Abstract
Background
: To identify risk factors for hypovitaminosis D in inflammatory bowel disease and conduct a comprehensive systematic review with meta-analysis to quantify the impact on vitamin D deficiency.
Methods
: We conducted a literature search of studies through PubMed, Embase, Cochrane Library, and Web of Science. In addition, relevant articles were searched manually. Studies were included if the odds ratios (OR) and 95% CI of each risk factor were reported or could be calculated. We will use the fixed-effects or random-effects model to estimate the pooled effect.
Results
: Out of 1018 articles, 25 eligible studies were identified, including 5826 participants. The risk factors associated with hypovitaminosis D were non-Caucasian (OR: 3.79, 95% CI: 2.68-5.34), Crohn’s disease (OR: 1.38, 95% CI: 1.21-1.56), disease activity (OR: 1.85, 95% CI: 1.61-2.13), inflammatory bowel disease-related surgery (OR: 1.61, 95% CI: 1.38-1.89), exposure to steroid (OR: 1.61, 95% CI: 1.28-2.03), and biologics (OR: 1.78, 95% CI: 1.48-2.14). In 30 ng/mL and adjusted OR subgroup, male (OR: 1.84, 95% CI: 1.47-2.31) and winter season (OR: 2.49, 95% CI: 1.69-3.67) also were risk factors, respectively. 5-aminosalicylic acid (OR: 1.10, 95% CI: 0.74-1.63) and smoking (OR: 1.19, 95% CI: 0.98-1.45) were unrelated to vitamin D deficiency.
Conclusions
: For vitamin D deficiency in inflammatory bowel disease, non-Caucasian, Crohn’s disease, disease activity, surgery, exposure to steroid and biologics, males are risk factors, while 5-aminosalicylic acid and smoking are not. The relationship between body mass index, winter season, exposure to immunomodulators, and vitamin D deficiency remains unclear.
Keywords: Inflammatory bowel disease, vitamin D deficiency, risk factors, meta-analysis
INTRODUCTION
Inflammatory bowel disease (IBD) refers to a chronic, immune-mediated disorder that mainly includes Crohn’s disease (CD) and ulcerative colitis (UC), which has become a global disease. At the turn of the 20th century, even though the incidence of IBD in Western countries has stabilized, the prevalence rate exceeds 0.3%. Additionally, the incidence is rising rapidly in newly industrialized countries.1 Epidemiological study predicts that the absolute number of IBD patients in developing countries is likely to catch up with that of western countries by the time of 2025.2 Up to now, the pathogenesis of IBD is not yet clear. However, it is speculated to result from a complex interaction between derangements in immune homeostasis, certain environmental factors, and genetic susceptibility.3,4
Increasing epidemiological studies have shown that vitamin D status plays a role in the pathogenesis of IBD.5 Vitamin D, signaling through the vitamin D receptor, repairing the gut epithelial barrier, and has anti-bacterial activity. As an immunomodulator, it suppresses the action of IL-12 on dendritic cells (DCs) by the binding of vitamin D receptors to DCs, which can downregulate Th1/Th17 and upregulate Th2 and Treg.6 Meanwhile, it can maintain the balance between immune homeostasis and inflammation by interaction above.6 Immunological studies reveal that CD is mainly affected by Th1/Th17 responses and Treg responses.7 Conversely, the pathogenesis of UC seems to be associated with mediated by Th2 and NK T cells.8
Micronutrient deficiencies are prevalent in IBD patients.9 Recently, clinical evidences suggest that vitamin D deficiency is involved in IBD,10,11 IBD patients exhibit an imbalance in vitamin D metabolism.12 A systematic review and meta-analysis of 14 studies, including 938 patients with IBD, found that approximately 37.85% of patients were classified as vitamin D deficiency.11 This high prevalence has caught our attention. Many observational studies tried to determine risk factors for vitamin D deficiency in IBD, including gender, ethnicity, season, disease activity, CD versu. UC diagnosis, smoking, body mass index (BMI), and concomitant medications.13-37 However, the conclusions among the above studies are still controversial and inconclusive. Potential reasons for such inconsistency may be attributed to the limited sample size and various definitions of vitamin D deficiency. Given that results from previous studies were conflicting and need to be clarified, we performed a systematic review and meta‐analysis to identify the association between risk factors and vitamin D deficiency in IBD.
MATERIALS AND METHODS
Study Protocol
The systematic review and meta-analysis protocol has been registered prospectively in PROSPERO (protocol number: PROSPERO CRD42020181938) and was prepared according to the PRISMA-P guidelines.38 A comprehensive search of major electronic databases was done to identify suitable studies from inception through June 2020 without language restrictions. Following databases were searched: PubMed, Embase, Cochrane Library, Web of Science. The search used the terms “vitamin D,” “ergocalciferol,” “calcifediol,” “inflammatory bowel disease,” “Crohn’s disease,” “ulcerative colitis,” and “risk factors” in different combinations. In addition, we searched relevant articles of all review articles manually.
Inclusion and Exclusion Criteria
Articles were initially screened by 2 authors who independently reviewed abstracts. Conflicts of opinion regarding eligibility were resolved through discussion or, if necessary, by involving a senior author. Population-based studies included prospective or retrospective cohort design, case–control, and cross-sectional design.
Inclusion criteria included:
Any observational study that reported risk factors of vitamin D deficiency in IBD patients (CD or UC) in all countries and regions was included.
Vitamin D outcomes were dichotomized into the deficient or non-deficient group.
The study evaluated the association of risk factors on vitamin D outcomes.
Exclusion criteria were the following:
Systematic reviews, meta-analyses, and other review articles.
In vitro studies.
Any risk factor did not be reported.
Combining other diseases that affect vitamin D levels.
Vitamin D cutoff value is not 20 ng/mL or 30 ng/mL, which is most often discussed.
The data of the vitamin D outcome statistical measures were incomplete (odds ratios (OR), and 95% CIs), or the data cannot be used to calculate the outcome measure.
Data Extraction
For included original reports, 2 authors independently carried out data extraction including the following information: author, study design, publication year, country, study site latitude, the number of vitamin D deficiency in IBD and non-deficiency patients, definition of vitamin D deficiency, and any possible risk factors.
Study Quality Assessment
Two authors independently assessed the quality of each study by using the Newcastle–Ottawa scale (NOS), which is primarily applied to cohort and case–control studies in systematic reviews.39 The NOS was used to assess the quality of retrieved studies on a scale of 0-9 via the 3 aspects: study selection, comparability, and the ascertainment of exposure or outcome. The quality of cross-sectional studies was evaluated using the Agency for Healthcare Research and Quality (AHRQ), which includes an 11-item checklist.40 The AHRQ was used to assess article quality as follows: 1-3 as low quality, 4-7 as moderate quality, and 8-11 as high quality.
Data Analysis
Adjusted OR were extracted if available; otherwise, we used unadjusted results from dichotomous outcome variables. Statistical analysis was conducted using the Stata 16.0 software to calculate the pooled odds (and 95% CI) for each potential risk factor among IBD patients with deficient versus insufficient/normal vitamin D levels. Statistical heterogeneity among the research results was to be assessed using the I-squared and Q-test’s P-value with the significance level set at 50% and .1, respectively. When a high degree of heterogeneity (P < .1 and/or I 2 > 50%) existed and contained enough available studies, we will use the random-effects model and further analyze the sources of heterogeneity by subgroup analysis, sensitivity analysis or meta-regression analysis. Publication bias was evaluated using funnel plots and a quantitative test (Egger’s test), when more than 10 studies were included. When the funnel plot shows an obvious asymmetry, the trim and fill method will be further analyzed.
RESULTS
Out of 1018 citations, 25 studies13-37 with a total of 5826 patients who fulfilled requirements for predefined inclusion and exclusion criteria were included. The study flowchart is presented in Figure 1. The baseline characteristics of the included studies and quality score are listed in Table 1. The qualities of cohort studies were ranged from 5 to 9, with a median score of 7. Cross-sectional studies with AHRQ scores ranged from 7 to 9, with a median score of 8. The patients included in this systematic review and meta-analysis came from 14 countries on 4 continents (Asia, North America, Europe, and Africa). The majority of included studies14, 16-21, 23, 24, 26-31, 33, 35 defined deficient status as serum vitamin D levels < 20 ng/mL. Eight studies13,15,22,25,32,34,36,37 used the cutoff value of 30 ng/mL for vitamin D deficiency.
Figure 1.
PRISMA flow diagram.
Table 1.
The Baseline Characteristics of the Studies
| Study | Study design | Country | Latitude | Disease | No. of Patients | No. of Deficiency | No. of Normal | Vitamin D Deficiency Definition (ng/mL) | QS |
|---|---|---|---|---|---|---|---|---|---|
| Olmedo et al.29 | CS | Magala, Spain | 36.72 | CD+UC | 224 | 65 | 159 | <20 | 9 |
| Domislovic et al.19 | CS | Zagreb, Croatia | 45.82 | CD+UC | 83 | 59 | 24 | <20 | 8 |
| Law et al.27 | CS | Chandigargh, India | 30.73 | UC | 80 | 46 | 34 | <20 | 9 |
| Pallav et al.30 | CS | Jackson, USA | 30.83 | CD+UC | 211 | 73 | 138 | <20 | 7 |
| Castro et al.17 | CS | Guimarães, Portugal | 41.43 | CD+UC | 76 | 23 | 53 | <20 | 8 |
| Mentella et al.28 | CS | Chicago, USA | 41.87 | CD+UC | 206 | 65 | 141 | <20 | 7 |
| Basson et al.13 | CS | Cape Town | 33.92 | CD | 185 | 106 | 79 | <30 | 9 |
| Blanck et al.15 | CS | Philadelphia, USA | 39.95 | UC | 34 | 19 | 15 | <30 | 9 |
| Bhagavathula et al.14 | PC | Atlanta, GA, USA | 33.75 | CD+UC | 148 | 80 | 68 | <20 | 5 |
| Tran et al.37 | PC | Cleveland, USA | 41.47 | CD+UC | 112 | 76 | 36 | <30 | 7 |
| Bours et al.16 | PC | Amersfoort, Netherlands | 52.92 | CD+UC | 281 | 160 | 121 | <20 | 9 |
| Frigstad et al.20 | PC | Norway | 59.28 | CD+UC | 408 | 213 | 195 | <20 | 7 |
| Kabbani et al.25 | PC | Pittsburgh, USA | 40.43 | CD+UC | 965 | 291 | 674 | <30 | 8 |
| Scotti et al.31 | RC | Rome, Italy | 41.9 | CD+UC | 300 | 186 | 114 | <20 | 6 |
| Torella et al.32 | RC | Buenos Aires, Argentina | 34.33 | CD+UC | 59 | 39 | 20 | <30 | 9 |
| Janssen et al.23 | RC | Freiburg, Germany | 53.85 | CD+UC | 384 | 232 | 152 | <20 | 8 |
| Bruyn et al.24 | RC | Amsterdam, Netherlands | 52.37 | CD | 101 | 55 | 46 | <20 | 8 |
| Ulitsky et al.33 | RC | Milwaukee, USA | 43.03 | CD+UC | 504 | 241 | 263 | <20 | 6 |
| Chatu et al.18 | RC | London, UK | 51.5 | CD+UC | 168 | 113 | 55 | <20 | 7 |
| Fu et al.21 | RC | Vancouver, Canada | 49.23 | CD+UC | 100 | 39 | 61 | <20 | 5 |
| Kyong et al.26 | RC | Seoul, Korea | 37.55 | CD+UC | 87 | 64 | 23 | <20 | 8 |
| Zullow et al.35 | RC | Baltimore, USA | 39.23 | CD+UC | 255 | 99 | 156 | <20 | 6 |
| Hausmann et al.22 | RC | Frankfurt, Germany | 50.03 | CD+UC | 470 | 281 | 188 | <30 | 7 |
| Juneja et al.36 | RC | Washington, USA | 38.88 | CD+UC | 156 | 122 | 34 | <30 | 5 |
| Zator et al.34 | RC | Boston, USA | 52.98 | CD+UC | 101 | 59 | 42 | <30 | 6 |
CS, cross-sectional; PC, prospective cohort; RC, retrospective cohort; QS, quality score.
Gender
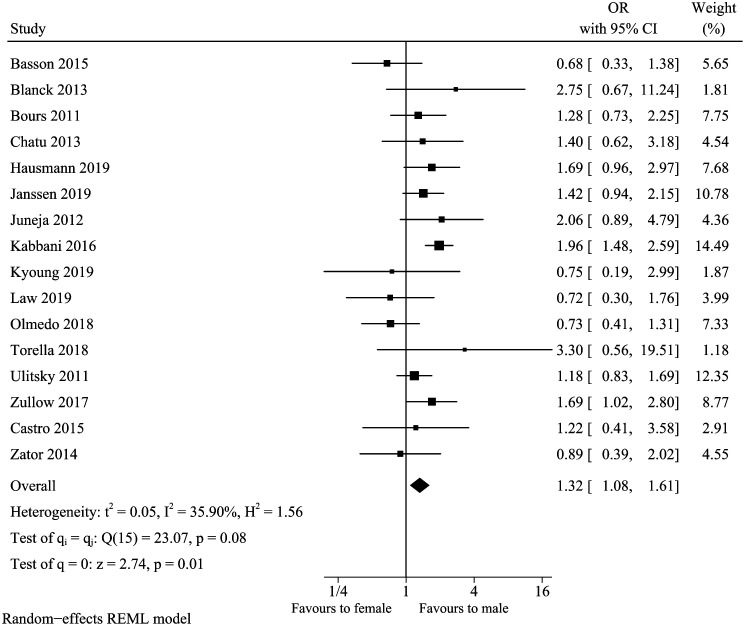
A total of 16 studies included in our review13,15-18,22,23,25-27,29,32-36 analyzed the association between gender and vitamin D deficiency in IBD. No obvious asymmetry was observed for the funnel plot, and Egger’s test further indicated no significant publication bias (Egger’s test P-value = .6044). The summary result of gender (Figure 2) showed that males were associated with increased odds of vitamin D deficiency (pooled OR: 1.32, 95% CI: 1.08-1.61). Mild heterogeneity existed in studies (I 2 = 35.90%, P = .08). To evaluate the stability of the combined outcomes, we carried out a sensitivity analysis. It indicated that excluding any single study did not change the overall result (data not shown). Subgroup analyses in participants with vitamin D deficiency cutoff point below 20 and 30 ng/mL showed that the higher cutoff concentration had nearly double risk (OR: 1.84, 95% CI: 1.47-2.31, I 2 = 0%) (Table 2). However, there was no statistical significance when the cutoff value was 20 ng/mL (OR: 1.16, 95% CI: 0.95-1.41, I 2 = 8.18%). In the meta-regression analysis, the sample size and vitamin D deficiency cutoff value could impact 95.37 and 100% of heterogeneity, respectively.
Figure 2.
Vitamin D deficiency and gender.
Table 2.
Subgroup Analysis of Studies Evaluating the Relationship Between Gender, Season, Immunomodulator, and Vitamin D Deficiency
| Risk factor-Subgroups | No. of Studies | OR (95% CI) | P-Value for Heterogeneity |
|---|---|---|---|
| Gender-Vitamin D cutoff value | |||
| 20 ng/mL | 10 | 1.16 (0.95-1.14) | .39 |
| 30 ng/mL | 6 | 1.84 (1.47-2.31) | .53 |
| Overall | 16 | 1.32 (1.08-1.61) | .08 |
| Season-unadjusted and adjusted OR | |||
| Unadjusted OR | 2 | 0.88 (0.47-1.65) | .95 |
| Adjusted OR | 4 | 2.49 (1.69-3.67) | .45 |
| Overall | 6 | 1.82 (1.16-2.87) | .05 |
| Immunomodulator-study design type | |||
| Cross-sectional study/retrospective cohort study | 7 | 0.78 (0.62-0.99) | .6 |
| Prospective cohort study | 2 | 1.83 (0.99-3.39) | .19 |
| Overall | 9 | 0.96 (0.70-1.32) | .01 |
BMI
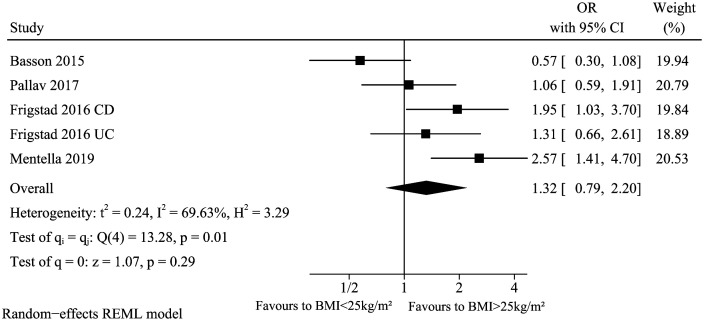
These studies13,20,28,30 analyzed the association between BMI (>25 kg/m2 vs. <25 kg/m2) and vitamin D deficiency in IBD. The summary result of BMI (Figure 3) showed that BMI did not significantly affect low vitamin D levels in IBD (pooled OR: 1.32, 95% CI: 0.79-2.20). However, there was significant heterogeneity across studies (I 2 = 69.63%, P = .01). Because Basson et al. reported unadjusted OR while others reported adjusted OR, we excluded this article in sensitivity analysis. The heterogeneity reduced to an acceptable level (I2 = 39.88%) which suggested the pool conclusion was not robust (OR: 1.63, 95% CI: 1.09-2.44).
Figure 3.
Vitamin D deficiency and BMI.
Race
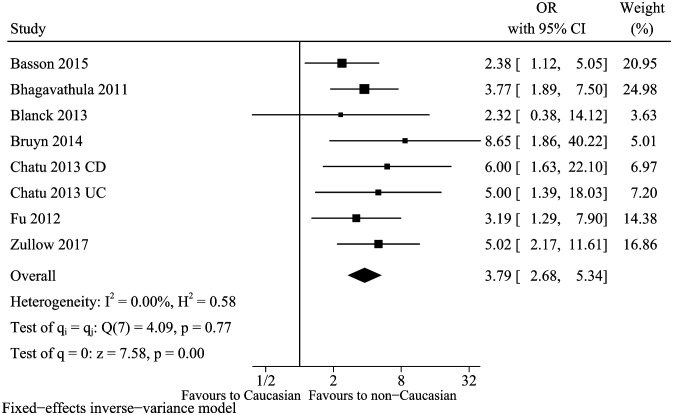
Seven articles13-15,18,21,24,35 were identified to describe association between race factor (non-Caucasian vs. Caucasian) and vitamin D deficiency in IBD. There was no heterogeneity (I 2 = 0%, P = .77), and a fixed-effects model was selected. The pooled OR was 3.79, and 95% CI was 2.68-5.34 (Figure 4). Therefore, non-Caucasian could be served as an independent risk factor. Meanwhile, sensitivity analysis did not find an obvious change in the overall result (data not shown).
Figure 4.
Vitamin D deficiency and race.
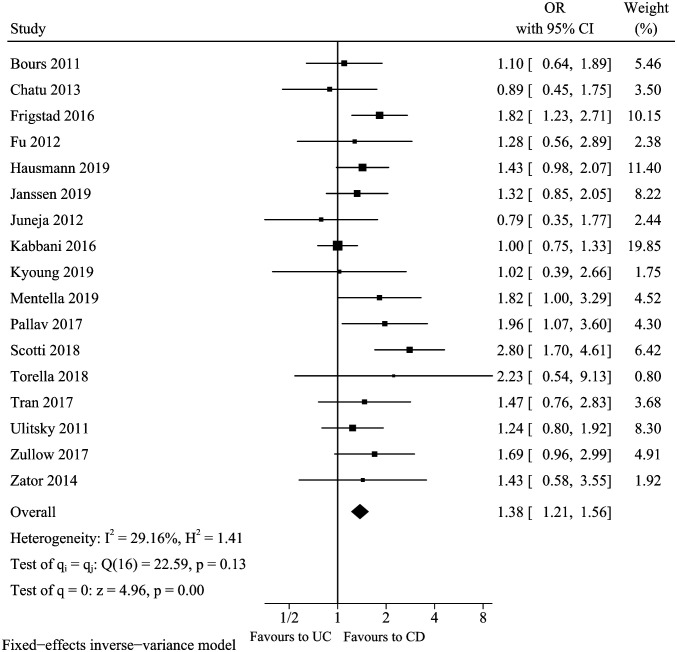
Disease Type
A total of 17 studies16,18,20-23,25,26,28,30-37 analyzed the association between type of IBD disease (CD vs. UC) and vitamin D deficiency. No obvious asymmetry was observed for the funnel plot, and Egger’s test further indicated there was no significant publication bias (Egger’s test P = .4764). The pooled result of disease type (Figure 5) suggested that CD was associated with an increased risk of vitamin D deficiency in patients with IBD (pooled OR: 1.38, 95% CI: 1.21-1.56). Mild heterogeneity was found (I 2 = 29.16%, P = .13), and fixed-effects model was selected. Sensitivity analysis suggested that the pool conclusion was robust (data not shown). Subgroup analysis and meta-regression analysis did not find the source of heterogeneity.
Figure 5.
Vitamin D deficiency and disease type.
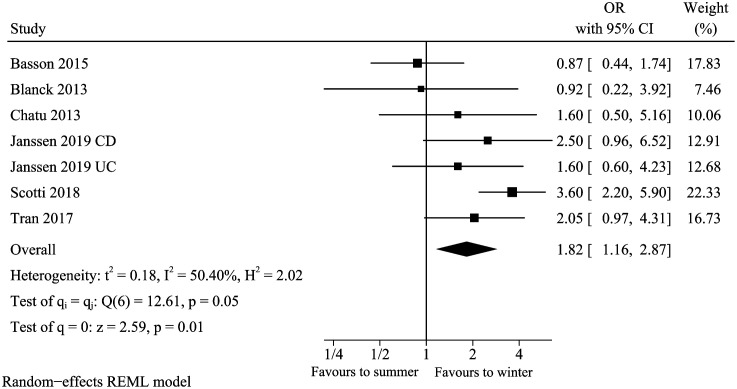
Season
A total of 6 studies13,15,18,23,31,37 analyzed the association between season (winter vs. summer) and vitamin D deficiency in IBD. The pooled result of the season (Figure 6) suggested winter was associated with vitamin D deficiency (pooled OR: 1.82, 95% CI: 1.16-2.87). Moreover, there was moderate heterogeneity reported (I 2 = 50.40%, P = .05). Sensitivity analysis suggested that the pooled conclusion was not robust after removing the study of Scotti et al. (OR: 1.49, 95% CI: 0.99-2.23, I2 = 13.20%). Subgroup analysis showed that winter was a risk factor (pooled OR: 2.49, 95% CI: 1.69-3.67, I2 = 17.34%) among studies with adjusted OR, but there was no statistical significance among studies with unadjusted OR (pooled OR: 0.88, 95% CI: 0.47-1.65, I 2 = 0%) (Table 2). There was a statistical difference between the subgroup (test for subgroup differences P < .05). Therefore, we preferred the cautious view that winter was an independent risk factor.
Figure 6.
Vitamin D deficiency and season.
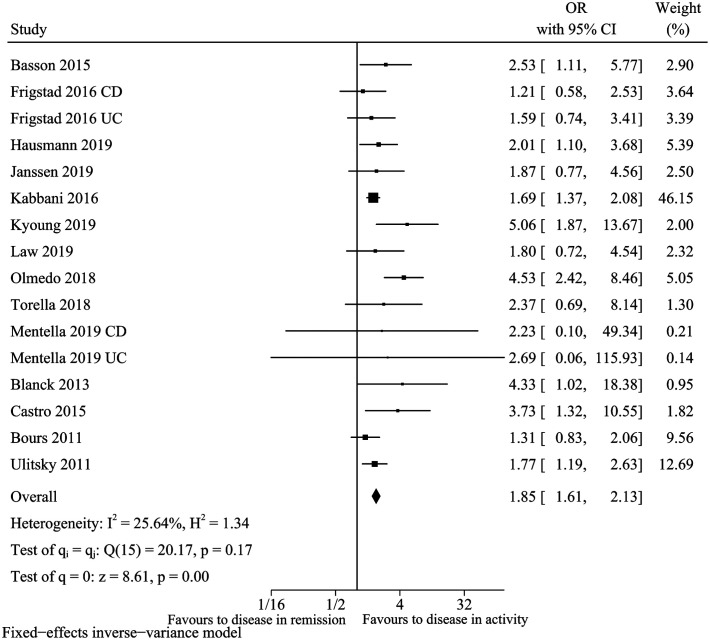
Disease Activity
For the factor of active disease, 14 clinical research studies13,15-17,20,22,23,25-29,32,33 fulfilled the inclusion criteria. We noted that active disease was associated with an increased risk of vitamin D deficiency (pooled OR: 1.85, 95% CI: 1.61-2.13, I 2 = 25.64%) (Figure 6, 7). Sensitivity analysis suggested the pooled conclusion was robust and not altered by sequential excluding individual study. In meta-regression analysis, covariate did not impact the risk of vitamin D deficiency. In addition, the funnel plot seems to be asymmetric with the P-value for the Egger’s test was .0435. After further trim and fill the funnel plot, the funnel plot became symmetrical, and the combined conclusion (pooled OR: 1.66, 95% CI: 1.46-1.90) still indicated statistical significance.
Figure 7.
Vitamin D deficiency and disease activity.
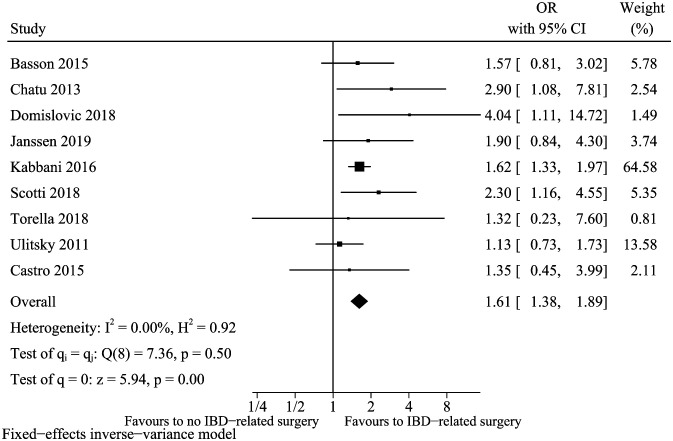
IBD-Related Surgery
For the factor of IBD-related surgery defined by ileocecal resection, small bowel resection, or subtotal/total colectomy, 9 clinical studies 13,17-19,23,25,31-33 fulfilled the inclusion criteria. The pooled result of surgery (Figure 8) showed that patients with a history of bowel resection had a higher risk of vitamin D deficiency (pooled OR: 1.61, 95% CI: 1.38-1.89, I 2 = 0%). In the sensitivity analysis, the conclusion was robust when we removed any singular clinical study (data not shown).
Figure 8.
Vitamin D deficiency and IBD-related surgery.
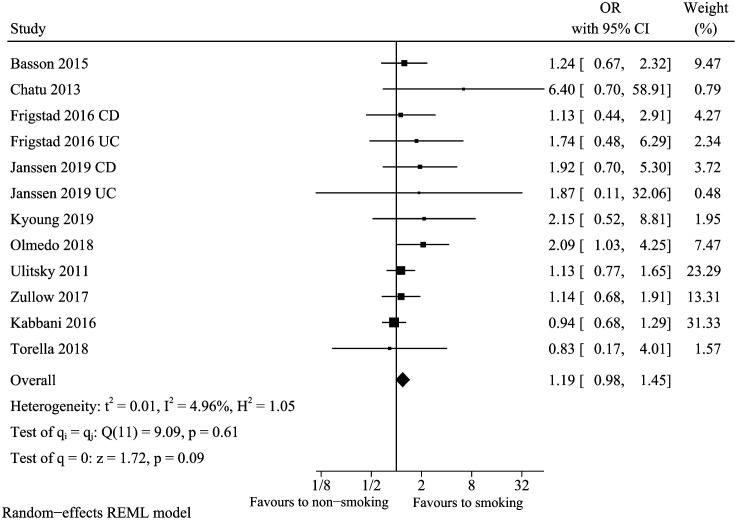
Smoking
Combining effect sizes calculated from 10 studies13,18,20,23,25,26,29,32,33,35 based on the comparison between the smoking group and non-smoking group demonstrated an overall non-significant effect size of 1.19 (95% CI: 0.98-1.45, I 2 = 4.96%) (Figure 9). Sensitivity analysis was conducted by omitting the study of Kabbia et al., and the pooled conclusion was changed (pooled OR: 1.31, 95% CI: 1.05-1.65). Not obvious heterogeneity or pooled conclusion change was detected in subgroup analysis. In meta-regression analysis, the sample size, which could explain 99.99% heterogeneity, may be a source of heterogeneity (P = .05). However, the funnel plot for smoking demonstrated the asymmetric distribution of studies, and the Egger’s test showed publication bias (P = .0403). After further trim and fill the funnel plot, the funnel plot became symmetrical, and the combined conclusion (pooled OR: 1.07, 95% CI: 0.90-1.27) still suggested that smoking was not a risk factor.
Figure 9.
Vitamin D deficiency and smoking.
Exposure to Drugs
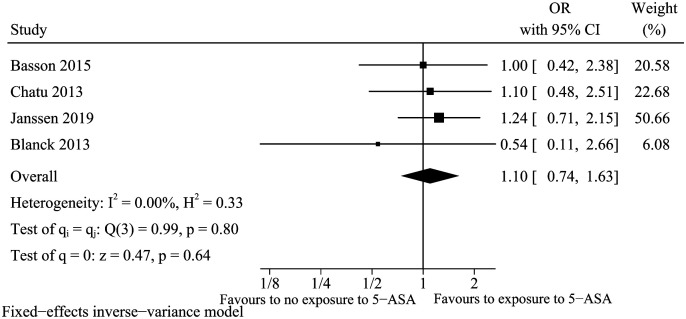
5-Aminosalicylic Acid (5-ASA)
A total of 4 studies13,15,18,23 were included for the factor of 5-ASA. Previous exposure to 5-ASA was not relevant to vitamin D deficiency in IBD (OR: 1.10, 95% CI: 0.74-1.63, I 2 = 0%) (Figure 10). Sensitivity analysis indicated that the result was robust (data not shown).
Figure 10.
Vitamin D deficiency and 5-ASA.
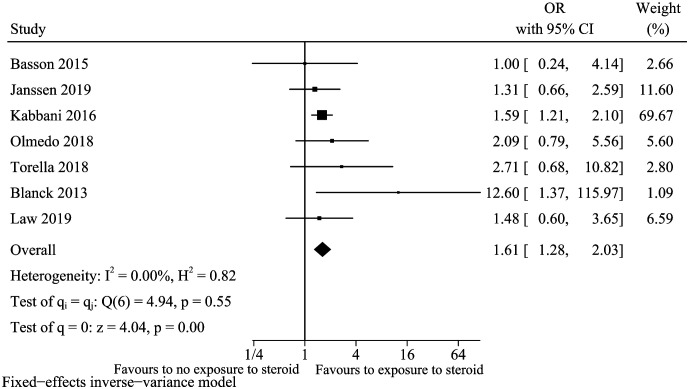
Steroid
A total of 7 studies13,15,23,25,27,29,32 were included for the factor of using steroid during the study procedure. We did not evaluate the publication bias because less than 10 studies were included. Exposure to steroids increased the risk of vitamin D deficiency in IBD (OR: 1.61, 95% CI: 1.28-2.03, I 2 = 0%) (Figure 11). Sensitivity analysis suggested that this model is robust (data not shown).
Figure 11.
Vitamin D deficiency and steroid.
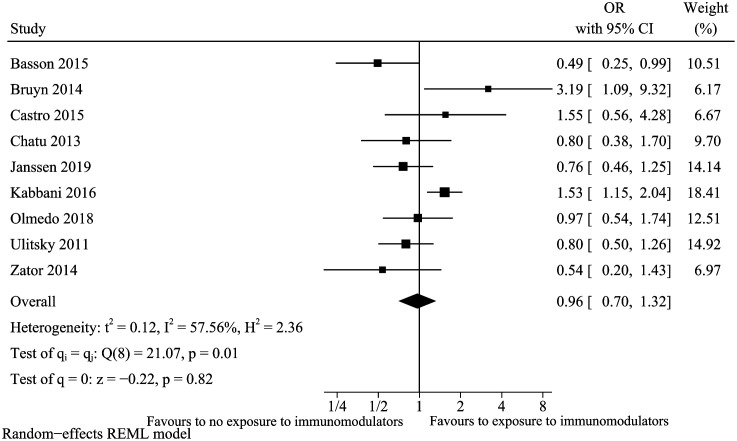
Immunomodulators
A total of 9 studies13,17,18,23-25,29,33,34 were included for the factor of immunomodulators. The result suggested that ever exposure to immunomodulators was not a risk factor for vitamin D deficiency (pooled OR: 0.96, 95% CI: 0.70-1.32) (Figure 12) with high heterogeneity (I 2 = 57.56%). The high observed heterogeneity disappeared (I 2 = 0%), but the combined conclusion did not change (pooled OR: 0.84, 95% CI: 0.66-1.05) after excluding the study of Kabbia et al. in the sensitivity analysis. Table 2 also summarizes subgroup analysis on study type for immunomodulators (cross-sectional study/retrospective cohort study vs. prospective cohort study). In cross-sectional study/retrospective cohort study subgroup, immunomodulators may be a protecting factor for vitamin D deficiency. Still, the upper limit of the 95% CI was 0.99, which was very close to 1.00, suggested we need to be very careful to explain the result. In meta-regression analysis, the study type could explain 100% heterogeneity.
Figure 12.
Vitamin D deficiency and immunomodulators.
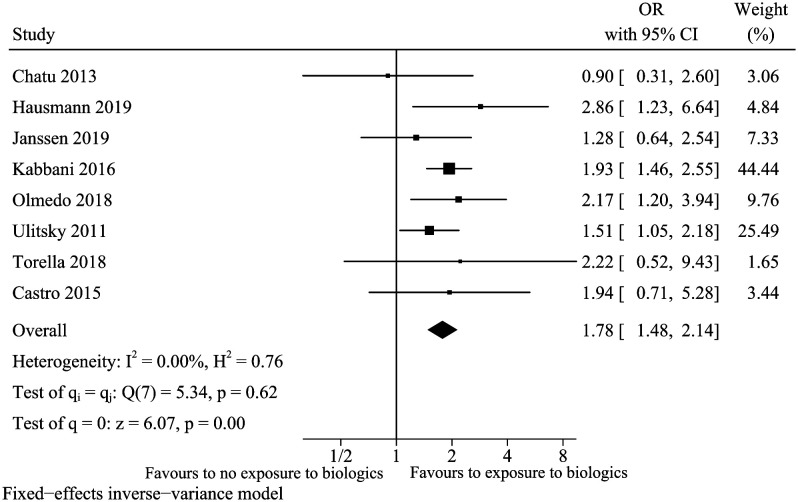
Biologics
A total of 8 studies17,18,22,23,25,29,32,33 were included for the factor of biologics. Previous exposure to biologics increased the risk of vitamin D deficiency in IBD patients (OR: 1.78,95% CI: 1.48-2.14, I 2 = 0%) (Figure 13). Sensitivity analyses indicated that the conclusion was robust (data not shown).
Figure 13.
Vitamin D deficiency and biologics.
DISCUSSION
This systematic review and meta-analysis provide a comprehensive overview and critical assessment of risk factors for vitamin D deficiency in IBD. A total of 12 factors, including general characteristics (gender, BMI, ethnicity), disease type, disease activity, season, surgery, smoking, and drug exposures, have been studied. Among these, we identified risk factors included non-Caucasian, CD, disease activity, IBD-related surgery, exposure to steroid, and biologics with moderate- to high-quality evidence. Also noted, males were a risk factor only in the 30 ng/mL cutoff value subgroup, but not in the 20 ng/mL subgroup. As for the season, winter presented as a risk factor in the adjusted OR subgroup but not in the unadjusted OR subgroup. 5-ASA and smoking were unrelated to vitamin D deficiency. For BMI, winter season, and immunomodulators, due to the high heterogeneity, it is still not clear whether there is an association with vitamin D deficiency, and more high-quality and large-sample studies needed to be included to draw a conclusion.
For the race, non-Caucasian have a higher risk of vitamin D deficiency. As we all know, vitamin D status is influenced by solar ultraviolet (UV) exposure of the skin, but that dark skin pigmentation can reduce the amount of UV permeation.41 Thus, people with darker skin have higher melanin content, which may have a negative effect on vitamin D synthesis. For disease type, a previous meta-analysis showed that UC had greater odds of vitamin D deficiency in comparison to CD (11), but our studies concluded the opposite result. Compared with UC, the relationship between vitamin D and CD may be more targeted. It has been reported that CD-sensitive genes (NOD2 and ATG16L1) can be induced by the active form of vitamin D.42,43 VDR is genetically polymorphic, which has 4 alleles of ApaI, BsmI, FokI, TaqI. Among these, Apal is believed to increase the risk of CD.44 CD most commonly involves the terminal ileum, which is the main site of vitamin D absorption. Still, UC mainly affects the rectum and the distal colon. This may also be one of the reasons. For surgery, the most included studies did not specify the type of surgery, so our meta-analysis did not further quantify the impact of different procedures on vitamin D deficiency. Some studies had involved ileectomy,13,18 which can disrupt the enterohepatic circulation of bile salts, resulting in fat-soluble vitamin deficiencies. Previous meta-analyses concluded that patients with active CD were more likely to enhance the risk of vitamin D deficiency for disease activity.12,45 Recently, a meta-analysis by Gubatan et al.46 concluded that the risk of vitamin D deficiency is high in patients with active IBD. A similar conclusion emerged from our study. Vitamin D deficiency may result from undernutrition or malabsorption. Also, at the active stage of IBD, people may feel discomfort and reduce outdoor activities. Some studies had shown that IBD patients with low 25(OH) D status are related to poor quality of life.33,46 On the other hand, when the condition worsens, it may lead to the activation of inflammatory factors, inhibit the proliferation of osteoblasts, and stimulate osteoclasts’ production, which leads to osteoporosis. Steroids are mainly used in patients with moderate to severe IBD to induce remission, and our research has proved that disease activity is a risk factor for vitamin D deficiency. The use of biologics is also a risk factor. Vitamin D, on the one hand, can regulate the TNF-mediated pro-inflammatory pathway.13 On the other hand, patients treated with biologics are more likely to be affected by the active severe disease, increasing the risk of vitamin D deficiency.
For women, it is less likely to lack vitamin D, because they have more estrogen to stimulate the secretion of parathyroid hormone, which can promote the production of active vitamin D. Therefore, premenopausal women are less likely to suffer from vitamin D deficiency. On the contrary, the probability of males suffering from vitamin D deficiency greatly increases. Such a theory was also applicable in patients with IBD. However, when the cutoff value of vitamin D deficiency is defined as below 20 ng/mL, males were not a risk factor in our study. The level of 20 ng/mL to define vitamin D deficiency is based on bone health. There is no current optimal cutoff of vitamin D for immune function. A meta-analysis has shown that the low level of vitamin D in patients with IBD is relative to poor clinical prognosis.46 However, this study did not clarify whether the threshold for vitamin D deficiency should be raised. Therefore, we should conduct more high-quality research to clarify the lack of cutoff value.
As for 5-ASA, we concluded the result from 4 studies that 5-ASA was not related to vitamin D deficiency; more relevant research should be included to increase the credibility of the conclusion. Smoking increases the recruitment of immune cells, upregulates the expression of various cytokines, and induces autophagy and apoptosis, which can affect immune regulation.47 In non-Jewish white individuals, smoking may be protective against UC, and elevate the risks of CD.10 There have been reported that concentrations of 25(OH)D in patients with CD were decreased in smokers compared with nonsmokers.48 However, we concluded the opposite conclusion in our meta-analysis. A threshold may exist in the amount smoked with regard to vitamin D deficiency. We hope more research in this field will be published to refine the further analysis.
Considering BMI, several studies suggested there was an inverse association between BMI and the 25(OH)D.49,50 The reason could be explained by the fat-soluble vitamin D, which has a greater distribution in the body for people with high BMI. However, the fact is that we were unable to identify the correlation between BMI and vitamin D concentrations due to the limited number of included studies. Considering immunomodulators, in the included studies, except for the study by Chatu et al., others were not adjusted for confounding factors. The conclusion in the non-prospective subgroup suggested that it is a protective factor, which needs to be treated with caution because the upper limit of 95% CI is nearly equal to one, and more studies need to be included. In addition, the dose and course of treatment of immunomodulators in the study were not unified. So we need to include more high-quality research for future analysis. For the winter season, as shorter sun exposure and clothing issues would generally lead to vitamin D deficiency. However, in our meta-analysis, heterogeneity existed may be due to the latitude of the geographic region and the baseline characteristics of the studied population. In addition, some included studies did not perform multivariate analysis for confounding factors or only adjusted for a few important factors. In future studies, more relevant confounders should be assessed to improve the credibility of our result.
The strengths of our meta-analysis lie in the large-sample subjects and comprehensive coverage of the risk of vitamin D deficiency in IBD. Additionally, in the meta-analysis we conducted, the heterogeneity of most results was mild or non-existent. However, several limitations of our meta-analysis need to be considered. First, in the included studies, vitamin D deficiency was defined in different cutoff levels. Different classification criteria may lead to a certain degree of heterogeneity, although we carried out a subgroup analysis based upon this. Second, some original studies did not adjust for potentially relevant confounding factors; some only adjust the major potential confounders, so the residual confounders may still exist, and any factors could result in biased estimates.
In conclusion, our meta-analysis identified 7 risk factors, 2 unrelated factors, and 3 uncertain factors. It appears important to monitors and takes vitamin D regularly when risk factors are present. Regarding the unresolved influencing factors in the article, research should be intensified. For the use of drugs, it is best to have research on drug dosage and duration of treatment to provide strong evidence for further guidance on clinical medication.
Funding Statement
The authors declared that this study has received no financial support.
Footnotes
Ethics committee approval: Ethics committee approval was received for this study.
Informed consent: Informed consent was obtained.
Peer review: Externally peer-reviewed.
Author contributions: Concept – S.S., J.F.; Design – S.S., J.F.; Supervision – H.S.; Resources – H.S.; Data Collection and/or Processing – S.S., J.F., L.Z., Y.L., H.S.; Analysis and/or Interpretation – S.S., J.F., L.Z., Y.L., H.S.; Literature Search – S.S., H.S.; Writing Manuscript – S.S., J.F.; Critical Review – S.S., J.F., L.Z., Y.L., H.S.
Conflict of interest: The authors have no conflict of interest to declare.
References
- 1. . Ng SC, Shi HY, Hamidi N. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet. 2017;390(10114):2769–2778.. 10.1016/S0140-6736(17)32448-0) [DOI] [PubMed] [Google Scholar]
- 2. . Kaplan GG. The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol. 2015;12(12):720–727.. 10.1038/nrgastro.2015.150) [DOI] [PubMed] [Google Scholar]
- 3. . Ananthakrishnan AN, Bernstein CN, Iliopoulos D. Environmental triggers in IBD: A review of progress and evidence. Nat Rev Gastroenterol Hepatol. 2018;15(1):39–49.. 10.1038/nrgastro.2017.136) [DOI] [PubMed] [Google Scholar]
- 4. . de Souza HSP, Fiocchi C, Iliopoulos D. The IBD interactome: An integrated view of aetiology, pathogenesis and therapy. Nat Rev Gastroenterol Hepatol. 2017;14(12):739–749.. 10.1038/nrgastro.2017.110) [DOI] [PubMed] [Google Scholar]
- 5. . Nielsen OH, Rejnmark L, Moss AC. Role of vitamin D in the natural history of inflammatory bowel disease. J Crohns Colitis. 2018;12(6):742–752.. 10.1093/ecco-jcc/jjy025) [DOI] [PubMed] [Google Scholar]
- 6. . Alhassan Mohammed HA, Mirshafiey A, Vahedi H. Immunoregulation of inflammatory and inhibitory cytokines by vitamin D3 in patients with inflammatory bowel diseases. Scand J Immunol. 2017;85(6):386–394. 10.1111/sji.12547) [DOI] [PubMed] [Google Scholar]
- 7. . Li J, Zuo L, Tian Y. Spontaneous colitis in IL-10-deficient mice was ameliorated via inhibiting glutaminase1. J Cell Mol Med. 2019;23(8):5632–5641.. 10.1111/jcmm.14471) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. . Baumgart DC, Sandborn WJ. Inflammatory bowel disease: Clinical aspects and established and evolving therapies. Lancet. 2007;369(9573):1641–1657.. 10.1016/S0140-6736(07)60751-X) [DOI] [PubMed] [Google Scholar]
- 9. . Ghishan FK, Kiela PR. VitaminS and minerals in inflammatory bowel disease. Gastroenterol Clin North Am. 2017;46(4):797–808.. 10.1016/j.gtc.2017.08.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. . Piovani D, Danese S, Peyrin-Biroulet L. Environmental risk factors for inflammatory bowel diseases: An umbrella review of meta-analyses. Gastroenterology. 2019;157(3):647-659.e4. 10.1053/j.gastro.2019.04.016) [DOI] [PubMed] [Google Scholar]
- 11. . Del Pinto RD, Pietropaoli D, Chandar AK, Ferri C, Cominelli F. Association Between inflammatory bowel disease and vitamin D deficiency: A systematic review and meta-analysis. Inflamm Bowel Dis. 2015;21(11):2708–2717.. 10.1097/MIB.0000000000000546) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. . Huang J, Chen T, Liu Y. How would serum 25(OH)D level change in patients with inflammatory bowel disease depending on intestinal mucosa vitamin D receptor (VDR) and vitamin D1-α hydroxylase (CYP27B1)? Turk J Gastroenterol. 2019;30(2):132–138.. 10.5152/tjg.2018.17828) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. . Basson AR, Swart R, Jordaan E, Mikatako M, Watermeyer G. Vitamin D deficiency increases the risk for moderate to severe disease activity in Crohn’s disease patients in South Africa, measured by the Harvey Bradshaw index. J Am Coll Nutr. 2015;35:1–12.. [DOI] [PubMed] [Google Scholar]
- 14. . Bhagavathula A, Gaye B, Alvarez J. Vitamin D deficiency is found in 2/3 of African American population regardless of underlying IBD or not and the vitamin D levels are not influenced by disease severity or location in AA population. Inflam Bowel Dis. 2011;17(suppl 6) . 10.1097/00054725-201112002-00015) [DOI] [Google Scholar]
- 15. . Blanck S, Aberra F. Vitamin D deficiency is associated with ulcerative colitis disease activity. Dig Dis Sci. 2013;58(6):1698–1702.. 10.1007/s10620-012-2531-7) [DOI] [PubMed] [Google Scholar]
- 16. . Bours PHA, Wielders JPM, Vermeijden JR, van de Wiel A. Seasonal variation of serum 25-hydroxyvitamin D levels in adult patients with inflammatory bowel disease. Osteoporos Int. 2011;22(11):2857–2867.. 10.1007/s00198-010-1484-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. . Castro FD, Magalhães J, Carvalho PB. Lower levels of vitamin D correlate with clinical disease activity and quality of life in inflammatory bowel disease. Arq gastroenterol. 2015;52(4):260–265. 10.1590/S0004-28032015000400003) [DOI] [PubMed] [Google Scholar]
- 18. . Chatu S, Chhaya V, Holmes R. Factors associated with vitamin D deficiency in a multicultural inflammatory bowel disease cohort. Frontline Gastroenterol. 2013;4(1):51–56.. 10.1136/flgastro-2012-100231) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. . Domislović V, Barišic A, Brinar M. High prevalence of untreated and undertreated vitamin D deficiency in patients with IBD. U Eur Gastroenterol J. 2018;6:A644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. . Frigstad SO, Høivik M, Jahnsen J. Vitamin D deficiency in inflammatory bowel disease: Prevalence and predictors in a Norwegian outpatient population. Scand J Gastroenterol. 2017;52(1):100–106.. 10.1080/00365521.2016.1233577) [DOI] [PubMed] [Google Scholar]
- 21. . Fu YT, Chatur N, Cheong-Lee C, Salh B. Hypovitaminosis D in adults with inflammatory bowel disease: potential role of ethnicity. Dig Dis Sci. 2012;57(8):2144–2148.. 10.1007/s10620-012-2130-7) [DOI] [PubMed] [Google Scholar]
- 22. . Hausmann J, Kubesch A, Amiri M, Filmann N, Blumenstein I. Vitamin D deficiency is associated with increased disease activity in patients with inflammatory bowel disease. J Clin Med. 2019;8(9):1319. 10.3390/jcm8091319) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. . Janssen CE, Globig AM, Busse Grawitz A, Bettinger D, Hasselblatt P. Seasonal variability of vitamin D status in patients with inflammatory bowel disease - A retrospective cohort study. PLOS ONE. 2019;14(5):e0217238. 10.1371/journal.pone.0217238) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. . de Bruyn JRd, van Heeckeren R, Ponsioen CY. Vitamin D deficiency in Crohn’s disease and healthy controls: A prospective case-control study in the Netherlands. J Crohns Colitis. 2014;8(10):1267–1273.. 10.1016/j.crohns.2014.03.004) [DOI] [PubMed] [Google Scholar]
- 25. . Kabbani TA, Koutroubakis IE, Schoen RE. Association of vitamin D level With clinical status in inflammatory bowel disease: A 5-year longitudinal study. Am J Gastroenterol. 2016;111(5):712–719.. 10.1038/ajg.2016.53) [DOI] [PubMed] [Google Scholar]
- 26. . Ko KH, Kim YS, Lee BK. Vitamin D deficiency is associated with disease activity in patients with Crohn’s disease. Intest Res. 2019;17(1):70–77.. 10.5217/ir.2018.00022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. . Law AD, Dutta U, Kochhar R. Vitamin D deficiency in adult patients with ulcerative colitis: Prevalence and relationship with disease severity, extent, and duration. Indian J Gastroenterol Off J Indian Soc Gastroenterol. 2019;38:6–14.. [DOI] [PubMed] [Google Scholar]
- 28. . Mentella MC, Scaldaferri F, Pizzoferrato M, Gasbarrini A, Miggiano GAD. The Association of Disease Activity, BMI and phase angle with vitamin D deficiency in patients with IBD. Nutrients. 2019;11(11):2583. 10.3390/nu11112583) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. . Olmedo Martín RV, González Molero I, Olveira Fuster G, Amo Trillo V, Jiménez Pérez M. Vitamin D deficiency in outpatients with inflammatory bowel disease: Prevalence and association with clinical-biological activity. Rev Esp Enferm Dig. 2019;111(1):46–54.. 10.17235/reed.2018.5714/2018) [DOI] [PubMed] [Google Scholar]
- 30. . Pallav K, Riche D, May WL, Sanchez P, Gupta NK. Predictors of vitamin D deficiency in inflammatory bowel disease and health: A Mississippi perspective. World J Gastroenterol. 2017;23(4):638–645.. 10.3748/wjg.v23.i4.638) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. . Scotti GB, Afferri MT, Carolis AD. Factors Affecting Vitamin D Deficiency in Active Inflammatory Bowel Diseases; vol 51. Elsevier Ltd; 2018:657–662.. [DOI] [PubMed] [Google Scholar]
- 32. . Torella MC, Rausch A, Lasa J, Zubiaurre I. Vitamin D deficiency among inflammatorybowel disease patients in Argentina: A cross-sectional study. Arq gastroenterol. 2018;55(3):216–220.. 10.1590/S0004-2803.201800000-57) [DOI] [PubMed] [Google Scholar]
- 33. . Ulitsky A, Ananthakrishnan AN, Naik A. Vitamin D deficiency in patients with inflammatory bowel disease: Association with disease activity and quality of life. JPEN J Parenter Enter Nutr. 2011;35(3):308–316.. 10.1177/0148607110381267) [DOI] [PubMed] [Google Scholar]
- 34. . Zator ZA, Cantu SM, Konijeti GG. Pretreatment 25-hydroxyvitamin D levels and durability of anti-tumor necrosis factor-alpha therapy in inflammatory bowel diseases. JPEN J Parenter Enter Nutr. 2014;38(3):385–391.. 10.1177/0148607113504002) [DOI] [PubMed] [Google Scholar]
- 35. . Zullow S, Jambaulikar G, Rustgi A, Quezada S, Cross RK. Risk factors for vitamin D deficiency and impact of repletion in a tertiary care inflammatory bowel disease population. Dig Dis Sci. 2017;62(8):2072–2078.. 10.1007/s10620-017-4614-y) [DOI] [PubMed] [Google Scholar]
- 36. . Juneja M, Jorge A, LeStrange A. Patterns of vitamin D deficiency in inflammatory bowel disease: Tertiary care referral study. Am J Gastroenterol. 2012;107:S671. 10.14309/00000434-201210001-01659) [DOI] [Google Scholar]
- 37. . Tran B-CR, Huang GS, Jantchou P. Risk factors for vitamin D deficiency in children with Crohn’s disease or ulcerative colitis in Canada. Gastroenterology. Elsevier Inc. 2017;152(5):S785. 10.1016/S0016-5085(17)32720-8) [DOI] [Google Scholar]
- 38. . Shamseer L, Moher D, Clarke M. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647. 10.1136/bmj.g7647) [DOI] [PubMed] [Google Scholar]
- 39. . Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605.. 10.1007/s10654-010-9491-z) [DOI] [PubMed] [Google Scholar]
- 40. . Zeng X, Zhang Y, Kwong JS. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: A systematic review. J Evid Based Med. 2015;8(1):2–10.. 10.1111/jebm.12141) [DOI] [PubMed] [Google Scholar]
- 41. . Holick MF, Binkley NC, Bischoff-Ferrari HA. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–1930.. 10.1210/jc.2011-0385) [DOI] [PubMed] [Google Scholar]
- 42. . Sun J. VDR/vitamin D receptor regulates autophagic activity through ATG16L1. Autophagy. 2016;12(6):1057–1058.. 10.1080/15548627.2015.1072670) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. . Wang TT, Dabbas B, Laperriere D. Direct and indirect induction by 1,25-dihydroxyvitamin D3 of the NOD2/CARD15-defensin beta2 innate immune pathway defective in Crohn disease. J Biol Chem. 2010;285(4):2227–2231. 10.1074/jbc.C109.071225) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. . Wang L, Wang ZT, Hu JJ. Polymorphisms of the vitamin D receptor gene and the risk of inflammatory bowel disease: A meta-analysis. Genet Mol Res. 2014;13(2):2598–2610.. 10.4238/2014.April.8.2) [DOI] [PubMed] [Google Scholar]
- 45. . Lu C, Yang J, Yu WL. Association between 25(OH)D level, ultraviolet exposure, geographical location, and inflammatory bowel disease activity: A systematic review and meta-analysis. PLOS ONE. 2015;10(7):e0132036. 10.1371/journal.pone.0132036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. . Gubatan J, Chou ND, Nielsen OH, Moss AC. Systematic review with meta-analysis: Association of vitamin D status with clinical outcomes in adult patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2019;50(11-12):1146–1158.. 10.1111/apt.15506) [DOI] [PubMed] [Google Scholar]
- 47. . Allais L, De Smet RD, Verschuere S. Transient receptor potential channels in intestinal inflammation: What is the impact of cigarette smoking? Pathobiology. 2017;84(1):1–15.. 10.1159/000446568) [DOI] [PubMed] [Google Scholar]
- 48. . Jørgensen SP, Hvas CL, Agnholt J. Active Crohn’s disease is associated with low vitamin D levels. J Crohns Colitis. 2013;7(10):e407–e413. 10.1016/j.crohns.2013.01.012) [DOI] [PubMed] [Google Scholar]
- 49. . Konradsen S, Ag H, Lindberg F, Hexeberg S, Jorde R. Serum 1,25-dihydroxy vitamin D is inversely associated with body mass index. Eur J Nutr. 2008;47(2):87–91.. 10.1007/s00394-008-0700-4) [DOI] [PubMed] [Google Scholar]
- 50. . Vimaleswaran KS, Berry DJ, Lu C. Causal relationship between obesity and vitamin D status: Bi-directional Mendelian randomization analysis of multiple cohorts. PLOS Med. 2013;10(2):e1001383. 10.1371/journal.pmed.1001383 [DOI] [PMC free article] [PubMed] [Google Scholar]