Abstract
High-flow nasal cannula oxygen therapy (HFNCOT) system consists of an air/oxygen supply system capable of delivering up to 100% humidified and heated oxygen at a flow rate of up to 80 L/min. The system includes a blender, active humidifier, single heated tube, and nasal cannula. HFNCOT has many physiological advantages compared with other standard oxygen therapies, such as anatomical dead space washout, more constant fraction of inspired oxygen, positive end-expiratory (PEEP) effect, supplement of adequate humidification and maintenance of muco-ciliary function. HFNCOT is mostly used for hypoxemic acute respiratory failure, although it also has other indications. HFNCOT is a common choice of physicians as its technology makes it more silent and comfortable. Though HFNCOT is used in many clinical settings, there is a lack of publications addressing devices and initial settings. We present a review on HFNCOT, with focus on device and application methodology.
Keywords: Respiratory failure, nasal cannula, high-flow oxygen, methodology
Main Points
High-flow nasal cannula oxygen therapy is an air/oxygen supply system capable of delivering up to 100% humidified and heated oxygen at a flow rate of up to 80 L/min.
Most studies on HFNCOT application in hypoxemic ARF patients used the Fisher & Paykel Healthcare™ technology, particularly the Optiflow®.
Frequent initial settings include higher flow rates (50 L/min) or more comfortable ones (30-40 L/min), and FiO2 between 50% and 100%, to maintain SpO2 > 90% or > 92%.
Introduction
High-flow Nasal Cannula Rationale
In acute respiratory failure (ARF), oxygen can be delivered in many ways, ranging from a simple oxygen facemask and other non-invasive methods to invasive mechanical ventilation (IMV) via an endotracheal tube. High-flow nasal cannula oxygen therapy (HFNCOT) is a non-invasive method that improves patient oxygenation when conventional oxygen therapy is not enough. HFNCOT provides humidity-enriched oxygen therapy. It provides flow rates exceeding patient inspiratory flow rates at various minute volumes, but is not a full substitute for invasive or non-invasive ventilation (NIV) therapy in ARF. HFNCOT may provide a bridge to NIV and may give some patients NIV-free hours.
The mechanisms of action of HFNCOT include a range of important and interdependent physiological effects on a variety of factors: (1) better control over FiO2 in comparison with conventional oxygen therapy; (2) provision of heated and humidified gas, increasing comfort and tolerability, and also improving muco-ciliary function and pulmonary mechanics; (3) washout of nasopharyngeal dead space, increasing alveolar ventilation; (4) reduction of the work of breathing, and (5) some positive airway pressure effect.1,2
The current clinical applications of HFNCOT include hypoxemic respiratory failure (mild ARF, pneumonia, interstitial pulmonary fibrosis, or cardiogenic pulmonary edema), pre-intubation oxygenation, post-extubation, postoperative applications, palliative care (do-not-intubate patients), bronchoscopy, respiratory failure in immunocompromised patients, bronchiectasis, and in selected cases of hypercapnic respiratory failure.1,2 The main clinical outcomes observed during HFNCOT are the improvements in oxygenation, reduced respiratory rate (RR), less dyspnea, better tolerated and improved patient comfort, and reduced risk for intubation.3
Mechanics and Devices of High-flow Nasal Cannula
The oxygen-air blender can deliver up to 60 L/min flow and an FiO2 between 21% and 100%. It also includes an active humidifier to heat delivered gases to 37°C with 100% relative humidity.4 The heated flow is delivered to the patient by flexible nasal prongs or a tracheostomy adapter, although, through tracheostomy, some of the benefits of HFNCOT may be lost due to bypassing the upper airways.5 The production of condensation droplets is reduced due to a specialized heating tube. The system of nasal prongs does not impair speaking and eating, as other systems do.4
There are 3 classes of independent stream generators
(1) Air-oxygen blenders (Fisher & Paykel Healthcare™, Auckland, New Zeeland, Optiflow®): The air-oxygen blender with stream meter is the most frequently used. The air-oxygen blender is supplied by air and oxygen from the divider, besides the flow meter, at a low flow; both assure the stable conveyance of FiO2 and gas stream.
(2) Turbines built in the device (Fisher and & Paykel Healthcare™ Airvo-2®): These are high-flow devices manufactured by Fisher & Paykel Healthcare™ and Vapotherm™ (New Hampshire, USA), which include accurate, built-in stream generators, mainly consisting of turbines to entrain room air and generate a high stream in the absence gas supply from the wall or the tank . These devices also contain low-pressure oxygen suppliers to deliver oxygen and can detect the concentration of oxygen in the provided gas. Higher oxygen concentrations cannot be delivered by these systems, even with the ignored gas loss.6
(3) Entrainment frameworks (Maxtec™, Utah, USA, Max-Venturi®): This class of stream generators can solve the previous limitation. A Maxtec™ Max-Venturi® with a medium flow uses an air-entrainment framework to deliver high flow and a higher concentration of oxygen. Moreover, air-entrainment generators can titrate the concentration of oxygen using a flowmeter.6,7
High-flow nasal cannula devices can be stand-alone units, such as the Optiflow® and the Airvo-2® models, or may be integrated within mechanical ventilators, such as the Mindray SV300™ and Air Liquide™ Monnal T75® models. Luo et al.8 compared the 3 devices––Airvo-2®, SV300®, and Monnal T75®––and they concluded that the mechanical ventilators performed better than Airvo-2® in providing positive end-expiratory pressure (PEEP), especially at higher flow rate. Yet, the most important factors which influence the PEEP effect in HFNCOT are the gas flow rate, the status of the patient’s mouth (open or closed), and lung compliance.
The Optiflow® is smaller compared to other non-invasive HFNCOT units (with the exception of Airvo-2®), making its use generally simpler to deal with.9 The integrated flowmeter allows setting the correct flow of the gas blend, with a typical maximum flow rate of 60 L/min, although flow rates up to 80 L/min are possible.
In all HFNCOT devices, the air is actively heated before the patient inspires. Mauri et al.10 conducted a prospective, randomized, cross-over study in which they evaluated whether a higher temperature of inspired gas would increase patient comfort. However, the authors concluded that patients were the most comfortable with HFNCOT temperatures slightly below body temperature (31°C).
Methods
We searched for publications and abstracts on PubMed, including the search terms (with synonyms and closely related words) “high-flow nasal cannula” and “hypoxemic ARF” from January 2000 to December 2019. We limited the publications to the English language and to publications on the adult population. We reviewed the bibliographies of selected studies for additiona
l references.
From 83 citations, we included 19 original publications comprising studies in which HFNCOT was used for the management of hypoxemic ARF. These studies were analyzed in relation to the included patients, the criteria to initiate HFNCOT, the devices used, initial set flow, FiO2 , and temperature. Studies on the pre and post-extubation use of HFNCOT were excluded, as also those in which HFNCOT was used during bronchoscopy. A flow chart of the study process is reported in Figure 1.
Figure 1.
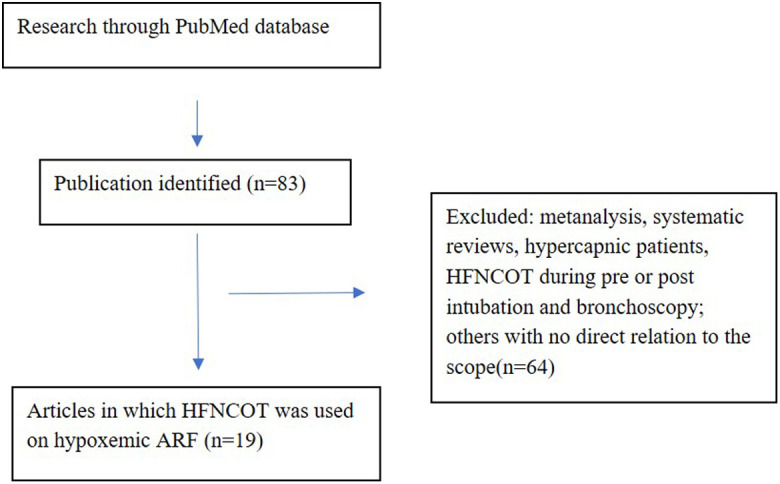
Flowchart of the study selection process (1).
We decided not to perform a formal statistical analysis, due to the subject and scope of the study.
Clinical and Research Consequences
Evidence on Initial Methodology of HFNCOT: The current literature on using HFNCOT therapy in ARF demonstrates different methodologies and approaches (Table 1). A summary of general HFNC indications and contraindications is presented in Table 2.
Table 1.
Analysis of HFNCOT Devices and Settings
| Study | Population | Device | Initial Flow | Initial FiO2 | Temperature |
|---|---|---|---|---|---|
| Frat et al. (11) | RR > 25 PaO2/FiO2 < 300 PaCO2 <45 mmHg (O2 ≥ 10 L/min) | Optiflow® | 50 L/min | 100% (SpO2 > 92%) |
37°C |
| Roca et al. (12) | SpO2 < 96%(FiO2 ≥ 50%) | Optiflow® | 20-30 L/min | Previous | No data |
| Schwablaeur et al. (13) | PaO2 < 55 mmHg(FiO2 21%) | Optiflow® | 55 L/min | 50% | No data |
| Parke et al. (14) | Mild-moderate hypoxemic ARF | Optiflow® | 35 L/min | No data (SpO2 ≥ 95%) |
No data |
| Cho et al. (15) | PaO2/FiO2 < 300RR > 24(O2 > 8 L/min) | Optiflow® | 30-40 L/min | 40-100% (SpO2 > 92% and PaO2 > 65 mmHg) |
No data |
| Sztrymf et al. (16) | SpO2 < 96%RR ≥ 25(FiO2 > 50%) | Optiflow® | No data (median 40 L/min) |
No data | No data |
| Jones et al.(17) | SpO2 ≤ 92% RR ≥ 22 | Airvo-1®Airvo-2® | 40 L/min | 28% | 37°C |
| Nagata et al. (18) | Neoplasia and need for any respiratory support | Optiflow® | 35-45 L/min | (SpO2 ≥ 95%) | No data |
| Lamiale et al. (19) | Immunosuppressed O2 > 6 L/min for SpO2 > 95 %RR > 30;respiratory distress |
No data | 40-50 L/min | 100% (SpO2 > 90%) |
No data |
| Roca et al. (20) | Lung transplant SpO2 ≤ 95%RR ≥ 25(FiO2 ≥ 50%) |
Optiflow® | No data | No data (SpO2 95%) |
37°C |
| Peters et al. (21) | Do-not-intubate status |
Optiflow® | 35 L/min (increased to 45-50 L/min) |
Previous (SpO2 > 90%) |
No data |
| Frat (22) | ARDS | Optiflow® | 50 L/min | 100% (SpO2 > 92%) |
37°C |
| Sztrymf et al. (3) | O2 > 9 L/min for SpO2 > 92%RR > 24;Respiratory distress | Optiflow® | No data | No data | 37°C |
| Rittayamai et al. (23) | SpO2 < 94%RR > 24 | Optiflow® | 35 L/min | No data (SpO2 ≥ 94%) |
37°C |
| Lenglet et al. (24) | O2 > 9 L/minRespiratory distress | Optiflow® | 40 L/min | ≥ 60% | No data |
| Rello et al. (25) | Influenza A H1N1 SpO2 ≤ 92%(O2 > 9 L/min) |
Optiflow® | 30 L/min | 100% (SpO2 95%) |
37°C |
| Messika et al. (26) | ARDS | Optiflow® | 60 /min | 100% * | 37°C |
| Coudroy et al. (27) | PaO2/FiO2 < 300RR > 24Respiratory distress | Optiflow® | 50 L/min | 60% | No data |
| Raesi et al. (28) | Moderate-severe asthma exacerbation | No data | 15-35 L/min | No data | 37°C |
*The majority of the patients.
ARDS, acute respiratory distress syndrome; FiO2, fraction of inspired oxygen; PaO2, partial pressure of oxygen; RR, respiratory rate; SpO2, peripheral capillary oxygen saturation.
Table 2.
General Indications and Contraindications of HFNCOT
| Indications | Contraindications |
|---|---|
| Hypoxemic acute respiratory failure: PaO2/FiO2 < 300 mmHg (40 kPa), and respiratory rate > 25 breaths/min, despite conventional oxygen therapy (with flow > 8 L/min) | Hypercapnia, PaCO2 ≥ 45 mmHg (6 kPa), hemodynamic instability, severe failure of ≥ 2 organs, Glasgow Coma Scale ≤ 13, urgent requirement of endotracheal intubation, non-cooperating patients, facial abnormalities preventing the use of nasal cannula, and recent facial or nasal surgeries |
Frat et al.11 conducted a study including patients with hypoxemic ARF but with no history of chronic lung disease or hypercapnia, in 23 intensive care units (ICU). The inclusion criteria for the study were: RR > 25 breaths/min when the patient was inhaling oxygen at a rate of 10 L/min or higher for at least 15 minutes, PaO2/FiO2 < 300 mmHg (40 kPa), and PaCO2 < 45 mmHg (6 kPa). The main exclusion criterion was PaCO2 being more than 45 mmHg (6 kPa). The other exclusion criteria were similar to those reported in other studies, namely, chronic respiratory failure or asthma exacerbation, cardiogenic pulmonary edema, hemodynamic instability, severe neutropenia, use of vasopressors, a Glasgow Coma Scale Score (GCSS) equal to 12 points or less, NIV contraindications, urgent requirement of endotracheal intubation, a do-not-intubate order, and the patient’s decision not to participate. They randomly assigned 310 patients to high-flow oxygen therapy, standard oxygen therapy, oxygen delivered through a face mask, or NIV, and evaluated the proportion of patients requiring intubation in each group over a 28-day period. HFNCOT (Fisher & Paykel Healthcare™ Optiflow®) was applied continuously through large-bore binasal prongs, with a gas-flow rate of 50 L/min and an initial FiO2 of 100%. Oxygen was passed through a heated humidifier (Fisher & Paykel Healthcare™ MR850®). The FiO2 was set at 100% and then adjusted to maintain SpO2 of 92% or more; the temperature was set to 37°C.
Roca et al.12 conducted a study with 20 ARF patients. The inclusion criteria were the following: SpO2 < 96% and receiving humidified oxygen via face mask, with FiO2 of 50% or more. The exclusion criteria were: an unstable clinical status, defined as significant changes in respiratory parameters in the last hour before inclusion in the study, the requirement of endotracheal intubation, GCSS < 14, severe hemodynamic instability in spite of receiving vasopressors and fluid therapy, severe failure of more than 2 organs other than respiratory failure, pregnancy, and non-cooperative patients. Oxygen was administered by 2 different modalities for sequential 30-minute periods. First, oxygen was administered via a standard face mask, and it was humidified with a bubble humidifier; the patient then received oxygen via HFNCOT (Fisher & Paykel Healthcare™ Optiflow®) at an initial flow of 20-30 L/min, with an FiO2 identical to that with a standard face mask.
Schwabbauer et al.13 similarly compared subjective respiratory parameters described by patients (PaO2 < 55 mmHg (7.3 kPa) at room air) who wore HFNCOT, a Venturi mask, and NIV, for sequential 30-minute periods in a randomized order. HFNCOT (Fisher & Paykel Healthcare™ Optiflow®) was administered with 55 L/min flow and FiO2 at 60%. Active humidification was provided by Fisher & Paykel Healthcare™ MR850®.
Parke et al.14 randomized patients with mild to moderate hypoxemic respiratory failure to receive HFNCOT oxygen therapy or standard high-flow face mask oxygen therapy in a cardiothoracic and vascular ICU. HFNCOT (Fisher & Paykel Healthcare™ Optiflow®, with MR880® humidifier, RT241® heated-delivery tube, RT033 large/RT034 small® wide-bore nasal cannula) was initiated with 35 L/min flow, and FiO2 was titrated to SpO2 ≥ 95%
In a retrospective analysis by Cho et al.,15 HFNCOT (Fisher & Paykel Healthcare™ Optiflow®) was used during acute hypoxemic respiratory failure (PaO2/FiO2 < 300 mmHg (40 kPa) or respiratory RR >24) despite the use of conventional oxygen therapy, with flow above 8 L/min. The initial flow was maintained at 30-40 L/min and FiO2 was 40-100%, to maintain SpO2 > 92% and PaO2 > 65 mmHg (8.7 kPa). The patients were intubated if HNFCOT at a flow of > 50 L/min and FiO2 100% was insufficient to maintain SpO2 > 90% or PaO2 > 60 mmHg (8 kPa).
In a study of patients with ARF by Sztrymf et al.,16 the inclusion criteria were SpO2 < 96% and/or RR ≥ 25 breaths/min, despite receiving oxygen via a facemask with an estimated FiO2 > 50%. The only exclusion criterion was an immediate need for intubation. HNFCOT (Fisher & Paykel Healthcare™ Optiflow®) was provided with a median flow of 40 L/min and a median duration of 26.5 hours.
In the HOT-ER study, the inclusion criteria were defined as SpO2 ≤ 92% and a, RR ≥ 22 breaths/min, while the exclusion criteria were the immediate need for mechanical ventilation in the emergency department, past intubation, pneumothorax, the presence of facial abnormalities preventing the use of nasal cannula, and recent facial or nasal surgeries.17 In this study, the devices used for HFNCOT were the Fisher & Paykel Healthcare™ Airvo-1® and Airvo-2®.
Nagata et al.18 performed a retrospective study in ARF patients who required any means of respiratory support (NIV, HFNCOT, or IMV). The only inclusion criterion in this study was the need for any respiratory support and patients with neoplastic disease; those requiring urgent management of airways (respiratory arrest, massive hemoptysis, or asphyxia), and those in comatose states were excluded. HFNCOT (Fisher & Paykel Healthcare™ Optiflow®) was started with 35-45 L/min flow and FiO2 was titrated to maintain SpO2 > 90%.
On the other hand, Lemiale et al.19 evaluated immunosuppressed patients and defined the inclusion criteria as the onset of respiratory symptoms within 72 hours prior to admission to ICU, and either a requirement of oxygen provided at flows greater than 6 L/min to maintain SpO2 > 95 %, or the presence of respiratory distress symptoms (RR > 30 breaths/min, labored breathing, and intercostal recession with or without dyspnea at rest). Patients were excluded if they were hypercapnic (PaCO2 > 45 mmHg, 6 kPa), received any type of mechanical ventilation before ICU admission, needed NIV or IMV, or refused to participate in the conducted study. HFNC was initiated with 40-50 L/min flow and FiO2 100%, which was titrated to maintain SpO2 ≥ 95%.
In another study, Roca et al.20 worked on 37 patients with lung transplant who needed readmission to ICU due to ARF (mainly due to infection), and were divided in 2 cohorts (conventional oxygen therapy vs. HFNCOT). HFNCOT was provided with flow and FiO2 titrated to a target FiO2 of 95% at a temperature of 37°C.
Peters et al.21 studied the efficacy of HFNCOT in 50 patients with hypoxemic ARF and do-not-intubate status admitted to the ICU. Patients with PaCO2 > 65 mmHg (8.7 kPa) and pH < 7.28 were excluded. The HFNCOT (Fisher & Paykel Healthcare™ Optiflow® system, using the MR850® respiratory humidifier with MR290® chamber; RT241® heated-delivery tubing, and RT033® or RT044® small or wide-bore nasal cannula) was initiated at a flow of 35 L/min (titrated to 45-50 L/min if tolerated) and FiO2 at the previous level, with titration to SpO2 > 90%. The mean flow was 42.6 L/min (30-60 L/min) and the mean FiO2 67% (30-100%).
Frat et al.22 assessed sequential HFNCOT and NIV application in ARDS patients. HFNCOT (Fisher & Paykel Healthcare™ Optiflow®, heated humidifier MR850®) was initially administered with 50 L/min flow and FiO2 100%, which was titrated to maintain SpO2 > 92%.
Sztrymf et al.3 performed a prospective study on HFNCOT in ICU patients with ARF. Thirty-eight patients were included, all requiring more than 9 L/min of oxygen output to achieve SpO2 > 92% or showing persisting signs of respiratory distress (defined when one or more of the following criteria were present: RR > 24 bpm, thoraco-abdominal asynchrony, and supraclavicular retraction) despite oxygen administration were eligible. Patients requiring immediate endotracheal intubation were excluded, as were those with hypercapnic respiratory failure. They used the Fisher & Paykel Healthcare™ Optiflow® HFNCOT device, the Fisher & Paykel Healthcare TM MR850® heated chamber, and the Fisher & Paykel Healthcare™ RT310® high-performance circuit.
Rittayamai et al.23 evaluated the effects of HFNCOT (Fisher & Paykel Healthcare™ Optiflow® at an inspiratory flow of 35 L/min), compared with conventional oxygen therapy (COT) in 40 subjects with acute dyspnea and hypoxemia in the emergency department. They included subjects who had developed acute dyspnea with hypoxemia (breathing frequency > 24 bpm and SpO2 < 94% in room air). Subjects with hemodynamic instability, the need for IMV, chronic respiratory failure, decreased level of consciousness, and lack of cooperation were excluded.
Lenglet et al.24 aimed to study the feasibility and efficacy of HFNCOT in patients exhibiting ARF in the emergency department. They performed a prospective, observational study including 17 patients with ARF requiring > 9 L/min oxygen or with ongoing clinical signs of respiratory distress despite oxygen therapy. The device of oxygen administration was then switched, from a non-rebreathing mask to HFNCOT (Fisher & Paykel Healthcare™ Optiflow®, initial flow of 40 L/min).
Rello et al.25 performed a cohort study to assess the effectiveness of HFNCOT in 25 ICU adult patients with ARF by confirmed 2009 influenza A/H1N1 virus infection. The exclusion criteria were age < 18 years and hypercapnia. HFNCOT (Fisher & Paykel Healthcare™ Optiflow®, heated humidifier Fisher & Paykel Healthcare™ MR850®) was indicated in the presence of ARF when the patient was unable to maintain a pulse oximetry SpO2 > 92%, with more than 9 L/min of oxygen using a standard face mask conventional delivery system. The median flow used was 30 L/min, the initial FiO2 was 100% with the target SpO2 of 95%, and temperature was set at 37°C. Twenty patients were unable to maintain SpO2 > 92% with conventional oxygen administration, and required HFNCOT.
Messika et al.26 conducted a 1-year observational study about the use of HFNCOT in subjects with ARDS. HFNCOT (Fisher & Paykel Healthcare™ Optiflow®) was used in 87 subjects, 45 of whom had ARDS. The initial oxygen flow was 60 L/min.
Coudroy et al.27 conducted an observational cohort study over an 8-year period, comparing HFNCOT (Fisher & Paykel Healthcare™ Optiflow®) and NIV in 115 immunocompromised patients with ARF. They included patients admitted for ARF, defined by the following criteria: a respiratory rate > 24 bpm or clinical signs of respiratory distress, and PaO2/FiO2 < 300 mmHg (40 kPa). Patients with acute-on-chronic respiratory failure, those treated with standard oxygen alone or needing immediate intubation, and those with a do-not-intubate order were excluded. In HFNCOT, the flow was set to 40-50 L/min and FiO2 60%.
In a randomized double-blind study, Raeisi et al.28 included 40 patients with moderate-to-severe asthma exacerbations. Patients were randomly assigned to receive either HFNCOT or conventional oxygen therapy (COT) for 24 hours. HFNCOT was provided at a flow rate of 15-35 L/min (37°C).
Discussion
HFNCOT is a simple system with clinical effects that mainly depend on flow rate, oxygen concentration, and temperature control. As presented in most conclusions of the publications included in this study, the use of HFNCOT in hypoxemic patients may avoid the use of other NIV techniques and minimize the risk of secondary intubation.
Most of the reviewed studies suggest that the inclusion criteria for HFNC include SpO2 between 92% and 96% , with an oxygen flow rate > 6-10 L/min, an RR > 24-30 breaths/min, and a breathing pattern suggestive of thoraco-abdominal asynchrony and supraclavicular retractions. The exclusion criteria suggested for HFNCOT are severe hemodynamic instability, GCSS <12-14 points, general contraindications to NIV, the urgent need for endotracheal intubation, and hypercapnic respiratory failure (PaCO2 > 45 mmHg, 6 kPa). Even if some studies excluded cancer and immunosuppressive patients, HFNCOT is used in these groups too.
Concerning devices, clearly the most used one was the Fisher & Paykel Healthcare™ Optiflow®, although not all publications indicated the humidifier devices used. In those who did, Fisher & Paykel Healthcare™ MR850® was the most common. Few studies applied the new Fisher & Paykel Healthcare™ Airvo-2®, which is presently used in several ICUs, emergency departments, and pulmonology departments.
Concerning the HFNCOT application methodology, Ischaki et al.29 suggested that in hypoxemic ARF, HFNCOT should be initiated with a flow rate of 40-60 L/min (preferably 60 L/min), 100% FiO2 , and a temperature of 37°C. Lower flow rates (35-40 L/min) allow for better comfort and initial adaptation, while a higher flow rate (60 L/min) provides a faster relief of dyspnea.29
As presented in Table 1, in most studies, the authors have usually opted for lower flow rates. However, quite often, the flow titration methodology is missing, and only 1 study utilized a 60 L/min flow which could provide optimal physiological advantages, although it might be associated with higher discomfort and less tolerability.
In patients with PaO2 /FiO2 < 300 mmHg (40 kPa), the studies tend to initiate HFNCOT at higher flows 50-60 L/min),11, 22, 26, 27 although others start with 30-40 L/min.14,15
In terms of FiO2, there were a considerable a variety of strategies, with only 5 studies deciding to initiate with 100%. There were groups who had opted to use the value of the estimated FiO2 that had been previously applied through conventional oxygen systems. Although not all studies gave special focus to FiO2 titration, the most common targets were SpO2 > 90%, > 92%, and ≥ 95% (in the cardiothoracic and vascular ICU patients and in immunosuppressive patients).
In patients with hypoxemic ARF, the temperature of the HFNCOT gas can affect the ease of use. At equal flow rates, it was proven that reducing the temperature to 31°C could be more comfortable than 37°C.10 However, most studies applied a temperature of 37°C. Moreover, the majority of studies did not provide data on initial temperature use (or did not clearly state it). Temperature titration was also generally absent.
Ischaki et al.29 also suggest that during weaning from HFNCOT, FiO2 should be reduced earlier than flow reduction. However, in the studies analyzed, the HFNCOT reduction strategy was not commonly mentioned.
Conclusions
Most studies on HFNCOT application in hypoxemic ARF patients used the Fisher & Paykel Healthcare™ technology, particularly the Optiflow®. The initial settings included higher flow rates (50 L/min) or more comfortable ones (30-40 L/min), as also FiO2 between 50% and 100%, to maintain SpO2 > 90% or > 92%. Information about the criteria to decide the initial flow rate and FiO2 level is virtually missing.
There is a need for more studies in this field, with a focus on the comparison of devices (also evaluating the efficacy of the new Airvo-2®) and HFNCOT methodologies, particularly on the settings titration.
Funding Statement
The authors declared that this study has received no financial support.
Footnotes
Peer Review: Externally peer-reviewed.
Author Contributions: Concept - M.G., A.E. Design: M.G., A.E.; Supervision - A.E.; - Resources - M.G., A.E.; Materials - M.G.; Data Collection and/or Processing - M.G., N.A., A.G., Y.M., M.A., H.S., H.H., G.G., B.C., J.A., M.E., M.G., A.F., L.D.C., H.K., E.P., M.S., P.S., W.L., I.B., J.W., M.D.A., A.P., A.N., S.S., G.G., A.P., A.E.; Analysis and/or Interpretation - M.G., N.A., A.G., Y.M., M.A., H.S., H.H., G.G., B.C., J.A., M.E., M.G., A.F., L.D.C., H.K., E.P., M.S., P.S., W.L., I.B., J.W., M.D.A., A.P., A.N., S.S., G.G., A.P., A.E.; Literature Search - M.G., N.A., A.G., Y.M., M.A., H.S., H.H., G.G., B.C., J.A., M.E., M.G., A.F., L.D.C., H.K., E.P., M.S., P.S., W.L., I.B., J.W., M.D.A., A.P., A.N., S.S., G.G., A.P., A.E.; Writing Manuscript - M.G., N.A., A.G., Y.M., M.A., H.S., H.H., G.G., B.C., J.A., M.E., M.G., A.F., L.D.C., H.K., E.P., M.S., P.S., W.L., I.B., J.W., M.D.A., A.P., A.N., S.S., G.G., A.P., A.E.; Critical Review - M.G., N.A., A.G., Y.M., M.A., H.S., H.H., G.G., B.C., J.A., M.E., M.G., A.F., L.D.C., H.K., E.P., M.S., P.S., W.L., I.B., J.W., M.D.A., A.P., A.N., S.S., G.G., A.P., A.E.
Conflict of Interest: The authors have no conflict of interest to declare.
References
- 1. Lodeserto FJ, Lettich TM, Rezaie SR. High-flow nasal cannula: mechanisms of action and adult and pediatric indications. Cureus. 2018;10(11):e3639. 10.7759/cureus.3639) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nishimura M. High-flow nasal cannula oxygen therapy in adults. J Intensive Care. 2015;3(1):15. 10.1186/s40560-015-0084-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sztrymf B, Messika J, Bertrand F, et al. Beneficial effects of humidified high flow nasal oxygen in critical care patients: a prospective pilot study. Intensive Care Med. 2011;37(11):1780–178. 6. 10.1007/s00134-011-2354-6) [DOI] [PubMed] [Google Scholar]
- 4. Roca O, Hernández G, Díaz-Lobato S, et al. Current evidence for the effectiveness of heated and humidified high flow nasal cannula supportive therapy in adult patients with respiratory failure. Crit Care. 2016;20(1):109. 10.1186/s13054-016-1263-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Corley A, Edwards M, Spooner AJ, et al. High-flow oxygen via tracheostomy improves oxygenation in patients weaning from mechanical ventilation: a randomised crossover study. Intensive Care Med. 2017;43(3):465–46. 7. 10.1007/s00134-016-4634-7) [DOI] [PubMed] [Google Scholar]
- 6. Nishimura M. High-Flow Nasal Cannula oxygen therapy devices. Respir Care. 2019;64(6):735–7. 42. 10.4187/respcare.06718) [DOI] [PubMed] [Google Scholar]
- 7. Mauri T, Alban L, Turrini C, et al. Optimum support by high-flow nasal cannula in acute hypoxemic respiratory failure: effects of increasing flow rates. Intensive Care Med. 2017;43(10):1453–14. 63. 10.1007/s00134-017-4890-1) [DOI] [PubMed] [Google Scholar]
- 8. Luo JC, Lu MS, Zhao ZH, et al. Positive end-expiratory pressure effect of 3 high-flow nasal cannula devices. Respir Care. 2017;62(7):888–8. 95. 10.4187/respcare.05337) [DOI] [PubMed] [Google Scholar]
- 9. Loo MV, Sottiaux T. High Flow Nasal Cannula oxygenation for adult patients in the ICU: a literature review. Acta Anaesthesiol Belg. 2016;67(2):63–72.. [PubMed] [Google Scholar]
- 10. Mauri T, Galazzi A, Binda F, et al. Impact of flow and temperature on patient comfort during respiratory support by high-flow nasal cannula. Crit Care. 2018;22(1):120. 10.1186/s13054-018-2039-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Frat JP, Thille AW, Mercat A, et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med. 2015;372(23):2185–21. 96. 10.1056/NEJMoa1503326) [DOI] [PubMed] [Google Scholar]
- 12. Roca O, Riera J, Torres F, Masclans JR. High-flow oxygen therapy in acute respiratory failure. Respir Care. 2010;55(4):408–4. 13. [PubMed] [Google Scholar]
- 13. Schwabbauer N, Berg B, Blumenstock G, et al. Nasal high-flow oxygen therapy in patients with hypoxic respiratory failure: effect on functional and subjective respiratory parameters compared to conventional oxygen therapy and non-invasive ventilation (NIV). BMC Anesthesiol. 2014;14:66. 10.1186/1471-2253-14-66) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Parke RL, McGuinness SP, Eccleston ML. A preliminary randomized controlled trial to assess effectiveness of nasal high-flow oxygen in intensive care patients. Respir Care. 2011;56(3):265–2. 70. 10.4187/respcare.00801) [DOI] [PubMed] [Google Scholar]
- 15. Hyun Cho W, Ju Yeo H, Hoon Yoon S, et al. High-flow nasal cannula therapy for acute hypoxemic respiratory failure in adults: a retrospective analysis. Intern Med. 2015;54(18):2307–23. 13. 10.2169/internalmedicine.54.4266) [DOI] [PubMed] [Google Scholar]
- 16. Sztrymf B, Messika J, Mayot T, et al. Impact of high-flow nasal cannula oxygen therapy on intensive care unit patients with acute respiratory failure: a prospective observational study. J Crit Care. 2012;27(3):324.e9–324.13.. 10.1016/j.jcrc.2011.07.075) [DOI] [PubMed] [Google Scholar]
- 17. Jones PG, Kamona S, Doran O, Sawtell F, Wilsher M. Randomized controlled trial of humidified high-flow nasal oxygen for acute respiratory distress in the Emergency Department: the HOT-ER study. Respir Care. 2016;61(3):291–29. 9. 10.4187/respcare.04252) [DOI] [PubMed] [Google Scholar]
- 18. Nagata K, Morimoto T, Fujimoto D, et al. Efficacy of high-flow nasal cannula therapy in acute hypoxemic respiratory failure: decreased use of mechanical ventilation. Respir Care. 2015;60(10):1390–139. 6. 10.4187/respcare.04026) [DOI] [PubMed] [Google Scholar]
- 19. Lemiale V, Mokart D, Mayaux J, et al. The effects of a 2-h trial of high-flow oxygen by nasal cannula versus Venturi mask in immunocompromised patients with hypoxemic acute respiratory failure: a multicenter randomized trial. Crit Care. 2015;19:380. 10.1186/s13054-015-1097-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Roca O, de Acilu MG, Caralt B, et al. Humidified high flow nasal cannula supportive therapy improves outcomes in lung transplant recipients readmitted to the intensive care unit because of acute respiratory failure. Transplantation. 2015;99(5):1092–109. 8. 10.1097/TP.0000000000000460) [DOI] [PubMed] [Google Scholar]
- 21. Peters SG, Holets SR, Gay PC. High-flow nasal cannula therapy in do-not-intubate patients with hypoxemic respiratory distress. Respir Care. 2013;58(4):597–600.. 10.4187/respcare.01887) [DOI] [PubMed] [Google Scholar]
- 22. Frat JP, Brugiere B, Ragot S, et al. Sequential application of oxygen therapy via high-flow nasal cannula and noninvasive ventilation in acute respiratory failure: an observational pilot study. Respir Care. 2015;60(2):170–17. 8. 10.4187/respcare.03075) [DOI] [PubMed] [Google Scholar]
- 23. Rittayamai N, Tscheikuna J, Rujiwit P. High-flow nasal cannula versus conventional oxygen therapy after endotracheal extubation: a randomized crossover physiologic study. Respir Care. 2014;59(4):485–4. 90. 10.4187/respcare.02397) [DOI] [PubMed] [Google Scholar]
- 24. Lenglet H, Sztrymf B, Leroy C, et al. Humidified high flow nasal oxygen during respiratory failure in the emergency department: feasibility and efficacy. Respir Care. 2012;57(11):1873–187. 8. 10.4187/respcare.01575) [DOI] [PubMed] [Google Scholar]
- 25. Rello J, Pérez M, Roca O, et al. High-flow nasal therapy in adults with severe acute respiratory infection: a cohort study in patients with 2009 influenza A/H1N1v. J Crit Care. 2012;27(5):434–43. 9. 10.1016/j.jcrc.2012.04.006) [DOI] [PubMed] [Google Scholar]
- 26. Messika J, Ben Ahmed K, Gaudry S, et al. Use of high-flow nasal cannula oxygen therapy in subjects With ARDS: A 1-year observational study. Respir Care. 2015;60(2):162–16. 9. 10.4187/respcare.03423) [DOI] [PubMed] [Google Scholar]
- 27. Coudroy R, Jamet A, Petua P, et al. High-flow nasal cannula oxygen therapy versus noninvasive ventilation in immunocompromised patients with acute respiratory failure: an observational cohort study. Ann Intensive Care. 2016;6(1):45. 10.1186/s13613-016-0151-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Raeisi S, Fakharian A, Ghorbani F, Jamaati HR, Mirenayat MS. Value and safety of high flow oxygenation in the treatment of inpatient asthma: a randomized, double-blind, pilot study. Iran J Allergy Asthma Immunol. 2019;18(6):615–6. 23. 10.18502/ijaai.v18i6.2174) [DOI] [PubMed] [Google Scholar]
- 29. Ischaki E, Pantazopoulos I, Zakynthinos S. Nasal high flow therapy: a novel treatment rather than a more expensive oxygen device. Eur Respir Rev. 2017;26(145). 10.1183/16000617.0028-2017) [DOI] [PMC free article] [PubMed] [Google Scholar]


