Abstract
Background
: The aim was to assess the clinical Glasgow–Blatchford score (GBS), Rockall score (CRS), and AIMS65 score in predicting outcomes (rebleeding, need for intervention, and length of stay) among patients with small bowel hemorrhage.
Methods
: We conducted a retrospective study of patients with small bowel bleeding (SBB). Rebleeding, need for intervention, and length of stay was investigated by 3 scoring systems. The area under the receiver operator characteristic curve was used to analyze the performance of 3 scoring systems.
Results
: Among 162 included patients, the scores of rebleeding, intervention, and length of stay ≥10 days groups were higher than no rebleeding, non-intervention, and length of stay <10 days groups, respectively (P < .05). The CRS, GBS, and AIMS65 scoring systems demonstrated statistically significant difference in predicting rebleeding (AUROC 0.693 vs. 0.790 vs. 0.740; all P < .01), intervention (AUROC: 0.726 vs. 0.825 vs. 0.773; all P < .01) and length of stay (AUROC 0.651 vs. 0.631 vs. 0.635; all P < .05). Higher cut-off scores achieved better sensitivity/specificity [rebleeding (CRS > 2, GBS > 7, AIMS65 > 0); need for intervention (CRS > 2, GBS > 7, AIMS65 > 0); length of stay (CRS > 0, GBS > 7, AIMS65 > 1)] in the risk stratification.
Conclusions
: The GBS system is reliable to be recommended for routine use in predicting rebleeding and the need for intervention for early decision making in patients with SBB. The 3 scoring systems are poorly useful in predicting length of stay.
Keywords: Glasgow–Blatchford score, clinical Rockall score, AIMS65 score, small bowel bleeding, ROC curve
INTRODUCTION
Small bowel bleeding (SBB) remains a relatively uncommon event in gastrointestinal (GI) bleeding.1,2 It can be a severe and potentially life-threatening condition, which is difficult to be resolved. Capsule endoscopy (CE) and double-ballon enteroscopy(DBE) could be used to detect and treat small bowel-specific bleeding lesions. However, rebleeding has still been reported in 13-20% of cases after cessation of small bowel bleeding.3,4 It is valuable to find high-risk patients with rebleeding and quickly treat them in these situations.
The risk scores are reported to be useful for GI bleeding patient risk stratification, triage, and management. The “PRSBB” score, comorbidity Index, and ORBIT score (Older age, Reduced hemoglobin/hematocrit, Bleeding history, Insufficient kidney function and Treatment with antiplatelets) were once used for the prediction of small bowel rebleeding in patients with SBB, but there were limitations for them.5-7 For example, no risk score was designed to distinguish between high-risk and low-risk patients with SBB after the first investigation and making early decisions such as the timing of intervention(blood transfusion, endoscopic, or surgical therapy) and time of discharge in patients. Therefore, it is useful to find a clinical prognosis score for the identification of high-risk patients with small bowel hemorrhage, such as rebleeding, to determine who requires early intervention.
For upper gastrointestinal bleeding (UGIB), some clinical prognosis scores are used to predict the risk of rebleeding, need for intervention, and length of stay in clinical practice, such as Glasgow–Blatchford score (GBS), clinical Rockall score (CRS), and AIMS65 score.8,9 Whether the 3 scoring systems could be used for SBB is still unknown. There are several risk factors in common between SBB and UGIB.5,6,7,10 For example, risk factors for SBB include age, the pulse rate and systolic blood pressure, comorbidities, sex, hemoglobin levels, INR, medication use on admission, melena, and so on.10 The risk factors in SBB are also shared by UGIB.5-7 Furthermore, the risk factors included in SBB are similar to risk factors that included in CRS, GBS, and AIMS65 score.5,6,7,10 Therefore, we aimed to determine 3 different prognosis scores designed for UGIB could be used in small bowel hemorrhage to estimate the risk of rebleeding and make further decisions for intervention and length of stay .
METHODS
Study Design and Setting
We conducted a retrospective study in patients who suffered SBB between January 1, 2016 and January 31, 2019 at our academic medical center. The medical center is a 2468-bed urban academic, tertiary care, and university hospital. Ethics committee approval was received for this study from the Ethics Committee of the academic medical center. Written informed consent was necessary to be signed for the patients who participated in this study.
Selection of Participants
In this study, 162 patients hospitalized with SBB were retrospectively analyzed. All patients 18 years of age or older were necessary for the study. Upper endoscopy and colonoscopy were negative in patients who got GI bleeding with signs (melena/hematochezia, hypotension, shock, orthostatic changes in systolic blood pressure and/or pulse, or repeated bleeding). For patients to be enrolled, they needed to have performed examinations of CE or/and DBE. The patients with pregnant and unable to be performed with CE or DBE were excluded.
Rebleeding is defined as bleeding again after 24 h of cessation of bleeding in the small bowel, including melena or/and hematochezia, a decrease of >2 g/dL in hemoglobin value, or need of a transfusion.
Scores
The 3 scoring systems (GBS, CRS, and AIMS65 score) chosen for SBB were independent from endoscopy. The clinical or laboratory findings were the basement of the 3 scoring systems. The Blatchford score, CRS, and AIMS65 score were calculated from clinical or laboratory variables.11-13
Statistical Analysis
All data were identified and entered into computer data files by experienced data managers. Statistical analysis was performed using SPSS19.0. Baseline characteristics and outcomes were summarized by frequency tabulation and means with standard deviations as appropriate. The discriminative ability of the scoring systems for predicting outcomes was evaluated by receiver–operator characteristic curve analysis. The area under the receiver–operating characteristic curve the area under the receiver-operating characteristic curve (AUROC) was calculated and compared for all scores using the DeLong test. A cut-off point was selected according to the maximal sum of each score’s sensitivity and specificity.
Estimates of sensitivity and specificity were calculated for each score. Comparison between rebleeding/intervention/length of stay ≥ 10 days groups and no rebleeding/non-intervention/length of stay <10 days groups for each score was performed using the chi-square test and Fisher’s exact test as appropriate. All statistical comparisons were 2 tailed, with P value <.05 considered statistically significant.
RESULTS
Population Characteristics
In this study, 162 patients with SBB were identified and suitable for our study to be analyzed. The median age of the patients was 56 years. Of these patients, 104 (64.81%) were males. At admission, melena or/and hematochezia were the most common presentation of SBB. The most common comorbidities were hypertension (37.0%) and cardiovascular disease (18.5%). Regarding medication, 46 (28.4%) patients were taking non-steroidal anti-inflammatory drugs when the bleeding episode occurred. Blood transfusion was required in nearly half of the patients (48.1%), while endoscopic therapy was required in almost 1/10 patients. Only 2 patients (2.5%) required surgery as rescue therapy. Rebleeding was observed in 76 patients (46.9%) over a median follow-up period of 4 weeks. Two patients died because of small bowel malignant tumor. The baseline demographic and clinical characteristics of these patients according to in-patient status are shown in Table 1.
Table 1.
Baseline Characteristics and Outcomes of Patients
| Characteristics of Patients at Baseline | |
|---|---|
| Age (mean ± SD) (years) | 56.27 ± 18.92 |
| Male/female | 104/58 |
| Length of stay (days) | 12.14 ± 6.40 |
| Mean course (months) (mean± SD) | 12.84 ± 27.27 |
| Previous bleeding, n (%) | 38 (23.5%) |
| Symptoms at admission, n (%) | |
| Melena | 110 (67.9%) |
| Hematochezia | 52 (32.1%) |
| Comorbidities, n (%) | |
| Hypertension | 60 (37.0%) |
| Cardiovascular disease | 30 (18.5%) |
| Diabetic mellitus | 14 (8.6%) |
| Malignant tumor | 8 (4.9%) |
| Chronic kidney disease | 6 (3.7%) |
| Medication, n (%) | |
| Non-steroidal anti-inflammatory drugs and/or aspirin use | 46 (28.4%) |
| Laboratory test, mean ± SD | |
| Heart rate | 73.83 ± 13.16 |
| Systolic blood pressure (mmHg) | 126.28 ± 20.63 |
| White blood cells (×109/L) | 6.83 ± 4.51 |
| Hemoglobin count (g/L) | 86.43 ± 28.42 |
| Platelet count (×109/L) | 247.67 ± 94.94 |
| PT-INR (prothrombin time, international normalized ratio) | 1.09 ± 0.16 |
| Albumin (g/L) | 37.27 ± 6.59 |
| Creatinine (µmol/L) | 71.59 ± 26.90 |
| Urea nitrogen (mmol/L) | 5.34 ± 3.05 |
| Intervention, n (%) | |
| Blood transfusion | 78 (48.1%) |
| Endoscopic therapy | 16 (9.9%) |
| Surgical therapy | 2 (2.5%) |
| Outcomes, n (%) | |
| Rebleeding | 76 (46.9%) |
| Final etiologic diagnosis, n (%) | |
| Angiodysplasia | 48 (29.63%) |
| Crohn’s disease | 22 (13.58%) |
| Small bowel neoplasia | 18 (11.11%) |
| Non-specific enteritis | 18 (11.11%) |
| Diverticular disease | 10 (6.17%) |
| Polyp | 10 (6.17%) |
| Non-steroidal anti-inflammatory drugs enteropathy | 4 (2.47%) |
| Other disease | 4 (2.47%) |
| Unknown | 28 (17.30%) |
| Total | 162 (100%) |
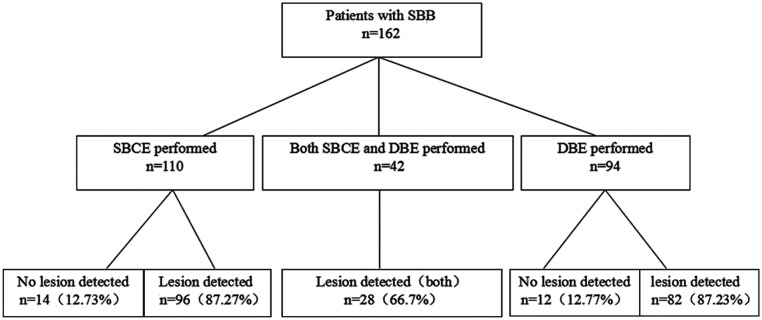
One hundred sixty-two cases involved in SBB were diagnosed by small bowel capsule endoscopy (SBCE) and/or DBE. Of all the patients who experienced SBB, 48 (29.63%) had lesions detected by SBCE, while 82 (87.23%) were detected by DBE. Twenty-eight (66.7%) lesions were found in both SBCE/DBE. Patients performed by SBCE/DBE can be found in Figure 1.
Figure 1.
Patient enrollment flow chart. SBCE, small bowel capsule endoscopy; SBB, small bowel bleeding; DBE, double-balloon endoscopy.
Etiology of SBB
After examination of SBCE/DBE, the 4 most frequent sources of SBB were angiodysplasia, Crohn’s disease, small bowel neoplasia, and non-specific enteritis, accounting for 29.63%, 13.58%, 11.11%, and 11.11% of the endoscopic diagnosis, respectively. In 28 (17.3%) patients, the cause of bleeding could not be detected. A complete list of etiologic diagnosis of SBB is shown in Table 1.
Performance of CRS, GBS, and AIMS65 Score in the Prediction of Rebleeding, Interventions, and Length of Stay
Rebleeding, interventions, and length of stay were observed in patients with SBB by CRS, GBS, and AIMS65 score systems. According to CRS, GBS, and AIMS65 score systems, the scores of rebleeding patients were significantly higher than no rebleeding patients (all P < .05). The scores of non-intervention patients were significantly lower than intervention patients (all P < .05).
Similarly, the scores of the length of stay ≥10 days were significantly higher than the length of stay <10 days in SBB. The above results suggested that rebleeding, interventions, and length of stay in SBB could be predicted by all the 3 scores (Table 2).
Table 2.
Performance of the CRS, GBS, and AIMS65 Scoring Systems in Predicting Clinical Outcomes in SBB
| Score | Outcomes | P | |
|---|---|---|---|
| Rebleeding | No Rebleeding | ||
| CRS | 2.92 ± 1.67 | 1.98 ± 1.39 | <.05 |
| GBS | 9.08 ± 2.66 | 5.98 ± 2.88 | <.05 |
| AIMS65 | 1.24 ± 0.97 | 0.47 ± 0.70 | <.05 |
| Intervention | Non-intervention | ||
| CRS | 2.94 ± 1.50 | 1.71 ± 1.45 | <.05 |
| GBS | 8.83 ± 3.07 | 5.50 ± 2.15 | <.05 |
| AIMS65 | 1.17 ± 0.92 | 0.35 ± 0.69 | <.05 |
| Length of stay ≥10 days | Length of stay <10 days | ||
| CRS | 2.76 ± 1.52 | 1.87 ± 1.57 | <.05 |
| GBS | 8.02 ± 3.27 | 6.48 ± 2.79 | <.05 |
| AIMS65 | 1.02 ± 1.02 | 0.52 ± 0.63 | <.05 |
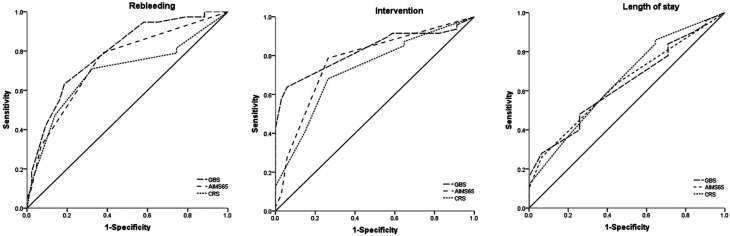
The receiver-operating characteristic (ROC) curves of the GBS, CRS, and AIMS65 scores for the prediction of rebleeding in SBB are shown in Table 3 and Fig. 2. The 3 scores were useful for the prediction of rebleeding in SBB. The area under curve (AUC) for the GBS was 0.790, that for the CRS was 0.693, and that for the AIMS65 score was 0.740 (P < .01). The rebleeding cut-off point that maximized the sum of sensitivity and specificity was 7 for GBS (sensitivity 63.16%, specificity 81.40%), 2 for CRS (sensitivity 71.05%, specificity 67.44%), and 0 for AIMS65 score (sensitivity 78.95%, specificity 62.79%). There was no statistically significant difference among 3 scores in predicting rebleeding (P > .05).
Table 3.
The ROC Results of Clinical Outcomes in SBB
| Outcomes | Score | AUROC (95% CI) | P | Cut-off | Se% | Sp% |
|---|---|---|---|---|---|---|
| Rebleeding | CRS | 0.693 (0.573-0.813) | .003 | >2 | 71.05 | 67.44 |
| GBS | 0.790 (0.692-0.888) | .000 | >7 | 63.16 | 81.40 | |
| AIMS65 | 0.740 (0.631-0.849) | .000 | >0 | 78.95 | 62.79 | |
| Intervention | CRS | 0.726 (0.616-0.819) | .001 | >2 | 68.09 | 73.53 |
| GBS | 0.825 (0.725-0.901) | .000 | >7 | 63.83 | 94.12 | |
| AIMS65 | 0.773 (0.667-0.859) | .000 | >0 | 78.72 | 73.53 | |
| Length of stay | CRS | 0.651 (0.529-0.771) | .023 | >0 | 86.00 | 35.48 |
| GBS | 0.631 (0.515-0.756) | .049 | >7 | 48.00 | 74.19 | |
| AIMS65 | 0.635 (0.521-0.740) | .041 | >1 | 26.00 | 93.55 |
Figure 2.
The receiver–operating characteristic (ROC) curves for the predictive value of clinical Rockall score (CRS), Glasgow–Blatchford score (GBS), and AIMS65 systems for the risk of clinical outcomes in SBB.
Similarly, GBS (AUC 0.825, 95% CI 0.725-0.901), CRS (AUC 0.726, 95% CI 0.616-0.819), and AIMS65 score (AUC 0.773, 95% CI 0.667-0.859) performed reliably in predicting the need for intervention in patients with SBB (all P < .01). There was no statistically significant difference among 3 scores in predicting the need for intervention (P > .05). The intervention cut-off point that maximized the sum of sensitivity and specificity was 7 for GBS (sensitivity 63.83%, specificity 94.12%), 2 for CRS (sensitivity 68.09%, specificity 73.53%), and 0 for AIMS65 score (sensitivity 78.72%, specificity 73.53%) (Table 3 and Figure 2).
CRS (AUC 0.651), GBS (AUC 0.631), and AIMS65 score (AUC 0.635) were similar and useful for prediction of the length of stay(all P < .05). There was a statistically significant difference among 3 scores in predicting the length of stay (P > .05). Regarding the length of stay, the cut-off point that maximized the sum of sensitivity and specificity was 7 for GBS (sensitivity 48%, specificity 74.19%), 0 for CRS (sensitivity 86%, specificity 35.48%), 1 for the AIMS65 score (sensitivity 26%, specificity 93.55%) (Table 3 and Figure 2).
DISCUSSION
Establishing the patient’s life support measures and stabilizing the patient’s physical situation is the first step when severe acute SBB was encountered. Next, the specific etiological diagnosis should be considered. If the origins of bleeding in GI could not be found after an initial evaluation using esophagogastroduodenoscopy (EGD) and colonoscopy, small bowel hemorrhage was always to be suspected. The specific etiology of bleeding in the small bowel might now be identified by DBE/CE. In the present study, DBE was performed after CE for some patients with SBB even if their small bowel CE findings were negative because small bowel CE might miss some lesions.14
The differential diagnosis of the SBB lesions identified by DBE/CE is broad and includes vascular lesions such as non-steroidal anti-inflammatory drug (NSAID)-induced ulcers and erosions, angioectasias, inflammatory diseases such as Crohn’s disease, small bowel tumors including lymphomas, gastrointestinal stromal tumors, carcinoid tumors, adenocarcinomas, and small bowel metastases.15,16 In the present cohort of 162 patients with SBB detected by DBE/CE, the 4 most frequent sources of SBB were angiodysplasia, Crohn’s disease, small bowel neoplasia, and non-specific enteritis. However, the specific etiologies of 28 (17.3%) patients were still not be found. There might be various causes for negative findings in suspected SBB detected by DBE/CE. For example, the lesion localized at a site in the small bowel that was easily overlooked or cessation of bleeding at the time of double-balloon endoscopy/CE. The results of etiology in the present study were not consistent with the previous study, which might attribute to various causes such as the age of the patients.17
The treatments of SBB have varied according to the situation of the patients with SBB. It is desirable to apply strategies such as scoring systems for optimizing patient outcomes in early hospitalization and minimize healthcare resources at the same time. Several scoring systems were used to predict small bowel rebleeding before, such as “PRSBB” score, comorbidity Index, and ORBIT score.5-7 However, all of them were not ideal scoring models for predicting the clinical outcomes and making early decisions in patients with SBB. For example, “PRSBB” score was only used as an appropriate follow-up strategy for small bowel hemorrhage; Comorbidity Index based on comorbidities and age was only designed to estimate recurrent bleeding and vascular disease for SBB. The ORBIT score was originally created to predict small bowel hemorrhage in patients with atrial fibrillation and chronic anticoagulation. Therefore, new computing risk scores such as CRS, GBS, and AIMS65 scores were needed to improve medical decision-making at the initial situation of the patient after admission, which got patient management and outcome improved.18
The present study found that the 3 scoring systems, especially the GBS scoring system, were valuable in small bowel hemorrhage for prediction of rebleeding and the need for intervention. However, 3 scoring systems were poor in predicting length of stay. And there was no statistically significant difference among the 3 scoring systems to predict clinical outcomes in small bowel hemorrhage.
Blood urea, hemoglobin, pulse, melena, systolic blood pressure, syncope on presentation, liver disease, and heart failure were all associated with the GBS, which was reliable for predicting rebleeding in small bowel hemorrhage. The clinical or laboratory variables except blood urea included in GBS could also be found in the risk factors in SBB, which might be attributed to the good prediction of rebleeding of SBB.10 Similar results could be detected in CRS and AIMS65 scores. The scores of rebleeding patients were significantly higher than no rebleeding patients according to CRS, GBS, and AIMS65 score, which was consistent with “PRSBB” score and ORBIT score.5,7 We suggested that patients with suspected SBB at high risk of rebleeding should be clinically interfered and closely observed after the first investigation. The present study also suggested that a threshold of GBS of more than 7 could be used as a decision cut-off for rebleeding in SBB. The results also suggested the usage of cut-off 2 be a risk factor for CRS, while cut-off 0 for AIMS65 score. Therefore, patients with a high score of GBS, CRS, and AIMS65 should be paid more attention because of the high risk of rebleeding.
In our study, through intervention and supportive care, nearly 60% of the patients with SBB stopped bleeding. According to CRS, GBS, and AIMS65 scoring systems, patients’ scores with the intervention were higher than patients with non-intervention, which could also be found in the previous study.6 Therefore, the 3 scoring systems could be used to identify the patients with a high risk of rebleeding and allow clinicians to develop the individualized intervention. As we all known, the GBS was developed to identify a patient’s need for treatment in UGIB.19 In the present study, the score of GBS was also reliable in predicting the need for intervention in SBB. According to the present results, the score of GBS more than 7 had a very high specificity, which resulted in a high AUROC of 0.825 for the prediction of this outcome. However, the score of GBS more than 7 had 63.83% sensitivity for the results. Therefore, patients with a low score of GBS might not need an intervention, should be followed up closely. Similar results were also found in CRS and AIMS65 scores. Although the scores of patients with a length of stay ≥10 days were higher than patients with a length of stay <10 days, the GBS (AUC 0.631), CRS (AUC 0.651) and AIMS65 score (AUC 0.635) performed poorly in small bowel hemorrhage for prediction of the length of stay (Table 3 and Figure 2). The results also suggested that the cut-off point that maximized the sum of sensitivity and specificity was 7 for GBS, 0 for CRS, and 1 for the AIMS65 score. It was poor for 3 scoring systems in the sensitivity and specificity. Therefore, the 3 scoring systems had a poor value for the prediction of the length of stay in small bowel hemorrhage.
Several limitations associated with the present study warranted a mention. First, this was a retrospective study with a small number of patients. Second, we did not validate our findings in a separate, prospective cohort study in patients with SBB. Thus further studies were required.
In conclusion, this study showed that the GBS system was reliable and accurate for predicting rebleeding and the need for interferon in patients with SBB. The 3 scoring systems were poorly useful in small bowel hemorrhage for the prediction of length of stay. Therefore, the GBS system was good enough to be recommended as routine use in clinical practice for early decision making in patients with SBB. Further studies are warranted to test the GBS systems in SBB.
Funding Statement
The author declared that this study has received no financial support.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of Tianjin Medical University General Hospital (IRB2019-wz-193).
Informed Consent: Written informed consent was obtained.
Peer Review: Externally peer-reviewed.
Author Contributions: Concept – W. B.; Design - W.Y.; Supervision - S.C.; Resource - S.S.; Materials - Z.Z.; Data Collection and/or Processing - J.K., J.H.; Analysis and/or Interpretation - S.S., Z.Z.; Literature Search - Z.Z.; Writing - S,S., Z.Z.; Critical Reviews - W.B.
Acknowledgments: Authors are thankful for the medical and nonmedical staff of the Tianjin Medical University General Hospital, Tianjin, China.
Conflict of Interest: The authors have no conflict of interest to declare.
References
- 1. . Lau WY, Fan ST, Wong SH. Preoperative and intraoperative localization of gastrointestinal bleeding of obscure origin. Gut. 1987;28(7):869–8. 77. 10.1136/gut.28.7.869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. . Longstreth GF. Epidemiology and outcome of patients hospitalized with acute lower gastrointestinal hemorrhage: a population-based study. Am J Gastroenterol. 1997;92(3):419–4. 24. 10.1136/gut.28.7.869 [PubMed] [Google Scholar]
- 3. . Mitsui K, Tanaka S, Yamamoto H. Role of double-balloon endoscopy in the diagnosis of small-bowel tumors: the first Japanese multicenter study. Gastrointest Endosc. 2009;70(3):498–504.. 10.1016/j.gie.2008.12.242 [DOI] [PubMed] [Google Scholar]
- 4. . Cangemi DJ, Patel MK, Gomez V. Small bowel tumors discovered during double-balloon enteroscopy: analysis of a large prospectively collected single-center database. J Clin Gastroenterol. 2013;47(9):769–7. 72. 10.1097/MCG.0b013e318281a44e [DOI] [PubMed] [Google Scholar]
- 5. . Uchida G, Hirooka Y, Nakamura M. Nomogram-based prediction of rebleeding in small bowel bleeding patients: the ‘PRSBB’ score. Sci Rep. 2018;8(1); Turk J Gastroenterol. 2019; 1: -. 10.1038/s41598-018-24868-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. . Ohmiya N, Nakamura M, Osaki H. Development of a comorbidity index to identify patients With small bowel bleeding at risk for rebleeding and small bowel vascular diseases. Clin Gastroenterol Hepatol. 2019;17(5):896–904.e4.. 10.1016/j.cgh.2018.08.034 [DOI] [PubMed] [Google Scholar]
- 7. . Cúrdia Gonçalves T, Arieira C, Monteiro S. ORBIT score: an useful predictor of small bowel rebleeding in patients under chronicanticoagulation. Scand J Gastroenterol. 2018;53(2):179–184.. 10.1080/00365521.2017.1410568 Epub 2017 Dec 7. [DOI] [PubMed] [Google Scholar]
- 8. . Rockall TA, Logan RF, Devlin HB, Northfield TC. Risk assessment after acute upper gastrointestinal haemorrhage. Gut. 1996;38(3):316–321.. 10.1136/gut.38.3.316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. . Blatchford O, Murray WR, Blatchford M. A risk score to predict need for treatment for upper-gastrointestinal haemorrhage. Lancet. 2000;356(9238):1318–1321.. 10.1016/S0140-6736(00)02816-6 [DOI] [PubMed] [Google Scholar]
- 10. . Micic D, Gaetano JN, Nigam N. Risk factors for small bowel bleeding in an overt gastrointestinal bleeding presentation after negative upper and lower endoscopy. PloS One. 2019 February 20;14(2):e0212509. 10.1371/journal.pone.0212509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. . Rockall TA, Logan RF, Devlin HB, Northfield TC. Risk assessment after acute upper gastrointestinal haemorrhage. Gut. 1996;38(3):316–3. 21. 10.1136/gut.38.3.316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. . Blatchford O, Murray WR, Blatchford M. A risk score to predict need for treatment for upper-gastrointestinal haemorrhage. Lancet. 2000;356(9238):1318–13. 21. 10.1016/S0140-6736(00)02816-6 [DOI] [PubMed] [Google Scholar]
- 13. . Saltzman JR, Tabak YP, Hyett BH. A simple risk score accurately predicts in-hospital mortality, length of stay, and cost in acute upper GI bleeding. Gastrointest Endosc. 2011;74(6):1215–12. 24. 10.1016/j.gie.2011.06.024 [DOI] [PubMed] [Google Scholar]
- 14. . Postgate A., Despott E, Burling D. Significant small-bowel lesions detected by alternative diagnostic modalities after negative capsule endoscopy. Gastrointest Endosc. 2008;68(6):1209–1214.. 10.1016/j.gie.2008.06.035 [DOI] [PubMed] [Google Scholar]
- 15. . Pioche M, Gaudin JL, Filoche B. Prospective, randomized comparison of two small-bowel capsule endoscopy systems in patients with obscure GI bleeding. Gastrointest Endosc. 2011;73(6):1181–118. 8. 10.1016/j.gie.2011.02.011 [DOI] [PubMed] [Google Scholar]
- 16. . Pioche M, Vanbiervliet G, Jacob P. Prospective randomized comparison between axial- and lateral-viewing capsule endoscopy systems in patients with obscure digestive bleeding. Endoscopy. 2014;46(6):479–4. 84. 10.1055/s-0033-1358832 [DOI] [PubMed] [Google Scholar]
- 17. . Song JH, Hong SN, Kyung Chang D. The etiology of potential small-bowel bleeding depending on patient’s age and gender. U Eur Gastroenterol J. 2018 October;6(8):1169–1178.. 10.1177/2050640618797841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. . Cipolletta L, Bianco MA, Rotondano G, Marmo R, Piscopo R. Outpatient management for low-risk nonvariceal upper GI bleeding: a randomized controlled trial. Gastrointest Endosc. 2002;55(1):1–5.. 10.1067/mge.2002.119219 [DOI] [PubMed] [Google Scholar]
- 19. . Blatchford O, Murray WR, Blatchford M. A risk score to predict need for treatment for upper-gastrointestinal haemorrhage. Lancet. 2000;356(9238):1318–13. 21. 10.1016/S0140-6736(00)02816-6 [DOI] [PubMed] [Google Scholar]