Abstract
Background:
Hepatocellular carcinoma (HCC) is male-predominant cancer, but the underlying mechanism remains unclear. This study aimed to investigate the expression of the age-suppressing gene klotho and estrogen receptors (ERs) in HCC patients and analyze their association with clinicopathological variables and their effects on prognosis.
Methods:
The expression patterns of klotho, ERα, and ERβ were determined by tissue microarray and immunohistochemical technique, and their correlations with clinicopathological characteristics were investigated using univariate and multivariate analysis.
Results:
Klotho expression was significantly lower in HCC than in the adjacent noncancerous tissues (52.7% (49/93) vs. 90.8% (79/87), P = .000), and its protein level in HCC tissue was negatively correlated with clinical staging, histological grade, and stage of the primary tumor (T) (P < .05). Whereas the expression of nuclear ERα and ERβ was higher in HCC than their corresponding non-neoplastic tissues (55.9% (52/93) vs. 35.6% (31/87), P = .006; 59.1% (55/93) vs. 43.7% (38/87), P = .038), and the level of nuclear ERα and ERβ in HCC tissue was inversely correlated with T stage, tumor size, and clinical staging (P < .05). Correlation analysis showed the expression level of klotho, which is positively correlated with that of nuclear ERα (r = 0.243, P = .019). Patients with klotho-positive tumors had longer survival than those with klotho-negative tumors (P = .002). Cox proportional hazards model analysis demonstrated that positive expression of klotho was an important factor indicating good prognosis (P = .003).
Conclusion:
Klotho, partially regulated by ERα-mediated estrogen pathway, acts as a tumor suppressor and might be a novel biomarker candidate for predicting progression and prognosis in HCC patients.
Keywords: Hepatocellular carcinoma (HCC), klotho, estrogen receptors (ERs), immunohistochemistry
INTRODUCTION
It has been considered that the final outcome of most liver diseases is hepatocellular carcinoma (HCC).1 In the past few decades, although some progress has been made in the field of surgery, there is still a lack of effective treatment programs for HCC.1 Because of the high degree of malignancy and the lack of effective drug therapy, HCC is 1 of the 4 highest mortality cancers worldwide.2 The common risk factors for HCC are viral hepatitis, nonalcoholic steatohepatitis, excessive drinking, liver cirrhosis, rare genetic conditions, and environmental exposure.3 Through comparative study, it has been found that there is a significant characteristic of HCC. In both areas with a low and high incidence of HCC, men have a higher incidence and worse prognosis than women.4 Moreover, according to the latest statistics of the World Health Organization (WHO), the death rate of men is 2.35 times that of women in primary liver cancer.2 Due to the great gender difference, sex hormones have long been considered to play a significant part in the development of HCC.1 Unlike androgen, which is considered to be one of the factors promoting tumor growth, estrogen plays various or even opposite roles in HCC.1,5-7 With the in-depth research in recent years, although there are still many vague aspects at present, increasing evidences have shown that estrogen may play a protective role in HCC progression through estrogen receptors (ERs).3
Initially, the klotho gene is defined as an assumed age inhibitory gene.8 Klotho gene-deficient mice showed symptoms similar to the human premature aging syndrome, including the reproductive system. Later, Sárvári et al. found that the expression of klotho in the hippocampus of middle-aged female rats had a strong response to estradiol replacement.9 Sinha et al. reported the reactivation of klotho was, at least in part, dependent on green tea polyphenols and sulforaphane-mediated reactivation of estrogen receptor alpha (ERα) in ERα-negative MDA-MB-231 cells.10 These results propose that klotho might be regulated by sex hormones, especially estrogen signaling pathways. Also, estrogen receptor binding sites are found in the promoter region of the klotho gene by software analysis, indicating that the klotho gene has a structural basis regulated by the estrogen signaling pathway.
In nearly a decade, increasing evidence suggests that klotho is related to the aging process of human beings and related to the development of cancers. Our previous observations also have shown that klotho functions as a tumor-suppressing gene in HCC.11 To further explore the mechanism of klotho’s function in HCC characterized by gender differences, taking estrogen signaling pathway as the cut-in point in the present study, we investigated the expression of estrogen receptors and klotho, analy-sed their relationship with clinicopathological parameters, and assessed their impact on prognosis in HCC patients.
MATERIALS AND METHODS
Patients
HCC tissue microarrays were purchased from Shanghai Outdo Biotechnology Co. Ltd (Shanghai, China). These arrays included 93 cancer tissues and 87 paracancerous tissue specimens from HCC patients who underwent surgery in Shanghai Outdo Biotechnology cooperation hospitals from 2007 to 2009. Before the operation, the study was approved by the ethics committee of the cooperative hospitals, and the written informed consent of each patient was also obtained. The patients did not receive any radiotherapy or chemotherapy before surgery. Ninety-three cases of tumors were diagnosed as HCC by histopathology. The following 2 specimens were collected: (1) paraffin-embedded diagnostic tumor biopsy specimens and (2) adjacent non-tumor specimens (distance ≥1.5 cm from the tumor). There were 10 female patients and 83 male patients with an average age of 53 years old (25-73 years). Clinical research results based on the tumor node metabolic system of the United States Joint Cancer Committee (AJCC) were evaluated. There were 12 patients in stage I, 34 patients in stage II, 42 patients in stage III, and 5 patients in stage IV. According to WHO standard histological classification, there were 7 patients with high differentiation, 72 patients with medium differentiation, and 14 patients with low differentiation. Survival was defined as the period from the day of surgery to the day of death or the last follow-up from the operation date. The patients were followed up for 2-80 months, with an average follow-up time of 33 months.
Immunohistochemistry Staining and Scoring
The construction of liver TMA is referred to the previous article.12 Generally, “two-step method” was used for immunohistochemical staining. First, the slices needed dewaxing, and then the slices were incubated with 3% hydrogen peroxide to inactivate endogenous peroxides. Then, antigen recovery involved treatment with 10 mmol/L citric acid buffer in an autoclave. Klotho goat polyclonal antibody (1:100 dilution; cat# sc-22218, Santa Cruz, USA), rabbit monoclonal to ERα (1:1000 dilution; cat# ab167611, Abcam, USA) and mouse monoclonal to estrogen receptor beta (ERβ, 1:1000 dilution; cat# ab212351, Abcam, USA) were used as the primary antibody. The reaction product was re-dyed with hematoxylin using diaminobenzidine as the developer. Tumor sections were scored under the light microscope.
For each antibody studied at present, the following 3 aspects were determined: (1) location of immunoreactivity, (2) percentage of stained cells, and (3) strength of stained cells. In order to evaluate the intensity of immunostaining, we used a numerical value of 0-3: 0, missing; 1, weak; 2, medium; and 3, strong dyeing. If at least 1% of tumor cells are moderately or strongly stained, or at least 10% of tumor cells are weakly stained, the expression of estrogen receptor (ERα or ERβ) was considered positive.13 By multiplying the immune response intensity and relative abundance value of klotho immune cells, the expression of klotho was semi-quantitatively evaluated using comprehensive scores. The abundance of klotho positive cells was divided into 0-4 points, and the detailed division rules were as follows: 0, less than 5% of positive cells; 1, 5-25% of positive cells; 2, 26-50% of positive cells; 3, 51-75% of positive cells; 4, 76-100% of positive cells. When the comprehensive score was greater than 4, klotho expression was considered positive. Cases with scores less than or equal to 4 were considered as tissues with negative klotho expression.
Statistical Analysis
A chi-square test was used for the analysis of the relationship between protein expression and clinicopathological parameters. The correlation between the 2 variables was determined by the Spearman rank test. The survival period was calculated from the operation date to the death date or the last follow-up from the operation date. The logarithmic rank test was used to estimate survival difference, and the Kaplan–Meier method was used to calculate the cumulative survival rate. When multivariate survival analysis was needed, Cox proportional hazards model was used. The P-value was considered significant when it was less than .05. All statistical analysis was conducted by SPSS 20.0v (SPSS, Chicago, IL, USA) for Windows software.
RESULTS
Expression of Klotho, ERα, and ERβ in HCC
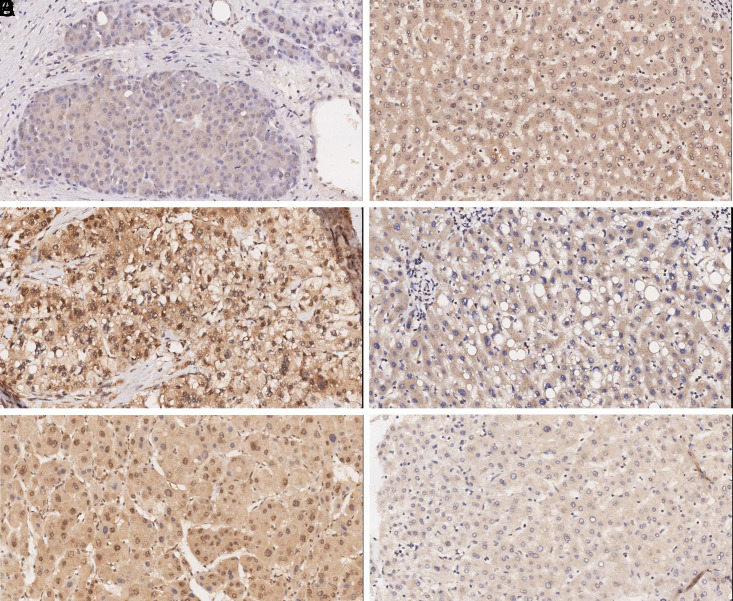
In order to explore the clinical meaning of klotho gene and estrogen signal pathway in the developement of HCC, the expression of klotho and ERs was studied by the immunohistochemical method using HCC tissue microarrays. Figure 1 shows an example of immunostaining for each protein evaluated in the present study. Klotho immunostaining was of cytoplasmic localization, whereas ERα and ERβ were located in both cytoplasm and nucleus. Forty-nine tumors were positively (52.7%) for klotho, 88 (94.6%) for cytoplasmic ERα, 52 (55.9%) for nuclear ERα, 76 (81.7%) for cytoplasmic ERβ, and 55 (59.1%) for nuclear ERβ. Statistical analysis demonstrated that the positive expression rate of klotho was significantly lower. Still, the expression rate of nuclear ERα and ERβ was higher in cancer tissue than that in the corresponding non-tumor tissue (P < .05, Table 1).
Figure 1.
Expression of klotho and ERs in hepatocellular carcinoma (HCC) tissues and adjacent non-tumorous tissues. Immunohistochemical staining revealed low klotho expression (A), high ERα (C), and ERβ (E) expression in HCC tissues and high klotho expression (B), low ERα (D), and ERβ (F) expression in adjacent non-neoplastic hepatic tissues. The original magnifications were ×200.
Table 1.
Expression of Klotho and ERs in HCC
| Group | Total | Klotho (+) | ERα (+) | ERβ (+) | ||
|---|---|---|---|---|---|---|
| Cytoplasm | Nuclear | Cytoplasm | Nuclear | |||
| HCC tissue | 93 | 49 (52.7%) | 88 (94.6%) | 52 (55.9%) | 76 (81.7%) | 55 (59.1%) |
| Normal tissue | 87 | 79 (90.8%) | 83 (95.4%) | 31 (35.6%) | 82 (94.3%) | 38 (43.7%) |
| P = .000* | P = .811 | P = .006* | P = .010* | P = .038* | ||
* P < .05 is considered statistically significant. ERs, estrogen receptors; HCC, hepatocellular carcinoma.
The possible relationship between klotho and ERs expression and clinicopathological characteristics of HCC patients was also one of our analysis focuses. As shown in Table 2, klotho expression was negatively correlated with clinical stage, stage of the primary tumor (T), and histological grade (P < .05), but klotho expression was not related to age, sex, tumor size, or liver cirrhosis. In addition, the expression of ERα and ERβ in the nucleus was significantly negatively correlated with T stage, tumor size, and clinical staging (P < .05, Table 2). The expression of ERα and ERβ in cytoplasm had no significant correlation with these clinicopathological characteristics (data not shown).
Table 2.
Correlation Between Klotho and ERs Expression and HCC Patient Characteristics
| Group | Total | Klotho (+) | P | Nuclear ERα (+) | P | Nuclear ERβ (+) | P |
|---|---|---|---|---|---|---|---|
| Gender | |||||||
| Male | 83 | 44 (53.0%) | .857 | 47 (56.6%) | .690 | 50 (60.2%) | .778 |
| Female | 10 | 5 (50.0%) | 5 (50.0%) | 5 (50.0%) | |||
| Age (year) | |||||||
| ≥60 | 25 | 16 (64.0%) | .185 | 16 (64.0%) | .341 | 18 (72.0%) | .126 |
| <60 | 68 | 33 (48.5%) | 36 (52.9%) | 37 (54.4%) | |||
| Clinical stage | |||||||
| I, II | 46 | 30 (65.2%) | .017* | 32 (69.6%) | .009* | 33 (71.7%) | .014* |
| III, IV | 47 | 19 (40.4%) | 20 (42.6%) | 22 (46.8%) | |||
| T stage | |||||||
| T1+T2 | 47 | 30 (63.8%) | .030* | 32 (68.1%) | .017* | 33 (70.2%) | .028* |
| T3+T4 | 46 | 19 (41.3%) | 20 (43.5%) | 22 (47.8%) | |||
| Histological grade | |||||||
| Well | 7 | 6 (85.7%) | .012* | 4 (57.1%) | .388 | 5 (71.4%) | .693 |
| Moderate | 72 | 40 (55.6%) | 41 (56.9%) | 41 (56.9%) | |||
| Poorly | 14 | 3 (21.4%) | 7 (50.0%) | 9 (64.3%) | |||
| Tumor size (cm) | |||||||
| ≤5 cm | 38 | 23 (60.5%) | .208 | 27 (71.1%) | .015* | 28 (73.7%) | .018* |
| >5 cm | 55 | 26 (47.3%) | 25 (45.5%) | 27 (49.1%) | |||
| Liver cirrhosis | |||||||
| Yes | 42 | 26 (61.9%) | .106 | 21 (50.0%) | .297 | 19 (45.2%) | .013* |
| No | 51 | 23 (45.1%) | 31 (60.8%) | 36 (70.6%) | |||
* P < .05 is considered statistically significant. ERs, estrogen receptors; HCC, hepatocellular carcinoma.
We made a further analysis on the potential relationship between klotho and ERs expression. As shown in Table 3, 35.5% (33/93) of tumors were klotho (+)/nuclear ERα (+), and 26.9% (25/93) were klotho (−)/nuclear ERα (−). Klotho expression was significantly positively correlated with nuclear ERα (r = 0.243, P = .019). Meanwhile, the relevance between klotho and cytoplasmic ERβ expression was similar (r = 0.220, P = .043).
Table 3.
Relationship Between Klotho and ERs Expression in HCC
| Klotho | Total | ERα | ERβ | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cytoplasm | Nuclear | Cytoplasm | Nuclear | ||||||
| (+) | (−) | (+) | (−) | (+) | (−) | (+) | (−) | ||
| (+) | 49 | 48 | 1 | 33 | 16 | 44 | 5 | 32 | 17 |
| (−) | 44 | 40 | 4 | 19 | 25 | 32 | 12 | 23 | 21 |
| Total | 93 | 88 | 5 | 52 | 41 | 76 | 17 | 55 | 38 |
| r = 0.156 | r = 0.243 | r = 0.220 | r = 0.132 | ||||||
| P = .135 | P = .019* | P = .034* | P = .206 | ||||||
* P < .05 is considered statistically significant. ERs, estrogen receptors; HCC, hepatocellular carcinoma.
Relationship Between Klotho and ERs Expression and Survival Time
In univariate survival analysis, the median survival time of klotho positive and negative HCC patients was 43.0 and 26.0 months, respectively, which indicated that patients with tumor negative immunostaining for klotho had a poor prognosis (P = .002, Table 4, Figure 2A). The median survival time of nuclear ERα positive patients was 39 months, while that of nuclear ERα negative patients was 29 months. There was no significant difference between them (P > .05, Table 4). Similarly, the expression of ERβ had no significant correlation with the overall survival (P > .05, Table 4). Significant parameters in univariate survival analysis of klotho positive and negative HCC patients included T stage, tumor size, clinical stage, and liver cirrhosis (Table 4).
Table 4.
Clinicopathological Parameters and Expression of Klotho and ERs for the Prognosis in HCC Patients
| Variable | Total | Mean Survival (Months) | Median Survival (Months) | P |
|---|---|---|---|---|
| Gender | ||||
| Male | 83 | 35.19 | 34.00 | .620 |
| Female | 10 | 31.50 | 31.00 | |
| Age (year) | ||||
| ≥60 | 25 | 39.60 | 41.00 | .205 |
| <60 | 68 | 33.03 | 29.50 | |
| Clinical stage | ||||
| I, II | 46 | 46.00 | 49.00 | .000* |
| III, IV | 47 | 23.83 | 24.00 | |
| T stage | ||||
| T1+T2 | 47 | 45.17 | 49.00 | .000* |
| T3+T4 | 46 | 24.20 | 24.50 | |
| Histological grade | ||||
| Well | 7 | 46.43 | 49.00 | .077 |
| Moderate | 72 | 35.67 | 34.00 | |
| Poorly | 14 | 24.50 | 14.00 | |
| Tumor size (cm) | ||||
| ≤5 cm | 38 | 40.37 | 43.00 | .042* |
| >5 cm | 55 | 30.95 | 29.00 | |
| Liver cirrhosis | ||||
| Yes | 42 | 28.00 | 28.00 | .006* |
| No | 51 | 40.39 | 40.00 | |
| Klotho | ||||
| Positive | 49 | 41.43 | 43.00 | .002* |
| Negative | 44 | 27.41 | 26.00 | |
| Cytoplasmic ERα | ||||
| Positive | 88 | 34.60 | 33.00 | .725 |
| Negative | 5 | 38.20 | 37.00 | |
| Nuclear ERα | ||||
| Positive | 52 | 38.67 | 39.00 | .056 |
| Negative | 41 | 29.88 | 29.00 | |
| Cytoplasmic ERβ | ||||
| Positive | 76 | 35.71 | 33.50 | .401 |
| Negative | 17 | 30.71 | 33.00 | |
| Nuclear ERβ | ||||
| Positive | 55 | 37.60 | 36.00 | .141 |
| Negative | 38 | 30.74 | 29.50 | |
* P < .05 is considered statistically significant. ERs, estrogen receptors; HCC, hepatocellular carcinoma.
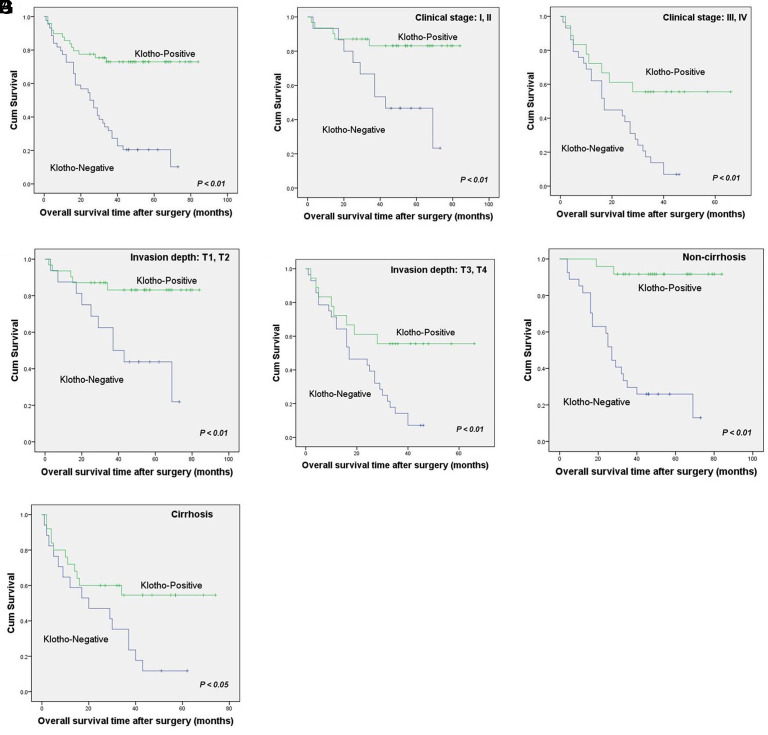
Figure 2.
Relationship between expression of klotho and survival time. (A) Kaplan–Meier survival analyses showed worse survival in HCC patients with klotho-negative (n = 44) than those with klotho-positive tumors (n = 49) (P < .01 by log-rank test). Klotho expression was related to better overall survival in the cases of clinical stage I/II (B) and stage III/IV (C), respectively. Klotho expression could differentiate patients’ overall survival time in stages T1/T2 (D) and T3/T4 (E). Patients with klotho expression had better overall survival in the subgroup with (F) or without cirrhosis (G).
In a further stratified survival analysis, we found that the expression of klotho was closely related to the better overall survival of clinical stage I/II invalids (P < .01, Figure 2B) and stage III/IV (P < .01, Figure 2C), respectively. Moreover, klotho expression could distinguish severally the total survival time of T1/T2 patients (P < .01, Figure 2D) and T3/T4 (P < .01, Figure 2E). The overall survival period of invalids with klotho positive expression was longer in subgroups with (P < .05, Figure 2G) or without cirrhosis (P < .01, Figure 2F). Nevertheless, the expression of klotho did not affect the overall survival in subgroups with different histological grades and tumor sizes (data not shown).
Based on the Cox proportional hazards model, multivariate survival analysis was carried out by inputting the following covariates: T stage, tumor size, clinical stage, liver cirrhosis, and klotho expression. The Backward Wald method was used to screen covariates step by step. The important prognostic factors affecting survival were clinical stage, liver cirrhosis, and klotho status (Table 5). Compared with patients with positive klotho expression, those with negative klotho expression had a higher risk of death (hazard ratio = 0.349, 95% CI: 0.173-0.702, P = .003). This indicates that the higher level of klotho expression is related to the longer overall survival time of HCC patients.
Table 5.
Multivariate Results in Cox Proportional Hazards Analysis for Patients With HCC
| Variable | P | Hazard Ratio | 95% CI | |
|---|---|---|---|---|
| Lower | Upper | |||
| Clinical stage | .041* | 11.480 | 1.104 | 119.436 |
| T stage | .303 | 0.310 | 0.033 | 2.876 |
| Tumor size | .832 | 1.089 | 0.495 | 2.395 |
| Liver cirrhosis | .002* | 2.469 | 1.381 | 4.416 |
| Klotho status | .003* | 0.349 | 0.173 | 0.702 |
* P < .05 is considered statistically significant. HCC, hepatocellular carcinoma.
DISCUSSION
Klotho was originally identified as a presumed senescence suppressor gene. Therefore, it was named after Klotho, the Greek goddess of fate.8 The deletion of klotho gene expression in mice led to the result of complex phenotype similar to the human premature aging syndrome, whereas the overexpression of klotho prolonged the life span of mice.14 Since then, the function of klotho has been extensively studied, and several new endocrine axes have been identified to regulate various metabolic processes.8 In particular, since Wolf et al. first discovered klotho had an inhibitory effect on breast cancer in 2008,15 the role of klotho in the pathogenesis, development, and prognosis of cancer has received more and more attention. At present, it has been shown that klotho functions as a tumor suppressor gene in most malignant tumors, such as lung cancer, gastric cancer, melanoma, pancreatic cancer, etc.16,17 However, some controversial results have also been reported. For instance, klotho’s effects on angiogenesis and anti-apoptosis may play a part in the progression of ovarian cancer.18 In HCC, klotho’s function is also contradictory. Shu et al. found that the restoration of klotho expression had an inducing effect in the process of apoptosis and autophagy of hepatoma cells.19 According to Xie et al., epigenetic silencing of klotho expression was closely related to the poor prognosis of HCC patients.20 Likewise, in our previous study, the results suggested that klotho had the antitumor effect and inhibited Wnt/β-catenin pathway in HCC cell line model and nude mice model.11 However, Chen et al. revealed klotho’s carcinogenic effect in promoting anoikis resistance, thus promoting tumor migration and invasion during HCC progression.21 These data also indicate that the role of klotho in the development of HCC may be quite complicated. In this work, based on our previous studies in vitro, we further explored the association between klotho expression and clinicopathological variables in HCC patients and analyzed its effect on prognosis. According to the immunohistochemical analysis of tissue microarray, the data showed that the expression of klotho in cancer tissue was lower than that in adjacent noncancerous tissue. Klotho expression was negatively correlated with T stage, histological grade, and clinical stage. More than these, survival analysis showed that the death risk of klotho-positive patients was lower than that of klotho-negative patients. According to the Cox proportional hazards model analysis, it could be concluded that klotho positive expression was an important predictor for a good prognosis. These results further confirm klotho’s protective effect on the development of HCC.
Compared with women, the incidence and invasiveness of HCC in men are much higher,3 suggesting that HCC is a hormone-reactive tumor.13 However, unlike prostate cancer and breast cancer, which are regulated by androgen and estrogen, respectively, HCC is likely to be regulated by both sex hormone simultaneously.1,5 Androgen, male hormones, is considered to serve roles in promoting sex disparity and hepatoma progression in male-predominant HCC.3 Contrary to the tumor-promoting effect of androgens, the role of estrogen in HCC has been controversial in many years of research. At the outset, it was found that estrogen could promote hepatocarcinogenesis in rats.1 Inspired by the success of tamoxifen, a competitive estrogen receptor antagonist, in the treatment of estrogen receptor-positive breast cancer, some clinical trials have studied the potential effect of tamoxifen on improving the survival of HCC patients. However, these studies show contradictory and unoptimistic results.22 With further research, it is found that estrogen can inhibit hepatocarcinogenesis,23 and this conclusion is supported by epidemiological data, which indicates that the incidence rate of HCC is higher in postmenopausal women, and estrogen replacement therapy can reverse this phenomenon.24,25 Moreover, the prognosis of females with HCC is significantly better than that of male invalids.26 These clinical phenomena are consistent with the animal studies that ovariectomy increased the susceptibility to HCC, and estrogen therapy decreased the incidence and metastasis of HCC in female mice.3,27 However, it should be noted that estrogen usually functions in females before HCC initiation, and estrogen may have adverse effects on patients, especially men, who constitute the majority of HCC cases.1 In the present study, we examined estrogenic activity in HCC patients, including 83 men and 10 women. Since the physiological function of estrogen in the target tissue is caused by the combination of estrogen and 2 different estrogen receptor subtypes, usually ERα and ERβ.28 Ligand-bound estrogen receptor recognizes estrogen-responsive element (ERE) and regulates transcription of target genes. Thus, the expression of ERα and ERβ in the nucleus reflects the activity of the estrogen pathway to some extent. Our results showed that the nuclear accumulation of ERα and ERβ in HCC tissues was more frequent than that in adjacent noncancerous tissues, suggesting that the carcinogenesis of estrogen might be involved in tumorigenesis because estrogen can promote the proliferation of hepatocytes.29 However, a significant negative correlation existed between nuclear ERα and ERβ expression and T stage, tumor size, and clinical staging, suggesting estrogen exerted a protective effect during HCC progression. These data suggested both faces of estrogen in HCC. No significant correlation was found between nuclear ERα or ERβ expression and the overall patient survival in further survival analysis. Therefore, nuclear ERα and ERβ expression might not be used for predicting prognosis in patients with HCC. This result may be related to multiple splice variants of ERα (vER-α) since studies found that HCC with vER-α positive expression presented faster growth and stronger tumor metastasis.30 Therefore, detecting the expression pattern of ER-α subtypes is of great significance for exploring the induction effect of estrogen on HCC.
It is well known that estrogen-induced expression of longevity-related genes is one of the potential mechanisms that estrogen is beneficial to longevity.31 Klotho gene was found to be an aging inhibitor gene, and klotho’s overexpression made the life span exceed that of wild-type mice.32 Therefore, we speculate that the function of klotho gene may be related to the estrogen signaling pathway. It is found by AliBaba 2.1 software analysis that the upstream promoter region of klotho contains ERE. The binding of the estrogen at the ERE might promote the recruitment of ER and other transcription factors to the upstream promoter region of klotho, suggesting that there is a structural basis for the expression of klotho gene regulated by the estrogen pathway. In addition, several studies have explored the relationship between klotho and estrogenic activity. Sárvári et al. found klotho expression was highly responsive to estradiol replacement in the hippocampal of middle-aged female rats.9 Sinha et al. reported green tea polyphenols and sulforaphane-mediated reactivation of tumor suppressor genes such as klotho was, at least in part, the dependence of ERα reactivation in MDA-MB-231 cells with ERα negative expression.10 Therefore, in this study, we tried to find out the correlation between klotho expression and estrogen pathway in HCC. The results showed that the expression of klotho had a significant positive correlation with nuclear ERα but not nuclear ERβ, in cancer tissues of patients with HCC, indicating that estrogen might regulate klotho expression through ERα rather than ERβ in the progress of HCC. However, the nuclear accumulation of ERα in HCC tissues was more frequent than that in adjacent non-neoplastic hepatic tissues, which was inconsistent with the pattern of klotho, suggesting that klotho expression is not completely affected by the estrogen pathway in hepatocarcinogenesis.
In conclusion, we have confirmed in this study that klotho often shows low expression in HCC, and it is at least partially regulated by ERα mediated estrogen signaling pathway. In addition, our results further confirm the anti-cancer effect of klotho, and klotho positive expression is an important predictor for a good prognosis in HCC. However, this study is only based on tissue microarray analysis and lacks relevant research on the exact molecular mechanism. Given the complex interaction between klotho and estrogen pathway in HCC, further elucidation is warranted.
Ethics Committee Approval:
This study was approved by the ethics committee of the cooperative hospitals.
Informed Consent:
Before initiating this study, written informed consent was obtained from all the subjects.
Author Contributions:
(I) Conception and design: J Lin; (II) Administrative support: All authors; (III) Provision of study materials: S Huang, M Wang; (IV) Collection and assembly of data: All authors; (V) Data analysis and interpretation: J Lin, W Wang; (VI) Manuscript writing: S Huang, W Wang, Y Cheng; (VII) Final approval of manuscript: All authors.
Funding Statement
This work is supported in part by the grants from Jiangsu Youth Medical Key Talents Cultivation (QNRC2016442) and Jiangsu dual creation project (2017-37).
Footnotes
Peer-review: Externally peer-reviewed.
Conflict of Interest: The authors have no conflict of interest to declare.
References
- 1. . Shi L, Feng Y, Lin H, Ma R, Cai X. Role of estrogen in hepatocellular carcinoma: is inflammation the key? J Transl Med. 2014;12:93. 10.1186/1479-5876-12-93) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. . Greten TF. Gender disparity in HCC: is it the fat and not the sex? J Exp Med. 2019;216(5):1014–101. 5. 10.1084/jem.20190441) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. . Li Y, Xu A, Jia S, Huang J. Recent advances in the molecular mechanism of sex disparity in hepatocellular carcinoma. Oncol Lett. 2019;17(5):4222–422. 8. 10.3892/ol.2019.10127) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. . Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends: an update. Cancer Epidemiol Biomarkers Prev. 2016;25(1):16–27.. 10.1158/1055-9965.EPI-15-0578) [DOI] [PubMed] [Google Scholar]
- 5. . Kalra M, Mayes J, Assefa S, Kaul AK, Kaul R. Role of sex steroid receptors in pathobiology of hepatocellular carcinoma. World J Gastroenterol. 2008;14(39):5945–59. 61. 10.3748/wjg.14.5945) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. . Pignata S, Daniele B, Gallo C. Endocrine treatment of hepatocellular carcinoma. Any evidence of benefit? Eur J Cancer. 1998;34(1):25–32.. 10.1016/s0959-8049(97)00317-1) [DOI] [PubMed] [Google Scholar]
- 7. . Shimizu I, Yasuda M, Mizobuchi Y. Suppressive effect of oestradiol on chemical hepatocarcinogenesis in rats. Gut. 1998;42(1):112–11. 9. 10.1136/gut.42.1.112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. . Kuro-o M. Klotho and the aging process. Korean J Intern Med. 2011;26(2):113–1. 22. 10.3904/kjim.2011.26.2.113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. . Sárvári M, Kalló I, Hrabovszky E. Hippocampal gene expression is highly responsive to estradiol replacement in middle-aged female rats. Endocrinology. 2015;156(7):2632–26. 45. 10.1210/en.2015-1109) [DOI] [PubMed] [Google Scholar]
- 10. . Sinha S, Shukla S, Khan S, Tollefsbol TO, Meeran SM. Epigenetic reactivation of p21CIP1/WAF1 and klotho by a combination of bioactive dietary supplements is partially ERα-dependent in ERα-negative human breast cancer cells. Mol Cell Endocrinol. 2015;406:102–1. 14. 10.1016/j.mce.2015.02.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. . Tang X, Wang Y, Fan Z. Klotho: a tumor suppressor and a modulator of the Wnt1/β-catenin pathway in human hepatocellular carcinoma. Lab Invest. 2016;96(2):197–205.. 10.1038/labinvest.2015.86) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. . Kononen J, Bubendorf L, Kallioniemi A. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. 1998;4(7):844–84. 7. 10.1038/nm0798-844) [DOI] [PubMed] [Google Scholar]
- 13. . Vizoso FJ, Rodriguez M, Altadill A. Liver expression of steroid hormones and apolipoprotein D receptors in hepatocellular carcinoma. World J Gastroenterol. 2007;13(23):3221–322. 7. 10.3748/wjg.v13.i23.3221) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. . Kuro-o M. Phosphate and klotho. Kidney Int. 2011;79:S20–S2. 3. 10.1038/ki.2011.26) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. . Wolf I, Levanon-Cohen S, Bose S. Klotho: a tumor suppressor and a modulator of the IGF-1 and FGF pathways in human breast cancer. Oncogene. 2008;27(56):7094–7. 105. 10.1038/onc.2008.292) [DOI] [PubMed] [Google Scholar]
- 16. . Xie B, Chen J, Liu B, Zhan J. Klotho acts as a tumor suppressor in cancers. Pathol Oncol Res. 2013;19(4):611–61. 7 10.1007/s12253-013-9663-8) [DOI] [PubMed] [Google Scholar]
- 17. . Camilli T C, Xu M, O’Connell MP. Loss of klotho during melanoma progression leads to increased filamin cleavage, increased Wnt5A expression, and enhanced melanoma cell motility. Pigment Cell Melanoma Res. 2011;24(1):175–1. 86. 10.1111/j.1755-148X.2010.00792.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. . Lu L, Katsaros D, Wiley A. Klotho expression in epithelial ovarian cancer and its association with insulin-like growth factors and disease progression. Cancer Investig. 2008;26(2):185–1. 92. 10.1080/07357900701638343) [DOI] [PubMed] [Google Scholar]
- 19. . Shu G, Xie B, Ren F. Restoration of klotho expression induces apoptosis and autophagy in hepatocellular carcinoma cells. Cell Oncol (Dordr). 2013;36(2):121–12. 9. 10.1007/s13402-012-0118-0) [DOI] [PubMed] [Google Scholar]
- 20. . Xie B, Zhou J, Yuan L. Epigenetic silencing of klotho expression correlates with poor prognosis of human hepatocellular carcinoma. Hum Pathol. 2013;44(5):795–801.. 10.1016/j.humpath.2012.07.023) [DOI] [PubMed] [Google Scholar]
- 21. . Chen L, Liu H, Liu J. Klotho endows hepatoma cells with resistance to anoikis via VEGFR2/PAK1 activation in hepatocellular carcinoma. PLoS ONE. 2013;8(3):e58413. 10.1371/journal.pone.0058413) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. . Barbare JC, Bouché O, Bonnetain F. Randomized controlled trial of tamoxifen in advanced hepatocellular carcinoma. J Clin Oncol. 2005;23(19):4338–43. 46. 10.1200/JCO.2005.05.470) [DOI] [PubMed] [Google Scholar]
- 23. . Villa E. Role of estrogen in liver cancer. Womens Health. 2008;4:41–50.. 10.2217/17455057.4.1.41) [DOI] [PubMed] [Google Scholar]
- 24. . Yu MW, Chang HC, Chang SC. Role of reproductive factors in hepatocellular carcinoma: Impact on hepatitis B- and C-related risk. Hepatology. 2003;38(6):1393–1. 400. 10.1016/j.hep.2003.09.041) [DOI] [PubMed] [Google Scholar]
- 25. . Shimizu I. Impact of oestrogens on the progression of liver disease. Liver Int. 2003;23(1):63–6. 9. 10.1034/j.1600-0676.2003.00811.x) [DOI] [PubMed] [Google Scholar]
- 26. . El-Serag HB, Mason AC, Key C. Trends in survival of patients with hepatocellular carcinoma between 1977 and 1996 in the United States. Hepatology. 2001;33(1):62–6. 5. 10.1053/jhep.2001.21041) [DOI] [PubMed] [Google Scholar]
- 27. . Bigsby RM, Caperell-Grant A. The role for estrogen receptor-alpha and prolactin receptor in sex-dependent DEN-induced liver tumorigenesis. Carcinogenesis. 2011;32(8):1162–116. 6. 10.1093/carcin/bgr094) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. . Webster R, Sheriff S, Faroqui R. Klotho/fibroblast growth factor 23- and PTH-independent estrogen receptor-α-mediated direct downregulation of NaPi-IIa by estrogen in the mouse kidney. Am J Physiol Ren Physiol. 2016;311(2):F249–F2. 59. 10.1152/ajprenal.00542.2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. . Kalaitzidis D, Gilmore TD. Transcription factor cross-talk: the estrogen receptor and NF-kappaB. Trends Endocrinol Metab. 2005;16(2):46–52.. 10.1016/j.tem.2005.01.004) [DOI] [PubMed] [Google Scholar]
- 30. . Villa E, Moles A, Ferretti I. Natural history of inoperable hepatocellular carcinoma: estrogen receptors’ status in the tumor is the strongest prognostic factor for survival. Hepatology. 2000;32(2):233–23. 8. 10.1053/jhep.2000.9603) [DOI] [PubMed] [Google Scholar]
- 31. . Hsu SC, Huang SM, Lin SH. Testosterone increases renal anti-aging klotho gene expression via the androgen receptor-mediated pathway. Biochem J. 2014;464(2):221–22. 9. 10.1042/BJ20140739) [DOI] [PubMed] [Google Scholar]
- 32. . Kurosu H, Yamamoto M, Clark JD. Suppression of aging in mice by the hormone klotho. Science. 2005;309(5742):1829–18. 33. 10.1126/science.1112766) [DOI] [PMC free article] [PubMed] [Google Scholar]