Abstract
Background/Aims:
Patients with achalasia have a high incidence of esophageal squamous cell carcinoma (ESCC), which may be associated with alterations in oral and esophageal microbiota caused by food stasis. This study compared the oral and esophageal microbiota of patients with achalasia before and after peroral endoscopic myotomy (POEM). It also compared patients with achalasia to those with ESCC.
Materials and Methods:
The study prospectively examined 6 patients with achalasia and 14 with superficial ESCC. Oral samples obtained from the buccal mucosa using a swab and esophageal samples obtained from the mid-esophagus using a brush via endoscopy were analyzed by 16S rRNA metagenome sequencing. Additionally, endoscopic and histological findings of patients with achalasia before and after POEM were prospectively compared.
Results:
In patients with achalasia, Streptococcus was most abundant in both the oral and the esophageal microbiota, and these microbiota were significantly different. Although the overall structure of the oral and esophageal microbiota did not change after POEM, the relative abundance rate of Haemophilus and Neisseria increased in the esophagus, and endoscopic findings of inflammation improved after POEM (P = .04). The relative abundance of microbiota was not different among patients with achalasia from those with ESCC.
Conclusions:
The oral and esophageal microbiota were significantly different in patients with achalasia, and some of the composition of the esophageal microbiota changed after POEM. However, these findings and disease-specific microbiota should be further evaluated in large-scale studies.
Keywords: Achalasia, esophageal squamous cell carcinoma, microbiota, myotomy
INTRODUCTION
Achalasia is a major esophageal motility disorder characterized by the failure of the lower esophageal sphincter to relax.1 Patients with achalasia often present with dysphagia, chest pain, and regurgitation, which can impair their quality of life. Peroral endoscopic myotomy (POEM), which showed favorable long-term efficacy (97.4%; 95% confidence interval, 95.2-99.7%) with less invasiveness, has recently gained popularity.2 Although most patients with achalasia have normal life expectancy with appropriate treatment,3,4 clinicians should be mindful of the high incidence of esophageal squamous cell carcinoma (ESCC) among patients with achalasia (0.25/100 person-years in the Japanese population),5 considering that advanced ESCC has a poor prognosis.6-9 Therefore, it is significant to identify the risk factors for the development of ESCC and to minimize them in patients with achalasia.
In patients with achalasia, endoscopic examination often demonstrates food stasis and esophagitis, and histopathological examination shows inflammatory cells in the esophageal mucosa.10,11 Chronic food stasis is thought to cause chronic inflammation in the esophageal epithelium, with subsequent dysphagia and ESCC.11-13 Furthermore, it is reported that the number of esophageal epithelial nuclei and Ki-67-positive cells decreases after POEM, and that POEM might reduce the risk of esophageal carcinogenesis by improving food stasis.11 Considering these previous reports, it seems reasonable to consider food stasis as a risk factor for ESCC. However, the mechanism by which chronic food stasis leads to chronic inflammation and subsequent ESCC remains to be investigated.
Recently, precise evaluations of microbial communities in the human body have become possible through 16S rRNA metagenome analyses using next-generation sequencing. To date, several reports have demonstrated that dysbiosis of the oral and esophageal microbiota is associated with ESCC.14,15 However, the esophageal microbiota in patients with achalasia or the effect of POEM on the microbiota has not been reported thus far. Because the esophagus is directly exposed to food and oral bacteria after swallowing, the esophageal microbiota might be altered, depending on the food that is swallowed and the time for which it stays in the esophagus in patients with achalasia.
Therefore, the rationale for this study lies in the hypothesis that the esophageal microbiota can be altered by long-term stasis of food and oral bacteria, resulting in chronic inflammation of the esophageal mucosa and increased risk of ESCC. On the basis of the results from a previous report showing reduced inflammation of the esophageal epithelium after POEM, we hypothesized that altered esophageal microbiota would be normalized after POEM through an improvement in the passage of esophageal contents.11 Analysis of the oral and esophageal microbiota and their association with esophageal mucosal inflammation may provide insight into the mechanisms leading to ESCC in patients with achalasia. Furthermore, comparing the oral and esophageal microbiota of patients with achalasia and those with ESCC may lead to the detection of disease-specific characteristics that are responsible for the development of ESCC. Therefore, we compared the oral and esophageal microbiota of patients with achalasia before and after POEM, and the microbiota of patients with achalasia to that of patients with ESCC.
MATERIALS AND METHODS
Study Design, Subjects, and Sample Collection
This single-center, prospective, observational study was approved by the ethics committee of the School of Medicine, Niigata University, Japan (Approval No. 2018-0073), and was registered in the University Hospital Medical Information Network Clinical Trial Registry (https://www.umin.ac.jp/ctr/ in Japan; UMIN000033544). Written informed consent was obtained from all participants before beginning the study.
Six patients with achalasia who underwent POEM between August 2018 and February 2019 at Niigata University Hospital in Japan were included in this study. Patients underwent esophagogastroduodenoscopy (EGD) after overnight fasting, one month before POEM. Before performing EGD, an oral sample was collected by gently brushing the buccal mucosa using a sterile swab. Subsequently, during EGD, an esophageal sample was collected using a brush within a sheath (ECB-5-180-3-S; Cytology Brushes; Cook Medical, Bloomington, IN, USA). The brush was first passed through the endoscopic accessory channel with the tip positioned within the sheath. Then, the tip was pushed out of the sheath, and the surface of the mid-esophagus was gently brushed (samples derived from the esophageal mucosa; Figure 1). Finally, the tip of the brush was retracted and pulled out of the endoscopic channel. The sheath of the catheter prevents contamination of the samples, while the brush collects samples more effectively than biopsy forceps.16 Regardless of the esophageal location, the esophageal microbiota was reported to be the same.17 Therefore, the esophageal microbiota of the mid-esophagus would sufficiently reflect the microbiota of the whole esophagus. Following sample collection, the tips of the swab and brush were cut using sterile scissors and placed separately in small sterile containers. The samples were promptly stored in a refrigerator at −80°C. After brushing, a biopsy of the mid-esophageal mucosa was performed with conventional biopsy forceps for histopathological evaluation of esophageal inflammation.
Figure 1.
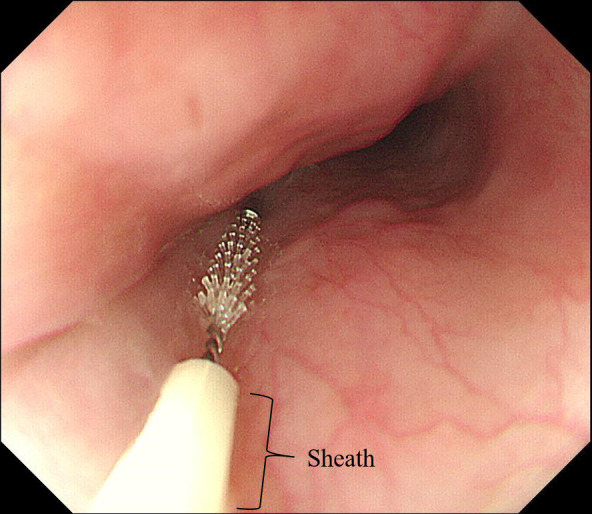
Collection of esophageal samples using a brush with a sheath. The surface of the mid-esophagus was gently brushed.
One month after the initial endoscopic evaluation, POEM was performed as described previously.18 Sulbactam/ampicillin (3.0 g) was injected twice per day for two days, starting from the day of POEM. Subsequently, sulbactam/ampicillin 375 mg was administered orally three times per day for three days. A proton pump inhibitor (PPI) was used for two weeks starting from the day of POEM. No other changes except for antibiotics and PPI were made to the medication regimen before or after POEM. The use of antibiotics and PPI was limited; therefore, the effects of these medications on the oral and esophageal microbiota were considered to be negligible. Two months after POEM, patients underwent a follow-up EGD. Oral and esophageal samples were collected as described above.
Fourteen patients with superficial ESCC who underwent endoscopic submucosal dissection (ESD) during the same period were also examined so that their results could be compared with those of patients with achalasia. These patients underwent EGD after overnight fasting, approximately one month before ESD. Oral and esophageal samples were collected and processed in the same manner as those obtained from patients with achalasia. Tumor sites were avoided during the collection of the oral and esophageal samples.
Evaluation of Endoscopic Findings
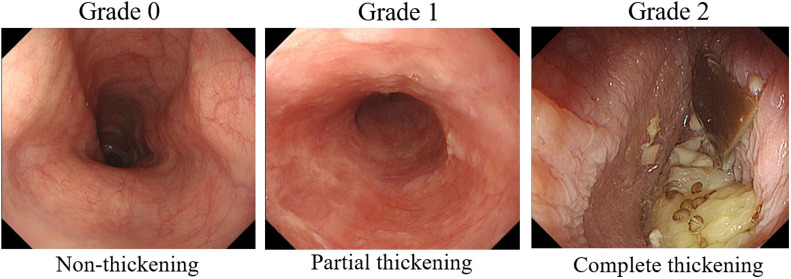
The endoscopic findings of the mid-esophagus were prospectively compared before and after POEM. In our previous report, mucosal thickening was defined as reduced visibility of the esophageal microvascular patterns and/or the presence of a white, cloudy mucosal surface in patients with achalasia.10 Based on this report, endoscopic findings were graded as follows: grade 0, non-thickening (normal mucosa); grade 1, partial thickening (intermediate between grade 0 and 1); and grade 2, complete thickening (loss of visibility of the esophageal microvascular patterns and/or the presence of a white, cloudy mucosal surface; Figure 2). Two experienced endoscopists independently evaluated the endoscopic findings, and the final diagnosis was made when the findings were in agreement.
Figure 2.
Grading of endoscopic findings. Endoscopic findings were graded as follows: grade 0, non-thickening; grade 1, partial thickening (intermediate between grade 0 and 2); and grade 2, complete thickening (disappearance of visibility of esophageal microvascular patterns and/or a white, cloudy surface on the mucosa).
Evaluation of Histological Findings
Biopsy specimens were stained with hematoxylin and eosin to assess the infiltration of the esophageal mucosa by inflammatory cells. Areas where inflammatory cells were most prominent were selected for histological evaluation by an expert pathologist. The evaluation was performed at 400× magnification, which was defined as the high-power field (HPF). Inflammatory cells were counted and graded based on our previous report as follows: I-0, presence of 0-9 inflammatory cells per HPF; I-1, presence of 10-19 inflammatory cells per HPF; and I-2, presence of 20 or more inflammatory cells per HPF.19 Comparisons of histological findings before and after POEM were performed.
Analysis of Mucosa-Associated Microbiota
After collecting esophageal and oral samples, DNA extraction was performed using the MORA-EXTRACT kit (Kyokuto Pharmaceutical, Japan). To purify DNA samples and obtain sequence libraries, two-step polymerase chain reactions were performed according to previously reported methods.20 During the first step, the V3-V4 hypervariable region of 16S ribosomal RNA (16S rRNA) was amplified using the following non-degenerate universal primers: 341F (5′-TCGTCGGCAGCG TCAGATGTGTATAAGAGA CAGCCTACGGGNGGCWGCAG-3′) and 806R (5′-GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGGACTACHVGGGTWTCTAAT-3′).21,22 During the second step, index sequencing using the Illumina sequencer (Illumina, San Diego, CA) and a barcode sequencing using sample-specific 8-bp barcode sequences (CTCTCTAT, TATCCTCT, GTAAGG AG, ACTGCATA, AAGGAGTA, CTAAGCCT, CGTCT AAT, TCTCTCCG, TCGACTAG, and TTCTAGCT) were performed.23 Then, the amplicons were sequenced using the Illumina MiSeq sequencing system with MiSeq Reagent Nano Kit version 3 (600 Cycle; Illumina). Overlapping paired-end reads were joined using the fastq-join script with the default settings. Only reads with quality value scores higher than 20 for more than 99% of the sequence were extracted for further analysis. All sequences with ambiguous base calls were discarded. Chimeras were removed using USEARCH 6.1.544_i86. Operational taxonomic units (OTUs) were defined using QIIME 1.8.0, and the obtained OTUs were assigned to each taxonomic group at the 97% similarity threshold in the Greengenes database.23 As a secondary analysis, a rarefaction analysis (Chao1 diversity index and Shannon diversity index) was utilized to assess α diversity, which indicated the mean species diversity between individuals. The UniFrac metric visualized by the principal coordinate analysis (PCoA) for the assessment of β diversity, which specifies the ratio of inter-individual species diversity, was determined using QIIME 1.8.0. A hierarchical cluster analysis was also used to evaluate the differences in the microbiota before and after POEM and to analyze inter-individual differences in the microbiota.
Statistical Analysis
Categorical data were expressed as numbers and percentages. Continuous data were expressed as means ± standard deviations or medians (range). To assess α diversity, the Chao1 diversity index and the Shannon diversity index were calculated using QIIME 1.8.0, and the non-parametric t-test was used for statistical testing. To assess β diversity, PCoA was performed using QIIME 1.8.0, and permutational multivariate analysis of variance was used for statistics.
The Wilcoxon rank sum test was used to compare the relative bacterial abundance before and after POEM; the Kruskal–Wallis test, the Mann–Whitney U test, and the χ 2 test were used to compare the abundance in patients with achalasia to that in patients with ESCC. Endoscopic and histological findings were evaluated using the Wilcoxon rank sum and χ 2 tests. These analyses were performed using SPSS Statistics for Windows (version 21.0; IBM Corp., Armonk, NY, USA). A P-value of <.05 was considered statistically significant.
RESULTS
Changes in the Microbiota in Patients with Achalasia Before and After POEM
The demographic data of the patients are summarized in Table 1. There were no significant differences between patients with achalasia and those with ESCC in terms of demographic characteristics. POEM was successfully performed, and oral and esophageal samples were successfully obtained without any adverse events for all patients.
Table 1.
Demographic Data of the 6 Patients with Achalasia and 14 Patients with Esophageal Squamous Cell Carcinoma
| Achalasia (n = 6) | ESCC (n = 14) | P-value | |
|---|---|---|---|
| Average age ± SD | 64.5 ± 24.8 | 71.6 ± 8.7 | .755 |
| Sex (male:female) | 4:2 | 12:2 | .329 |
| Antibiotics, n/N (%) | 0/6 (0) | 0/14 (0) | – |
| PPI, n/N (%) | 2/6 (33.3) | 7/14 (50) | .492 |
| Alcohol >20 g/day (n/N) (%) | 3/6 (50) | 10/14 (71.4) | .357 |
| Smoking (n/N) (%) | 3/6 (50) | 11/14 (78.6) | .201 |
| Median disease duration, years (range) | 2 (0.5-20) | – | – |
| Type of achalasia on barium swallow (straight:sigmoid) | 3:3 | – | – |
| Location of ESCC (Ce/Ut/Mt/Lt) | – | 1/1/8/4 | – |
| Invasion depth of ESCC (EP/LPM/MM/SM) | – | 5/6/2/1 | – |
SD, standard deviation; PPI, proton pump inhibitor; ESCC, esophageal squamous cell carcinoma; Ce, cervical esophagus; Ut, upper thoracic esophagus; Mt, middle thoracic esophagus; Lt, lower thoracic esophagus; EP, epithelium; LPM, lamina propria muscularis; MM, muscularis mucosae; SM, submucosa.
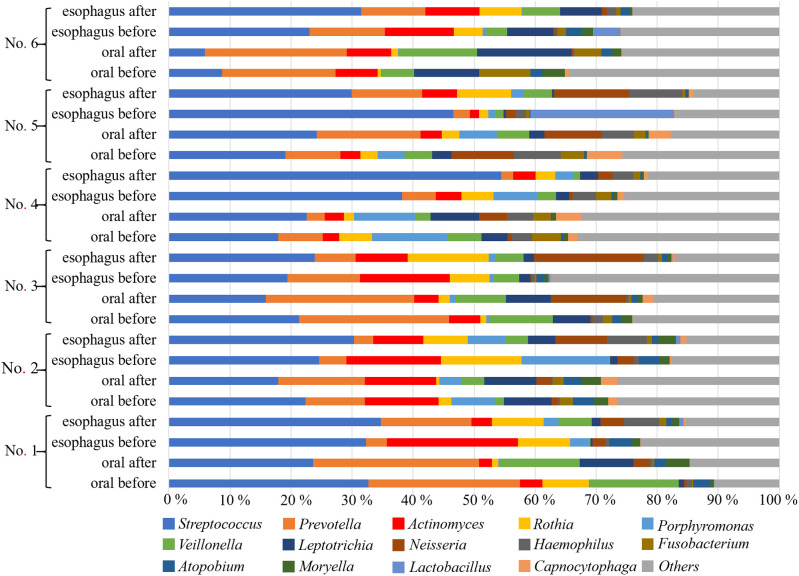
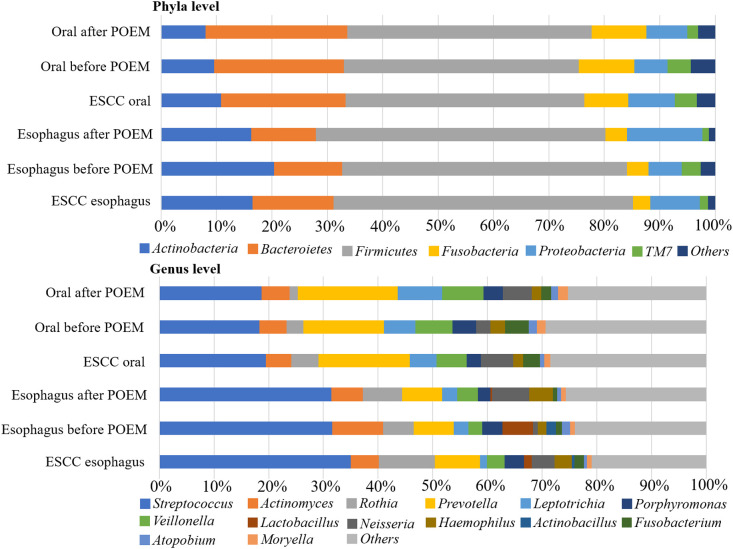
Streptococcus was the most abundant genera in all groups: 18.3% and 31.7% in the oral and esophageal samples, respectively, before POEM, and 18.8% and 34.5% in the oral and esophageal samples, respectively, after POEM (Figure 3). The Chao1 (Figure 4) and Shannon (Figure 5) diversity indices were not significantly different for the oral and esophageal samples. However, the PCoA demonstrated significant differences between the oral and esophageal microbiota both before (weighted: P < .01; unweighted: P = .02) and after POEM (weighted: P < .01; unweighted: P = .02), indicating that the oral and esophageal microbiota had different compositions in patients with achalasia (Figure 6). In contrast, no statistically significant differences were found between the oral microbiota before and after POEM (weighted: P = .68; unweighted: P = .60), or between the esophageal microbiota before POEM and after POEM (weighted: P = .27; unweighted:P = .28).
Figure 3.
Relative abundance of oral and esophageal microbiota (genus level) in patients with achalasia before and after POEM. Streptococcus, Prevotella, Actinomyces, Rothia, and Veillonella were the most common genera. POEM, peroral endoscopic myotomy.
Figure 4.
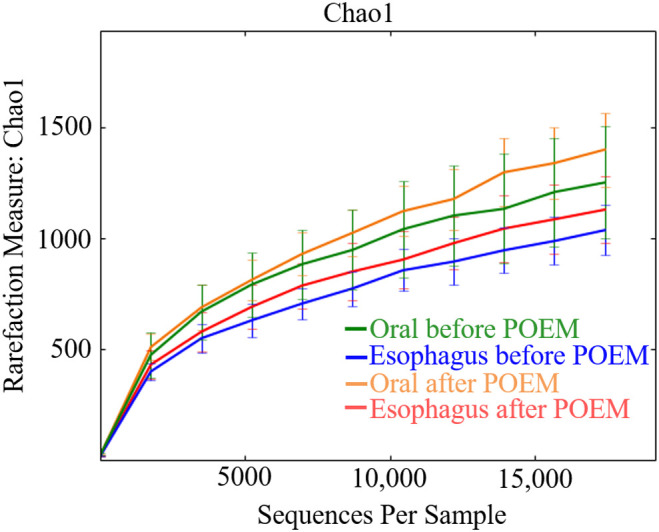
Chao1 index of the oral and esophageal microbiota of six patients with achalasia before and after POEM. There were no statistically significant differences. POEM, peroral endoscopic myotomy.
Figure 5.
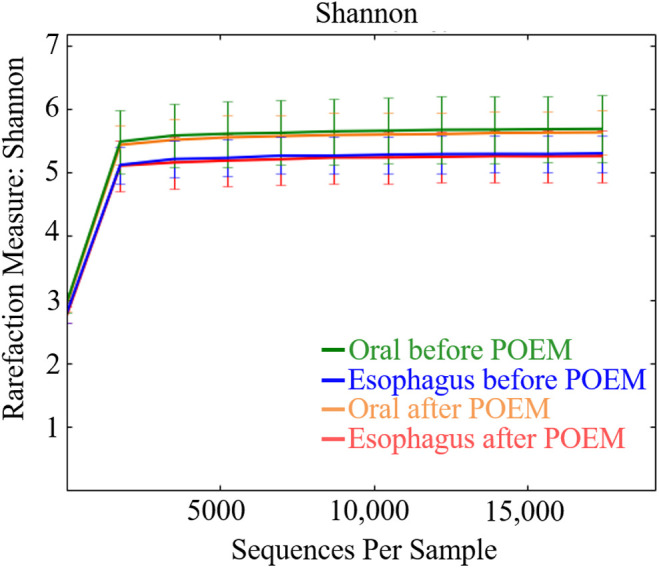
Shannon index of the oral and esophageal microbiota of six patients with achalasia before and after POEM. There were no statistically significant differences. POEM, peroral endoscopic myotomy.
Figure 6.
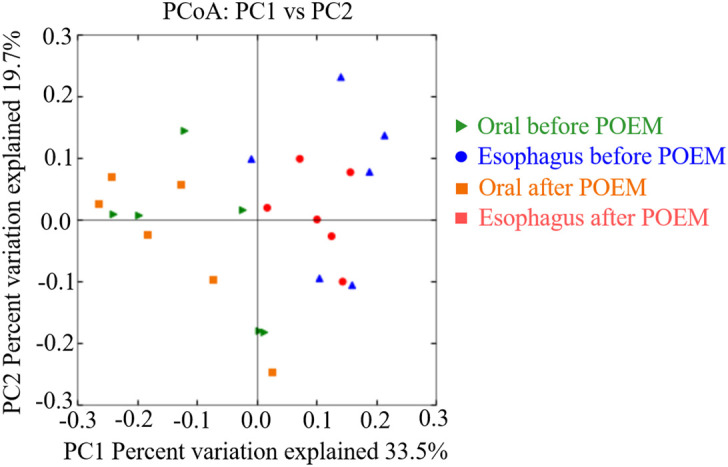
Principal coordinate analysis of the oral and esophageal microbiota of six patients with achalasia before and after POEM. It showed significant differences between the oral and esophageal microbiota both before and after POEM. POEM, peroral endoscopic myotomy.
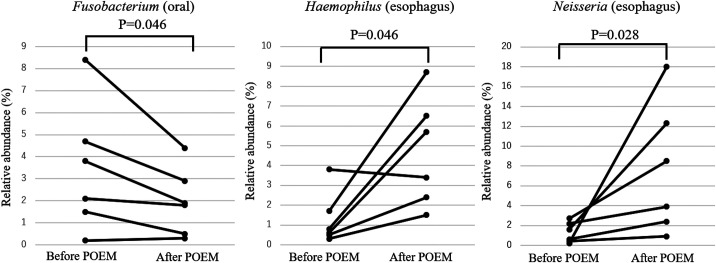
Although β diversity was not significantly different between groups, the relative abundance of several genera changed by more than 1% after POEM (Table 2). Among these, the statistically significant differences (before vs after POEM) were observed for F u sobacterium (3.5 ± 2.9% vs 2.0 ± 1.5%; P = .046) in the oral samples and for Haemophilus (1.3 ± 1.3% vs 4.7 ± 2.7%; P = .046) and Neisseria (1.3 ± 1.0% vs 7.7 ± 6.6%; P = .028) in the esophageal samples (Figure 7).
Table 2.
Relative Abundance of Genera in Patients with Achalasia Changed by More than 1% After POEM
| Before POEM | After POEM | P-value | |
|---|---|---|---|
| Oral | |||
| Fusobacterium | 3.5 ± 2.9% | 2.0 ± 1.5% | .046 |
| Haemophilus | 2.3 ± 2.9% | 1.8 ± 2.4% | .293 |
| Leptotorichia | 5.5 ± 3.5% | 8.4 ± 1.7% | .058 |
| Neisseria | 2.1 ± 4.0% | 5.3 ± 4.6% | .075 |
| Prevotella | 15.7 ± 8.0% | 18.15 ± 8.9% | .173 |
| Rothia | 3.2 ± 2.7% | 1.5 ± 0.8% | .345 |
| Veillonella | 7.0 ± 4.6% | 7.7 ± 4.6% | .917 |
| Esophagus | |||
| Actinobacillus | 1.4 ± 3.3% | 0.5 ± 1.2% | .654 |
| Actinomyces | 11.5 ± 7.5% | 6.4 ± 2.5% | .116 |
| Haemophilus | 1.3 ± 1.3% | 4.7 ± 2.7% | .046 |
| Lactobacillus | 4.8 ± 9.4% | 0.3 ± 0.3% | .500 |
| Neisseria | 1.3 ± 1.0% | 7.7 ± 6.6% | .028 |
| Porphyromonas | 4.5 ± 5.4% | 2.5 ± 2.1% | .249 |
| Rothia | 6.6 ± 4.0% | 8.0 ± 3.3% | .345 |
| Streptococcus | 30.7 ± 10.4% | 34.1 ± 10.5% | .345 |
| Veillonella | 2.1 ± 1.7% | 4.3 ± 1.8% | .075 |
Data are expressed as mean ± standard deviation.
POEM, peroral endoscopic myotomy.
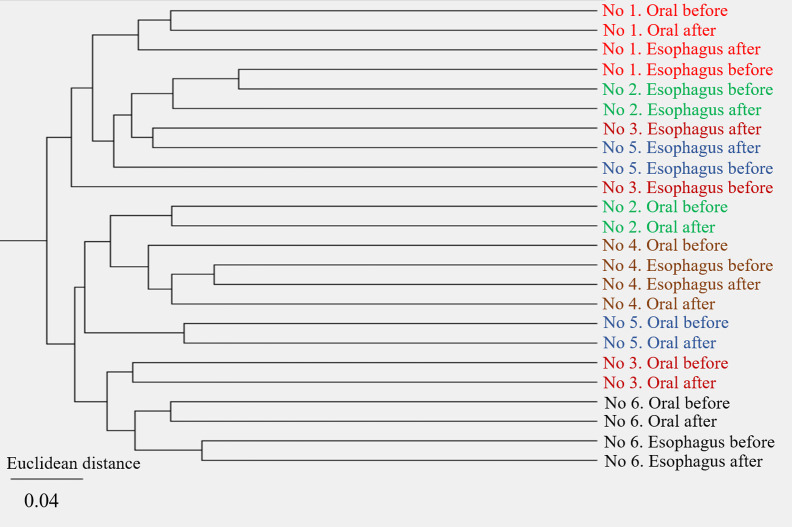
Figure 7.
Hierarchical cluster analysis showed that each cluster contained oral or esophageal specimens from the same individual regardless of POEM. Different individuals were in different clusters.
Hierarchical cluster analysis revealed that each cluster contained the oral or esophageal specimens from the same individual regardless of POEM and that individuals were in different clusters, indicating a small effect of POEM on the oral and esophageal microbiota with inter-individual variability in the microbiota (Figure 8).
Figure 8.
The significant differences in the relative abundances before and after POEM. The relative abundances of Fusobacterium (3.5 ± 2.9% vs 2.0 ± 1.5%; P = .046) in the oral samples and of Haemophilus (1.3 ± 1.3% vs 4.7 ± 2.7%; P = .046) and Neisseria (1.3 ± 1.0% vs 7.7 ± 6.6%; P = .028) in the esophageal samples were significantly different before and after POEM.
Comparison of the Microbiota Between Patients with Achalasia and Those with ESCC
The most abundant genera in the oral and esophageal microbiota in patients with ESCC were Streptococcus (19.5% in the oral samples and 35% in the esophageal samples). (19.5% in the oral samples and 35% in the esophageal samples). Bar graphs depicting data from these patients were similar to those of patients with achalasia; bacterial compositions were not statisticallydifferent between patients with achalasia and those with ESCC (phyla level: P = .953, oral; P = .956, esophagus; genus level: P = .997, oral; P = .918, esophagus; Figure 9). Because no significant differences were found between the two groups, further analyses were not performed.
Figure 9.
Comparisons of the relative abundance of patients with achalasia and those with ESCC. Both phyla and genus levels showed similar patterns of composition among groups, with no statistically significant differences.
Changes in Endoscopic and Histopathological Findings of the Esophagus in Patients with Achalasia Before and After POEM
Importantly, the mucosal thickness significantly improved after POEM (non-thickening/partial thickening/complete thickening: 0/3/3 before POEM vs 4/2/0 after POEM; P = .02). The grade significantly improved from 1.5 ± 0.5 before POEM to 0.3 ± 0.5 after POEM (P = .04). In contrast, the numbers of inflammatory cells in the esophageal epithelium observed histologically before and after POEM were 34.0 ± 32.0 and 21.5 ± 19.0, respectively (P = .345). Inflammatory cell grades were not significantly different (I-0/I-2/I-3 = 2/0/4 before POEM vs 1/3/2 after POEM; P = .135). The results are summarized in Table 3.
Table 3.
Endoscopic and Histopathological Findings of the Esophagus in Patients with Achalasia Before and After POEM
| Before POEM | After POEM | P-value | |
|---|---|---|---|
| Endoscopic findings | |||
| Normal/partial thickening/complete thickening | 0/3/3 | 4/2/0 | .02 |
| Grade score | 1.5 ± 0.5 | 0.3 ± 0.5 | .04 |
| Histological findings | |||
| No. of inflammatory cells | 34.0 ± 32.0 | 21.5 ± 19.0 | .345 |
| I-0/I-1/I-2 | 2/0/4 | 1/3/2 | .135 |
POEM, peroral endoscopic myotomy.
DISCUSSION
In this study, we compared the oral and esophageal microbiota of patients with achalasia before and after POEM. The results of the Chao1 and Shannon diversity indices in the present study showed that the diversity (richness and evenness) of oral and esophageal microbiota was not significantly different before and after POEM. With regard to the composition of the esophageal microbiota, Streptococcus was dominant and accounted for approximately 30% of the esophageal microbiota in patients with achalasia. This is similar to the composition of the esophageal microbiota in healthy individuals reported previously, showing that Streptococcus was the dominant genus in the esophagus and accounted for 30-40% of the entire composition, followed by Prevotella orActinobacillus.17,24-26 Other abundant genera in the esophagus of the patients with achalasia in this study (Rothia, Prevotella, and Actinomyces) have also been seen in the healthy esophagus in the previous reports.17,26 Initially, we hypothesized that achalasia involved alterations in the esophageal microbiota, as with other esophageal diseases such as increased Gram-negative anaerobes and microaerophiles in patients with Barret’s esophagus and gastroesophageal reflux disease or increased Haemophilus in patients with untreated eosinophilic esophagitis.24,25,27 Furthermore, we assumed that comparing patients with achalasia and ESCC would reveal disease-specific characteristics of the oral and esophageal microbiota that could contribute to the development of ESCC. However, although we compared esophageal microbiota among patients with achalasia, patients with ESCC, and healthy individuals in previous reports, we could not reveal any disease-specific characteristics that might lead to the development of ESCC.17,24-26 This negative result implied that the esophageal microbiota might be stable against food stasis, and the dysbiosis of the esophageal microbiota might not be the main factor for the development of ESCC in patients with achalasia. Hence, esophageal microbiota might not affect the patients’ prognosis. We speculated that direct stimulation to the esophageal epithelium by food stasis or individual habits (e.g., smoking and drinking) would be possible factors for the development of ESCC in patients with achalasia.
On the other hand, PCoA revealed that, regardless of POEM, the composition of oral microbiota was significantly different from that of esophageal microbiota among patients with achalasia; further, Streptococcus was more dominant in the esophagus than in the oral buccal mucosa. Although the same tendencies were reported in healthy individuals,17 this is the first report that demonstrated the difference between the oral and the esophageal microbiota in patients with achalasia. While dysbiosis of oral microbiota is reported to have an association with the development of ESCC.15,28 reports on the association between dysbiosis of esophageal microbiota and the development of ESCC are few.14 Given that oral and esophageal microbiota were apparently different from each other, the association between esophageal microbiota and the development of ESCC should be more thoroughly investigated.
We also investigated the effect of POEM on the oral and esophageal microbiota of patients with achalasia. Our results showed that POEM did not produce significant changes to the overall structure of the oral and esophageal microbiota. However, considering each genus, the prevalence of Fusobacterium in the oral microbiota decreased significantly, while that of Haemophilus and Neisseria in the esophagus increased significantly after POEM despite the limited sample size. While the reason for the decrease in Fusobacterium after POEM was not clear, an increased proportion of Gram-negative taxa, including Haemophilus and Neisseria, in the esophagus was reported to be associated with gastroesophageal reflux disease and Barret’s esophagus.24,28 Furthermore, acid reflux is the most common post-surgical complication of POEM.29 In this study, endoscopic images obtained two months after POEM indicated that three of six patients had reflux esophagitis (grade A, 2; grade B, 1). Therefore, a significant increase in these taxa might have been due to increased acid reflux after POEM. Further large-scale studies involving pH monitoring and long-term follow-up are warranted to investigate the relationship between these variables and disease risk.
Although it has been suggested that chronic stasis of food leads to chronic inflammation, epithelial hyperplasia, multifocal dysplasia, and ESCC,11-13 the association between the development of ESCC and the oral and esophageal microbiota could not be elucidated in this study. However, the significant improvements in endoscopic findings might imply the potential of POEM to decrease the risk of ESCC by reducing food stasis and thereby contributing to a better prognosis in patients with achalasia. In clinical practice, we often encounter prominent endoscopic changes in patients with a long history of achalasia. As the present study included only six patients with relatively short disease durations, an analysis of more patients with a longer disease history may lead to more definitive conclusions.
There were two major limitations to this study. First, this was the preliminary pilot study with a limited sample size. The results of the hierarchical cluster analysis revealed that each individual stayed within the same cluster, indicating inter-individual variabilities in the oral and esophageal microbiota of patients with achalasia, which increased the difficulty of interpreting the results of the small sample. Therefore, this study did not have enough power to reach definitive conclusions. Nonetheless, there were apparent tendencies for several results, including differences between oral and esophageal microbiota, increased Haemophilus and Neisseria in the esophagus, and improvements in endoscopic findingsafter POEM. We believe these findings can be stepping-stones for future large-scale studies. Second, this study lacked direct comparisons between healthy individuals and patients with achalasia and ESCC. Although we used data from healthy individuals in the previous studies, this data would not have been sufficient for revealing disease-specific characteristics of the esophageal microbiota in patients with achalasia and ESCC.
In conclusion, the compositions of microbiota were significantly different between the buccal mucosa and the esophageal mucosa in patients with achalasia. Haemophilus and Neisseria in the esophagus significantly increased after POEM, which might have been associated with acid reflux after POEM. Furthermore, inflammation of the esophageal mucosa endoscopically improved after POEM, implying that POEM might reduce the risk of ESCC. However, the overall structure of the oral and esophageal microbiota did not change before or after POEM, and disease-specific characteristics of the oral and esophageal microbiota in patients with achalasia and ESCC could not be detected. Further large-scale studies with healthy controls are warranted to determine definitive conclusions.
Funding Statement
This work was supported by JSPS Grants-in-Aid for Scientific Research (grant number 18K15776).
Footnotes
Ethics Committee Approval: This study was approved by the ethics committee of the School of Medicine, Niigata University, Japan (Approval No. 2018-0073).
Informed Consent: Written informed consent was obtained from the patient who participated in this study.
Peer-review: Externally-peer reviewed.
Author Contributions: Concept - K.T.; Design - K.T., H.S.; Supervision - S.T., J.Y.; Resource - K.T.; Materials - K.T.; Data Collection and/or Processing - H.S., T.M., K.T., S.I., K.H., KI.M., S.H.; Analysis and/or Interpretation - K.T., H.S.; Literature Search - K.T.; Writing - K.T.; Critical Reviews - H.S., S.T.
Acknowledgments: We thank Techno Suruga Laboratory (Shizuoka, Japan) for technical support.
Conflict of Interest: The authors have no conflict of interest to declare.
References
- 1.. Sato H, Takahashi K, Mizuno KI. Esophageal motility disorders: new perspectives from high-resolution manometry and histopathology. J Gastroenterol. 2018;53(4):484–493.. (doi: 10.1007/s00535-017-1413-3) [DOI] [PubMed] [Google Scholar]
- 2.. Shiwaku H, Inoue H, Sato H. Peroral endoscopic myotomy for achalasia: a prospective multicenter study in Japan. Gastrointest Endosc. 2020;91(5):1037–1044.e2.. 10.1016/j.gie.2019.11.020) [DOI] [PubMed] [Google Scholar]
- 3.. Patel DA, Kim HP, Zifodya JS. Idiophathic (primary) achalasia: a review. Orphanet J Rare Dis. 2015;10(1):1–14.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.. Eckardt VF, Hoischen T, Bernhard G. Life expectancy, complications, and causes of death in patients with achalasia: results of a 33-year follow-up investigation. Eur J Gastroenterol Hepatol. 2008;20(10):956–960.. 10.1097/MEG.0b013e3282fbf5e5) [DOI] [PubMed] [Google Scholar]
- 5.. Sato H, Yokomichi H, Takahashi K. Epidemiological analysis of achalasia in Japan using a large-scale claims database. J Gastroenterol. 2019;54(7):621–627.. 10.1007/s00535-018-01544-8) [DOI] [PubMed] [Google Scholar]
- 6.. Jung HK, Tae CH, Lee HA. Treatment pattern and overall survival in esophageal cancer during a 13-year period: a nationwide cohort study of 6,354 Korean patients. PLoS ONE. 2020;15(4):e0231456. 10.1371/journal.pone.0231456) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.. Abnet CC, Arnold M, Wei WQ. Epidemiology of esophageal squamous cell carcinoma. Gastroenterology. 2018;154(2):360–373.. 10.1053/j.gastro.2017.08.023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.. Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet. 2013;381(9864):400–412.. 10.1016/S0140-6736(12)60643-6) [DOI] [PubMed] [Google Scholar]
- 9.. Lordick F, Mariette C, Haustermans K, Obermannová R, Arnold D.ESMO Guidelines Committee on behalf of the ESMO Guidelines Committee Clinical Guidelines. Oesophageal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(Suppl 5):v50–v57.. 10.1093/annonc/mdw329) [DOI] [PubMed] [Google Scholar]
- 10.. Sato H, Takahashi K, Nakajima N. Full-layer mucosal histology in achalasia: histological epithelial wave is characteristic in “pinstripe pattern”-positive achalasia. Neurogastroenterol Motil. 2018;30(1). 10.1111/nmo.13168) [DOI] [PubMed] [Google Scholar]
- 11.. Minami H, Yamaguchi N, Matsushima K. Improvement of endocytoscopic findings after per oral endoscopic myotomy (POEM) in esophageal achalasia; does POEM reduce the risk of developing esophageal carcinoma? Per oral endoscopic myotomy, endocytoscopy and carcinogenesis. BMC Gastroenterol. 2013;13:22. 10.1186/1471-230X-13-22) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.. Leeuwenburgh I, Scholten P, Alderliesten J. Long-term esophageal cancer risk in patients with primary achalasia: a prospective study. Am J Gastroenterol. 2010;105(10):2144–2149.. 10.1038/ajg.2010.263) [DOI] [PubMed] [Google Scholar]
- 13.. Lehman MB, Clark SB, Ormsby AH, Rice TW, Richter JE, Goldblum JR. Squamous mucosal alterations in esophagectomy specimens from patients with end-stage achalasia. Am J Surg Pathol. 2001;25(11):1413–141. 8. 10.1097/00000478-200111000-00009) [DOI] [PubMed] [Google Scholar]
- 14.. Shao D, Vogtmann E, Liu A. Microbial characterization of esophageal squamous cell carcinoma and gastric cardia adenocarcinoma from a high-risk region of China. Cancer. 2019;125(22):3993–4002.. 10.1002/cncr.32403) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.. Peters BA, Wu J, Pei Z. Oral microbiome composition reflects prospective risk for esophageal cancers. Cancer Res. 2017;77(23):6777–6787.. 10.1158/0008-5472.CAN-17-1296) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.. Gall A, Fero J, McCoy C. Bacterial composition of the human upper gastrointestinal tract microbiome is dynamic and associated with genomic instability in a Barrett’s esophagus cohort. PLoS ONE. 2015;10(6):e0129055. 10.1371/journal.pone.0129055) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.. Dong L, Yin J, Zhao J. Microbial similarity and preference for specific sites in healthy oral cavity and esophagus. Front Microbiol. 2018;9:1603. 10.3389/fmicb.2018.01603) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.. Inoue H, Sato H, Ikeda H. Per-oral endoscopic myotomy: a series of 500 patients. J Am Coll Surg. 2015;221(2):256–264.. 10.1016/j.jamcollsurg.2015.03.057) [DOI] [PubMed] [Google Scholar]
- 19.. Nakajima N, Sato H, Takahashi K. Muscle layer histopathology and manometry pattern of primary esophageal motility disorders including achalasia. Neurogastroenterol Motil. 2017;29(3):1–8.. 10.1111/nmo.12968) [DOI] [PubMed] [Google Scholar]
- 20.. Takahashi S, Tomita J, Nishioka K, Hisada T, Nishijima M. Development of a prokaryotic universal primer for simultaneous analysis of bacteria and archaea using next-generation sequencing. PLoS ONE. 2014;9(8):e105592. 10.1371/journal.pone.0105592) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.. Muyzer G, de Waal EC, Uitterlinden AG. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microbiol. 1993;59(3):695–700.. http://www.ncbi.nlm.nih.gov/pubmed/7683183%0Ahttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC202176. 10.1128/AEM.59.3.695-700.1993) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.. Caporaso JG, Lauber CL, Walters WA. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4516–45. 22. 10.1073/pnas.1000080107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.. Hisada T, Endoh K, Kuriki K. Inter- and intra-individual variations in seasonal and daily stabilities of the human gut microbiota in Japanese. Arch Microbiol. 2015;197(7):919–934.. 10.1007/s00203-015-1125-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.. Yang L, Lu X, Nossa CW. Inflammation and intestinal metaplasia of the distal esophagus are associated with alterations in the microbiome. Gastroenterology. 2010;137:588–597.. 10.1053/j.gastro.2009.04.046.Inflammation) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.. Harris JK, Fang R, Wagner BD. Inflammation and intestinal metaplasia of the distal esophagus are associated with alterations in the microbiome. Gastroenterology. 2010;137(2):588–597.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.. Fillon SA, Harris JK, Wagner BD. Novel device to sample the esophageal microbiome-the esophageal string test. PLoS ONE. 2012;7(9):e42938. 10.1371/journal.pone.0042938) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.. Blackett KL, Siddhi SS, Cleary S. Oesophageal bacterial biofilm changes in gastro-oesophageal reflux disease, Barrett’s and oesophageal carcinoma: association or causality? Aliment Pharmacol Ther. 2013;37(11):1084–1092.. 10.1111/apt.12317) [DOI] [PubMed] [Google Scholar]
- 28.. Chen X, Winckler B, Lu M. Oral microbiota and risk for esophageal squamous cell carcinoma in a high-risk area of China. PLoS ONE. 2015;10(12):e0143603. 10.1371/journal.pone.0143603) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.. Shiwaku H, Inoue H, Sasaki T. A prospective analysis of GERD after POEM on anterior myotomy. Surg Endosc. 2016;30(6):2496–2504.. 10.1007/s00464-015-4507-0) [DOI] [PMC free article] [PubMed] [Google Scholar]