcomes of entecavir therapy in patients with chronic hepatitis B: a real life experience. J Infect Chemother. 2019;25(1):12–16. 10.1016/j.jiac.2018.09.014) [DOI] [PubMed] [Google Scholar]
- 9. . Rivino L, Le Bert N, Gill US.et al. Hepatitis B virus-specific T cells associate with viral control upon nucleos(t)ide-analogue therapy discontinuation. J Clin Invest. 2018;128(2):668–681. 10.1172/JCI92812) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. . Hu Y, Wang X, Yu S, Hou Y, Ma D, Hou M. Neutralizations of IL-17A and IL-21 regulate regulatory T cell/ T-helper 17 imbalance via T-helper 17-associated signaling pathway in immune thrombocytopenia. Expert Opin Ther Targets. 2015;19(6):723–7 32. 10.1517/14728222.2015.1016499) [DOI] [PubMed] [Google Scholar]
- 11. . Zhou L, Ivanov II, Spolski R.et al. IL-6 programs TH-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8(9):967–974. 10.1038/ni1488) [DOI] [PubMed] [Google Scholar]
- 12. . Gensous N, Charrier M, Duluc D.et al. T follicular helper cells in autoimmune disorders. Front Immunol. 2018;9:1637. 10.3389/fimmu.2018.01637) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. . Vogelzang A, McGuire HM, Yu D, Sprent J, Mackay CR, King C. A fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity. 2008;29(1):127–1 37. 10.1016/j.immuni.2008.06.001) [DOI] [PubMed] [Google Scholar]
- 14. . Ma SW, Huang X, Li YY.et al. High serum IL-21 levels after 12 weeks of antiviral therapy predict HBeAg seroconversion in chronic hepatitis B. J Hepatol. 2012;56(4):775–7 81. 10.1016/j.jhep.2011.10.020) [DOI] [PubMed] [Google Scholar]
- 15. . Qiu YW, Huang LH, Yang WL.et al. Hepatitis B surface antigen quantification at hepatitis B e antigen seroconversion predicts virological relapse after the cessation of entecavir treatment in hepatitis B e antigen-positive patients. Int J Infect Dis. 2016;43:43–48. 10.1016/j.ijid.2015.10.019) [DOI] [PubMed] [Google Scholar]
- 16. . Song MJ, Song DS, Kim HY.et al. Durability of viral response after offtreatment in HBeAg positive chronic hepatitis B. World J Gastroenterol. 2012;18(43):6277–6283. 10.3748/wjg.v18.i43.6277) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. . Ridruejo E, Marciano S, Galdame O. Relapse rates in chronic hepatitis B naive patients after discontinuation of antiviral therapy with entecavir. J Viral Hepat. 2014;21(8):590–596. 10.1111/jvh.12200) [DOI] [PubMed] [Google Scholar]
- 18. . Spolski R, Leonard WJ. Interleukin-21: a double-edged sword with therapeutic potential. Nat Rev Drug Discov. 2014;13(5):379–3 95. 10.1038/nrd4296) [DOI] [PubMed] [Google Scholar]
- 19. . Leavy O. B cells:IL-21 promotes B10 cell population expansion. Nat Rev Immunol. 2012;12(12):808–80 9. 10.1038/nri3346) [DOI] [PubMed] [Google Scholar]
- 20. . Shoraka S, Mohebbi SR, Hosseini SM.et al. Association between interleukin-21 and interleukin-21 receptor gene polymorphisms with susceptibility to chronic hepatitis B virus infection and HBV spontaneous clearance in Iranian population. Microb Pathog. 2019;128:263–267. 10.1016/j.micpath.2019.01.008) [DOI] [PubMed] [Google Scholar]
- 21. . Li HJ, Kang FB, Li BS, Yang XY, Zhang YG, Sun DX. Interleukin-21 inhibits HBV replication in vitro. Antivir Ther. 2015;20(6):583–5 90. 10.3851/IMP2950) [DOI] [PubMed] [Google Scholar]
- 22. . McGavern DB. A little ‘help’ from IL-21 during persistent viral infection. J Mol Cell Biol. 2010;2(1):8–10. 10.1093/jmcb/mjp021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. . Jiang Y, Li W, Yu L.et al. Enhancing the antihepatitis B virus immune response by adefovir dipivoxil and entecavir therapies. Cell Mol Immunol. 2011;8(1):75–82. 10.1038/cmi.2010.37) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. . Elsaesser H, Sauer K, Brooks DG. IL-21 is required to control chronic viral infection. Science. 2009;324(5934):1569–15 72. 10.1126/science.1174182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. . Bertoletti A, Ferrari C. Innate and adaptive immune responses in chronic hepatitis B virus infections: towards restoration of immune control of viral infection. Gut. 2012;61(12):1754–17 64. 10.1136/gutjnl-2011-301073) [DOI] [PubMed] [Google Scholar]
- 26. . Yi JS, Du M, Zajac AJ. A vital role for interleukin-21 in the control of a chronic viral infection. Science. 2009;324(5934):1572–157 6. 10.1126/science.1175194) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. . Hu X, Ma S, Huang X.et al. Interleukin-21 is upregulated in hepatitis B-related acute-on-chronic liver failure and associated with severity of liver disease. J Viral Hepat. 2011;18(7):458–4 67. 10.1111/j.1365-2893.2011.01475.x) [DOI] [PubMed] [Google Scholar]