Abstract
Objective:
The aim of the study was to assess the impact of non-surgical periodontal therapy (NSPT) on periodontal clinical parameters, spirometric indices, and salivary MMP-8 levels in patients with chronic obstructive pulmonary disease (COPD) with concurrence of chronic periodontitis (CP) compared with systemically healthy CP.
MATERIAL AND METHODS:
In this prospective clinico-biochemical study, a total of 75 patients belonging to various socioeconomic strata were randomly divided into cases, that is, COPD patients as per the Global Initiative for Obstructive Lung Disease (GOLD) criteria with concurrence of CP [at least ≥ 20 teeth with ≥ 2 tooth sites having pocket probing depth (PPD) or clinical attachment loss (CAL) ≥ 4mm and bleeding on probing (BOP)] and controls (systemically healthy CP). Both groups underwent NSPT and were evaluated for plaque index (PI), gingival index (GI), PPD, CAL, and BOP and spirometry (FEV1/forced vital capacity (FVC)) values at baseline, 3, 6, and 12 months and for salivary MMP-8 levels at baseline and 3 months.
Results:
Statistical results showed that cases (COPD with CP; n = 37) were significantly older (mean age 56.16 ± 9.01 years), ex-smokers (48.6%) with male preponderance (78.4%), and belonged to the upper middle class (40.5%) as compared to controls (systemically healthy CP; n = 38). After NSPT, significant improvement in mean PI, GI, PPD, CAL, and BOP was observed in both groups at 3, 6, and 12 months with better results in controls. FEV1/FVC was significantly improved (P < .001) in cases with insignificant change in controls at 12 months. After 3 months, MMP-8 levels were significantly reduced in cases (P = .002) and controls (P < .001).
Conclusion:
The present study provided substantial evidence that COPD patients have poorer periodontal health as compared to systemically healthy counterparts. Further, these patients showed improvement in FEV1/FVC, however, with higher salivary MMP-8 levels despite NSPT at the end of the study, indicating a possible role of systemic inflammatory overburden of pulmonary disease.
Keywords: Chronic periodontitis, chronic obstructive pulmonary disease, non-surgical periodontal therapy, matrix metalloproteinases-8
Main points
COPD patients have poor periodontal heath in terms of increased plaque, deepened pockets, and higher clinical attachments loss.
COPD patients with CP demonstrated higher salivary MMP-8 levels.
Non-surgical periodontal therapy improved not only periodontal health but also spirometric indices.
A possible association could be considered between COPD and CP.
Introduction
Over the past 20 years, compelling evidence has supported a significant association between periodontal and systemic diseases like cardiovascular disease, respiratory disease, diabetes, rheumatoid arthritis, etc.1 Although the association between periodontitis and chronic obstructive pulmonary disease (COPD) is considered as casual but with possible bidirectionality,2,3,4 a biologically persuasive reason behind such an association could be a similarity in the chronic progressive nature of these immune-inflammatory diseases and a common anatomic structure for accessibility inside the body that could be responsible for modifying the progression of these diseases in an identical manner.5
Lower respiratory tract infections have caused a death toll of around 430 000 worldwide with COPD accounting for 2.2 million mortality alone.6 Earlier it was presumed that by 2030, COPD will attain third rank in terms of death rate worldwide as per WHO Global Burden of Disease Study (GBD) 2010.7 However, in the United States COPD has hit this mark sooner in 2008, thereby surpassing stroke.8 The peak in age-normalized death rates and deaths by COPD in entire Asia with respect to GBD was topped by East Asia in 1994, whereas in South Asia it was from 2013 to 2023 and is expected to rise again up to 2040 accounting for approximately 1.6 million deaths.9 In this pandemic era, the prevalence of COPD was not found to be high in COVID-19 established cases, but it is worth noting that COVID-19 has resulted in considerable burden on such patients intensifying the severity of the disease.10
Clinically, COPD is characterized by continuous shortness of breath and airflow limitation because of overinflation of alveoli mainly precipitated by noxious aerosols (smoking and pollution) which is much higher in low and middle income countries like India. It has a sex predilection with a slight male preponderance affecting 2-22% men and 1.2-19% women and is variable among different states across the nation.11
An area of concern is especially hospitalized and critically ill patients with poor neglected oral care. Such patients have increased oral microbial load promoting interaction between indigenous plaque microorganism and recognized pulmonary pathogens.12 Probably, it could be due to aspiration of oropharyngeal or gastric contents,13 inhalation of liquids and solids (dust, mist, aerosols, etc.),14 and rolling out of infections through blood via sources other than lungs.15
Dental plaque shed into saliva through various masticatory movements may result in respiratory epithelial alteration for intensified adherence and expansion of colonies of pulmonary pathogens. Alternatively, local inflammation in the periodontium causes elaboration of a diversified range of proinflammatory cytokines (interleukins IL-6, IL-1α, IL-1β; tumor necrosis factor (TNF-α); and interferon IFN-γ) and other robust biomolecules into systemic circulation.16 These mediators not only cause cellular alterations (hyperactive neutrophils) but also distant inflammatory sequelae in the lungs of COPD patients. Moreover, these patients usually have an exalted salivary exoglycosidases (i.e., fucosidase, sialidase, etc.).17 Since COPD patients have high aspiration risk during swallowing due to incoordination between respiratory phases and deglutition,18 there are greater chances of inhaling pharyngeal products laden with these enzymes and pathogenic microorganisms. Additionally, these enzymes are also known for degrading protective mucins over the respiratory epithelium. Hence, the primary pulmonary defense gets compromised partly due to enzymatic action and partly due to aspirating putative pathogens.19 Thus, periodontal disease could probably act as an important risk factor for COPD.
NSPT is the cornerstone therapy when it comes to managing chronic periodontitis (CP) patients. It is a preliminary procedure that encompasses manual and ultrasonic instrumentation together with supragingival plaque controlling measures for removal of dental biofilm/plaque, calculus, and infected cementum. This reduces overall periodontal pathogen load from various oral niches (shedding/non-shedding surfaces) ultimately preserving and improving periodontal health. Hence, being a noninvasive procedure with better patient compliance, it could be very advantageous in systemically compromised patients with COPD due to the aforementioned reasons.
Matrix metalloproteinase 8 (MMP-8) is known to cause architectural disruption of the periodontium and lungs during inflammatory diseases. It has been found to be elevated in the saliva of periodontitis patients in comparison to those having healthy gums.20 Notable reduction of salivary MMP-8 levels after scaling and root planing stipulate that it can be used for monitoring periodontal disease severity. The pathophysiology of COPD shows that these endopeptidases (MMP-8, MMP-2, MMP-9, and MMP-12) are primary proteolytic enzymes degrading lung parenchyma and its extracellular matrix, leading to disease development.21 Collagen I and III delimiting to interstitial tissues and pulmonary vessels as well as type II collagen in cartilaginous parts of bronchi and trachea are essential for providing tensile strength. These collagen types act as substrates for MMP-8 which on activation disrupt the architectural integrity of lungs.21 Cigarette smoke, a key causative risk factor for COPD, elicits an inflammatory cellular response with the production of a high concentration of latent MMPs in the lungs. These latent pro-enzymes get converted into activated forms through proteolytic conversion and in turn release various proinflammatory cytokines continuing the vicious cycle of tissue destruction.21 Data on the relationship of MMP-8 and COPD is although relatively sparse, its elevated levels have been observed in induced sputum22 during exacerbations23 and is found to be correlative with airway obstruction.22
Therefore, our hypothesis states that NSPT reduces periodontal microbial colonies by removal of dental plaque and will surely benefit COPD patients with CP by improving their periodontal health as well as pulmonary functions. Thus, the present study aimed to evaluate the impact of non-surgical periodontal therapy (NSPT) on periodontal health, pulmonary function, and salivary MMP-8 levels in COPD patients with CP.
Material and Methods
Study Population
In this prospective, clinico-biochemical study, 85 participants (aged 40-70 years) were screened from the patients who were referred to the Department of Internal Medicine and Periodontology, King George’s Medical University, Lucknow, UP, India, during the period starting from July 2017 to July 2018 after obtaining written informed consent. Cases and controls were selected through a computer-assisted randomization method. Cases were from among the patients diagnosed with COPD by an experienced physician (Department of Internal Medicine) with concurrent presence of CP by a periodontist. Cases were COPD patients with history of recent exacerbation (last 1 month) in terms of worsening of symptoms, that is, breathlessness, increased severity and frequency of cough, and change in color and/or amount of sputum. Systemically healthy periodontitis patients were chosen as controls from the outpatient clinic of the Department of Periodontology. Ethical clearance (Ref. no: 82nd ECM II B-Thesis/P13) for the study protocol was obtained by the Institutional Ethical Committee, King George’s Medical University, Lucknow, UP, India. The study was conducted in accordance with ethical principles as per the Declaration of Helsinki, 2000.
All participants were clinically evaluated for CP as per criteria of having at least ≥20 teeth with ≥2 tooth sites having pocket probing depth (PPD) or clinical attachment loss (CAL) ≥ 4 mm and bleeding on probing (BOP).24 Participants from the Department of Internal Medicine were physician confirmed cases of COPD with FEV1/forced vital capacity (FVC) ratio less than 0.70, symptomatic presence of breathlessness, recurrent cough and sputum production, or wheezing and history of being exposed to considerable noxious stimuli (as per Global Initiative for Obstructive Lung Disease (GOLD) guidelines)25 and were assessed for CP as per the above criteria.
Participants who were edentulous, mentally disabled, pregnant and lactating females, suffering from any other systemic diseases except COPD, who underwent any periodontal treatment in the past 3 months, with inability to perform lung function test, and who received any drug known to have an impact on periodontal tissues in the last 6 months were excluded from the study. The present observational study was framed and executed according to the STROBE (Strengthening the reporting of observational studies in epidemiology) guidelines.
The sample size was calculated on the basis of maximum variation in change of periodontal diseases within the follow-up period by using SD (Standard Deviation) of GI as 0.4919 in both groups, which was considered to be statistically significant. Considering 20% loss to follow-up, 40 patients were required in each group to provide 90% power with 5% alpha error and 10% beta error and CI of 95%. All analyses were performed on Statistical Package for Social Sciences (SPSS) version 21.0 software (IBM Corp.; Armonk, NY, USA).
Study Design
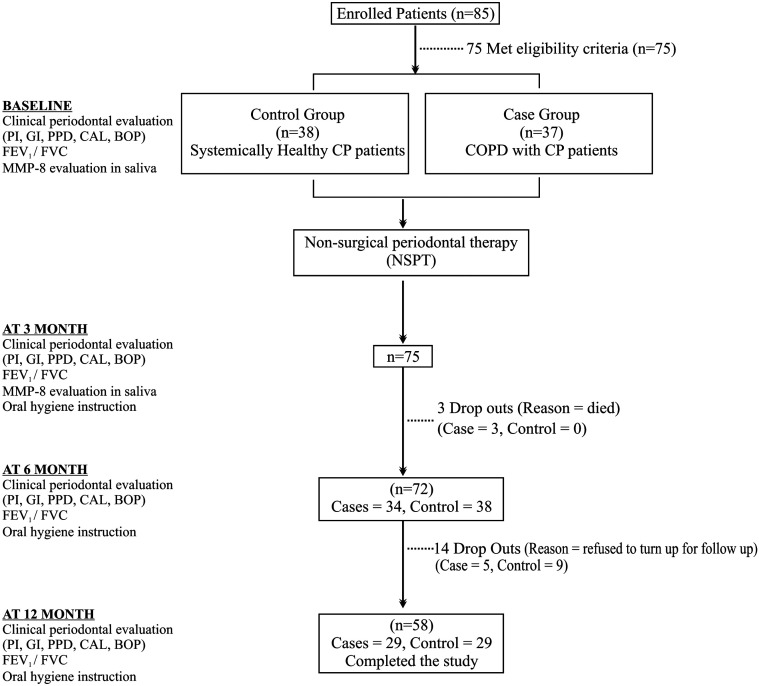
After recruitment, a detailed case history (demographic and behavioral variables) was recorded followed by clinical, spirometric (pulmonary function test (PFT)), and biochemical analysis at baseline followed by NSPT in both groups. NSPT comprised oral hygiene instructions to the patients and professional full mouth scaling and root planing (SRP) using an ultrasonic scaler (Satalec Suprasson P5 Booster, Aceton India Private Limited, Gujrat, India) and periodontal hand instruments (Curette Columbia # 2R/2L & #4R/4L, Hu-Friedy Mfg. Co., LLC 3232 N. Rockwell St., Chicago, IL) without local anesthesia in both groups immediately after recording of baseline parameters. Reassessment was done at 3, 6, and 12 months (clinical and spirometry) and biochemical salivary MMP-8 levels at 3 month by an investigator (blinded). Out of 75 patients, 3 cases died and 14 (5 cases and 9 controls) did not turn up for follow-up. Study design is depicted in a flow diagram (Figure 1).
Figure 1.
Flowchart depicting the study design.
Data Collection
Demographic and Clinical Data
Assessment of demographic data, that is, age, sex, socioeconomic status (determined using Updated Kuppuswamy scale for 2016),26 and behavioral habits including history of cigarette smoking and tobacco chewing was done on the basis of a questionnaire-based method. A full mouth periodontal clinical examination was done in terms of plaque index (PI), gingival index (GI), PPD, clinical attachment level (CAL), and BOP. PPD, CAL, and BOP were measured using the University of North Carolina Probe (UNC-15, Hu-Friedy). PI and GI were determined by using Wilkin’s explorer #17/23 at 6 sites per tooth.
Spirometric Data
Case definition of COPD was defined on the basis of clinical symptoms as patient who had dyspnea, long standing unresolved cough, and/or sputum production and/ or history of subjection to risk factors. Pulmonary function test using an Easy One Plus Spirometer (NDD Medical Technologies, Andover, MA) was used to confirm the diagnosis with the presence of post-bronchodilator FEV1/FVC < 0.70, and without reversibility to bronchodilators this diagnosis usually confirms the presence of persistent airflow limitation. The same procedure was also performed in controls to get the baseline spirometric parameters for better comparison with cases since temporal variations do occur in lung function indices over time, space, within countries, within people, among ethnic groups, and between different spirometers.
Biochemical Data
For the evaluation of MMP-8 levels, unstimulated salivary samples of all patients were collected in sterile Eppendorf tubes at baseline and at 3 months according to the draining method.27 To determine the concentration of salivary MMP-8 levels (ng/mL), enzyme linked immune-sorbent assay was performed utilizing QAYEE Human-MMP-8 kit (QAYEE-BIO for Life Sciences, Shanghai, China, catalog no.QY-E02979, LOT:07/2018(96T)).
Statistical Analysis
The softwares used were The Statistical Package for Social Sciences version 21.0 software (IBM Corp.; Armonk, NY, USA) and MS-Excel (Microsoft Corporation, Redmond, WA). The results were analyzed using descriptive statistics and making comparisons among various groups. Categorical data were summarized as proportions and quantitative data as mean ± SD. Demographic data of study participants were compared between 2 groups using chi-square test for categorical variables and Student’s unpaired t-test (parametric test) for quantitative variables (age). Student’s unpaired t-test (parametric test) and repeated measures analysis of variance test were used to analyze baseline as well as change in clinical periodontal parameters, FEV1/FVC, and MMP-8 values. The cases and controls were matched on the basis of behavioral habits as well as clinical and biochemical parameters.
Results
The demographics of controls (n = 38) and cases (n = 37) are summarized in Table 1. The control group had comparatively younger individuals than cases (mean age 42.87 ± 11.86 vs. 56.16 ± 9.01 years; P < .001). There was equal gender distribution in controls than cases (50.0% males and rest 50.0% females vs. 78.4% males and rest 21.6% females) (P = .010). In the control group, majority of participants belonged to the upper class (34.2%) while in cases majority belonged to the upper middle class (40.5%) followed by the upper lower class (37.8%) (P = .012), which was responsible for intergroup significance. While both groups were almost similar in smoking status, most of them were ex-smokers (47.4% and 48.6%) in control and cases, respectively (P = .362).
Table 1.
Demographic Distribution of Participants
| Demographic Details | Control (n = 38) | Cases (n = 37) | Chi-Square/t-Value | P |
|---|---|---|---|---|
| Age (years, mean ± SD) | 42.87 ± 11.86 | 56.16 ± 9.01 | 5.45 | <.001 |
| Gender | ||||
| Male | 19 | 29 | 6.55 | .010 |
| Female | 19 | 8 | ||
| Socioeconomic status | ||||
| Lower class | 3 | 3 | 12.82 | .012 |
| Lower middle class | 6 | 3 | ||
| Upper class | 13 | 2 | ||
| Upper lower class | 8 | 14 | ||
| Upper middle class | 8 | 15 | ||
| Behavioral habit | ||||
| Ex-smoker | 18 | 18 | 5.46 | .36 |
| Former smoker | 12 | 12 | ||
| Indoor exposure | 3 | 4 | ||
| No habit | 0 | 1 | ||
| NS | 4 | 0 | ||
| Tobacco chewer | 1 | 2 |
P < .05 = significant, P < .001 = highly significant.
NS, non-significant.
Clinical periodontal parameters are depicted in Table 2. Mean periodontal parameters, that is, PI, GI, PPD, CAL, and BOP, at baseline were insignificantly higher in cases than in controls. After 3, 6, and 12 months, all clinical periodontal parameters were statistically improved in both controls and cases. Also, there was a statistically significant difference in both groups at each follow-up visit (P < .001) from baseline. In the intragroup comparison from baseline to 12 months in terms of PI, GI, PPD, CAL, and BOP, controls showed better improvement than cases, that is, F values 510.28 versus 95.6, 524.9 versus 73.2, 256.6 versus 96.9, 301.9 versus 89.8, 350.9 versus 213.8, respectively.
Table 2.
Intergroup and Intragroup Comparison of Clinical Periodontal Parameters at Baseline and After NSPT at 3, 6, and 12 Months
| Parameter | Time | Control (n = 38) | Cases (n = 37) | P | ||
|---|---|---|---|---|---|---|
| Mean ± SD | % Improvement from BL | Mean ± SD | % Improvement from BL | |||
| PI | Baseline | 50.66 ± 7.39 | - | 55.00 ± 11.93 | - | .061 |
| 3 month | 21.82 ± 4.06 | 56.92 | 34.75 ± 5.87 | 36.82 | <.001 | |
| 6 month | 21.23 ± 3.77 | 58.10 | 30.55 ± 5.52 | 44.45 | <.001 | |
| 12 month | 21.21 ± 3.21 | 58.12 | 30.83 ± 5.00 | 43.95 | <.001 | |
| Sig. | F = 510.28, P < .001 | F = 95.6, P < .001 | ||||
| GI | Baseline | 50.60 ± 7.37 | - | 53.74 ± 11.67 | - | .167 |
| 3 month | 21.83 ± 3.99 | 33.58 ± 5.03 | 37.51 | <.001 | ||
| 6 month | 21.26 ± 3.88 | 57.98 | 30.89 ± 5.94 | 42.52 | <.001 | |
| 12 month | 21.20 ± 3.61 | 58.09 | 31.12 ± 5.41 | 42.08 | <.001 | |
| Sig. | F = 524.9, P < .001 | F = 73.2, P < .001 | ||||
| PPD | Baseline | 3.83 ± 0.29 | - | 3.92 ± 0.35 | - | .219 |
| 3 month | 2.71 ± 0.29 | 29.09 | 3.30 ± 0.29 | 15.79 | <.001 | |
| 6 month | 2.66 ± 0.32 | 30.42 | 3.02 ± 0.29 | 23.03 | <.001 | |
| 12 month | 2.64 ± 0.28 | 31.07 | 3.03 ± 0.29 | 22.63 | <.001 | |
| Sig. | F = 256.6, P < .001 | F = 96.9, P < .001 | ||||
| CAL | Baseline | 3.90 ± 0.32 | - | 4.06 ± 0.38 | - | .054 |
| 3 month | 2.82 ± 0.34 | 27.76 | 3.49 ± 0.31 | 13.97 | <.001 | |
| 6 month | 2.80 ± 0.33 | 28.10 | 3.22 ± 0.28 | 20.63 | <.001 | |
| 12 month | 2.79 ± 0.29 | 28.45 | 3.23 ± 0.23 | 20.46 | <.001 | |
| Sig. | F = 301.9, P < .001 | F = 89.8, P < .001 | ||||
| BOP | Baseline | 82.89 ± 12.33 | - | 85.46 ± 8.24 | - | .294 |
| 3 month | 33.05 ± 7.93 | 60.13 | 50.35 ± 12.50 | 41.08 | <.001 | |
| 6 month | 30.92 ± 6.89 | 62.70 | 41.30 ± 9.53 | 51.68 | <.001 | |
| 12 month | 30.25 ± 6.59 | 63.51 | 41.43 ± 8.70 | 51.52 | <.001 | |
| Sig. | F = 350.9, P < .001 | F = 213.8, P < .001 | ||||
P < .05 = significant, P < .001 = highly significant.
PI, plaque index; GI, gingival index; PPD, pocket probing depth; CAL, clinical attachment level; BOP, bleeding on probing.
Mean FEV1/FVC values were significantly higher in controls than cases at baseline (82.49 ± 5.29 vs. 63.39 ± 11.65; P < .001) and remained almost constant as displayed in Table 3. Unlike controls, cases showed a statistically significant improvement in mean FEV1/FVC value in each follow-up visit, that is, 12.36%, 16.91%, and 15.85% at 3, 6, and 12 months, respectively. In the intragroup comparison from baseline to 12 months, controls showed insignificant improvement (F = 0.821, P = .485) compared to highly significant improvement in cases (F = 15.02, P < .001). Controls were almost stable in terms of pulmonary function throughout the study, and none of them developed any obstructive airway disease.
Table 3.
Intergroup and Intragroup Comparison of FEV1/FVC Values at Baseline and After NSPT at 3 and 6 Months
| FEV1/FVC | Control (n = 38) | Cases (n = 37) | t-value | P | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | % Improvement from BL | Mean | SD | % Improvement from BL | |||
| Baseline (BL) | 82.49 | 5.29 | - | 63.39 | 11.65 | - | 9.17 | <.001 |
| 3 month | 81.52 | 3.59 | −1.17 | 71.22 | 13.11 | 12.36 | 4.67 | <.001 |
| 6 month | 81.52 | 3.59 | −1.17 | 74.10 | 10.56 | 16.91 | 4.08 | <.001 |
| 12 month | 81.49 | 3.82 | −1.21 | 73.43 | 10.51 | 15.85 | 4.04 | <.001 |
| Sig. | F = 0.821, P = .485 | F = 15.02, P < .001 | ||||||
P < 0.05 = significant, P < .001 = highly significant.
Baseline mean salivary MMP-8 levels between controls and cases were almost comparable with no statistically significant difference between the 2 (Table 4). In intragroup comparison, cases showed significant improvement (t = 3.34, P = .002) while controls showed highly significant improvement (t = 5.15, P < .001) from baseline. Moreover, in the intergroup comparison between cases and controls there was an insignificant difference at baseline but significant difference at the 3rd month.
Table 4.
Intergroup and Intragroup Comparison of Salivary MMP-8 Levels at Baseline and 3 Months after NSPT
| Parameter | Time | Control (n = 38) | Cases (n = 37) | t-value | P | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | % Improvement from BL | Mean | SD | % Improvement from BL | ||||
| MMP-8 (ng/mL) | Baseline (BL) | 456.97 | 121.96 | - | 465.35 | 91.76 | - | −0.34 | 0.738 |
| 3 month | 382.34 | 62.54 | 16.33 | 427.68 | 79.15 | 8.10 | −2.76 | 0.007 | |
| Sig. | t = 5.15, P < .001 | t = 3.34, P = .002 | |||||||
P < 0.05 = significant, P < .001 = highly significant.
Discussion
In the present study, we assessed the role of NSPT on pulmonary functions, periodontal health, and salivary MMP-8 levels of COPD patients with CP. Through our study it was demonstrated that COPD patients had poorer periodontal health with greater attachment loss compared to systemically healthy counterparts. Besides, such patients got benefited from NSPT in terms of reduction of salivary MMP-8 levels and to some extent in matters of improvement in lung function (FEV1/FVC). Previous studies have reported elevated levels of MMP-8 in sputum,23 plasma,28 and BAL (Bronchoalveolar Lavage)29 of COPD patients, but none has evaluated its salivary concentration. Also, MMP-8 has been strongly associated with high levels of periodontitis in the oral fluids of patients with periodontitis (saliva and gingival crevicular fluid).30 Therefore, assessment of its levels through saliva as in our study could be a preferable noninvasive alternative for assessing both systemic and local inflammatory burden.
Our results demonstrated significantly improved periodontal parameters, that is, PI, GI, PPD, CAL, and BOP after NSPT at all intervals from baseline in both groups, with better results in controls than in cases. The results in controls were agreeable with a 12-month follow-up study focusing on the same indices.31 The findings in cases were in accordance with a study on periodontally diseased COPD patients demonstrating improved periodontal parameters after periodontal treatment from baseline to 6-month, 1-year, and 2-year follow-up periods.32
Although intergroup comparison showed statistically comparable baseline periodontal parameters, cases had comparatively poorer baseline periodontal health than controls, which could be due to the cumulative effect of inflammatory mediators33 and impaired systemic pulmonary function. Literature shows COPD patients having relatively elevated serum cytokines (TNF-α, MMPs, and IL-8) and destructive mediators (CRP) compared to periodontitis patients.34 Another probable factor could be the adverse effects of medications given in COPD, for example, glucocorticoids, salbutamol, and tiotropium bromide,35 which could accelerate periodontal disease in such patients.
Further improvements in these parameters were less pronounced in cases at 3, 6, and 12 months intervals compared to controls. It could be due to impaired migrating ability of monocytes and lack of balance in subsets of CD8+peripheral blood T cells seen in COPD,36,37 leading to delayed periodontal healing despite NSPT.
Spirometry, a gold standard for diagnosing COPD, consists of FVC, forced expiratory volume in 1 second (FEV1), and FEV1/FVC ratio. Due to narrowing or airway inflammation, exhaled air gets hindered leading to reduction in FEV1. Diagnosis of COPD is made when FEV1 is disproportionately decreased to FVC.
Spirometric analysis of controls was done to ensure that they have healthy lungs. Also, we got the mean FEV1/FVC baseline values exclusively for population entering in hospital. Further, PFT was performed at various time intervals to rule out the chances of controls developing any pulmonary disease during the course of the study and affecting the results. Intergroup comparison at baseline showed a highly significant difference in mean FEV1/FVC between both groups which could be attributable to reduced lung efficiency and poor periodontal health in obstructive lung disease patients (cases). Although they had significant improvement (P < .001) between baseline and other follow-up periods, which could be partially due to the effect of medications given in COPD as well as a beneficial role of NSPT on pulmonary health, the mechanistic explanation is that NSPT reduces biofilm and associated pathogens, such as Pseudomonas spp. and Klebsiella pneumoniae, which infect the lower respiratory tract, in turn reducing the chances of colonization in the lungs.38
Current literature suggests periodontitis as a possible risk factor for COPD. Supporting evidence demonstrates a favorable role of periodontal treatment (SRP) in COPD patients with CP with improvement in periodontal health, spirometric parameters (FEV1), lowered intensity of pulmonary infections, and exacerbation frequency.32 Interestingly, dental plaque may act as a reservoir for respiratory microbes in hospitalized patients with chronic lung disease.39 Moreover, Brook and Frazier40 showed elevated Ig A antibodies against pathogens (Fusobacterium nucleatum and Prevotella intermedia) in the sputum of acute exacerbation of chronic bronchitis while Takahashi et al.41 regarded that an IgG titer for periodontitis-related antibody can be an independent predictor of frequent COPD exacerbations. Thus, this explains the concept of shared microbiological etiology of both diseases, that is, CP and COPD. Hence, the concurrence of several peripheral inflammatory triggers may be an important consideration in the development of more severe lung disease.
MMP-8 has been known to be a promising biomarker for assessing disease activity in both periodontitis42 and COPD,43 and therefore the reason for including it in our study. A study on salivary MMP-8, 13, and the tissue inhibitor of matrix metalloproteinase-1 (TIMP-1) were compared in mild COPD versus non-COPD individuals and were found to be comparable in both groups (P > .05) at baseline28 which was similar to the findings of our study (P = .738).
After NSPT both controls and cases had shown reduced salivary MMP-8 levels but it was statistically highly significant in controls (t = 5.15, P < .001) whereas cases showed significant results (t = 3.34, P = .002). Supportive studies are numerous showing reduced salivary and plasma MMP-8 levelsin periodontitis subjects after oral prophylaxis.44 A question ponders on why cases obtained lesser reduction in salivary MMP-8 compared to controls despite NSPT while their baseline values were comparable. Contradictory to this, significant periodontal improvement (P < .001) was noticed after NSPT in cases at all time intervals. It clearly suggests that although local periodontal inflammation got subsided after NSPT, concurrence of COPD disease in cases causes still high concentrations of MMP-8 in systemic circulation ultimately percolating in salivary fluid. To best of our knowledge, this is the first study that has evaluated MMP-8 levels in saliva of COPD with CP. Also, saliva can be an easy noninvasive alternative against plasma and BAL to assess pulmonary inflammatory overburden through salivary MMP-8 levels.
Additionally cases were significantly older than controls, and therefore aging might play a pivotal role for poor lung function in such patients. Moreover, medications for COPD could be considered as an effect modifier due to its direct impact on FEV1/FVC and indirectly on periodontal health by reducing salivary flow and increasing dental plaque accumulation resulting in severe periodontal disease. Hence, the sole effect of NSPT on FEV1/FVC and periodontal health of cases could not be assessed. Considering the shortcomings, long-term observational and clinical studies with larger sample sizes are the need of the hour to validate the findings of the current study. The results of the present study can be applied to individuals, groups, or populations that differed from those enrolled in the study according to age, sex, ethnicity, behavioral habits (smoking, tobacco chewing, etc.), and severity of disease. Although the nature and level of exposure in other countries will be different, this depends on the number of factors such as socioeconomic status, education level, awareness toward health, and genetic susceptibly, which can affect disease severity and outcome variables.
Conclusion
This study supports the fact that COPD patients have greater plaque accumulation, more inflamed and bleeding gums, deeper pockets, and greater CAL compared to systemically healthy counterparts. In addition, such patients had comparatively higher salivary MMP-8 levels even after NSPT due to systemic overburden of inflammatory mediators. However, they were benefited in terms of improvement in FEV1/FVC. Consequently, it could be contemplated that these patients will have reduced exacerbation frequency, hospitalization, and a better quality of life just by a small measure of maintaining their proper oral hygiene. Also, saliva with ease of its collection by noninvasive methods can be utilized as a potential biological fluid for assessing the status of not only periodontitis but also COPD by measuring MMP-8 levels which was evaluated for the first time in our study.
Moreover, studies should be targeted in finding the role of NSPT on pulmonary MMP-8 levels for better association between COPD and CP. Hence, our hypothesis was partially proven that NSPT does improve the pulmonary function of COPD patients, and salivary MMP-8 is associated with both CP and COPD.
Funding Statement
The authors declared that this study has received no financial support.
Footnotes
Ethics Committee Approval: This study was approved by Ethics committee of King George’s Medical University, (Approval No: 82 nd ECM II B-Thesis/P13).
Informed Consent: Written informed consent was obtained from the patients who agreed to take part in the study.
Peer Review: Externally peer-reviewed.
Author Contributions: Supervision – A.V., A.P., S.V., S.C., S.K., N.L., S.K., U.V.; Design – U.V., A.V., S.V.; Concept– U.V., A.V.; Resources – S.S., A.V., S.V., S.C., S.K., U.V.; Materials – S.S., A.G., A.V., S.V., U.V.; Data Collection and/or Processing – S.S., A.G.; Analysis and/or Interpretation – S.S., A.G., A.V., A.P., S.V., S.C., S.K., N.L., S.K., U.V.; Literature Search – S.S., A.G.; Writing Manuscript – S.S., A.G., A.V., S.C., U.V.; Critical Review – S.S., A.G., A.V., S.C., U.V.
Conflict of Interest: The authors have no conflict of interest to declare.
References
- 1. Otomo-Corgel J, Pucher JJ, Rethman MP, Reynolds MA. State of the science: Chronic periodontitis and systemic health. J Evid Based Dent Pract. 2012;12(3)(suppl):20–28.. 10.1016/S1532-3382(12)70006-4) [DOI] [PubMed] [Google Scholar]
- 2. Chung JH, Hwang HJ, Kim SH, Kim TH. Associations between periodontitis and chronic obstructive pulmonary disease: The 2010 to 2012 Korean national health and nutrition examination survey. J Periodontol. 2016;87(8):864–871.. 10.1902/jop.2016.150682) [DOI] [PubMed] [Google Scholar]
- 3. Shi Q, Zhang B, Xing H. Patients with chronic obstructive pulmonary disease suffer from worse periodontal health-evidence from a meta-analysis. Front Physiol. 2018;9(9:33):33. 10.3389/fphys.2018.00033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Scannapieco FA, Stewart EM, Mylotte JM. Colonization of dental plaque by respiratory pathogens in medical intensive care patients. Crit Care Med. 1992;20(6):740–745.. 10.1097/00003246-199206000-00007) [DOI] [PubMed] [Google Scholar]
- 5. Mojon P. Oral health and respiratory infection.J Can Dent Assoc. 2002;68(6):340–345.. [PubMed] [Google Scholar]
- 6. Murray CJ, Lopez AD. Mortality by cause for eight regions of the world: Global burden of disease study. Lancet. 1997;349(9061):1269–1276.. 10.1016/S0140-6736(96)07493-4) [DOI] [PubMed] [Google Scholar]
- 7. Lozano R, Naghavi M, Foreman K. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010:A systematic analysis for the Global burden of disease study 2010. Lancet. 2012;380(9859):2095–2128.. 10.1016/S0140-6736(12)61728-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. May SM, Li JT. Burden of chronic obstructive pulmonary disease: Healthcare costs and beyond. Allergy Asthma Proc. 2015;36(1):4–10.. 10.2500/aap.2015.36.3812) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Soriano JB, Ancochea J, Celli BR. The most beautiful COPD chart in the world: All together to end COPD!. Eur Respir J. 2019;54(6):1902047. 10.1183/13993003.02047-2019) [DOI] [PubMed] [Google Scholar]
- 10. Alqahtani JS, Oyelade T, Aldhahir AM. Prevalence, severity and mortality associated with COPD and Smoking in patients with COVID-19: A rapid systematic review and meta-analysis. PLOS ONE. 2020;15(5):e0233147. 10.1371/journal.pone.0233147) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jindal SK, Aggarwal AN, Gupta D. A review of population studies from India to estimate national burden of chronic obstructive pulmonary disease and its association with smoking. Indian J Chest Dis Allied Sci. 2001;43(3):139–147.. [PubMed] [Google Scholar]
- 12. Komiyama K, Tynan JJ, Habbick BF, Duncan DE, Liepert DJ. Pseudomonas aeruginosa in the oral cavity and sputum of patients with cystic fibrosis. Oral Surg Oral Med Oral Pathol. 1985;59(6):590–594.. 10.1016/0030-4220(85)90187-2) [DOI] [PubMed] [Google Scholar]
- 13. Gorguner M, Akgun M. Acute inhalation injury. Eurasian J Med. 2010;42(1):28–35.. 10.5152/eajm.2010.09) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Falcon-Rodriguez CI, Osornio-Vargas AR, Sada-Ovalle I, Segura-Medina P. Aeroparticles, composition, and lung diseases. Front Immunol. 2016;7:3. 10.3389/fimmu.2016.00003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fiddian-Green RG, Baker S. Nosocomial pneumonia in the critically ill: Product of aspiration or translocation? Crit Care Med. 1991;19(6):763–769.. 10.1097/00003246-199106000-00006) [DOI] [PubMed] [Google Scholar]
- 16. Reddi K, Wilson M, Nair S, Poole S, Henderson B. Comparison of the pro-inflammatory cytokine- stimulating activity of the surface-associated proteins of periodontopathic bacteria. J Periodont Res. 1996;31(2):120–130.. 10.1111/j.1600-0765.1996.tb00473.x) [DOI] [PubMed] [Google Scholar]
- 17. Quinn MO, Miller VE, Dal Nogare AR. Increased salivary exoglycosidase activity during critical illness. Am J Respir Crit Care Med. 1994;150(1):179–183.. 10.1164/ajrccm.150.1.8025747) [DOI] [PubMed] [Google Scholar]
- 18. Shaker R, Li Q, Ren J. Coordination of deglutition and phases of respiration: Effect of aging, tachypnea, bolus volume, and chronic obstructive pulmonary disease. Am J Physiol. 1992;263(5 Pt 1):G750–G755.. 10.1152/ajpgi.1992.263.5.G750) [DOI] [PubMed] [Google Scholar]
- 19. Prasanna SJ. Causal relationship between periodontitis and chronic obstructive pulmonary disease. J Indian Soc Periodontol. 2011;15(4):359–365.. 10.4103/0972-124X.92570) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ebersole JL, Schuster JL, Stevens J. Patterns of salivary analytes provide diagnostic capacity for distinguishing chronic adult periodontitis from health. J Clin Immunol. 2013;33(1):271–279.. 10.1007/s10875-012-9771-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Churg A, Zhou S, Wright JL. Series “Matrix metalloproteinases in lung health and disease”: Matrix metalloproteinases in COPD. Eur Respir J. 2012;39(1):197–209.. 10.1183/09031936.00121611) [DOI] [PubMed] [Google Scholar]
- 22. Vornooy JHJ, Lindeman JHN, Jacobs JA. Increased activity of matrix metalloproteinase −8 and matrix metalloproteinase − 9 in induced sputum from patients with COPD. Chest. 2004;126(6):1802- 1810. (doi: 10.1378/chest.126.6.1802) [DOI] [PubMed] [Google Scholar]
- 23. Ilumets H, Rytilä P, Demedts I. Matrix metalloproteinases-8,-9 and-12 in smokers and patients with stage 0 COPD. Int J Chron Obstruct Pulmon Dis. 2007;2(3):369–379.. [PMC free article] [PubMed] [Google Scholar]
- 24. Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4(1):1–6.. 10.1902/annals.1999.4.1.1) [DOI] [PubMed] [Google Scholar]
- 25. Mirza S, Clay RD, Koslow MA, Scanlon PD. COPD Guidelines: A review of the 2018 GOLD Report. Mayo Clin Proc. 2018;93(10):1488–1502.. 10.1016/j.mayocp.2018.05.026) [DOI] [PubMed] [Google Scholar]
- 26. Shaikh Z, Pathak R. Revised kuppuswamy and BG Prasad socio-economic scales for 2016. Int J Commun Med Public Health. 2017;4(4):997–999.. 10.18203/2394-6040.ijcmph20171313) [DOI] [Google Scholar]
- 27. Navazesh M, Christensen CM. A comparison of whole mouth resting and stimulated salivary measurement procedures. J Dent Res. 1982;61(10):1158–1162.. 10.1177/00220345820610100901) [DOI] [PubMed] [Google Scholar]
- 28. Koo HK, Hong Y, Lim MN, Yim JJ, Kim WJ. Relationship between plasma matrix metalloproteinase levels, pulmonary function, bronchodilator response, and emphysema severity. Int J Chron Obstruct Pulmon Dis. 2016;11:1129–1137.. 10.2147/COPD.S103281) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ostridge K, Williams N, Kim V. Relationship between pulmonary matrix metalloproteinases and quantitative CT markers of small airways disease and emphysema in COPD. Thorax. 2016;71(2):126–132.. Epub 2015 Dec 8. 10.1136/thoraxjnl-2015-207428) [DOI] [PubMed] [Google Scholar]
- 30. Sorsa T, Gursoy UK. Analysis of matrix metalloproteinases, especially MMP-8, in gingival crevicular fluid, mouthrinse and saliva for monitoring periodontal diseases. Periodontol 2000. 2016;70(1):142–163.. 10.1111/prd.12101) [DOI] [PubMed] [Google Scholar]
- 31. Badersten A, Nilveus R, Egelberg J. Effect of nonsurgical periodontal therapy. II. Severely advanced periodontitis. J Clin Periodontol. 1984;11:63–76.. 10.1111/j.1600-051x.1981.tb02024.x) [DOI] [PubMed] [Google Scholar]
- 32. Zhou X, Han J, Liu Z. Effects of periodontal treatment on lung function and exacerbation frequency in patients with chronic obstructive pulmonary disease and chronic periodontitis: a 2-year pilot randomized controlled trial. J Clin Periodontol. 2014;41(6):564–572.. 10.1111/jcpe.12247) [DOI] [PubMed] [Google Scholar]
- 33. Hobbins S, Chapple ILC, Sapey E, Stockley RA. Is periodontitis a comorbidity of COPD or can associations be explained by shared risk factors/behaviors? Int J Chron Obstruct Pulmon Dis. 2017;12:1339–1349.. 10.2147/COPD.S127802) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hoenderdos K, Condliffe A. The neutrophil in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2013;48(5):531–539.. 10.1165/rcmb.2012-0492TR) [DOI] [PubMed] [Google Scholar]
- 35. Sousa LHT, Moura EV, Queiroz AL. Effects of glucocorticoid-induced osteoporosis on bone tissue of rats with experimental periodontitis. Arch Oral Biol. 2017;77:55–61.. 10.1016/j.archoralbio.2017.01.014) [DOI] [PubMed] [Google Scholar]
- 36. Chen L, Chen G, Zhang MQ. Imbalance between subsets of CD8+ peripheral blood T cells in patients with chronic obstructive pulmonary disease. PeerJ. 2016;4:1–14.. 10.7717/peerj.2301) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cardoso EM, Arosa FA. CD8+ T cells in chronic periodontitis: Roles and rules. Front Immunol. 2017;8:145. 10.3389/fimmu.2017.00145) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Senpuku H, Sogame A, Inoshita E. Systemic diseases in association with microbial species in oral biofilm from elderly requiring care. Gerontology. 2003;49(5):301–309.. 10.1159/000071711) [DOI] [PubMed] [Google Scholar]
- 39. Didilescu AC, Skaug N, Marica C, Didilescu C. Respiratory pathogens in dental plaque of hospitalized patients with chronic lung diseases. Clin Oral Investig. 2005;9(3):141–147.. 10.1007/s00784-005-0315-6) [DOI] [PubMed] [Google Scholar]
- 40. Brook I, Frazier EH. Immune response to Fusobacterium nucleatum and Prevotella intermedia in the sputum of patients with acute exacerbation of chronic bronchitis. Chest. 2003;124(3):832–833.. 10.1378/chest.124.3.832) [DOI] [PubMed] [Google Scholar]
- 41. Takahashi T, Muro S, Tanabe N. Relationship between periodontitis-related antibody and frequent exacerbationsin chronic obstructive pulmonary disease. PLOS ONE. 2012;7(7):e40570. 10.1371/journal.pone.0040570) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rangbulla V, Nirola A, Gupta M, Batra P, Gupta M. Salivary IgA, interleukin-1β and MMP-8 as salivary biomarkers in chronic periodontitis patients. Chin J Dent Res. 2017;20(1):43–51.. 10.3290/j.cjdr.a37741) [DOI] [PubMed] [Google Scholar]
- 43. Yıldırım E, Kormi I, Başoglu ÖK. Periodontal health and serum, saliva matrix metalloproteinases in patients with mild chronic obstructive pulmonary disease. J Periodontal Res. 2013;48(3):269–275.. 10.1111/jre.12004). [DOI] [PubMed] [Google Scholar]
- 44. Romero AM, Mastromatteo-Alberga P, Escalona L, Correnti M. MMP-3 and MMP-8 levels in patients with chronic periodontitis before and after non-surgical periodontal therapy. Investig Clin. 2013;54(2):138–148.. [PubMed] [Google Scholar]