Abstract
Objective
: Interruption or reduction in airflow and desaturation is a theoretically expected result in bronchiectasis accompanied by excessive secretions in the airways, bronchial wall thickening, and destruction of the wall structure. The same mechanism of interruption or reduction in airflow and desaturation is valid for obstructive sleep apnea (OSA). However, data on the association of bronchiectasis with OSA are scarce. We aimed to investigate the frequency of OSA and related parameters in patients with non-cystic fibrosis bronchiectasis (NCFB).
Material and Methods
: All 43 consecutive patients who presented to the outpatient clinic for bronchiectasis follow-up between January 1, 2018 and January 1, 2019 were included. The polysomnography (PSG) data of the 43 patients were reviewed. Groups of patients with and without OSA, as detected using PSG, were compared in terms of clinical, demographic, and polysomnographic parameters.
Results
: The mean age of the 43 patients was 50 ± 15 years; 28 (65.2%) were female. OSA was detected in 24 (55.8%) of 43 patients, of whom 14 (32.6%) had mild, 5 (11.6%) had moderate, and 5 (11.6%) had severe OSA. Three (7.0%) patients were REM-dependent and 7 (16.3%) were position-dependent. When evaluated using logistic regression analysis, REM percentage (16.8% vs. 11.8%, P = .03) and presence of witnessed apnea (33.3% vs. 15.7%, P = .01) were observed to be significantly higher in the patients with OSA. The age factor was found at the significance limit (P = .05).
Conclusion
: The frequency of OSA in patients with NCFB is 55.8%. Investigating OSA using PSG is important in patients with NCFB, especially at advanced ages.
Keywords: Non-cystic bronchiectasis, OSA, risk factors
MAIN POINTS
The frequency of OSA in patients with non-cystic fibrosis bronchiectasis is 55.8%.
The frequency of OSA in patients with non-cystic fibrosis bronchiectasis increases with age.
Investigating OSA using PSG is important in patients with non-cystic fibrosis bronchiectasis, especially at advanced ages.
Introduction
Interruption or reduction in airflow and desaturation is a theoretically expected result in bronchiectasis accompanied by excessive secretions in the airways, bronchial wall thickening, and destruction of the wall structure. The same mechanism of interruption or reduction in airflow and desaturation is valid for obstructive sleep apnea (OSA). However, data on the association of bronchiectasis with OSA are scarce.
OSA is characterized by recurrent partial or full airway obstruction resulting in hypoxia and arousals during sleep.1 This condition, associated with increased cerebrovascular and cardiovascular mortality and morbidity rates, affects a substantial part of the population.2 Studies have shown that the prevalence of OSA ranges between 23 and 50%.3 OSA is a societal burden that increases direct and indirect costs by causing loss of labor force, in addition to altering the social life and quality of life of people because it is quite a common chronic condition.2 However, OSA is a condition for which treatment is possible. Continuous positive airway pressure (CPAP) treatment is known to decrease mortality, morbidity, and other consequences of OSA.2 In this regard, early diagnosis and treatment of a common condition like OSA are of great importance, both individually and socially.
Bronchiectasis is a chronic condition characterized by permanent dilation of the bronchi, accompanied by inflammatory changes in the bronchial walls and lung parenchyma adjacent to the bronchi.4 Recurrent inflammation in the bronchus and fibrosis occurring in the parenchyma surrounding it are the fundamental basis of the pathogenesis of bronchiectasis.4 Although the exact prevalence of bronchiectasis is unknown, an epidemiologic study in 2013 found that the prevalence of non-cystic fibrosis bronchiectasis (NCFB) was 139 per 100 000 capita after the age of 18, and that its prevalence increased prominently with age.5 Being one of the obstructive airway diseases, but not investigated as much as asthma and chronic obstructive pulmonary disease (COPD), bronchiectasis currently has many unknown features.6,7 The data about the association of bronchiectasis with OSA are very limited. Faria Junior et al.4 reported that the prevalence of OSA in patients with non-cystic fibrosis was 40.82%.4 In most of the studies, especially in those conducted before 2015, polysomnography (PSG) which is the gold-standard in the diagnosis of OSA, was not used.8-10 In a study in 2015, hypoxemia, as detected using PSG, was found to be related to indexes of life quality of patients with NCFB, and another study in 2017 linked PSG results to several parameters such as the colonization of sputum, number of attacks, and respiratory function tests.4,11
In the present study, we aimed to investigate the frequency of OSA and the parameters related to this in patients with NCFB.
Material and Methods
The present study included the patients presenting to the outpatient clinic for bronchiectasis of the Respiratory Diseases Department of our university between January 1, 2018, and January 1, 2019. High-resolution computed tomography (HRCT) was used in making the diagnosis of bronchiectasis.12 In all patients, the presence and extent of bronchiectasis were reported by a radiologist based on the guiding criteria of the British Thoracic Society for bronchiectasis in adult patients.13 The study included patients who had a negative cystic fibrosis sweat test (chloride concentration < 30 mmol or negative cystic fibrosis transmembrane conductance gene analysis) and patients aged 18 years or over.14 Patients with a diagnosis of cystic fibrosis, those who had an infectious attack in the last 1 month, those using an oxygen concentrator or a non-invasive mechanical ventilator, and those with co-morbidities that might be risky for OSA such as hypothyroidism, asthma, COPD, malignancy, or cardiac failure, were excluded.
The study was approved by the local Ethics Committee (Approval number: 23786442-604.01.01-102359) and written informed consent was taken from all participants.
It was planned to include all of the volunteer consecutive patients who presented to the outpatient clinic for bronchiectasis follow-up between January 1, 2018, and January 1, 2019, and met the inclusion criteria. Seventy-five patients were included, demographic and anthropometric characteristics of the patients in the study were recorded, and their essential OSA symptoms were enquired (snoring, witnessed apnea, excessive daytime sleepiness). They underwent a spirometric examination. The Epworth Sleepiness Scale was used. The status of sputum colonization was assessed. The extent and type of bronchiectasis were examined using HRCT. Twenty-four patients did not accept to participate in the study, and the PSG test was not available for 6 patients. A total of 45 patients underwent PSG in the sleep laboratory; 2 patients were excluded because sufficient sleep time was not achieved during PSG. A total of 43 patients were included in the final analysis (Figure 1).
Figure 1.
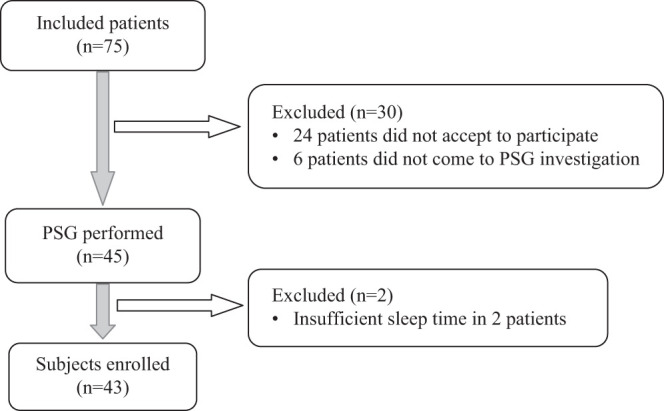
Flow chart.
PSG
Overnight PSG recordings of the patients included in the study were made using the DOMINO software (version 2.5.0, Somnomedics). Two-channel electroencephalography (EEG) (EEG cables; C4-A1, C3-A2), two-channel electrooculography (EOG), electromyogram (EMG) both from the chin and the tibialis anterior, two-bullet electrocardiography (ECG), respiratory sounds, oxygen saturation, as well as thoracic and abdominal respiratory movements were recorded. Synchronized video recording was also made using a body position detector and nasal pressure sensor. Abnormal respiratory events related to sleep were scored using epochs of 30 seconds based on the 2012 criteria of the American Academy of Sleep Medicine (AASM).15 Apnea was defined as an interruption of the airflow for 10 seconds. In defining hypopnea, an accompaniment of a 30% decrease of flow to 3% desaturation was used. Using this definition of hypopnea, apnea and hypopnea indexes (AHI) per sleep-hour were estimated to define OSA. Patients with AHI ≥ 5 were considered as having OSA, and those with AHI of 5-14, 15-29, and ≥30 were classified as having mild, moderate, and severe OSA, respectively. REM-dependent OSA was described as AHI ≥ 5 and AHI < 5 during the non-REM period, and AHI during the REM period being twice as much as during the non-REM period.16 Similarly, position-dependent OSA was described as AHI ≥ 5 and AHI < 5 in the supine position, and AHI in the supine position was more than twice as much as in the non-supine position.16,17
Epworth Sleepiness Scale (ESS)
The ESS is a questionnaire consisting of 8 items, and is used to demonstrate daytime sleepiness status. The patient answers each question by giving points ranging from 0 to 3. This questionnaire enquires the possibility of sleeping in certain situations on an ordinary day during which the patient is not very tired. The enquiry method is the same for all questions, and 0 points are given if there is no possibility of sleeping, 1 point if the possibility of sleeping is of low, 2 points if there is an intermediate possibility of sleeping, and 3 points are given if there is a high possibility of sleeping in the daytime. A total score of 10 or more indicates excessive daytime sleepiness.1 The Turkish version of the ESS has been validated.18
Sputum Colonization
Sputum colonization was defined as the growth of the same pathogen bacteria in 3 sputum samples obtained with time intervals of at least 1 month in the last year.
Respiratory Function Test
All patients underwent spirometric evaluation during the stable phase using a Sensor Medics Vmax device (SensorMedics Series 22, USA) in the pulmonary function laboratory of our university. Spirometry was performed according to the criteria of the American Thoracic Society/European Respiratory Society (ATS/ERS) guidelines.19
Statistical Analysis
Statistical analyses were performed using statistical software (SPSS, version 10.0; SPSS Inc., Chicago, IL, USA). Mean and standard deviation (SD) were calculated for the variables with normal distribution, and medians were calculated for variables not showing normal distribution. Categorical variables were expressed as percentages. The Mann–Whitney U-test and chi–square tests were used to compare the groups, and Spearman’s test for assessing correlations. The significance level was set at P < .05.
Results
The clinical, demographic, and anthropometric characteristics of the 43 patients included in the study and the findings related to bronchiectasis are given in Table 1.
Table 1.
Clinical, Demographic, and Anthropometric Features
| Parameter | n = 43 |
|---|---|
| Age (years) (mean ± SD) | 50 ± 15 |
| Male n (%) | 15 (34.8) |
| Female n (%) | 28 (65.2) |
| Age of onset (years) (mean ± SD) | 21 ± 20 |
| Duration of disease (years) (mean ± SD) | 31 ± 19 |
| BMI (kg/m2) (mean ± SD) | 26.2 ± 5.6 |
| Essential symptoms n (%) | 37 (86.0) |
| Snoring | 30 (69.8) |
| Excessive daytime sleepiness | 32 (74.4) |
| Witnessed apnea | 11 (25.6) |
| Sputum colonization n (%) | 16 (37.2) |
| Pseudomonas aeruginosa | 10 (23.0) |
| Candida albicans | 1 (2.0) |
| Escherichia coli | 1 (2.0) |
| Haemophlius influenzae | 2 (5.0) |
| Pseudomonas alcaligenes | 1 (2.0) |
| Klebsiella | 1 (2.0) |
| Brochiectasis localization n (%) | |
| Right upper lobe | 17 (39.0) |
| Right middle lobe | 19 (44.0) |
| Right lower lobe | 24 (56.0) |
| Left upper lobe | 12 (27.0) |
| Lingula | 20 (46.0) |
| Left lower lobe | 27 (62.0) |
| Type of bronchiectasis n (%) | |
| Cystic | 12 (28.0) |
| Cylindrical | 17 (40.0) |
| Varicose | 7 (16.0) |
| Traction | 5 (12.0) |
| Number of lobes with bronchiectasis n (mean ± SD) | 2.3 ± 1.6 |
| Spirometry | |
| FVC (%) (mean ± SD) | 78.9 ± 20.0 |
| FEV1 (%) (mean ± SD) | 64.5 ± 24.1 |
| FEV1/FVC (%) (mean ± SD) | 66.7 ± 13.9 |
| FEV1/FVC<%75 n (%) | 29 (67.4) |
BMI, body mass index; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; SD, standard deviation.
Twenty-five (58.1%) of the patients were aged over 50 years. The duration of the disease was more than 30 years in 22 (51.2%) patients. Disease symptoms started before the age of 20 years in 20 (46.5%) patients. In the spirometry, an obstructive pattern was found in 29 (67%) patients, and a restrictive pattern was seen in 19 (44%) participants.
The mean ESS score was 6.3 ± 4.7 in the patients. OSA was found in 24 (55.8%) patients. Of them, 14 (32.6%) had mild, 5 (11.6%) had moderate, and 5 (11.6%) had severe OSA. Three (7.0%) patients were REM-dependent and seven (16.3%) were position-dependent.
The PSG data of the patients are given in Table 2.
Table 2.
Polysomnographic Parameters
| AHI (mean ± SD) | 13.8 ± 21.05 (median: 6.0) |
| Sleep duration (hours) (mean ± SD) | 5.3 ± 1.1 |
| Stage 1 (min) (mean ± SD) | 21 ± 12 |
| Stage 1 (%) (mean ± SD) | 7.1 ± 4.6 |
| Stage 2 (min) (mean ± SD) | 185 ± 70 |
| Stage 2 (%) (mean ± SD) | 55.5 ± 14.1 |
| Stage 3 (min) (mean ± SD) | 65 ± 35 |
| Stage 3 (%) (mean ± SD) | 21.7 ± 12.3 |
| REM (min) (mean ± SD) | 46.7 ± 26.7 |
| REM (%) (mean ± SD) | 14.6 ± 7.5 |
| Sleep efficiency (%) (mean ± SD) | 71.7 ± 14.6 |
| Apnea index (mean ± SD) | 3.6 ± 11.9 (median: 0.5) |
| Hypopnea index (mean ± SD) | 7.7 ± 8.9 (median: 4.5) |
| Maximum apnea duration (seconds) (mean ± SD) | 16.9 ± 19.3 (median: 14) |
| Max hypopnea duration (seconds) (mean ± SD) | 66.6 ± 37.8 |
| Number of desaturations (n) (mean ± SD) | 73 ± 130 (median: 34) |
| Minimum SaO2 (%) (mean ± SD) | 86.2 ± 7.5 |
| SaO2 under 90% (n) (mean ± SD) | 47 ± 130 (median: 4) |
| SaO2 under 80% (n) (mean ± SD) | 10 ± 42 (median: 0) |
AHI, apnea hypopnea index; min, minute; REM, rapid eye movement; SaO2, oxygen saturation; SD, standart deviation; sec, second.
Eighty-six percent of the patients had at least one of the essential symptoms of OSA, with the most frequently reported symptom being daytime sleepiness at a rate of 74.4%. The rate of patients with both snoring and daytime sleepiness was 86%. Among the main complaints, only the presence of witnessed apnea was statistically significantly higher in patients with OSA (33.3% vs. 15.7%, P = .01).
The features of the patients with and without OSA and their comparison are given in Table 3. A significant relationship of OSA was found with age (P = .01), duration of disease
Table 3.
Comparison of Patients with and Without OSA
| OSA (+) | OSA (−) | P | |
|---|---|---|---|
| M/F | 9/15 | 6/13 | .76 |
| Age (years) (mean ± SD) | 58 ± 13 | 45 ± 15 | .01 |
| Duration of disease (years) (mean ± SD) | 37.3 ± 19.8 | 23.7 ± 15.2 | .03 |
| BMI (kg/m2) (mean ± SD) | 26.8 ± 5.4 | 25.4 ± 5.9 | .93 |
| FVC (%) (mean ± SD) | 78 ± 21 | 80 ± 19 | .633 |
| FEV1 (%) (mean ± SD) | 64 ± 26 | 65 ± 22 | .760 |
| FEV1/FVC (mean ± SD) | 66 ± 14 | 67 ± 14 | .816 |
| Obstruction, n (%) | 15 (62.5) | 14 (73.7) | .523 |
| Restriction, n (%) | 11 (45.8) | 8 (42.1) | 1.000 |
| Main complaints, n (%) | 21 (87) | 16 (84) | .3 |
| Snoring | 18(75) | 12(63.1) | .82 |
| Witnessed apnea | 8(33.3) | 3(15.7) | .01 |
| Excessive daytime sleepiness | 19(79.1) | 13(68.4) | .33 |
| Colonization, n (%) | 11 (45.8) | 5 (26.3) | .161 |
| Bronchiectasis localization, n (%) | |||
| Upper lobe | 11 (45.8) | 9 (47.4) | 1.000 |
| Lower lobe | 14 (58.3) | 15 (78.9) | .325 |
| Number of lobes with bronchiectasis, n (mean ± SD) | 2.3 ± 1.7 | 2.4 ± 1.3 | .624 |
| Type of bronchiectasis, n (%) | |||
| Cystic | 4 (16.6) | 8 (42.1) | .08 |
| Cylindrical | 10 (41.6) | 7 (36.8) | .96 |
| Varicose | 3 (13.0) | 4 (21.0) | .51 |
| Traction | 4 (16.6) | 1 (5.3) | .12 |
| Epworth score (mean ± SD) | 6.1 ± 4.9 | 6.6 ± 4.4 | .50 |
| Stage 1 (%) (mean ± SD) | 7.8 ± 4.3 | 6.3 ± 4.8 | .12 |
| Stage 2 (%) (mean ± SD) | 53.0 ± 12.0 | 58.6 ± 16.1 | .31 |
| Stage 3 (%) (mean ± SD) | 22.2 ± 13.9 | 21.2 ± 10.4 | .81 |
| REM (%) (mean ± SD) | 16.8 ± 8.5 | 11.8 ± 5.0 | .03 |
BMI, body mass index; F, female; FVC, forced vital capacity; M, male; REM, rapid eye movement; SD, standard deviation.
(P = .03), and percentage of REM sleep (P = .03). The results of the analyses are given in Table 3.
A positive correlation of AHI with age (r = 0.51, P = .01) and duration of disease (r = 0.34, P = .03) was found in patients with bronchiectasis. When the factors found to be related to OSA in univariate analysis were examined, only the percentage of REM was significant (P = .03). The factor of age remained at the significance limit (P = .05). Significance disappeared for disease duration as for all other factors associated with bronchiectasis.
Discussion
In the present study, we found that the frequency of OSA in patients with bronchiectasis was 55.8% and related with age. In the study conducted by Junior et al.4 in 2017, the prevalence of OSA in patients with non-cystic fibrosis was found as 40.82%, lower than in our study. Similar to our study, they found a high frequency of OSA compared with the normal population.4 It has been reported in the literature that the prevalence of OSA increases with age.20 Faria Junior et al.4 found a significant relationship between age and the presence of OSA.4 In our study, we found the same relationship at the limit of significance. Also, the positive correlation between AHI and age supports the opinion that there may be a relationship between age and the presence of OSA. Moreover, it has been emphasized that the prevalence of OSA increased after the age of 65 years; the mean age of patients with OSA was found as 58 years in our study, indicating that OSA may occur in this group of patients at earlier ages, as reported in the literature.4
In the HypnoLaus study published in 2015, the prevalence of OSA was reported as 23% in women and 50% in men.3 The HypnoLaus study contains data about the general population, thus it can represent the OSA prevalence of the general population. In our study, OSA prevalence in patients with NCFB was reported as 55.8%. Considering the total prevalence of women and men in the HypnoLaus study, it can be said that OSA is more common in patients with NCFB. In the HypnoLaus study, the average AHI in patients aged between 40 and 60 years was 7.6, and the average AHI in those aged over 60 years was 14.3; a statistically significant difference was found between these groups.3 Similarly, in our study, the prevalence of OSA increased with age. The percentage of REM sleep is 20-25% in the normal population, whereas we found that the duration of REM sleep decreased in patients with bronchiectasis (mean: 14.6%).21 It is known that the duration of REM sleep decreases in patients with OSA.22 In our study, the percentage of REM sleep, however, was statistically significantly higher in the OSA group than in the group without OSA (16.8% vs. 11.8%). We believe that further studies are warranted to interpret how this effect occurs, because there are many uncertainties about REM sleep.
Regarding sputum colonization in the present study, the most commonly found pathogen was Pseudomonas aeruginosa with a rate of 23%. This rate was similar to that of 20.6% found in 121 patients with NCFB in our clinic in 2016.23 In the study by Faria Junior et al.,4 a relationship was found between the colonization of P. aeruginosa and the presence of OSA; however, we found no relationship.4
Faria Junior et al.4 measured daytime levels of sleepiness using the Berlin Scale and emphasized that it was more common compared with the normal population.4 In our study, essential symptoms of OSA were enquired individually (snoring, witnessed apnea, excessive daytime sleepiness) and their relationship with OSA was examined. Among the main complaints in our study, only witnessed apnea was more common in patients with OSA. There was no significant difference in excessive daytime sleepiness and snoring. Based on this, we consider that it is important to evaluate patients at advanced ages for the presence of OSA regardless of whether the paient has symptoms.
In studies on patients with bronchiectasis, the relationship of spirometric values with several parameters has been reported. No significant relationship was found between quality of sleep and FEV1% in the study by Gao et al.24 in 2014 on patients with bronchiectasis.24 In a 2017 study by Faria Junior et al.,4 a significant relationship of FEV1% was found with snoring time, oxygen desaturation index per hour, and peripheral oxygen saturation, but no significant relationship was found with other sleep parameters.4 In our study, we found no significant relationship between the presence of OSA and spirometric parameters (FEV1, FVC, and FEV1/FVC).
In the available studies, no emphasis has been made on the relationship between the extent of bronchiectasis and the presence of OSA in patients with bronchiectasis. In our study, no significant relationship was found between the presence of OSA and the type and extent of bronchiectasis.
One of the limitations of the present study was that the study was a single-center study. However, patients with bronchiectasis from many cities of Turkey present to our center, which has been a specialized follow-up center for bronchiectasis since 1996. Thus, it may be considered that the patient population in the present study might have comprised a homogenous group to represent the general population of Turkey. Another limitation is the absence of a control group. However, the results of studies conducted in the normal population were used while making comparisons.
Sleep disorders in patients with primary ciliary dyskinesia and cystic fibrosis have been assessed previously using several surveys and scales.9,10 By contrast, our study included patients with NCFB in whom PSG was used, which is the gold-standard in the diagnosis of OSA, and this was used to make the diagnosis. This was one of the strengths of our study.
In conclusion, the frequency of OSA in patients with NCFB is 55.8% and increases with age. The relationship of OSA with REM sleep and the response of these patients to CPAP treatment is another unknown topic. Future studies on these topics will be guiding.
Funding Statement
The authors declared that this study has received no financial support.
Footnotes
Ethical Committee Approval: This study was approved by Ethics committe of Istanbul University-Cerrahpasa, Cerrahpasa Medical Faculty, (Approval No: 23786442-604.01.01-102359).
Informed consent: Written informed consent was obtained from the patients who agreed to take part in the study.
Peer Review: Externally peer-reviewed.
Author Contributions: Supervision - S.B., Y.S., B.M.; Design – S.B., Y.S., B.M.; Resources – S.B., Y.S., D.O.H.; Materials – S.B., Y.S., D.O.H., B.M.; Data Collection and/or Processing – S.B., Y.S., D.O.H.; Analysis and/or Interpretation – S.B, BM.; Literature Search- S.B., Y.S., D.O.H.; Writing Manuscript – S.B., Y.S., D.O.H., B.M.; Critical Review - S.B., B.M.
Conflict of interest: The authors have no conflict of interest to declare.
References
- 1. Cao MT, Guilleminault C, Kushida CA. Clinical features and evaluation of obstructive sleep apnea and upper airway resistance syndrome. In: Principles and Practice of Sleep Medicine. Elsevier, Canada, 5th ed; 2011:1206–1218.. [Google Scholar]
- 2. Jennum P, Tønnesen P, Ibsen R, Kjellberg J. All-cause mortality from obstructive sleep apnea in male and female patients with and without continuous positive airway pressure treatment: a registry study with 10 years of follow-up. Nat Sci Sleep. 2015;7:43–50.. (doi:10.2147/NSS.S75166) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Heinzer R, Vat S, Marques-Vidal P. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 2015;3(4):310–318.. 10.1016/S2213-2600(15)00043-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Faria Júnior NS, Urbano JJ, Santos IR. Evaluation of obstructive sleep apnea in non-cystic fibrosis bronchiectasis: a cross-sectional study. PloS One. 2017;12(10):e0185413. 10.1371/journal.pone.0185413) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Weycker D, Hansen GL, Seifer FD. Prevalence and incidence of noncystic fibrosis bronchiectasis among US adults in 2013. Chron Respir Dis. 2017;14(4):377–384.. 10.1177/1479972317709649) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mysliwiec V, Pina JS. Bronchiectasis: the ‘other’ obstructive lung disease. Postgrad Med. 1999 July;106(1):123–6, 128., 128–131.. 10.3810/pgm.1999.07.607) [DOI] [PubMed] [Google Scholar]
- 7. Martinez-Garcia MA, Polverino E, Aksamit T. Bronchiectasis and chronic airway disease: it is not just About asthma and COPD. Chest. 2018;154(4):737–739.. 10.1016/j.chest.2018.02.024) [DOI] [PubMed] [Google Scholar]
- 8. Jankelowitz L, Reid KJ, Wolfe L. Cystic fibrosis patients have poor sleep quality despite normal sleep latency and efficiency. Chest. 2005;127(5):1593–1599.. 10.1378/chest.127.5.1593) [DOI] [PubMed] [Google Scholar]
- 9. Santamaria F, Esposito M, Montella S. Sleep disordered breathing and airway disease in primary ciliary dyskinesia. Respirology. 2014;19(4):570–575.. (doi:10.1111/resp.12273) [DOI] [PubMed] [Google Scholar]
- 10. Milross MA, Piper AJ, Norman M. Subjective sleep quality in cystic fibrosis. Sleep Med. 2002;3(3):205–212.. (doi:10.1016/s1389-9457(01)00157-5) [DOI] [PubMed] [Google Scholar]
- 11. Faria Júnior NSF, Oliveira LVF, Perez EA. Observational study of sleep, respiratory mechanics and quality of life in patients with non-cystic fibrosis bronchiectasis: a protocol study. BMJ Open. 2015;5(7):e008183. 10.1136/bmjopen-2015-008183) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Milliron B, Henry TS, Veeraraghavan S, Little BP. Bronchiectasis: mechanisms and imaging clues of associated common and uncommon diseases. RadioGraphics. 2015;35(4):1011–1030.. 10.1148/rg.2015140214) [DOI] [PubMed] [Google Scholar]
- 13. Hill AT, Sullivan AL, Chalmers JD. British Thoracic Society guideline for bronchiectasis in adults. BMJ Open Respir Res. 2018;5(1):e000348. 10.1136/bmjresp-2018-000348) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gramegna A, Aliberti S, Seia M. When and how ruling out cystic fibrosis in adult patients with bronchiectasis. Multidiscip Respir Med. 2018;13(suppl 1):29. 10.1186/s40248-018-0142-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Berry RB, Budhiraja R, Gottlieb DJ. Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8(5):597–619.. 10.5664/jcsm.2172) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Uzer F, Toptas AB, Okur U. Comparison of positional and rapid eye movement-dependent sleep apnea syndromes. Ann Thorac Med. 2018;13(1):42–47.. 10.4103/atm.ATM_184_17) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Omobomi O., Quan S.F. Positional therapy in the management of positional obstructive sleep apnea-a review of the current literature. Sleep Breath. 2018;22(2):297–304.. 10.1007/s11325-017-1561-y) [DOI] [PubMed] [Google Scholar]
- 18. Izci B, Ardic S, Firat H. Reliability and validity studies of the Turkish version of the Epworth Sleepiness Scale. Sleep Breath. 2008;12(2):161–168.. 10.1007/s11325-007-0145-7) [DOI] [PubMed] [Google Scholar]
- 19. Miller MR, Hankinson J, Brusasco V.ATS/ERS TASK FORCE. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338.. [DOI] [PubMed] [Google Scholar]
- 20. Jennum P, Riha RL. Epidemiology of sleep apnoea/hypopnoea syndrome and sleep-disordered breathing. Eur Respir J. 2009;33(4):907–914.. 10.1183/09031936.00180108) [DOI] [PubMed] [Google Scholar]
- 21. Carskadon MA, Dement WC. Normal human sleep. In: Principles and Practice of Sleep Medicine. 5th ed; 2011:16–26.. [Google Scholar]
- 22. Roche J, Gillet V, Perret F, Mougin F. Obstructive sleep apnea and sleep architecture in adolescents With severe obesity: effects of a 9-month lifestyle modification program based on regular exercise and a balanced diet. J Clin Sleep Med. 2018;14(6):967–976.. 10.5664/jcsm.7162) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Borekci S, Halis AN, Aygun G, Musellim B. Bacterial colonization and associated factors in patients with bronchiectasis. Ann Thorac Med. 2016;11(1):55–59.. 10.4103/1817-1737.172297) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gao Y, Guan W, Xu G. Sleep disturbances and health-related quality of life in adults with steady-state bronchiectasis. PloS One. 2014;9(7):e102970. 10.1371/journal.pone.0102970) [DOI] [PMC free article] [PubMed] [Google Scholar]


