Abstract
Objective:
To detect the maternal and fetal problems experienced by patients with non-cystic fibrosis (non-CF) bronchiectasis during pregnancy.
Material and Methods:
A total of 185 women aged over 18 years with medical records available, who were diagnosed as having non-CF bronchiectasis and followed in the outpatient clinic for bronchiectasis, were interviewed by phone between November 1, 2019 and December 31, 2019. Forty-seven women who accepted to participate, were able to understand and answer the survey, and had experienced at least 1 pregnancy, were included in the study, The survey questions were read and the answers were recorded. The same survey was administered to a control group of 95 women.
Results:
It was found that the number of patients experiencing an increase in at least 1 of the symptoms of cough, sputum production, and dyspnea during pregnancy, and the number of visits to emergency departments for respiratory conditions, were statistically significantly higher (P < .001 and P < .001, respectively), and the rate of live births was significantly lower (P = .009) in the non-CF bronchiectasis group compared with the control group. No significant difference was found between the groups in the number of miscarriages, preterm births, cesarean section, extra visits to the obstetrics department, and the presence of anomalies in the infants.
Conclusion:
Among patients with non-CF bronchiectasis, it should be kept in mind that an increase may be seen in respiratory symptoms and the number of emergency department visits during pregnancy, and a decrease may be seen in the ratio of live births. These patients should be followed closely for these issues and measures should be taken accordingly.
Keywords: Bronchiectasis, pregnancy, risk factors
MAIN POINTS
An increase may be seen in respiratory symptoms and also in the number of visits to the emergency department during pregnancy.
A decrease might be seen in the ratio of live births in the patients with non-CF bronchiectasis during pregnancy.
These patients should be followed closely for these issues and measures should be taken accordingly.
Introduction
Bronchiectasis is abnormal and permanent dilation that occurs as a consequence of the destruction of elastic tissue and muscles in the bronchial wall due to chronic recurrent infection/inflammation.1,2 Considered as an orphan disease until recently, bronchiectasis is now gaining interest and attention, and is no longer ignored or considered rare. Recent studies have emphasized that the prevalence of bronchiectasis has increased by 40% compared with previous years, and has risen to high values of 566 per 100 000 due to increased awareness, as well as advances in the diagnostic methods. The prevalence rate increases by age and female sex, and the incidence of bronchiectasis in women has increased from 21.2 to 35.2 per 100 000 women.1,2 There are many uncertainties regarding bronchiectasis, one of which is the relationship between bronchiectasis and pregnancy. Theoretically, reduced respiratory function due to abnormal and permanent dilation associated with recurrent infections/inflammation in the presence of bronchiectasis is expected to affect pregnancy negatively; however, a few studies conducted to date have not completely supported this theory.3 Similarly, very few studies have been conducted on how pregnancy affects pre-existing bronchiectasis, with most being conducted on patients with cystic fibrosis (CF).3-12 There are far fewer studies on pregnancy in patients with non-CF bronchiectasis, and the majority are case reports.13-17 The aim of the present study was to detect maternal and fetal problems experienced during pregnancy in the patients with non-CF bronchiectasis.
Material and Methods
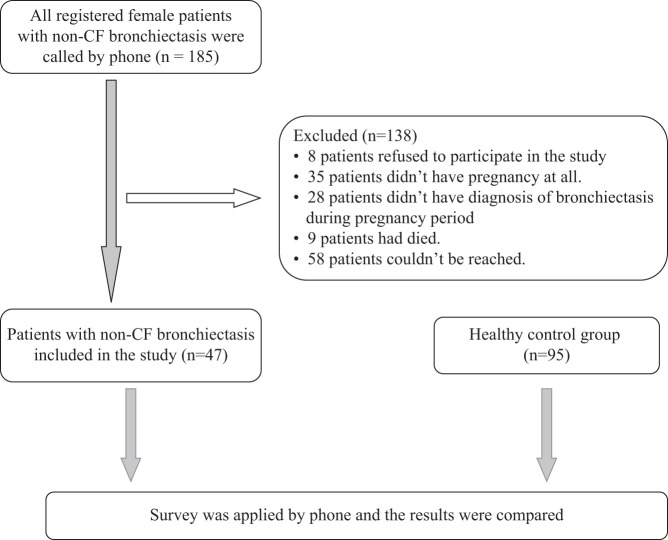
We obtained the contact details of 185 female patients with non-CF bronchiectasis, who were registered in the outpatient clinic of bronchiectasis and diagnosed as having bronchiectasis using pulmonary computed tomography (CT) or high-resolution CT between January 1, 1996, and January 1, 2019, with CF excluded through sweat tests or CF gene mutation analysis. All patients were contacted by phone, and their answers to the questions in the survey were recorded. The questions covered the diagnostic history of bronchiectasis before their pregnancy between November 1, 2019 and December 31, 2019, and maternal and fetal problems they experienced during these pregnancies. Of the 185 women, 47 expressed that they had a diagnosis of bronchiectasis before the pregnancy, and were inclduded in the study; the others were excluded. Eight women did not accept to participate in the study, 35 did not have any pregnancy, 28 experienced pregnancy before the diagnosis of bronchiectasis, 58 could not be reached, and 9 were deceased. The questions of the survey were read to 47 patients with non-CF bronchiectasis by one person in such a way that the patients would understand easily. The survey questions are presented in Table 1. The same questions were asked by phone to the control group of 95 healthy women and the results were compared between the 2 groups (Figure 1). The study was performed according to the principles of the Helsinki Declaration. The study protocol was approved by the Ethics Board (Approval no: 83045809/604.01.02).
Table 1.
Survey Questions
| Name and Surname:___________________________ Age:______ Job:________________ |
| For how many years have you known of your diagnosis of bronchiectasis?For how many years have you had cough and sputum complaints?How many pregnancies have you had, knowing your diagnosis of bronchiectasis?Number of pregnancies:Have you had a stillbirth? If the answer is “yes”: How many times, in which year, in which pregnancy (pregnancies)?Have you had a miscarriage? If the answer is yes: how many times, in which year, in which pregnancy (pregnancies)?Number of live births:Years of birth: |
| For Live Births |
| Method of birth: Normal ( ) Cesarean ( )Birth term: Mature ( ) Premature ( ) Posmature ( )Your baby's birth weight: (g)Does your baby have any anomalies or disabilities? If the answer is “yes”, please explain.Have your complaints increased during pregnancy? cough ( ) sputum production ( ) hemoptysis ( ) chest pain ( ) shortness of breath ( ) Have you presented to the emergency department due to respiratory distress or due to an increase in your symptoms during pregnancy? If the answer is “yes”: In which month of the pregnancy and how many times?Did you need an extra obstetric procedure in the routine obstetrics control during pregnancy? If the answer is “yes”: In which month of pregnancy and how many times?Did you have any other health problems during pregnancy? If the answer is “yes,” please explain. |
Figure 1.
Flow chart.
Statistical Analysis
Statistical analyses were performed using statistical software the Statistical Package for Social Sciences, version 10.0 software (SPSS Inc.; Chicago,IL, USA). For variables with normal distribution, mean and standard deviation (SD) were calculated. Categorical variables were expressed as percentages. Student’s t-test and the chi-square test were used to compare the groups. The significance level was taken as P < .05.
Results
In the group of 47 patients with non-CF bronchiectasis, the mean age was 45.5 ± 14.0 years, the mean number of pregnancies was 2.57 ± 1.31, and the mean number of live births was 1.87 ± 1.03. In the healthy control group of 95 women, the mean age was 51.0 ± 13.7 years, the mean number of pregnancies was 2.54 ± 1.38, and the mean number of live births was 2.21 ± 1.18.
When the 2 groups were compared, it was found that the number of patients with an increase in at least 1 of the symptoms of cough, sputum production, or dyspnea during pregnancy (34% vs. 1%; P < .001) and the number of emergency department visits for respiratory symptoms (for at least one of cough, sputum production, or dyspnea) (34% vs. 1%; P < .001) were statistically significantly higher, and the rate of live births were significantly lower (0.77 vs. 0.90; P = .002) in the non-CF bronchiectasis group compared with the healthy controls. No significant difference was found between the groups in the number of miscarriages (56% vs. 36%; P = .15), preterm births (20% vs. 9%; P = .09), cesarian sections (52% vs. 69%; P = .33), extra visits for obstetric examinations (12% vs. 4%: P = .12), and the presence of anomalies in the infants (10% vs. 2%; P > .05) (Table 2).
Table 2.
Comparison of the CF Bronchiectasis Group and the Control Group
| Non-CF Bronchiectasis (n = 47) |
Control (n = 95) | P | |
|---|---|---|---|
| Age (mean year ± SD) | 45.5 ± 14.0 | 51.0 ± 13.7 | .029 * |
| Number of prengnancies (mean ± SD) | 2.57 ± 1.31 | 2.54 ± 1.38 | .911 |
| Number of live births (mean ± SD) | 1.87 ± 1.03 | 2.21 ± 1.18 | .088 |
| Rate of live birth (% ± SD) | 0.77 ± 0.30 | 0.90 ± 0.18 | .002 * |
| Live birth weight (mean ± SD) | 3058 ± 642 | 3216 ± 349 | .061 |
| Patients with/without miscarriage (n) | 17/30 (56%) | 25/70 (36%) | .15 |
| Patients with/without C-section (n) | 16/31 (52%) | 39/56 (69%) | .33 |
| Patients with/without preterm birth (n) | 8/39 (%20) | 8/87 (9%) | .09 |
| Patients with/without anomaly in the infant (n) | 4/40 (10%) | 2/91 (2%) | >.05 |
| Patients with/without extra obstetric examinations (n) | 5/42 (12%) | 4/91 (4%) | .12 |
| Patients with/without recently appearing respiratory symptoms or increase in pre-existing symptoms (n) | 12/35 (34%) | 1/94 (1%) | <.001* |
| Patients with/without visit to the emergency service for the respiratory symptoms (n) | 12/35 (34%) | 1/94 (1%) | <.001* |
* P < .05.
Anomalies were found in 4 (8%) infants in the non-CF bronchiectasis group (congenital cardiac valvulopathy n = 3, sudden infant death n = 1), and 2 infants (2%) in the control group (mental retardation n = 1, motor retardation n = 1); the difference was not statistically significant.
No patients required long-term oxygen therapy at home or non-invasive mechanical ventilation before or during pregnancy.
Discussion
In the present study, it was found that respiratory symptoms and the number of visits to the emergency department increased and the rate of live births decreased during pregnancy in patients with non-CF bronchiectasis compared with healthy controls.
The first data about problems in pregnancy of patients with non-CF bronchiectasis were from a case report by Templeton et al.13 in 1977, who shared the results of 2 pregnancies of a patient with bronchiectasis and reported the presence of intrauterine growth retardation considered to be due to maternal hypoxemia during both pregnancies. In contrast to that case report, our study has a higher number of patients and includes a control group. Our study reported anomalies in a total of 4 infants in the non-CF bronchiectasis group, congenital cardiac valvulopathy in 3, and sudden infant death in 1. In the control group, mental retardation was found in 1 infant and motor retardation in 1 infant. The difference between the 2 groups in terms of the presence of anomalies was not significant. This may be because patients with non-CF bronchiectasis are clinically stable and do not require oxygen.
There is no study in the literature giving the rate of live births among women with non-CF bronchiectasis. In the present study, the rate of live births was found to be statistically significantly lower in the non-CF bronchiectasis group compared with the control group (live birth rates: 0.77 ± 0.30 in the non-CF group and 0.90 ± 0.18 in the controls). The rate of live births was lower, despite the fact that the patients included in the present study were stable and did not require oxygen therapy at home. This reveals that all patients with non-CF bronchiectasis should be closely followed from the beginning of the pregnancy to the time of delivery.
In 1979, in a report on the results pregnant patients with bronchiectasis, Howie et al. stated that the pregnancy was well tolerated and no complications were experienced during regular checks.14 Their study was a case report and included only 3 patients without a control group. In our study, a statistically significant increase was found in at least 1 of the symptoms of cough, sputum production, or dyspnea, and the number of visits to the emergency department for these symptoms in the non-CF bronchiectasis group compared with the controls (12/47 patients in the non-CF bronchiectasis group, and 1/95 subjects in the control group). This may be an indication to be cautious about respiratory symptoms in patients with non-CF bronchiectasis during pregnancy.
Since 1979, scant data have been published on the topic related to pregnancy in patients with non-CF bronchiectasis.15 In recent years, data in the form of case reports have begun to be published, possibly due to increased awareness of bronchiectasis. In a case report of a patient with non-CF bronchiectasis with forced expiratory volume in 1 second (FEV1) of 850 mL (29%) during pregnancy and at delivery, Udhayakumar et al. reported that in severe patients, intrauterine growth retardation might be seen and delivery might be possible with close follow-up, low-dose spinal anesthesia, and non-invasive mechanical ventilation.16 In 2017, Taylor et al. reported that pregnancy was well tolerated in a case report of 10 patients, of whom 9 patients had non-CF bronchiectasis with a pre-pregnancy FEV1 value of 70.2% and sputum colonization with a bacterium in 60% of them.17 We could not draw a firm conclusion on this topic because our study did not include results of respiratory function and sputum culture during pregnancy. On the other hand, no significant difference was found in our study between the non-CF bronchiectasis and control groups in terms of the number of miscarriages, cesarian sections, and extra visits for obstetric examinations. This may be because none of our patients with non-CF bronchiectasis was critically ill or required oxygen therapy or non-invasive mechanical ventilation, either before or during the pregnancy. The patients included in the present study did not have thoracic CT during the pregnancy, thus radiological severity could not be determined.
The strengths of the present study are the inclusion of the highest number of patients with non-CF bronchiectasis during pregnancy compared with the literature, and the presence of a control group. Its limitations are that the information on the pregnancy and delivery were based on self-reports by the patients and no data were available from respiratory function tests or radiological imaging.
It should be kept in mind that among patients with non-CF bronchiectasis, there may be an increase in respiratory symptoms and also in the number of emergency department visits during pregnancy, and a decrease in the ratio of live births. These patients should be followed closely for these issues and measures should be taken accordingly.
Funding Statement
The authors declared that this study has received no financial support.
Footnotes
Ethics Committee Approval: This study was approved by Ethics committe of Istanbul University-Cerrahpasa, Cerrahpasa Medical Faculty, (Approval No: 83045809/604.01.02).
Informed Consent: All patients were contacted by phone and asked whether they were willing to participate in this study.
Peer Review: Externally peer-reviewed.
Author Contributions: Supervision - S.B., B.K., B.M.; Design – S.B., B.K., B.M.; Resources – S.B., B.K., G.S.; Materials – S.B., B.K., G.S., B.M.; Data Collection and/or Processing – S.B., B.K., G.S.; Analysis and/or Interpretation – S.B, BM.; Literature Search- S.B., B.K.; Writing Manuscript – S.B., B.K., G.S., B.M.; Critical Review - S.B., B.M.
Conflict of Interest: The authors have no conflict of interest to declare.
References
- 1. Hill AT, Sullivan AL, Chalmers JD. British Thoracic Society guideline for bronchiectasis. Thorax. 2019;74:1–3.. 10.1136/thoraxjnl-2018-212468) [DOI] [PubMed] [Google Scholar]
- 2. Polverino E, Goeminne PC, McDonnell MJ. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur Respir J. 2017;50(3). 10.1183/13993003.00629-2017) [DOI] [PubMed] [Google Scholar]
- 3. Tonelli MR, Aitken ML. Pregnancy in cystic fibrosis. Curr Opin Pulm Med. 2007;13(6):537–540.. 10.1097/MCP.0b013e3282f01120) [DOI] [PubMed] [Google Scholar]
- 4. Whitty JE. Cystic fibrosis in pregnancy. Clin Obstet Gynecol. 2010;53(2):369–376.. 10.1097/GRF.0b013e3181deb448) [DOI] [PubMed] [Google Scholar]
- 5. Heltshe SL, Godfrey EM, Josephy T, Aitken ML, Taylor-Cousar JL. Pregnancy among cystic fibrosis women in the era of CFTR modulators. J Cyst Fibros. 2017;16(6):687–694.. 10.1016/j.jcf.2017.01.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. FitzSimmons SC, Fitzpatrick S, Thompson B. A longitudinal study of the effects of pregnancy on 325 women with cystic fibrosis. Pediatr Pulmonol Suppl. 1996;13:99–101.. [Google Scholar]
- 7. Edenborough FP, Mackenzie WE, Stableforth DE. The outcome of 72 pregnancies in 55 women with cystic fibrosis in the United Kingdom 1977-1996. BJOG. 2000;107(2):254–261.. 10.1111/j.1471-0528.2000.tb11697.x) [DOI] [PubMed] [Google Scholar]
- 8. Goss CH, Rubenfeld GD, Otto K, Aitken ML. The effect of pregnancy on survival in women with cystic fibrosis. Chest. 2003;124(4):1460–1468.. 10.1378/chest.124.4.1460) [DOI] [PubMed] [Google Scholar]
- 9. McMullen AH, Pasta DJ, Frederick PD. Impact of pregnancy on women with cystic fibrosis. Chest. 2006;129(3):706–711.. 10.1378/chest.129.3.706) [DOI] [PubMed] [Google Scholar]
- 10. Lau EM, Barnes DJ, Moriarty C. Pregnancy outcomes in the current era of cystic fibrosis care: A 15-year experience. Aust N Z J Obstet Gynaecol. 2011;51(3):220–224.. 10.1111/j.1479-828X.2010.01287.x) [DOI] [PubMed] [Google Scholar]
- 11. Geake J, Tay G, Callaway L, Bell SC. Pregnancy and cystic fibrosis: Approach to contemporary management. Obstet Med. 2014;7(4):147–155.. 10.1177/1753495X14554022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Edenborough FP, Borgo G, Knoop C. Guidelines for the management of pregnancy in women with cystic fibrosis. J Cyst Fibros. 2008;7(Suppl 1):S2–S32.. 10.1016/j.jcf.2007.10.001) [DOI] [PubMed] [Google Scholar]
- 13. Templeton A. Intrauterine growth retardation associated with hypoxia due to bronchiectasis. Br J Obstet Gynaecol. 1977;84(5):389-390. 10.1111/j.1471-0528.1977.tb12605.x) [DOI] [PubMed] [Google Scholar]
- 14. Howie AD, Milne JA. Pregnancy in patients with bronchiectasis. Br J Obstet Gynaecol. 1978;85(3):197–200.. 10.1111/j.1471-0528.1978.tb10480.x) [DOI] [PubMed] [Google Scholar]
- 15. de Swiet M. Respiratory disease in pregnancy. Postgrad Med J. 1979;55(643):325–328.. 10.1136/pgmj.55.643.325) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Udhayakumar G, Mahadasu S, Meneni D. Severe Bronchiectasis in Pregnancy: A Case Report. Birmingham, UK: RCOG World Congress; 2016, June 20-22. [Google Scholar]
- 17. Taylor SEG, Flight WG. Outcomes of pregnancy in women with bronchiectasis. Thorax. 2017;72:A1–A278.. Available at: https://thorax.bmj.com/content/thoraxjnl/72/Suppl_3/A114.1.full.pdf. [Google Scholar]