Abstract
Background
Data on specific gastrointestinal (GI) motility disorders, such as gastroparesis (GP), chronic intestinal pseudo-obstruction (CIPO), and colonic inertia (CI), as well as awareness among doctors about these disorders are scanty in Asia.
Method
Prospectively maintained records of 60 patients were retrospectively analyzed, and knowledge, attitude, and practice (KAP) of 66 Indian physicians were surveyed electronically.
Results
A total of 60 (age 37.7 ± 18.4 years, 25 female) patients were included in the study (13 [21.7%] GP, 25 [41.7%] CIPO, 14 [23.3%] CI, and 8 [13.3%] overlap of GP and either CIPO [5] or CI [3]), of whom 40 had primary disorders and 20 had secondary disorders due to diabetes mellitus (n = 6), systemic sclerosis (n = 4), paraneoplastic (n = 2), infection (n = 3), Parkinson’s disease (n = 1), hypothyroidism (n = 1), hyperparathyroidism (n = 1), celiac disease (n = 1), and amyloidosis (n = 1). Primary disorders were more often misdiagnosed as functional GI disorders, causing diagnostic delays and complications, than secondary disorders. More patients in the primary disorder group underwent surgery compared with those in the secondary group (25/40, 62.5% vs 1/20, 5%). A few rare infectious causes of GI motility disorders due to Strongyloides stercoralis, herpesvirus, and unidentified viruses were found. Of four patients treated with pyridostigmine with (n = 3) or without prucalopride (n = 1), three responded. Awareness about GI motility disorders, particularly the primary disorders, among 66 doctors participating in the KAP survey was inadequate.
Conclusion
Awareness regarding specific GI motility disorders among physicians is lacking, which leads to delay in diagnosis and results in more complications in patients, such as surgery, particularly in those with primary disorders.
Keywords: Gastroparesis, pseudo-obstruction, colonic transit study, Hirschsprung disease
MAIN POINTS
Primary gastrointestinal (GI) motility disorders, such as gastroparesis and chronic intestinal pseudo-obstruction, are more often misdiagnosed as functional GI disorders, particularly during the initial stage of the disease, causing diagnostic delays, complications, and surgery than secondary disorders.
Awareness about GI motility disorders, particularly the primary disorders, among 66 doctors participating in the knowledge, attitude, and practice survey was inadequate.
A few rare infectious causes of GI motility disorders, such as Strongyloides stercoralis, herpesvirus, and unidentified viruses, were found in this study.
INTRODUCTION
Chronic gastrointestinal (GI) dysmotility may involve any part of the GI tract including the esophagus, stomach, small intestine, and colorectum. Achalasia cardia, gastroparesis (GP), colonic inertia (CI; a severe form of slow transit constipation), and pseudo-obstruction are a few of the well-defined motility disorders of the esophagus, stomach, and intestines, respectively. GP is a syndrome characterized by delayed emptying of gastric contents in the absence of mechanical obstruction with cardinal symptoms of vomiting, early satiety, bloating, and abdominal pain,1 while pseudo-obstruction is a syndrome characterized by signs and symptoms of mechanical obstruction of the small or large bowel in the absence of an anatomic lesion that obstructs the flow of intestinal contents.2 Pseudo-obstruction may be acute or chronic and is characterized by the presence of dilation of the bowel on imaging.2 When there is evidence of chronic small intestinal motility disorder in the absence of bowel dilatation, the preferred term is chronic intestinal dysmotility. CI, which presents with chronic constipation, is the failure of the colon to propel stool toward the rectum, including the failure to produce a mass movement around the time of defecation.3 The pathophysiology of these disorders involves compromise in one or more of the following components of neuromuscular coordination: extrinsic and intrinsic nervous system, smooth muscles, and the interstitial cells of Cajal (ICC).4-8 These alterations can be idiopathic or may be secondary to many different conditions that include neurological, endocrine, metabolic, autoimmune, and paraneoplastic syndromes and infectious diseases.2 While patients with achalasia cardia typically present with dysphagia and regurgitation, and the diagnosis is easily confirmed by the absence of mechanical obstruction on upper endoscopy and a typical pattern on esophageal manometry,9 a test that is widely available in India, awareness about GP, CI, and chronic intestinal pseudo-obstruction (CIPO) is less and the availability of the diagnostic tests and the necessary technical expertise for these disorders such as gastric emptying study, electrogastrography, colonic transit study, and manometry of antroduodenum, colon, and anorectum are scanty. Since some of these disorders, particularly when mild, may present with symptoms similar to those of functional GI disorders (FGID),2 possible misdiagnosis and inappropriate treatment are quite likely, which may increase diagnostic delay, morbidity, and unnecessary healthcare expenditure.
Data on these disorders from Asia, in general, and India, in particular, are scanty. Therefore, we undertook this retrospective study on prospectively maintained data to evaluate: (i) the clinical and etiological spectrum of GI motility disorders in a multilevel teaching hospital in northern India, (ii) how often these disorders are misdiagnosed as FGID and the reasons for that, and (iii) their management and outcome. We also surveyed Indian healthcare professionals to know the prevalent knowledge, attitude, and practice (KAP) concerning these disorders.
METHODS
Patients
Consecutive patients visiting the gastroenterology outpatient or inpatient facilities of a multilevel teaching hospital in northern India from January 2000 to January 2019 and satisfying the following criteria for GI motility disorders were analyzed retrospectively: (i) GP—suggestive symptoms and objective evidence of delayed gastric emptying obtained via radionuclide scan with or without bradygastria on electrogastrography/low amplitude duodenal contraction or antral hypomotility on antroduodenal manometey (ADM), (ii) CIPO—one or more episodes of intestinal obstruction in the absence of evidence of mechanical obstruction (on imaging and/or laparotomy), and (iii) CI (an extreme form of slow transit constipation)—chronic constipation with delayed colon transit time (CTT) as evidenced by retention of radio-opaque markers with normal anorectal manometry and defecography.10
Each patient’s data including sociodemographic variables, medical and surgical history, symptom onset and its progression, number of attacks, treatments received, and laboratory tests including imaging (barium studies, computed tomography, magnetic resonance imaging), endoscopy (esophagogastroduodenoscopy, colonoscopy, and balloon enteroscopy), motility studies, hematological and biochemical investigations, and histology (full-thickness biopsies of the bowel) were recorded. Patients were classified as primary or idiopathic CIPO (if an underlying cause of the disease was not identified) and as secondary CIPO (with an identifiable cause of the motility disorder).2 The study was performed in a manner that conforms with the Helsinki Declaration of 1975, as revised in 2000 and 2008, concerning human rights. GP patients’ symptoms were graded retrospectively using Abell grading system as described earlier.11 Briefly, mild intermittent symptoms were graded as 1, moderately severe symptoms without weight loss were graded as 2, and refractory symptoms with inability to maintain nutrition with frequent hospital visits were graded as 3.11
Investigations
In addition to hematological and biochemical investigations, the patients underwent specific motility tests based on the clinical evaluation suggesting specific organ involvement and on the discretion of the treating clinicians.
Esophageal manometry
If dysphagia was one of the symptoms, conventional (before October 2010) or high-resolution water perfusion esophageal manometry was performed and interpreted using a standard technique.9
Gastric emptying study
Radionuclide gastric-emptying study for solids was performed with Tc-99m sulfur colloid-labeled Indian bread standardized earlier at our center, considering vegetarianism as common practice in our population.12 Images were obtained on a camera, and time-activity curves were generated as per the standard method. Emptying was considered delayed as per standard criteria.12,13
Antroduodenal manometry
In patients with symptoms suggestive of GP/pseudo-obstruction, ADM was performed after an overnight fast using a water perfusion system by a standard technique (RedTech, Calabasas CA, USA). A low compliance polyvinyl catheter with eight side holes, placed 3 cm apart, was passed through the nose via a guidewire and two upper ports were placed in the antrum of the stomach and the remaining in the duodenum under fluoroscopic guidance.14 Normal and abnormal manometric contraction patterns were defined according to previously defined criteria.2
Colonic transit study
CTT assessment using indigenous radio-opaque markers was performed in patients with chronic constipation using a protocol developed and validated by us for the Indian population.15 Briefly, 20 markers were administered each at 0, 12, and 24 h and abdominal radiographs were obtained at 36 and 60 h. Retention of more than 30 and 14 markers at 36 and 60 h, respectively, was considered abnormal.15
Anorectal function tests
In patients with chronic constipation, in addition to CTT assessment, anorectal manometry, balloon expulsion test, and defecography were performed using standard techniques.16,17
Etiological work-up
Evaluation for the cause of motility disorders was undertaken based on the discretion of the clinicians. Most patients included in this study underwent tests for blood sugar, HbA1C, thyroid function, and serum calcium. Work-up for underlying malignancy, amyloidosis (examination of duodenal/rectal/abdominal fat pad after congo red staining), celiac disease (anti-tissue transglutaminase or anti-endomysial antibodies and histology of duodenal biopsy), and mitochondrial myopathy was undertaken on the discretion of the clinician.
Investigations for complications
Appropriate investigations for possible complications of GI dysmotility such as small intestinal bacterial overgrowth (SIBO, by glucose hydrogen breath test and/or quantitative upper gut aspirate culture)18 and intestinal malabsorption (urinary d-xylose test and fecal fat estimation) were undertaken as indicated.19
Treatment and Follow-up
Treatment was based on the predominant symptoms and their severity. In addition to nutritional and supportive measures (such as treating the underlying disease due to secondary causes including diabetes mellitus, scleroderma, Parkinson’s disease, hypothyroidism, celiac disease, hyperparathyroidism, and various infections), specific treatment included prokinetics (e.g., metoclopramide, domperidone, itopride, mosapride, cisapride, and prucalopride) with or without proton pump inhibitors. GP patients not responding to single or multiple standard prokinetic therapy were treated with endoscopic pyloric botulinum toxin injection and repeated as and when needed based on symptoms. Cholinesterase inhibitors, including intravenous (IV) neostigmine and oral pyridostigmine, were used in the acute presentation of pseudo-obstruction and maintenance purposes in patients with CIPO, respectively. Those patients who presented with acute pseudo-obstruction were subjected to colonic decompression if they failed to respond with IV neostigmine. The parameters were evaluated for a response during follow-up visits or telephonically, which included relief from abdominal distension, sub-acute intestinal obstruction, vomiting, constipation, avoidance of surgery. Patients were followed-up at the Luminal Gastroenterology Clinic of the Department of Gastroenterology with appropriate investigations as indicated.
Classification of Patients
Patients were categorized into upper (GP), lower (CIPO), and pan-GI disease groups and then into primary and secondary GI motility disorder groups based on the involvement of the organs and on whether causes could be identified.
Survey on Knowledge, Attitude, and Practice About GI Motility Disorders Among Doctors
From February to March 2020, a survey was undertaken among Indian healthcare professionals using a questionnaire on an electronic platform (SurveyMonkey® Enterprise, San Mateo, CA) and the data were analyzed.
Statistical Analysis
The data on demographic variables including age, duration of symptoms, follow-up, and the total number of investigations that the patients underwent before the diagnosis was made were recorded. Continuous data were expressed as mean and standard deviation if normally distributed or median and range, if not. Continuous data were analyzed by unpaired t-test or Mann–Whitney U-test depending on the distribution. Categorical variables were compared using the Chi-squared test or Fisher’s exact test, as applicable. Data were analyzed using R, Epicalc, and R-Studio software (R development core team, Vienna, Austria) and Statistical Package for the Social Sciences (SPSS), version 15 (SPSS, Inc., Chicago, IL, USA).
RESULTS
Demographic, Clinical, and Etiological Spectrum
Of the 60 (mean age 37.7 ± 18.4 years, 25 [41.7%] female) patients included, 13 (21.7%), 25 (41.7%), 14 (23.3%), and 8 (13.3%) had GP only, CIPO only, CI/slow transit constipation, overlap between gastroparesis and either CIPO (n = 5) or CI (n = 3), respectively. Most patients had idiopathic (primary; n = 40 [66.7%]) and the remaining (n = 20 [33.3%]) had secondary dysmotility (Table 1). Although most patients with primary dysmotility had idiopathic disease (30), 8, and 1 each had Hirschsprung’s disease (HD), GI neuronal dysplasia, mitochondrial neuro-GI encephalopathy (reported earlier) (20), respectively (Figure 1). The causes of secondary dysmotility are also shown in Figure 1.
Table 1.
Differences in Demographic and Clinical Parameters of Patients with Primary and Secondary Gastrointestinal Motility Disorders
| Primary (n = 40) | Secondary (n = 20) | P | |
|---|---|---|---|
| Age (years, mean, SD) | 31.5 (15) | 50.2 (18.0) | <.001 |
| Sex (female) | 14/40 (35%) | 11/20 (55%) | .14 |
| Duration of symptom (years, mean, IQR) | 7.71 (2, 12.7) | 4.1 (0.87, 7.5) | .045 |
| Previous diagnosis of functional gastrointestinal disease | 25 (62.5%) | 5 (25%) | .01 |
| Functional dyspepsia | 1 (2.5%) | 1 (5%) | |
| IBS | 2 (5%) | 2 (10%) | |
| Cyclical vomiting syndrome | 1 (2.5%) | 0 (0%) | |
| Functional constipation | 21 (52.5%) | 2 (10%) | |
| Same diagnosis as before | 2 (5%) | 11 (55%) | <.0001 |
| Prior diagnosis of mechanical obstruction | 9 (22.5) | 2 (10) | NS |
| Number of investigations prior to diagnosis (mean, SD) | 6.46 (2.28) | 4.6 (1.9) | .003 |
| Associated symptoms/disorders | 15 (37.5%) | 18 (90%) | .00003 |
| Dysphagia | 5 (12.5%) | 4 (20%) | |
| Psychiatric co-morbidities | 2 (5%) | 1 (5%) | |
| Urinary symptoms | 2 (5%) | 0 (0%) | |
| Seizure | 1 (2.5%) | 0 (0%) | |
| Mental retardation | 2 (5%) | 0 (0%) | |
| Polyneuropathy | 3 (7.5%) | 8 (40%) | |
| Extra-pyramidal involvement | 0 (0%) | 1 (5%) | |
| Raynaud’s | 0 (0%) | 4 (20%) | |
| Complications | 12/25 (48%) | 2/12 (16.6%) | .06 |
| Biochemical malabsorption |
6/10 (60%) | 1/4 (25%) |
NS |
| Small intestinal bacterial overgrowth |
6/15 (40%)* | 1/8 (12.5%)** | NS |
*4 by GHBT and 2 by upper gut aspirate culture; **by GHBT only.
SD, standard deviation; IQR, inter-quartile range; IBS, irritable bowel syndrome; NS, not significant; GHBT, glucose hydrogen breath test.
Figure 1.
Outline showing the symptoms, associated disorders, and causes of secondary and primary gastrointestinal motility disorders. SAIO, subacute intestinal obstruction; EJG, esophago-gastric junction; HD, Hirschsprung’s disease; GND, gastrointestinal neuronal dysplasia; MNGE, mitochondrial neuro-gastrointestinal encephalopathy; VN, visceral neuropathy; VM, visceral myopathy; DM, diabetes mellitus; PSS, progressive systemic sclerosis; PN, paraneuoplastic.
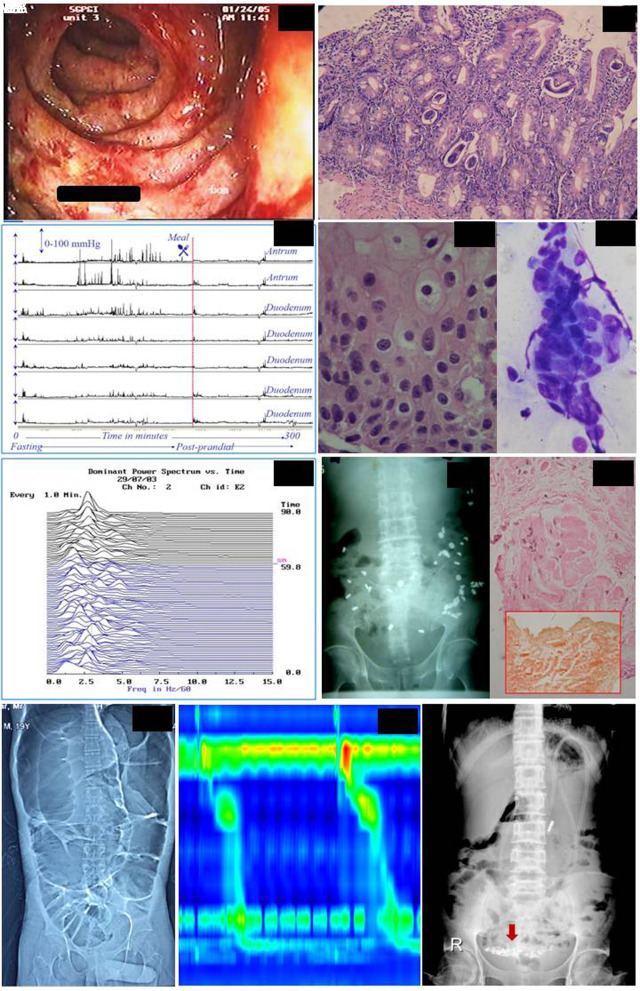
A few patients with secondary dysmotility, some of whom have been reported earlier, were unique; these included three patients who had infection-related conditions. An immunocompetent patient with varicella-zoster and multiple cranial nerve paralysis had marked esophageal and antroduodenal hypomotility (Figure 2);21 he improved with supportive treatment, prokinetics, and IV acyclovir. A 32-year-old man with lepra reaction while on treatment for lepromatous leprosy treated with prednisolone (70 mg/day) for one month presented with chronic constipation, severe lower abdominal pain, and distension.22 The patient had palpable bowel loops and visible peristalsis on abdominal examination and dilated small and large bowel loops on radiography without evidence of mechanical obstruction. His full-length colonoscopy was normal; esophagogastroduodenoscopy revealed ulcerated edematous duodenal mucosa, and the biopsy showed multiple adults and larval forms of Strongyloides stercoralis (Figure 2). The patient was treated with ivermectin 12 mg/day for two days and prokinetics, following which his symptoms resolved over three months. A repeat duodenal biopsy three months later did not show any worm. The third patient (a 62-year-old female) presented with GP and CI following an acute viral-like illness with fever six months ago. Investigation revealed delayed gastric emptying, normal esophagogastroduodenoscopy, and slow colon transit. She improved over the next three months with prokinetic treatment. Yet another patient with leprosy with slow-transit constipation had fatal systemic amyloidosis, which is a known complication of leprosy.
Figure 2.
A 32-year-old male with constipation, abdominal pain, distension, palpable bowel loops, visible peristalsis, dilated bowel loops on radiography, ulcerated duodenal mucosa on upper gastrointestinal endoscopy (IA) and Strongyloides stercoralis on biopsy (IB). Patient with varicella-zoster and multiple cranial nerve paralysis had marked esophageal and antroduodenal hypomotility. A 50-year-old man with varicella-zoster and multiple cranial nerve paralysis had marked esophageal and antroduodenal hypomotility with failure of conversion to fed pattern, suggestive of visceral neuropathy (IIA). Tzanck smear from esophageal tissue showed intra-nuclear inclusion bodies (IIB, IIC; H&E, ×400). Percutaneous electrogastrography showing bradygastria in patients presenting with gastroparesis and the cause later turned who turned out to be cholangiocarcinoma (III; X-axis: frequency of gastric myoelectrical activity in cpm, Y-axis: time in minute. “SM” = start meal). Colon transit study in a patient with chronic constipation showing slow-transit (IVA). Rectal biopsy shows amyloidosis (IVB). Computerized tomography of a patient with primary chronic intestinal pseudo-obstruction showing dilated bowel loops (VA). High-resolution esophageal manometry (Multiplex, Alacer Biomedica, São Paulo, Brazil) in the same patient showing long peristaltic break (VB). Abdominal radiograph of a patient with mitochondrial neuro-gastrointestinal encephalopathy showing coiled feeding tube in the hugely dilated stomach (VI).
Differences Between Primary and Secondary GI Dysmotility
As shown in Table 1, patients with primary dysmotility were younger, had a longer duration of symptoms, more often misdiagnosed as FGIDs by primary and secondary care doctors, underwent more investigations compared to those with secondary dysmotility, though their symptom profile was comparable.
Differences Between Upper and lower GI Motility Disorders
As shown in Table 2, patients with upper GI motility disorders (primarily GP) had a shorter duration of symptoms before diagnosis, less often diagnosed as FGIDs, underwent a lesser number of investigations, and underwent surgery less often as compared to those with lower GI motility disorders. The clinical and laboratory parameters of patients with GP are summarized in Table 4.
Table 2.
Differences in Clinical Parameters and Management in Patients with Upper and Lower Gut Disorders
| Upper Gut Disorders (n = 18) | Lower Gut Disorders (n = 42) | P | |
|---|---|---|---|
| Main symptom | Vomiting (13/18) | Constipation (26/42) | <.001 |
| Duration of symptom (year, mean, SD) | 3.3 (3.36) | 7.1 (6.55) | .018 |
| Labeled as FGID | 6 (33%) | 22 (52.4%) | .001 |
| Number of investigations prior to diagnosis (mean, SD) | 4.45(2) | 6.5 (2.07) | .001 |
| Response to medical management | 7/18 (50%) | 14/42 (41%) | .898 |
| Neostigmine (IV) | 1/3 | ||
| Pyridostigmine | 3/4 | ||
| Prucalopride | 7/8 | ||
| Pyridostigmine+prucalopride | 3/3 | ||
| Itopride | 3/7 | 0/1 | |
| Mosapride | 0/1 | 1/1 | |
| Cisapride | 1/2 | ||
| Metoclopramide | 1/1 | ||
| Domperidone | 1/1 | ||
| Levosulpiride | 2/2 | ||
| Botulinum toxin | 2/2 | ||
| NJ feed | 1/1 | ||
| Enema | 0/2 | ||
| Laxatives | 3/11 | ||
| GFD | 1/1 | ||
| Number of surgery (mean, SD) | 20 (0.20) | 40 (1.4) | .006 |
| Response to surgery | 0/3 (0%) | 14/23 (60.8%) | .012 |
SD, standard deviation; FGID, functional gastrointestinal disorder; NJ, nasojejunal; IV, intravenous; GFD, gluten-free diet.
Table 4.
Clinical and Laboratory Parameters of Patients with Gastroparesis
| Age (years) | Sex | Clinical Setting | Vomiting | Weight Loss | Basis for Diagnosis | Abell Score | Treatment |
|---|---|---|---|---|---|---|---|
| 28 | M | GJ | Yes | Yes | Hypomotility on ADM | 3 | Single prokinetic |
| 58 | M | Diabetes mellitus with multiple complications, CAD | Yes | Yes | Hypomotility on ADM, GE study | 3 | Single prokinetic* |
| 30 | M | GJ | Yes | Yes | Bradygastria on EGG, Hypomotility on ADM | 3 | GJ dismantling |
| 58 | F | Diabetes mellitus with TIA | Yes | No | GE study | 2 | Pyloric botulinum toxin |
| 13 | M | Idiopathic | Yes | No | Hypomotility on ADM | 1 | Single prokinetic |
| 14 | F | Idiopathic | Yes | No | Hypomotility on ADM, GE study | 2 | Multiple prokinetics |
| 49 | M | Paraneoplastic | Yes | No | Hypomotility on ADM | 2 | Single prokinetic* |
| 12 | F | Idiopathic | Yes | No | Hypomotility on ADM, GE study | 2 | Single prokinetic |
| 29 | F | Cerebral palsy, mental retardation, Barter syndrome | Yes | yes | GE study | 3 | Multiple prokinetics |
| 42 | F | Hypothyroidism | Yes | No | GE study | 2 | Pyloric botulinum toxin |
| 50 | M | Varicella-zoster cranial polyneuropathy, esophageal hypomotility** | Yes | No | Hypomotility on ADM | 1 | Multiple prokinetics, acyclovir, PEG feeding, parenteral nutrition |
| 39 | F | Diabetes mellitus with neuropathy | Yes | No | GE study | 2 | Single prokinetic |
| 82 | F | Diabetes mellitus with neuropathy | Yes | Yes | Hypomotility on ADM | 3 | Single prokinetic |
*Died of the illness. **Published previously Ref.21 None of the patients had structural lesions explaining recurrent vomiting on upper gastrointestinal endoscopy.
M, male; F, female; CAD, coronary artery disease; TIA, transient ischemic attack; ADM, antroduodenal manometry; GE, gastric emptying; EGG, electrogastrography; GJ, gastrojejunostomy; PEG, percutaneous endoscopic gastrostomy.
Investigations
Table 5 shows the results of endoscopy and motility tests undertaken in these patients. Four patients had achalasia on esophageal manometry (three of them had CI and one had HD, an association rarely reported earlier); one other patient with HD had esophagogastric junction outflow obstruction; eight patients showed large peristaltic breaks. Six, five, and one each had antral hypomotility, absent migratory motor complex, and myopathy and neuropathy on ADM, respectively. Electrogastrography showed bradygastria in four patients. Anorectal manometry showed absent recto-anal inhibitory reflex in seven and pubo-rectal dyssynergia in one patient.
Table 5.
Different Investigations and Their Results
| Investigations | Primary (n = 40) | Secondary (n = 20) |
|---|---|---|
| Upper Endoscopy (54) Normal |
33 |
18 |
| Abnormal | 2 | 1 |
| Colonoscopy (33) Normal |
21 |
12 |
| Gastric emptying for solid (25) Normal |
7 |
2 |
| Abnormal | 7 | 9 |
| Esophageal manometry (26) Normal |
4 |
5 |
| Abnormal | 10 | 7 |
| Antroduodenal manometry (26) Normal |
7 |
6 |
| Abnormal | 8 | 5 |
| CTT (37) Normal |
0 |
2 |
| Abnormal | 23 | 12 |
| Anorectal manometry (35) Normal |
16 |
11 |
| Abnormal | 7 | 1 |
| ANA (27) Negative |
14 |
4 |
| Positive | 4 | 5 |
| Uroflowmetry (9) Normal |
5 |
0 |
| Abnormal | 4 | 0 |
CTT, colon transit time; ANA, antinuclear antibody.
Treatment and Outcome
Patients were followed-up for a mean period of 4 ± 3.2 years. Acute episodes of pseudo-obstruction were treated initially with IV neostigmine 2 mg; those in whom it failed or was contraindicated, endoscopic colonic decompression and surgery were undertaken based on the physician’s discretion. Medical treatment included prokinetics (domperidone, metoclopramide, itopride, mosapride, cisapride [before it was withdrawn], pyridostigmine, prucalopride, laxatives, and enemas). SIBO, if detected, was treated with rifaximin. In patients with secondary motility disorders, specific treatments such as a gluten-free diet for celiac disease, specific anti-infective agents for infective conditions, anti-diabetic medication, and thyroid hormone replacement were given. The various treatment methods administered are summarized in Figure 3. Only two patients in the primary group required parenteral nutritional support while the rest were on either oral or enteral feeding.
Figure 3.

The outline of treatment and outcome of the patients.
Four patients were treated with pyridostigmine, three in the primary lower GI motility disorder group and one in the secondary group. Three of them received IV neostigmine initially to treat an acute attack of intestinal pseudo-obstruction. Of these four patients, three received a combination with prucalopride. Two out of three patients receiving a combination of pyridostigmine and prucalopride and one patient receiving pyridostigmine alone responded (two primary CIPO, one primary CI, and one secondary CI). Three patients received prucalopride alone (two primary and one secondary, all responded) while four received combination with either pyridostigmine (3) or after failure of octreotide (all responded except one in the pyridostigmine combination group).
Complications
Complications, including SIBO and malabsorption, tended to be commoner among patients with primary than secondary motility disorders (Table 1). Only two patients in the primary group required parenteral nutrition support while the rest were either on oral or enteral feeding.
A total of seven patients (four in the secondary and three in the primary groups) died during follow-up. All three patients in the primary group died of surgical complications (two with CI due to perforation peritonitis following the development of adhesion within one year of colectomy, and the third with mitochondrial neuro-gastrointestinal encephalopathy died of post-operative sepsis). In the secondary group, all four patients died of complications of the primary disease.
Survey on Knowledge, Attitude, and Practice About GI Motility Disorders Among Doctors
In total, 66 doctors (60 male, 6 female) completed the survey; of them, 25 were gastroenterologists, 10 trainee gastroenterologists, 25 general physicians, and 7 unspecified. A total of 23 out of 66 (35%) reported that they did not consider the diagnosis of GP in their practice during the last two years. A total of 20 out of 60 (33.3%) did not even consider the diagnosis of GP in patients presenting with dyspepsia-like symptoms though they did consider it among patients with diabetes mellitus, hypothyroidism, and neurological illnesses. In response to the question “What would be your diagnosis when someone comes to you with a one-year history of occasional vomiting, epigastric pain, epigastric fullness without significant weight loss?” 32/60 (53.3%) answered functional dyspepsia (FD), 13 (21.7%) gastroesophageal reflux disease, and only 15 (25%) idiopathic GP, but when the same doctor was asked to diagnose if the same patient had long-standing diabetes mellitus with a syncopal attack, 58/60 (96.7%) diagnosed GP, and only two (3.3%) diagnosed FD. Similarly, in response to the question, “In a patient who presents with chronic constipation, how often do you consider the diagnosis of CI/visceral myopathy/visceral neuropathy?” 26/60 (43.3%) answered “never” or “hardly ever”; 18/60 doctors did not consider the assessment of CTT important in such patients.
DISCUSSION
In the present study, we report the spectrum of GI motility disorders in a tropical and subtropical region and found a few causes of secondary disorders due to unusual infections. The study also revealed that specific primary disorders are commonly misdiagnosed as FGIDs, particularly during the early stage of the disease, delaying their diagnosis for a long period due to lack of awareness among physicians. The efficacy of pyridostigmine in CIPO supporting our data was reported earlier.23
Data on the spectrum of specific GI motility disorders, particularly from tropical and subtropical countries, are scanty. In a British study on 20 patients, all of whom had primary motility disorders, 80% were initially thought to have mechanical obstruction or refractory constipation.24 Most patients in that series required surgery (90%), while in our study, only 63% (25/40 in the primary group) underwent surgery. In a national survey from Japan on a large number of patients (n = 103; primary, 86 [83.5%]; secondary, 15 [14.5%]), the primary group was younger and surgical treatment was common for lower GI motility disorders; these findings are somewhat similar to our data.25 In another Chilean series on 64 patients (54 primary), 32% presented with chronic constipation, a proportion much lower than in our series.26 Seventy-six percent in that series underwent surgery compared to an overall rate of 43% in our study. Eight out of 60 (13%) patients in our series had HD. HD has been rarely reported in adults.27
In our series, we found unusual infections in three patients causing GI dysmotility; these include Herpes zoster, S. stercoralis, and an unidentified viral infection. Debinski et al. reported that three of 13 patients with CIPO had viral DNA (two had Epstein–Barr virus and one had cytomegalovirus) in resected intestinal tissue.28 In our series, we had a patient in whom Varicella zoster, which involves nerves, was associated with evidence of GI dysmotility. There are only occasional case reports on such an association.29 Our case of S. stercoralis infestation presenting as pseudo-obstruction is not entirely unexpected as this parasite invades the muscle wall. S. stercoralis has been reported rarely to present with intestinal dysmotility.30,31
As the symptoms in the early phase of GI motility disorders are quite nonspecific and may mimic those of FGIDs, misdiagnosing these as FGIDs is quite expected. Although about one-third of patients with FD may have some degree of delayed gastric emptying ,32 clinical presentation such as repeated vomiting, weight loss, the presence of underlying disease predisposing to GP, and the course of our patients clearly show that they had GP and not FD (Table 4). Recurrent vomiting and weight loss are somewhat uncommon in patients with FD. A study on 20 patients, 13 of whom needed parenteral nutrition in later stages, showed a considerable delay in diagnosis; these patients had to literally struggle to convince others that their symptoms were organic rather than functional.33 The authors concluded that healthcare professionals need to be aware of these specific GI motility disorders for early diagnosis. Our survey among doctors revealed the same conclusion, particularly for patients with primary GI motility disorders; for example, most physicians did not consider the possibility of GP in a patient presenting with dyspeptic symptoms but the moment they came to know that the patient was suffering from diabetes mellitus for a long time and had a syncopal attack, 97% considered GP as the most likely diagnosis. Similarly, in patients with chronic constipation, 43% of doctors did not consider CI and visceral myopathy or neuropathy to be the possibilities. Almost one-third did not consider that an assessment of CTT is needed. It is important to note that the Rome criteria are known to help in the diagnosis of FGIDs with a reasonable degree of accuracy. In addition to the lack of awareness about primary motility disorders such as GP and pseudo-obstruction, nonadherence and lack of familiarity about well-accepted criteria for a diagnosis of FGIDs34 might have led to misdiagnosis of the specific GI motility disorders as FGIDs at least in the early stages. Though familiarity with Rome criteria is reasonable among gastroenterologists, the same is not true for physicians in India.34 Delay in recognizing specific motility disorders and structural abnormalities causing chronic constipation have also been shown in a pediatric population.35 However, it is important to mention here that chronic idiopathic constipation and CI may be within the spectrum of the same disorder, and unless clinicians undertake an assessment of CTT, particularly in patients with difficult to treat patients, it would be difficult to differentiate.
Acetylcholine, an excitatory neurotransmitter of the enteric nervous system, is an important molecule for GI motility. Acetylcholinesterase inhibitors (CIs) inhibit the degradation of acetylcholine in the synaptic cleft and, hence, are effective in patients with GI hypomotility.36 IV neostigmine is effective in acute intestinal pseudo-obstruction.37 However, the data on the use of its oral congener, pyridostigmine, are limited to a few case reports and series.23,38 Pyridostigmine is also useful in patients with SIBO due to human immunodeficiency virus-related autonomic neuropathy, though the study was uncontrolled.39 In the current study, three of four patients receiving pyridostigmine with or without prucalopride improved. Interestingly, most of them responded to the initial acute episode of pseudo-obstruction to parenteral neostigmine. Prucalopride is known to work synergistically with pyridostigmine.40 Randomized controlled trials comparing pyridostigmine with and without prucalopride and placebo in patients with CIPO are urgently needed.
The retrospective design is a limitation of the study. Though the data are derived primarily from clinical experience, which by itself provides good clinical insight, we further analyzed the data statistically to generate a hypothesis of clinical significance. However, this is real-life data of a reasonably large cohort of patients from tropical and subtropical regions showing a few unusual causes. Our findings showing specific primary motility disorders to be often misdiagnosed as FGIDs delaying their diagnosis, possibly due to lack of awareness among physicians, may be an eye-opener for increasing awareness about these disorders. Our experience showing the efficacy of pyridostigmine highlights the need for randomized controlled trials on this issue.
Table 3.
Differences in the Treatment Employed and Outcome in Patients with Primary Versus Secondary Gastrointestinal Motility Disorders
| Primary (n = 40) | Secondary (n = 20) | P | |
|---|---|---|---|
| Response to medical management | 11/40 | 10/20 | NS |
| Colonic decompression | 4 | 0 | NS |
| Surgery | 25/40 | 1/20 | <.001 |
| Response to surgical management | 13/40 | 1/20 | .001 |
| Duhamel’s procedure (Hirschprung’s disease) | 7 (absent ganglion cell) | 0 | |
| Left hemicolectomy | 2 (1 absent ganglion cell, 1 normal) | 0 | |
| Subtotal colectomy | 1 (visceral neuropathy) | 0 | |
| Total colectomy | 5 (3: normal, 1: intestinal neuronal dysplasia) | 1 (normal) | |
| Segmental resection | 2 (histopathology not available) | 0 | |
| Gastrojejunostomy | 1 | 0 | |
| Duodenojejunostomy | 2 | 0 | |
| Exploratory laparotomy | 3 (1-visceral myopathy, rest not available) | 0 | |
| Diagnostic laparotomy | 2 ( non-diagnostic) | 0 | |
| Mortality | 3 | 4 | NS |
NS, not significant.
Funding Statement
The authors declared that this study has received no financial support.
Footnotes
Ethics Committee Approval: The authors declare that the study was performed to conform to the Helsinki Declaration of 1975, as revised in 2000 and 2008, concerning human and animal rights, and the identity of each study participant was kept anonymous.
Informed Consent: N/A.
Peer Review: Externally peer-reviewed.
Author Contributions: Concept – U.C.G.; Design – U.C.G.; Data Collection and/or Processing – U.C.G., B.V.; Analysis and/or Interpretation – U.C.G.; Technical Support – A.M.; Writing – U.C.G., B.V.
Acknowledgments: The authors thank the Departments of Pathology and Microbiology for their support in diagnostic work-up of patients.
Conflict of Interest: The authors have no conflict of interest to declare.
References
- 1. . Camilleri M, Parkman HP, Shafi MA.et al. Clinical guideline: management of gastroparesis. Am J Gastroenterol. 2013;108(1):18–37.. 10.1038/ajg.2012.373). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. . Ghoshal UC. Small intestinal motility disorders. In: Rao SSC, Lee YY, Ghoshal UC.eds. Clinical and Basic Neurogastroenterology and Motility. San Diego: Academic Press Elsevier; 2020:319–329.. [Google Scholar]
- 3. . Tillou J, Poylin V. Functional disorders: slow-transit constipation. Clin Colon Rect Surg. 2017;30(1):76–86.. 10.1055/s-0036-1593436). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. . De Giorgio R, Sarnelli G, Corinaldesi R, Stanghellini V. Advances in our understanding of the pathology of chronic intestinal pseudo-obstruction. Gut. 2004;53(11):1549–1552.. 10.1136/gut.2004.043968). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. . Bassotti G, Villanacci V. Slow transit constipation: a functional disorder becomes an enteric neuropathy. World J Gastroenterol. 2006;12(29):4609–4613.. 10.3748/wjg.v12.i29.4609). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. . Bassotti G, Villanacci V, Creţoiu D, Creţoiu SM, Becheanu G. Cellular and molecular basis of chronic constipation: taking the functional/idiopathic label out. World J Gastroenterol. 2013;19(26):4099–4105.. 10.3748/wjg.v19.i26.4099). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. . Battaglia E, Bassotti G, Bellone G.et al. Loss of interstitial cells of Cajal network in severe idiopathic gastroparesis. World J Gastroenterol. 2006;12(38):6172–6177.. 10.3748/wjg.v12.i38.6172). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. . Cohen M, Cazals-Hatem D, Duboc H.et al. Evaluation of interstitial cells of Cajal in patients with severe colonic inertia requiring surgery: a clinical-pathological study. Colorectal Dis. 2017;19(5):462–467.. 10.1111/codi.13511). [DOI] [PubMed] [Google Scholar]
- 9. . Kahrilas PJ, Bredenoord AJ, Fox M.et al. The chicago classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil. 2015;27(2):160–174.. 10.1111/nmo.12477). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. . Bassotti G, Roberto GD, Sediari L, Morelli A. Toward a definition of colonic inertia. World J Gastroenterol. 2004;10(17):2465–2467.. 10.3748/wjg.v10.i17.2465). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. . Abell TL, Bernstein RK, Cutts T.et al. Treatment of gastroparesis: a multi-disciplinary review. Neurogastroenterol Motil. 2006;18(4):263–283.. 10.1111/j.1365-2982.2006.00760.x). [DOI] [PubMed] [Google Scholar]
- 12. . Mittal BR, Dhiman RK, Maini A, Sewatkar AB, Das BK. Gastric emptying in patients with non ulcer dyspepsia (dysmotility type). Trop Gastroenterol. 1997;18(2):67–69.. [PubMed] [Google Scholar]
- 13. . Tougas G, Eaker EY, Abell TL.et al. Assessment of gastric emptying using a low fat meal: establishment of international control values. Am J Gastroenterol. 2000;95(6):1456–1462.. 10.1111/j.1572-0241.2000.02076.x). [DOI] [PubMed] [Google Scholar]
- 14. . Ghoshal UC, Paliwal M, Das K.et al. Antroduodenal manometry: experience from a tertiary care center. Indian J Gastroenterol. 2008;27(2):53–57.. [PubMed] [Google Scholar]
- 15. . Ghoshal UC, Gupta D, Kumar A, Misra A. Colonic transit study by radio-opaque markers to investigate constipation: validation of a new protocol for a population with rapid gut transit. Natl Med J India. 2007;20(5):225–229.. [PubMed] [Google Scholar]
- 16. . Ghoshal UC. Chronic constipation in Rome IV era: the Indian perspective. Indian J Gastroenterol. 2017;36(3):163–173.. 10.1007/s12664-017-0757-1). [DOI] [PubMed] [Google Scholar]
- 17. . Ghoshal UC, Sachdeva S, Pratap N.et al. Indian consensus on chronic constipation in adults: A joint position statement of the Indian Motility and Functional Diseases Association and the Indian Society of Gastroenterology. Indian J Gastroenterol. 2018;37(6):526–544.. 10.1007/s12664-018-0894-1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. . Ghoshal UC, Baba CS, Ghoshal U.et al. Low-grade small intestinal bacterial overgrowth is common in patients with non-alcoholic steatohepatitis on quantitative jejunal aspirate culture. Indian J Gastroenterol. 2017;36(5):390–399.. 10.1007/s12664-017-0797-6). [DOI] [PubMed] [Google Scholar]
- 19. . Ghoshal UC, Mehrotra M, Kumar S.et al. Spectrum of malabsorption syndrome among adults & factors differentiating celiac disease & tropical malabsorption. Indian J Med Res. 2012;136(3):451–459.. [PMC free article] [PubMed] [Google Scholar]
- 20. . Karyampudi A, Srivastava P, Mandal K.et al. Novel sequence variations in the thymidine phosphorylase gene causing mitochondrial neurogastrointestinal encephalopathy. Clin Dysmorphol. 2016;25(4):156–162.. 10.1097/MCD.0000000000000137). [DOI] [PubMed] [Google Scholar]
- 21. . Paliwal M, Prasanna KS, Saraswat VA.et al. Varicella zoster cranial polyneuropathy presenting with Dysphagia, esophagitis and gastroparesis. J Neurogastroenterol Motil. 2011;17(2):192–194.. 10.5056/jnm.2011.17.2.192). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. . Agrawal V, Agarwal T, Ghoshal UC. Intestinal strongyloidiasis: a diagnosis frequently missed in the tropics. Trans R Soc Trop Med Hyg. 2009;103(3):242–246.. 10.1016/j.trstmh.2008.08.009). [DOI] [PubMed] [Google Scholar]
- 23. . Di Nardo G, Viscogliosi F, Esposito F.et al. Pyridostigmine in Pediatric intestinal Pseudo-obstruction: case Report of a 2-year old Girl and Literature Review. J Neurogastroenterol Motil. 2019;25(4):508–514.. 10.5056/jnm19078). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. . Mann SD, Debinski HS, Kamm MA. Clinical characteristics of chronic idiopathic intestinal pseudo-obstruction in adults. Gut. 1997;41(5):675–681.. 10.1136/gut.41.5.675). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. . Masaki T, Sugihara K, Nakajima A, Muto T. Nationwide survey on adult type chronic intestinal pseudo-obstruction in surgical institutions in Japan. Surg Today. 2012;42(3):264–271.. 10.1007/s00595-011-0115-3). [DOI] [PubMed] [Google Scholar]
- 26. . Pérez de Arce E, Landskron G, Hirsch S, Defilippi C, Madrid AM. Chronic intestinal pseudo-obstruction: clinical and manometric characteristics in the Chilean population. J Neurogastroenterol Motil. 2017;23(2):273–280.. 10.5056/jnm16101). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. . Shair KA, Edwards E. Hirschsprung’s disease in an adult. Am J Med. 2020;133(11):e622–e624.. 10.1016/j.amjmed.2020.02.022). [DOI] [PubMed] [Google Scholar]
- 28. . Debinski HS, Kamm MA, Talbot IC.et al. DNA viruses in the pathogenesis of sporadic chronic idiopathic intestinal pseudo-obstruction. Gut. 1997;41(1):100–106.. 10.1136/gut.41.1.100). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. . Kebede D, Barthel JS, Singh A. Transient gastroparesis associated with cutaneous herpes zoster. Dig Dis Sci. 1987;32(3):318–322.. 10.1007/BF01297060). [DOI] [PubMed] [Google Scholar]
- 30. . Figueira CF, Gaspar MT, Cos LD.et al. Strongyloides stercoralis hyperinfection associated with impaired intestinal motility disorder. Autops Case Rep. 2015;5(2):27–34.. 10.4322/acr.2015.005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. . Greenberg J, Greenberg J, Helmstetter N. Chronic intestinal pseudo-obstruction due to Strongyloides stercoralis . IDCases. ID. 2018;13:e00425. 10.1016/j.idcr.2018.e00425). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. . Karamanolis G, Caenepeel P, Arts J, Tack J. Association of the predominant symptom with clinical characteristics and pathophysiological mechanisms in functional dyspepsia. Gastroenterology. 2006;130(2):296–303.. 10.1053/j.gastro.2005.10.019). [DOI] [PubMed] [Google Scholar]
- 33. . Twist K, Ablett J, Wearden A.et al. Gastrointestinal dysmotility: a qualitative exploration of the journey from symptom onset to diagnosis. Neurogastroenterol Motil. 2018;30(8):e13339. 10.1111/nmo.13339). [DOI] [PubMed] [Google Scholar]
- 34. . Schmulson M, Corazziari E, Ghoshal UC.et al. A four-country comparison of healthcare systems, implementation of diagnostic criteria, and treatment availability for functional gastrointestinal disorders: a report of the Rome Foundation Working Team on cross-cultural, multinational research. Neurogastroenterol Motil. 2014;26(10):1368–1385.. 10.1111/nmo.12402). [DOI] [PubMed] [Google Scholar]
- 35. . Noviello C, Nobile S, Romano M.et al. Functional constipation or redundancy of the colon? Indian J Gastroenterol. 2020;39(2):147–152.. 10.1007/s12664-020-01034-x). [DOI] [PubMed] [Google Scholar]
- 36. . Law NM, Bharucha AE, Undale AS, Zinsmeister AR. Cholinergic stimulation enhances colonic motor activity, transit, and sensation in humans. Am J Physiol Gastrointest Liver Physiol. 2001;281(5):G1228–G1237.. 10.1152/ajpgi.2001.281.5.G1228). [DOI] [PubMed] [Google Scholar]
- 37. . Mehta R, John A, Nair P.et al. Factors predicting successful outcome following neostigmine therapy in acute colonic pseudo-obstruction: a prospective study. J Gastroenterol Hepatol. 2006;21(2):459–461.. 10.1111/j.1440-1746.2005.03994.x). [DOI] [PubMed] [Google Scholar]
- 38. . O’Dea CJ, Brookes JH, Wattchow DA. The efficacy of treatment of patients with severe constipation or recurrent pseudo-obstruction with pyridostigmine. Colorectal Dis. 2010;12(6):540–548.. 10.1111/j.1463-1318.2009.01838.x). [DOI] [PubMed] [Google Scholar]
- 39. . Robinson-Papp J, Nmashie A, Pedowitz E.et al. The effect of pyridostigmine on small intestinal bacterial overgrowth (SIBO) and plasma inflammatory biomarkers in HIV-associated autonomic neuropathies. J Neurovirol. 2019;25(4):551–559.. 10.1007/s13365-019-00756-9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. . Broad J, Kung VW, Boundouki G.et al. Cholinergic interactions between donepezil and prucalopride in human colon: potential to treat severe intestinal dysmotility. Br J Pharmacol. 2013;170(6):1253–1261.. 10.1111/bph.12397). [DOI] [PMC free article] [PubMed] [Google Scholar]