Abstract
Backgrounds/Aims:
Interval gastric cancers (GCs) can be encountered during screening gastroscopy. This study investigated the rate of interval GCs and their risk factors.
Materials and Methods:
We retrospectively investigated subjects who underwent screening gastroscopy from 2005 to 2017 in a university hospital and were diagnosed with GC. Subjects were grouped based on their endoscopic images and descriptive results into interval GC and initially diagnosed GC groups. Interval GCs were defined when endoscopic results within the previous 3 years were negative for GC. The clinico-pathological characteristics of the groups and risk factors for interval GCs were evaluated.
Results:
Of 54 724 subjects who underwent screening gastroscopy, 234 were diagnosed with GC, of which 43 were interval GCs. The rate of interval GCs was 18.4% (43/234, mean age 61.6 years). Interval GCs were smaller than initially diagnosed GCs (1.6 vs 1.9 cm, P = .011). They were located in the low-to-mid-body in 44.2%, antrum in 48.8%, and high body and cardia in 7%. Their observation time was shorter (248.74 vs 410.64 sec, P = .032). In multivariate analysis, they were associated with short observation time (odds ratio [OR] 0.99, 95% CI 0.994-0.998, P < .001) and location in the low-to-mid-body (OR 2.12, 95% CI 1.071-4.181, P = .031), although differentiation, ulcerated type, metaplasia, Helicobacter pylori infection, and endoscopists’ experience were not associated with interval GCs.
Conclusions:
The rate of interval GCs was significant during screening gastroscopy. They might be reduced by increasing observation time, focusing on smaller lesions, and observing the low-to-mid-body of the stomach more carefully.
Keywords: Gastric cancer, rates, risk factors, gastroscopy, screening
INTRODUCTION
Gastric cancer (GC) is the fifth most common cancer worldwide and the third most common cause of cancer-related deaths. Globally in 2018, there were 1 million new cases and 783 000 cancer deaths.1 GC still continues to be prevalent in East Asia.2,3 Furthermore, GC is the leading cause of cancer deaths in Korea with a 5-year survival rate below 63.1%.4 Since its causes are variable, and clinical features and prognosis differ according to GC type, complete prevention seems difficult.
In terms of early GC, however, the prognosis is excellent with a 5-year survival rate of 99% with no lymph node metastasis; therefore early detection and treatment are very important.5 For early detection of GC, gastric endoscopy or an upper gastrointestinal (GI) series is recommended every 2 years for adults aged 40 years or older in Korea, where the prevalence of GC is relatively high.6 Applying this recommendation has improved its prognosis owing to earlier detection.7 Screening has been shown to be more effective by endoscopy than by upper GI series.8
Currently, gastroscopy with tissue biopsy is considered the gold standard for diagnosing GC with a sensitivity of about 69% and a specificity of about 96%.9,10 However, sometimes gastroscopy fails to detect not only early GC but also advanced GC lesions.11 In other words, interval GC, defined as GC that develops between the previous endoscopy and the next intended endoscopy,12 can be encountered after previous negative endoscopy. Thus, the issue of interval GC detection seems to be emerging in Korea where there is nation-wide screening gastroscopy of the population for GC. Surprisingly, the rate of interval GC is significantly high. In a meta-analysis of 22 studies evaluating missed GC, the average rate of missed GC was reported to be 9.4%.13 Further, the proportion of interval cancers in Korea was reported as 8.6% in a previous study14 and 7.3% in a US study,15 whereas it was as high as 18.3% in the UK.16
Nonetheless, we have insufficient information about the clinical characteristics of interval GCs and what is the real problem of screening endoscopy for GC. Therefore, we aimed to investigate the rate of interval cancers occurring in GC screening, the causes of these cancers, and the clinical characteristics of patients with these cancers. We compared the interval cancer group and the initially diagnosed cancer group of patients diagnosed with GC by screening gastroscopy for the purpose of health examination from 2005 to 2017 in a single university hospital.
MATERIALS AND METHODS
Study Design and Data Source
This was a retrospective study of endoscopic and electronic chart records in a university medical center from January 2005 to December 2017. We reviewed patients over 18 years of age who underwent gastroscopy for health screening and were diagnosed with GC by endoscopic biopsy. This study was conducted in accordance with the Declaration of Helsinki and was approved by the University Hospital Institutional Review Board that waived the requirement to obtain written informed consent.
Definitions of Interval GCs
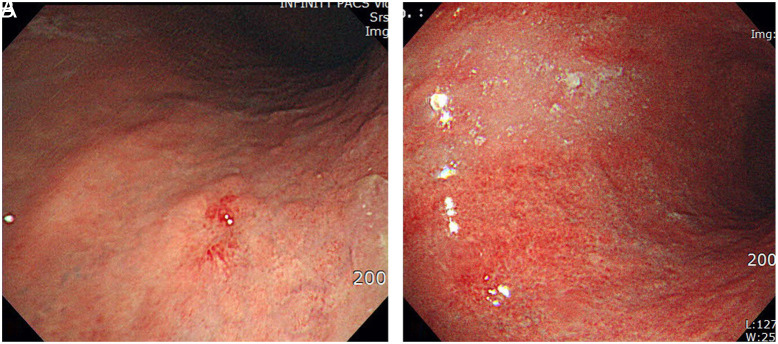
Interval cancer was defined when the previous endoscopic results obtained within 3 years were negative for GCs. True interval cancer was defined if endoscopic images had been taken at the location of a cancer lesion but no lesion had been found (Figure 1). Missed cancer was defined if no endoscopic images were obtained at the location of a cancer lesion (Figure 2); unnoticed cancer was defined if cancer lesions were visible in the earlier images but a biopsy was not performed (Figure 3); and as insufficient biopsies case if the cancer was not diagnosed by biopsy (Figure 4).14
Figure 1.
(A and B) Endoscopic images of a typical true interval cancer: (A) Reddish nodular lesion was found on the posterior wall of the high body stomach during screening gastroscopy. Endoscopic resection revealed differentiated adenocarcinoma of 4 mm in size. (B) Previous endoscopy images had not shown any suspicious lesions at this location 2 years prior.
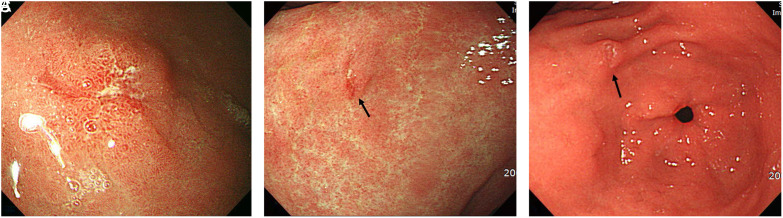
Figure 2.
(A and B) Endoscopic images of a typical missed cancer: (A) Slightly depressed lesion is found on the anterior wall of the gastric lower body. After endoscopic resection, the lesion was to be signet ring cell type intramucosal gastric cancer of 5 mm in size. (B) The previous endoscopic images 26 months prior had not included the same location of this lesion.
Figure 3.
(A and B) Endoscopic images of a typical unnoticed lesion: (A) A depressed lesion is shown at the gastric angle and was confirmed as differentiated adenocarcinoma by endoscopic resection. (B) The previous gastroscopy 3 years prior showed a slightly depressed lesion covered by mucus, indicating that the lesion could have been noticed if the mucosal visibility had been improved by washing the mucus away.
Figure 4.
(A-C) Endoscopic images of a typical case of insufficient biopsy. (A) Gastroscopy showing a small erosive lesion at the lesser curvature of the gastric antrum, confirmed as a differentiated adenocarcinoma by endoscopic resection. (B) The pathology report of the tissue biopsy was atypical epithelial cells 2 years prior, and (C) erosive gastritis 4 years prior.
Clinical, Endoscopic, and Pathologic Features
Based on the endoscopic images and descriptive results of the patients diagnosed with GC at screening, subjects were divided into the interval GC and the initially diagnosed GC group. Age, sex, screening history from other institutions, location/size/histologic classification of GC, experience of the endoscopists, and observation times obtained from chart reviews and their relationships with interval cancer were analyzed. The endoscopic images and descriptive results were reviewed and analyzed by 2 expert endoscopists.
The location of the GC was classified as upper (cardia, fundus, upper body), middle (mid-to-lower body, and angle), or lower (antrum and prepylorus). The circumferential location of GC was classified as the anterior wall, posterior wall, greater curvature, and lesser curvature. Experience of endoscopists was classified as more than 5 years and less than 5 years. Observation time was measured in seconds from the time when the duodenum was reached to the time when the endoscopic examination was completed.
Gross type of GC tissue was defined as elevated (I and IIa), flat (IIb), and depressed (IIc and III) by the Paris classification. Histologic type was defined as differentiated (well or moderately differentiated adenocarcinoma) or undifferentiated (poorly differentiated or signet ring cell adenocarcinoma) by the WHO classification 2010. Tumor size was measured as the longest diameter. The presence or absence of intestinal metaplasia was evaluated histologically, and Helicobacter pylori infection was investigated using the rapid urease test. Ulcerated type was histologically defined and not by endoscopic appearance.
Statistical Analysis
Data were processed with Microsoft Office Excel 2010 (Microsoft, Redmond, WA, USA) and IBM SPSS Statistics version 25.0 (SPSS Inc., Chicago, IL, USA) and were expressed as mean and median values. Differences in categorical variables between the 2 groups were analyzed using the chi-squared test. Mean differences in continuous variables were analyzed using Student’s t -test. Correlations between interval GC and various factors were estimated by logistic regression. The Hosmer-Leme-show chi-squared test was used to determine appropriateness before analysis. Odds ratios (ORs) and 95% CIs were calculated for each variable. All reported P values were 2-tailed and the significance level was set at .05.
RESULTS
Clinical Characteristics of Interval and Initially Diagnosed GC
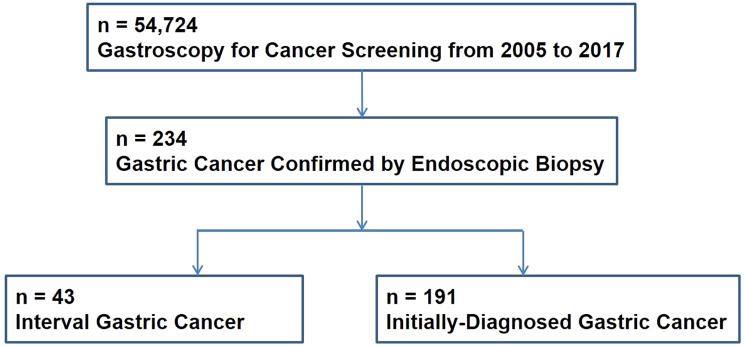
Between 2005 and 2017, 234 subjects were diagnosed with GC among the 54 724 individuals who underwent gastroscopy for health screening at the university medical center (Figure 5). Of these 234 subjects, 43 (18.4%) were identified as interval GC (Table 1). The mean time interval between diagnosis and previous endoscopy was 15.7 months. The overall number of male subjects was higher than that of female subjects (153 males: interval GC, 29; initially diagnosed GC, 124 and 81 females: interval GC, 14; initially diagnosed GC, 67), but the sex ratios of the 2 groups were not different. There was no statistically significant difference in the mean age of patients in the interval GC (61.6 ± 11.4 years) and initially diagnosed GC (59.8 ± 11.1 years) groups. The rates of gastrectomy in the interval GC and initially diagnosed GC groups were 37.8% (14/37) and 67.2% (80/119), respectively.
Figure 5.
Flow chart of study population.
Table 1.
Clinical Characteristics of Interval and Initially Diagnosed Gastric Cancer
| Interval | Initially Diagnosed | P | |
|---|---|---|---|
| Age (year) | 61.6 ± 11.4 | 59.8 ± 11.1 | .952 |
| Sex | |||
| Male | 29 | 124 | .754 |
| Female | 14 | 67 | |
| Observation Time (s) | 248.7 ± 203.4 | 410.6 ± 251.9 | .032 |
| Endoscopists’ experience | |||
| ≥5 years | 22 | 98 | .986 |
| <5 years | 21 | 93 |
Causes of Interval GC
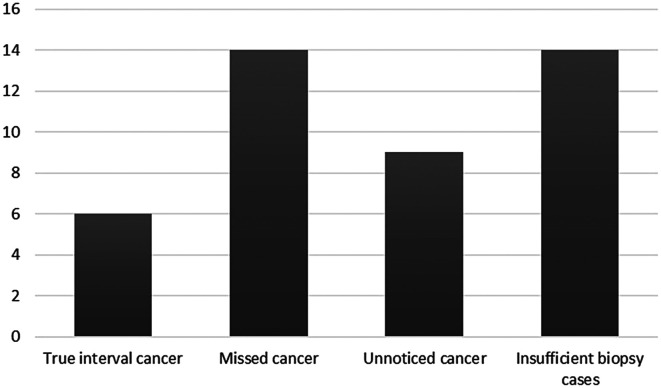
Of the 43 lesions in the interval GC group, 14 were missed lesions, that is, not captured in previous endoscopic examination and 9 were unnoticed lesions that could be seen in the previous endoscopy but biopsy was not performed. Fourteen of the 43 lesions were insufficient biopsies, meaning that the tissue had been previously biopsied at the same location but the lesions were not confirmed. Only the remaining 6 lesions could be considered true interval GC (Figure 6).
Figure 6.
The rate and cause of interval gastric cancer. True interval cancer, defined if endoscopic images had been taken at the location of a cancer lesion but no lesion had been found; missed cancer, defined if no endoscopic images had been made at the location of a cancer lesion; unnoticed cancer, defined if cancer lesions were visible in the earlier images but a biopsy was not performed; insufficient biopsy cases if the cancer was not diagnosed by biopsy.
Endoscopic Manifestation of Interval and Initially Diagnosed GC
Interval GC was more frequently located in the low-to-mid-body than initially diagnosed GC. Overall, GC lesions were most frequently located in the antrum: the interval GCs and initially diagnosed GCs were located in the antrum in 48.8% (21/43) and 56.6% (108/191) of the cases, respectively, in the low- to mid-body in 44.2% (19/43) and 27.2% (52/191) of the cases, respectively, and in the high body and cardia in 7% (3/43) and 16.2% (31/191), respectively (Table 2). However, when the location of the lesion was classified based on the anterior or posterior wall, and the greater or lesser curvatures, GCs were more frequently located in the lesser curvature and anterior wall, although there was no significant statistical difference between the 2 groups. Interval GC was significantly smaller than initially diagnosed GC (1.6 cm vs 1.9 cm, P = .011). However, with regard to intestinal metaplasia, histologic type, Helicobacter pylori infection, and ulcerated type, there were no significant differences between the 2 groups (Table 2). On the other hand, when the features of interval GCs were compared between the current and previous endoscopies, a difference was found in endoscopy time (371.9 ± 183.7 vs 248.7 ± 203.4 sec; P = .028) but not in endoscopists’ experience. In addition, when we analyzed the interval GCs based on the mean interval time of 15.7 months, no differences were found in location, size, metaplasia, H. pylori infection, and ulcerate type (Table 3).
Table 2.
Endoscopic Manifestation of Interval and Initially Diagnosed Gastric Cancer
| Interval (n = 43) | Initially Diagnosed Cancer (n = 191) | P | |
|---|---|---|---|
| Location | |||
| Upper | 3 (7.0%) | 31 (16.2%) | .056 |
| Mid | 19 (44.2%) | 52 (27.2%) | |
| Lower | 21 (48.8%) | 108 (56.6%) | |
| Circumference | |||
| Anterior wall | 10 (23.3%) | 31 (16.2%) | .109 |
| Posterior wall | 8 (18.6%) | 42 (22.0%) | |
| Greater curvature | 5 (11.6%) | 52 (27.2%) | |
| Lesser curvature | 25 (46.5%) | 66 (34.6%) | |
| Size (cm) | 1.63 ± 1.42 | 1.91 ± 2.73 | .011 |
| Metaplasia | |||
| Presence | 13 (30.2%) | 34 (17.8%) | .603 |
| Absence | 15 (34.9%) | 31 (16.2%) | |
| Not checked | 15 (34.9%) | 126 (66.0%) | |
| Differentiation | |||
| Well/Moderate | 26 (60.5%) | 76 (39.8%) | .108 |
| Poor | 15 (34.9%) | 77 (40.3%) | |
| Not Checked | 2 (4.6%) | 38 (19.9%) | |
| Helicobacter pylori | |||
| Presence | 10 (23.3%) | 42 (22.0%) | .144 |
| Absence | 31 (72.1%) | 117 (61.3%) | |
| Not checked | 2 (4.6%) | 32 (16.7%) | |
| Ulcerated | |||
| Yes | 21 (48.8%) | 112 (58.6%) | .241 |
| No | 22 (51.2%) | 79 (41.4%) |
Table 3.
Endoscopic Manifestation of Interval Gastric Cancer According to Mean Interval Time
| Below 15.7 months (n = 16) | Above 15.7 months (n = 27) | P | |
|---|---|---|---|
| Location | |||
| Upper | 1 (6.3%) | 2 (7.4%) | .728 |
| Mid | 8 (50.0%) | 11 (40.7%) | |
| Lower | 7 (43.7%) | 14 (51.9%) | |
| Circumference | |||
| Anterior wall | 4 (25.0%) | 6 (22.2%) | .803 |
| Posterior wall | 3 (18.8%) | 5 (18.5%) | |
| Greater curvature | 2 (12.5%) | 3 (11.1%) | |
| Lesser curvature | 7 (43.7%) | 13 (48.2%) | |
| Size (cm) | 1.49 ± 0.87 | 1.73 ± 0.87 | .424 |
| Metaplasia | |||
| Presence | 6 (37.5%) | 7 (25.9%) | .927 |
| Absence | 3 (18.8%) | 12 (44.5%) | |
| Not checked | 7 (43.7%) | 8 (29.6%) | |
| Differentiation | |||
| Well/moderate | 9 (56.3%) | 17 (63.0%) | .969 |
| Poor | 7 (43.7%) | 8 (29.6%) | |
| Not checked | 0 (0.0%) | 2 (7.4%) | |
| Helicobacter pylori | |||
| Presence | 1 (6.3%) | 9 (33.3%) | .217 |
| Absence | 15 (93.7%) | 16 (59.3%) | |
| Not checked | 0 (0.0%) | 2 (7.4%) | |
| Ulcerated | |||
| Yes | 8 (50.0%) | 13 (48.2%) | .910 |
| No | 8 (50.0%) | 14 (51.8%) |
Risk Factors for Interval GCs
Significant associations were found in a multivariate analysis between interval GCs and various factors: interval GCs were associated with their location in the low- to-mid-body (OR 2.12, 95% CI 1.071-4.181, P = .031) and short observation time (OR 0.99, 95% CI 0.994-0.998, P < .001) (Table 4). Observation time was substantially shorter in the interval GC group than the initially diagnosed GC group (248.74 ± 203.44 vs. 410.64 ± 251.91, P = .032). However, endoscopists’ experience, histologic type, metaplasia, H. pylori infection, and ulcerated type did not differ between the 2 groups.
Table 4.
Multivariate Analysis for Factors Related to Interval Gastric Cancer
| Variable | Odds Ratio (95% CI) | P |
|---|---|---|
| Observation time (s) | 0.99 (0.994-0.998) | <.001 |
| Location | ||
| Upper, lower | Reference | .031 |
| Mid | 2.12 (1.071-4.181) |
DISCUSSION
In a single institution of South Korea where individuals undergo regular screening gastroscopy, the rate of interval GC within the previous 3 years was 18.4% (43 of the 234 patients diagnosed with GC during screening from 2005 to 2017). The causes of interval GCs in the present study included missed, unnoticed, and insufficient biopsies as well as true interval GC. These results suggest that true interval GC occurs but that most interval GCs are actually caused by missed lesions, unnoticed lesions, and insufficient biopsies (see “Methods” section for definitions). Furthermore, interval GCs were smaller, more frequently located in the low- to-mid-body, and were associated with a shorter observation time than initially diagnosed GC.
The rate of interval GCs of 18.4% in the present study is similar to that of previous ones. The rates in previous studies differ between them, ranging from 4.6% to 25.8%.13,17 These differences may be attributed to various factors such as the definition of interval GC, time interval used, study design and study population, and even the prevalence of GC in a specific population. Among these factors, study design was of 3 different types: first, in studies of subjects with negative gastroscopy followed up over time, the rate of interval GCs was 10.0% when endoscopic examinations were performed 1-3 years later; second, in studies of patients diagnosed with GC who had normal endoscopies 1-3 years previously, the rate was 8.3%; third, in studies of those who underwent endoscopic or surgical treatment for GC, it was 23.3% when the proportion of missed synchronous lesions were evaluated.13 In addition, regarding the prevalence of GC, the rate is different in Asian and Western studies. In Asian studies that mostly investigated the development of synchronous cancer within 3 years of negative endoscopy, the rate was 19%, whereas it was as low as 1.2-9.7% in Western studies.13,16,18 Particularly in studies evaluating the incidence of gastric superficial neoplasia, which is a very early lesion, the miss rate of these lesions was reported to be as high as 75.2%.19 Furthermore, the rate depends on the time interval of negative endoscopy used to define the interval GC. The rate for the 1-year interval was 6.4% (95% CI, 4.3%-9.5%), compared with 11.3% (95% CI, 7.5%–16.6%) for a 3-year interval according to a meta-analysis of 10 studies.17 In addition, the rate of false-negative results by endoscopy at 3 years was 25.8% in an Asian study.20
In the present study, the rate of true interval GC was only 2.6%. In contrast to true interval GC, there were more missed lesions (without previous endoscopic images in that location), unnoticed lesions (with no recorded descriptions and therefore, no biopsy performed), and lesions considered to be negative on biopsy results (insufficient biopsies). This finding is consistent with that of the previous study reporting that unnoticed (or disregarded) lesions were the major causes of missed synchronous GCs.14 As for true interval GCs, although the assumption that more aggressive biology in undifferentiated GC plays a role in their development and progression is widely accepted, true interval cancers in the present study were all early GCs of small size rather than aggressive ones. Unfortunately, the long-term follow-up data of these true interval GCs were unavailable; thus, definitive conclusions on whether these GCs would behave aggressively could not be reached. Considering the low rate of true interval GCs, reducing those missed or unnoticed lesions and insufficient biopsy cases will be imperative since early detection is vital in managing GC.4,5
We defined missed lesions as those for which no endoscopic images of the lesion sites were obtained in the previous endoscopy, or only distant images were available and not close-up images. Close-up images are particularly important for detecting small and flat lesions. Thus, this result suggests that interval GCs could be reduced by closer observation in a systematic and consistent manner, thereby minimizing blind spots as well as finding small and flat lesions. We also found that missed lesions included synchronous GCs, since some GCs were encountered shortly after endoscopic resection of gastric adenoma during follow-up gastroscopy. Significantly high numbers of these missed synchronous GCs have also been reported by other investigators.21,22 All these findings indicate that when a lesion is identified during endoscopy, another lesion may easily be overlooked because more attention is focused on the first lesion. Therefore, it is strongly recommended that once a lesion is identified it is equally important to search for additional lesions as a study reported an incidence of synchronous GC of as high as 23%. Similarly, interval GCs are more common when gastric adenoma or ulcer is present.21,22 It is well known that if any missed synchronous GC needs surgery, patients would be subjected to additional economic burden and suffering. Therefore, it is important to pay extra attention to the possibility of the presence of synchronous GCs if gastroscopy finds any lesions such as gastric adenomas, ulcers, or even cancers. On the other hand, it may be possible to lower the rate of missing synchronous GCs using advanced technology like artificial intelligence in endoscopy.23 Therefore, the rate of neglected GCs may be reduced through machine learning for GCs found in endoscopy.24
In this study, we defined unnoticed lesions as those in which the mucosal surfaces of the lesion sites were covered by mucus or bubbles and thus could not be recognized by examiners. Indeed, the authors in a previous study reporting an endoscopic miss rate of GC of 19% argued that approximately 15% of the missed lesions failed to be detected due to being covered with mucus, emphasizing the importance of direct mucosal visualization.25 Mucosal visualization can be achieved by washing off mucus and bubbles during endoscopy. Given the heavy burden of work in health screening gastroscopy, however, repeated mucosal washing by endoscopists could not be sufficiently performed. Alternatively, mucosal visualization can be improved by administering mucolytics or defoamers to the examinees before performing endoscopy. On the other hand, concerning the heavy burden in conjunction with GC screening gastroscopy, an individualized screening strategy should be applied based on risk factors for GC instead of performing indiscriminate 2-year interval-gastroscopy for adults over 40 years of age.26 Finally, when it comes to the identification of early gastric lesions of small and flat type, the threshold for performing tissue biopsies should be lowered by improving the ability to recognize earlier lesions consistently and systematically.27
Of the 43 cases of interval GCs, 14 cases were attributed to insufficient biopsies, which may be due to either small number of biopsy specimens or errors by pathologists. This result also supports that of a prior study demonstrating that most missed cancers at gastroscopy are in the same locations as that of previously documented endoscopic abnormalities.28 A sufficient number of biopsy specimens (more than 4) is definitely important for GC diagnosis.29 However, for a correct diagnosis, decision of the first biopsy site, or which site to sample, is more important.30 As for pathologist error, their role in missed GC was reported to be 27% compared with endoscopist errors of 73% in a meta-analysis.17 In 1 of our cases, where gastroscopy with biopsies had been performed every year with erosive gastritis repeatedly reported by pathologists, a GC was confirmed several years later. It is also true that we may encounter cases where the pathologic results differ before and after endoscopic resection of GC.31 Therefore, it is important to decide whether to repeat biopsy, to follow-up gastroscopy earlier, or to perform endoscopic resection for both diagnosis and treatment, while considering the cost and possible complications of each decision. To make our decisions more accurate and consistent, a well-defined communication system needs to be established between endoscopists and pathologists. Hopefully in the future, advances in technology will allow endoscopists to make decisions by endoscopic findings alone, with no need to perform biopsies and pathologic examinations. This could be achieved by image-enhanced techniques such as magnifying endoscopy, confocal microscopic endoscopy, and blue laser imaging.32 Nonetheless, currently a large number of biopsy specimens are needed, particularly if the pathologic results are inconsistent with previous endoscopic findings.
In the present study, factors associated with interval GCs, such as lesion size, location, and observation time, were identified. Previously, a variety of factors have been reported to be associated with interval GCs in gastroscopy. Our study showed that interval GCs were significantly smaller in size, were associated with a shorter observation time, and were frequently located in the low-to-mid-body of the stomach than initially diagnosed GCs. Conversely, they were not associated with sex or with metaplasia, differentiation, H. pylori infection, and circumferential location. The association of interval GCs with short observation time was also reported in a previous study.33 However, no difference was found in the rate of interval GCs between examiners having more than or less than 5 years’ experience, whereas a higher rate of interval GCs in a US study was shown in non-gastroenterology specialists than specialists, and in outpatients than inpatients. In addition, there was a report showing a higher rate of interval GC in health screening tests than in an outpatient unit.16 The association of interval GCs with sex and age (higher in women and those under 55 years) was demonstrated in a UK study reporting a rate of 8.3%.18 A meta-analysis of studies concluded that there was an association between interval GCs and the presence of marked gastric atrophy, gastric adenoma or ulcer, although this association was not analyzed in the present study.13
There are some limitations to this study. First, there may have been a selection bias because this is a single-center study with a small sample. Second, a recall bias may have occurred due to the retrospective nature of the study. Indeed, complete information on the previous endoscopic results of the study patients was not obtained, and the development of GCs was investigated only during the period of this study and not followed up fully to the end. Third, gastrointestinal symptoms were not evaluated in the study as the subjects were all examinees who underwent screening gastroscopies. Fourth, a considerable proportion of the records did not include information on metaplasia or H. pylori infection; Therefore, the results regarding the association of these factors with interval GCs need to be carefully interpreted. Finally, an important risk factor of GC, the presence of gastric atrophy, was not evaluated because of unsatisfactory reporting of results.
In summary, despite these limitations, this study has the advantage of investigating the factors associated with interval GCs as well as the rate of interval GCs over a relatively long period of 13 years. In conclusion, although the rate of interval GC at screening endoscopy is significant, it should be possible to reduce the rate of interval GCs by carefully examining blind spots in gastroscopy and searching meticulously for any synchronous lesions particularly when any gastric lesion is found. If mucosal visibility is poor, mucosal visualization should be improved by clearing mucus and bubbles off the mucosal surface. If the findings of endoscopic and pathologic results are inconsistent, we should be able to determine whether to repeat the gastroscopy or to follow it up after a short time. Having identified the risk factors associated with interval GCs, the intervals between screening gastroscopies could be adjusted according to the number of risk factors presented by an examinee. This would be more cost-effective than indiscriminate biennial screening of all individuals. Multi-institutional prospective studies are needed to confirm the risk factors identified in the present study and the availability of interval GC rate as an indicator of gastroscopy quality.
MAIN POINTS
Gastric endoscopy for cancer screening fails to detect interval GC at a rate of 18.4% after 2-3 years of negative endoscopy.
Interval GCs are attributed to missed, unnoticed, or insufficient biopsy cases as well as true interval cancer and are associated with location in the low-to-mid-body of the stomach and shorter observation time.
Interval GCs could be reduced by paying attention to blind spots, improving mucosal visibility, and searching for synchronous lesions.
Funding Statement
The authors declare that this study has received no financial support.
Footnotes
Ethics Committee Approval: Ethics committee approval for this study was received from Hanyang University Institutional Review Board (2019-05-043-002).
Informed Consent: Hanyang University Hospital Institutional Review Board waived the requirement to obtain written informed consent (2019-05-043-002).
Peer-review: Externally peer-reviewed.
Author Contributions: Concept: K.N.L.; Design: K.N.L., J.H.P.; Supervision: K.N.L., H.L.L.; Resources: K.N.L., H.L.L., D.W.J., O.Y.L., B.C.Y., H.O.C.; Materials: K.N.L., J.H.P.; Data Collection and/or Processing – K.N.L., J.H.Y., H.L.L., O.Y.L., B.C.Y., H.O.C.; Analysis and/or Interpretation: J.H.P., K.N.L., J.H.Y., D.W.J.; Literature Search: J.H.P., K.N.L.; Writing Manuscript: J.H.P., K.N.L.; Critical Review: H.L.L., O.Y.L., B.C.Y.
Acknowledgments: The authors thank YS Song for his opinions on pathologic findings and processing of data. This work was supported by the research fund of Hanyang University (HY-2015).
Conflict of Interest: The authors have no conflict of interest to declare.
References
- 1. . Bray F, Ferlay J, Soerjomataram I. Global Cancer Statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.. 10.3322/caac.21492) [DOI] [PubMed] [Google Scholar]
- 2. . Leung WK, Wu MS, Kakugawa Y. et al. Screening for gastric cancer in Asia: current evidence and practice. Lancet Oncol. 2008;9(3):279–287.. 10.1016/S1470-2045(08)70072-X) [DOI] [PubMed] [Google Scholar]
- 3. . Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. 2006;12(3):354–362.. 10.3748/wjg.v12.i3.354) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. . Lee JH, Kim JG, Jung HK. et al. Clinical practice guidelines for gastric cancer in Korea: an evidence-based approach. J Gastric Cancer. 2014;14(2):87–104.. 10.5230/jgc.2014.14.2.87) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. .Japanese Gastric Cancer Association Registration C, Maruyama K, Kaminishi M.et al. Gastric cancer treated in 1991 in Japan: data analysis of nationwide registry. Gastric Cancer. 2006;9:51–66.. [DOI] [PubMed] [Google Scholar]
- 6. . Yoo KY. Cancer control activities in the Republic of Korea. Jpn J Clin Oncol. 2008;38(5):327–333.. 10.1093/jjco/hyn026) [DOI] [PubMed] [Google Scholar]
- 7. . Gong EJ, Ahn JY, Jung HY. et al. Risk factors and clinical outcomes of gastric cancer identified by screening endoscopy: a case-control study. J Gastroenterol Hepatol. 2014;29(2):301–309.. 10.1111/jgh.12387) [DOI] [PubMed] [Google Scholar]
- 8. . Choi KS, Jun JK, Park EC. et al. Performance of different gastric cancer screening methods in Korea: a population-based study. PLoS ONE. 2012;7(11):e50041. 10.1371/journal.pone.0050041) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. . Tashiro A, Sano M, Kinameri K, Fujita K, Takeuchi Y. Comparing mass screening techniques for gastric cancer in Japan. World J Gastroenterol. 2006;12(30):4873–4874.. 10.3748/wjg.v12.i30.4873) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. . Choi KS, Jun JK, Lee HY. et al. Performance of gastric cancer screening by endoscopy testing through the National Cancer Screening Program of Korea. Cancer Sci. 2011;102(8):1559–1564.. 10.1111/j.1349-7006.2011.01982.x) [DOI] [PubMed] [Google Scholar]
- 11. . Voutilainen ME, Juhola MT. Evaluation of the diagnostic accuracy of gastroscopy to detect gastric tumours: clinicopathological features and prognosis of patients with gastric cancer missed on endoscopy. Eur J Gastroenterol Hepatol. 2005;17(12):1345–1349.. 10.1097/00042737-200512000-00013) [DOI] [PubMed] [Google Scholar]
- 12. . Kaminski MF, Regula J, Kraszewska E. et al. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med. 2010;362(19):1795–1803.. 10.1056/NEJMoa0907667) [DOI] [PubMed] [Google Scholar]
- 13. . Pimenta-Melo AR, Monteiro-Soares M, Libânio D, Dinis-Ribeiro M. Missing rate for gastric cancer during upper gastrointestinal endoscopy: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2016;28(9):1041–1049.. 10.1097/MEG.0000000000000657) [DOI] [PubMed] [Google Scholar]
- 14. . Gong EJ, Lee JH, Jung K. et al. Characteristics of missed simultaneous gastric lesions based on double-check analysis of the endoscopic image. Clin Endosc. 2017;50(3):261–269.. 10.5946/ce.2016.056) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. . Wang YR, Loftus EV, Jr, Judge TA, Peikin SR. Rate and predictors of interval esophageal and gastric cancers after esophagogastroduodenoscopy in the United States. Digestion. 2016;94(3):176–180.. 10.1159/000452794) [DOI] [PubMed] [Google Scholar]
- 16. . Cho YS, Chung IK, Kim JH. et al. Risk factors of developing interval early gastric cancer after negative endoscopy. Dig Dis Sci. 2015;60(4):936–943.. 10.1007/s10620-014-3384-z) [DOI] [PubMed] [Google Scholar]
- 17. . Menon S, Trudgill N. How commonly is upper gastrointestinal cancer missed at endoscopy? A meta-analysis. Endosc Int Open. 2014;2(2):E46–E50.. 10.1055/s-0034-1365524) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. . Chadwick G, Groene O, Riley S. et al. Gastric cancers missed during endoscopy in England. Clin Gastroenterol Hepatol. 2015;13(7):1264-1270.e1. 10.1016/j.cgh.2015.01.025) [DOI] [PubMed] [Google Scholar]
- 19. . Shimodate Y, Mizuno M, Doi A. et al. Gastric superficial neoplasia: high miss rate but slow progression. Endosc Int Open. 2017;5(8):E722–E726.. 10.1055/s-0043-110076) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. . Hosokawa O, Hattori M, Douden K. Difference in accuracy between gastroscopy and colonoscopy for detection of cancer. Hepatogastroenterology. 2007;54(74):442–444.. [PubMed] [Google Scholar]
- 21. . Lee HL, Eun CS, Lee OY. et al. When do we miss synchronous gastric neoplasms with endoscopy? Gastrointest Endosc. 2010;71(7):1159–1165.. 10.1016/j.gie.2010.01.011) [DOI] [PubMed] [Google Scholar]
- 22. . Kim HH, Cho EJ, Noh E. et al. Missed synchronous gastric neoplasm with endoscopic submucosal dissection for gastric neoplasm: experience in our hospital. Dig Endosc. 2013;25(1):32–38.. 10.1111/j.1443-1661.2012.01339.x) [DOI] [PubMed] [Google Scholar]
- 23. . Min JK, Kwak MS, Cha JM. Overview of deep learning in gastrointestinal endoscopy. Gut Liver. 2019;13(4):388–393.. 10.5009/gnl18384) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. . Zhu Y, Wang QC, Xu MD. et al. Application of convolutional neural network in the diagnosis of the invasion depth of gastric cancer based on conventional endoscopy. Gastrointest Endosc. 2019;89(4):806.e1-815.e1. 10.1016/j.gie.2018.11.011) [DOI] [PubMed] [Google Scholar]
- 25. . Hosokawa O, Tsuda S, Kidani E. et al. Diagnosis of gastric cancer up to three years after negative upper gastrointestinal endoscopy. Endoscopy. 1998;30(8):669–674.. 10.1055/s-2007-1001386) [DOI] [PubMed] [Google Scholar]
- 26. . Kim GH, Liang PS, Bang SJ, Hwang JH. Screening and surveillance for gastric cancer in the United States: is it needed? Gastrointest Endosc. 2016;84(1):18–28.. 10.1016/j.gie.2016.02.028) [DOI] [PubMed] [Google Scholar]
- 27. . Veitch AM, Uedo N, Yao K, East JE. Optimizing early upper gastrointestinal cancer detection at endoscopy. Nat Rev Gastroenterol Hepatol. 2015;12(11):660–667.. 10.1038/nrgastro.2015.128) [DOI] [PubMed] [Google Scholar]
- 28. . Raftopoulos SC, Segarajasingam DS, Burke V, Ee HC, Yusoff IF. A cohort study of missed and new cancers after esophagogastroduodenoscopy. Am J Gastroenterol. 2010;105(6):1292–1297.. 10.1038/ajg.2009.736) [DOI] [PubMed] [Google Scholar]
- 29. . Vradelis S, Maynard N, Warren BF, Keshav S, Travis SP. Quality control in upper gastrointestinal endoscopy: detection rates of gastric cancer in Oxford 2005-2008. Postgrad Med J. 2011;87(1027):335–339.. 10.1136/pgmj.2010.101832) [DOI] [PubMed] [Google Scholar]
- 30. . Hatfield AR, Slavin G, Segal AW, Levi AJ. Importance of the site of endoscopic gastric biopsy in ulcerating lesions of the stomach. Gut. 1975;16(11):884–886.. 10.1136/gut.16.11.884) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. . Kim JM, Sohn JH, Cho MY. et al. Pre- and post-ESD discrepancies in clinicopathologic criteria in early gastric cancer: the NECA-Korea ESD for Early Gastric Cancer Prospective Study (N-Keep). Gastric Cancer. 2016;19(4):1104–1113.. 10.1007/s10120-015-0570-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. . Zhenming Y, Lei S. Diagnostic value of blue laser imaging combined with magnifying endoscopy for precancerous and early gastric cancer lesions. Turk J Gastroenterol. 2019;30(6):549–556.. 10.5152/tjg.2019.18210) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. . Park JM, Huo SM, Lee HH. Longer observation time increases proportion of neoplasms detected by esophagogastroduodenoscopy. Gastroenterology. 2017;153(2):460.e1-469.e1. 10.1053/j.gastro.2017.05.009) [DOI] [PubMed] [Google Scholar]