Abstract
SCH 56592 is a new broad-spectrum azole antifungal agent that is in phase 3 clinical trials for the treatment of serious systemic fungal infections. The pharmacokinetics of this drug candidate were evaluated following its intravenous (i.v.) or oral (p.o.) administration as a solution in hydroxypropyl-β-cyclodextrin (HPβCD) or oral administration as a suspension in 0.4% methylcellulose (MC) in studies involving mice, rats, rabbits, dogs, and cynomolgus monkeys. SCH 56592 was orally bioavailable in all species. The oral bioavailability was higher with the HPβCD solution (range, 52 to ∼100%) than from the MC suspension (range, 14 to 48%) and was higher in mice (∼100% [HPβCD] and 47% [MC]), rats (∼66% [HPβCD] and 48% [MC]), and dogs (72% [HPβCD] and 37% [MC]) than in monkeys (52% [HPβCD] and 14% [MC]). In rabbits, high concentrations in serum suggested good oral bioavailability with the MC suspension. The i.v. terminal-phase half-lives were 7 h in mice and rats, 15 h in dogs, and 23 h in monkeys. In rabbits, the oral half-life was 9 h. In species given increasing oral doses (mice, rats, and dogs), serum drug concentrations were dose related. Food produced a fourfold increase in serum drug concentrations in dogs. Multiple daily doses of 40 mg of SCH 56592/kg of body weight for eight consecutive days to fed dogs resulted in higher concentrations in serum, indicating accumulation upon multiple dosing, with an accumulation index of approximately 2.6. Concentrations above the MICs and minimum fungicidal concentrations for most organisms were observed at 24 h following a single oral dose in MC suspension in all five species studied (20 mg/kg for mice, rats, and rabbits and 10 mg/kg for dogs and monkeys), suggesting that once-daily administration of SCH 56592 in human subjects would be a therapeutically effective dosage regimen.
The incidence of fungal infections has substantially increased over the past 2 decades, and invasive forms are important causes of morbidity and mortality (1). Of the estimated 100,000 known species of fungi, about 180 have been shown to cause disease in humans, and only about 10% of these are encountered in most clinical settings (2). Disseminated candidiasis, pulmonary aspergillosis, and infections caused by emerging opportunistic fungi are the most common of the serious mycoses (12). Aggressive immunosuppression, myelotoxic therapeutic regimens, AIDS, cancer, and organ transplantation have opened the door for these organisms. Although amphotericin B has been the gold standard in antifungal therapy for half of a century, newer chemical agents, such as azoles, have emerged in recent decades. These entities are generally active by more than one route of administration, have a broader spectrum of activity, and are less toxic than amphotericin B. The ideal antifungal agent would be broad spectrum, fungicidal, active against resistant strains, and active by various routes of administration and would have a good safety profile.
SCH 56592 has been shown to be active against both yeasts and filamentous fungi, including Aspergillus, Candida (including fluconazole-resistant Candida krusei), Cryptococcus, Blastomyces, Coccidioides, and other opportunistic fungi (5, 7–11; V. M. Girijavallabhan, A. K. Saksena, R. G. Lovey, F. Bennett, R. E. Pike, H. Wang, P. Pinto, Y. T. Liu, N. Patel, and A. K. Ganguly, Abstr. 35th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F61, 1995). The MIC at which 90% of the isolates are inhibited and the minimal concentrations at which 90% of the isolates are killed for various strains of A. flavus, A. fumigatus, C. albicans, and C. tropicalis were in the ranges of 0.03 to 0.25 and 0.5 to 2.0 μg/ml, respectively. Preliminary studies suggest that SCH 56592 binds to the plasma protein of rats, dogs, and humans. SCH 56592 is expected to be clinically effective in both immunocompetent and immunocompromised patients as prophylactic, therapeutic, and maintenance treatment for fungal infections.
The objectives of the present studies were to characterize the pharmacokinetics of SCH 56592 following intravenous (i.v.) and/or oral (p.o.) administration to mice, rats, rabbits, dogs, and cynomolgus monkeys. In addition, the effect of dose on serum drug concentrations was evaluated in mice, rats, and dogs.
MATERIALS AND METHODS
Animals.
Male CF-1 mice (18 to 20 g), male CRL-CD-BR rats (180 to 220 g), and male New Zealand White rabbits (approximately 3 kg) were purchased from Charles River Laboratories (Wilmington, Mass.). Male beagle dogs (7 to 15 kg) and male cynomolgus monkeys (3 to 9 kg) were obtained from the Schering-Plough Research Institute colony. Animals were kept in temperature-, humidity-, and light cycle-controlled rooms. Animals were randomly assigned to each treatment group and were subjected to fasting (water allowed) for 18 h prior to dosing, unless otherwise indicated.
Bioavailability and pharmacokinetics.
SCH 56592 was administered i.v. to mice, rats, dogs, and monkeys as a solution in 40% (wt/vol) aqueous hydroxypropyl-β-cyclodextrin (HPβCD) and orally as a solution in HPβCD or as a suspension in an aqueous solution containing 0.4% methylcellulose, 0.5% polysorbate 80, and 0.9% NaCl (MC). Rabbits received SCH 56592 only p.o. in the MC suspension. Mice, rats, and rabbits received a single dose of 20 mg/kg of body weight; dogs and cynomolgus monkeys received a single 10-mg/kg dose. Blood was collected at various intervals following dosing, and serum was frozen at −20°C pending analysis.
Effect of oral dose on serum drug concentrations.
Mice were given 20-, 40-, 80-, and 160-mg/kg doses at a constant volume of 5 ml/kg of body weight from MC suspension formulations prepared at 4, 8, 16, and 32 mg/ml, respectively. Additional animals received only the vehicle. Three animals were sacrificed at each time point to determine concentrations of SCH 56592 in serum. In a similar experimental design, rats were given 10-, 20-, 40-, 80-, and 120-mg doses of SCH 56592/kg in MC, and 3 animals were sacrificed at each time point.
An initial study was carried out with dogs to evaluate the effect of food on the oral bioavailability of SCH 56592 following a single 10-mg/kg dose. This was a parallel group study design in which 6 dogs were dosed following an 18-h fast and another 6 animals were dosed following feeding. The results showed that food significantly improved the bioavailability of SCH 56592 (see Table 2); therefore, a study was carried out in fed dogs to evaluate the effect of dose on concentrations of SCH 56592 in serum. Six animals were used in a crossover study design with a 2-week washout period. The oral dose formulation was prepared as a suspension in MC at a concentration of 16 mg/ml, and the animals were dosed at 40, 80, and 120 mg/kg.
TABLE 2.
Mean pharmacokinetic parameters of SCH 56592 following p.o. administration in MC suspensions
| Species (n) | Dose (mg/kg) | Cmax (μg/ml) | Tmax (h) | AUC0–∞ (μg · h/ml) |
|---|---|---|---|---|
| Mice (6) | 20 | 4.9 (20)a | 3.0 | 82.0 |
| 40 | 11.5 (45) | 7.0 | 146.0 | |
| 80 | 12.1 (20) | 7.0 | 363.0 | |
| 160 | 17.1 (14) | 12.0 | 508.0 | |
| Rats (3) | 10 | 2.7 (51) | 4.0 | 31.4 |
| 20 | 3.7 (12) | 6.0 | 65.1 | |
| 40 | 6.0 (19) | 4.0 | 119.0 | |
| 80 | 10.2 (26) | 6.0 | 189.9 | |
| 120 | 10.1 (11) | 4.0 | 262.6 | |
| Rabbits (6) | 20 | 4.2 (12) | 5.1 (21) | 77.5 (16) |
| Dogs (fasting) (5) | 10 | 0.2 (22) | 7.6 (121) | 7.5 (45) |
| Dogs (fed) (6) | 10 | 0.7 (28) | 8.3 (93) | 26.6 (42) |
| 40 | 2.1 (31) | 9.2 (90) | 105.0 (30) | |
| 80 | 3.1 (20) | 9.0 (82) | 167.0 (18) | |
| 120 | 3.3 (27) | 15.0 (66) | 192.0 (34) |
Values in parentheses are CVs (in percent).
A third study was performed to investigate the pharmacokinetics of SCH 56592 in fed dogs following multiple dosing. Six animals were dosed with an MC suspension of SCH 56592 (16 mg/ml) at 40 mg/kg once daily for 8 consecutive days. Blood was collected at various time intervals on days 1 and 8, just prior to the administration of SCH 56592 on days 2 to 7, and once daily on days 9 to 13.
Analysis of serum samples.
Serum samples were analyzed for SCH 56592 by high-performance liquid chromatography (HPLC). The HPLC system used consisted of a Hitachi model L-620 pump, a Waters model 715 WISP sample processor, and a Waters 484 tunable absorbance detector set at 262 nm. Two sequential 5-μm Beckman octadecyl silane Ultrasphere 4.6-mm by 15-cm columns, preceded by a 5-μm Beckman octadecyl silane Ultrasphere 4.6-mm by 4.5-cm guard column, were used. The mobile phase was a mixture of 500 ml of acetonitrile, 500 ml of 0.1 M ammonium phosphate monobasic, 5 ml of methylene chloride, and 0.5 ml of triethylamine at a flow rate of 1.5 ml/min. The retention times for SCH 56592 and the internal standard (SCH 56894, a diastereomer analogue of SCH 56592) were approximately 10 and 7.5 min, respectively. Serum was prepared for analysis by the addition of 0.04 ml of the internal standard in methanol (10 μg/ml) and 0.56 ml of methanol to 0.2 ml of serum, mixed for about 1 min, and then centrifuged at 3,000 × g for 4 min. The supernatant was transferred into polypropylene tubes and was kept in a refrigerator (4°C) overnight. Samples were then recentrifuged as previously described, and 0.2 ml of the supernatant was analyzed by HPLC. The method was cross validated in all species studied prior to sample analysis. The limit of quantitation was 0.05 μg/ml. Intraday precision (coefficient of variation [CV]) and accuracy (bias) were in the ranges of 2 to 4 and 0 to 6%, respectively. The corresponding interday values were 3 to 5 and 0 to 5%, respectively, indicating that the method was satisfactory. With each analytical run, six quality control samples were analyzed, along with two standard curves prepared in serum.
Pharmacokinetic evaluation.
Concentrations of SCH 56592 in serum that were equal to and above the limit of quantitation were used for pharmacokinetic analysis by model-independent methods (3). The maximum concentration in serum (Cmax), time of Cmax (Tmax), and final quantifiable sampling time (tf) were the observed values. The terminal-phase rate constant (k) was the slope of the serum concentration-time curve. The half-life (t1/2) was estimated as 0.693/k. The area under the serum concentration-time curve from time zero to tf (AUCtf) was calculated by the trapezoidal rule and was extrapolated to infinity as follows: AUC0–∞ = AUCtf + Ctf/k, where Ctf is the estimated concentration at tf.
RESULTS
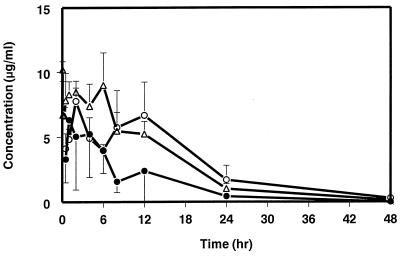
Mean serum concentration-time profiles of SCH 56592 following i.v. and p.o. administration to mice are shown in Fig. 1, with mean pharmacokinetic parameters presented in Table 1. SCH 56592 was completely bioavailable (100%) from the HPβCD solution; from the MC suspension, its bioavailability was 47%. Following p.o. administration at 20 mg/kg, the Cmax range was 6.3 to 7.8 μg/ml, which was attained at a Tmax range of 1 to 2 h after dosing. It is interesting that serum concentrations remained almost unchanged between 1 and 6 h following i.v. or p.o. administration (Fig. 1). Also, following p.o. administration in MC, mean concentrations of SCH 56592 in serum were >2 μg/ml for at least 12 h after dosing. The t1/2 following i.v. administration was 7 h. Table 2 shows the mean Cmax, Tmax, and AUC0–∞ following p.o. administration in MC at doses of 20, 40, 80, and 160 mg/kg to mice. There was a dose-related increase in both Cmax and AUC0–∞ within the dose range studied. Also, as the dose increased, Tmax shifted toward longer times.
FIG. 1.
Serum concentration-time profiles of SCH 56592 in mice following the i.v. or p.o. administration of a 20-mg/kg dose of SCH 56592. ▵, i.v. as a solution in HPβCD; ○, p.o. as a solution in HPβCD; ●, p.o. as a suspension in MC. Each vertical bar represents 1 standard deviation from the mean.
TABLE 1.
Mean pharmacokinetic parameters of SCH 56592 in mice, rats, dogs, and cynomolgus monkeysa
| Species (n) | Parameter | i.v. in HPβCD | p.o. in HPβCD | p.o. in MC |
|---|---|---|---|---|
| Mouse (3) | Cmax (μg/ml) | 7.8 (13) | 6.3 (11) | |
| Tmax (h) | 2.0 | 1.0 | ||
| AUC0–∞ (μg · h/ml) | 137.0 | 143.0 | 63.7 | |
| t1/2 (h) | 7.0 | |||
| Bioavailability (%) | 104.0 | 46.5 | ||
| Rat (3) | Cmax (μg/ml) | 3.5 (13) | 3.0 (20) | |
| Tmax (h) | 2.0 | 4.0 | ||
| AUC0–∞ (μg · h/ml) | 89.4 | 58.6 | 43.0 | |
| t1/2 (h) | 7.0 | |||
| Bioavailability (%) | 65.6 | 48.0 | ||
| Dog (2) | Cmax (μg/ml) | 3.5 | 3.0 | |
| Tmax (h) | 2.0 | 4.0 | ||
| AUC0–∞ (μg · h/ml) | 89.4 | 58.6 | 43.0 | |
| t1/2 (h) | 7.0 | |||
| Bioavailability (%) | 65.6 | 48.0 | ||
| Monkey (2) | Cmax (μg/ml) | 1.1 | 0.4 (18)b | |
| Tmax (h) | 14.0 | 5.0 (31) | ||
| AUC0–∞ (μg · h/ml) | 115.0 | 59.4 | 15.9 (20) | |
| t1/2 (h) | 22.7 | |||
| Bioavailability (%) | 51.7 | 13.8 |
Values in parentheses are CVs (in percent).
n = 6.
Mean serum concentration-time profiles of SCH 56592 in rats following i.v. and p.o. administration are shown in Fig. 2, with mean pharmacokinetic parameters shown in Table 1. The oral bioavailability values were approximately 66 and 48% for the HPβCD solution and the MC suspension, respectively. Following p.o. administration, the Cmax range was 3.0 to 3.5 μg/ml, which was attained at a Tmax range of 2 to 4 h after dosing. The t1/2 following i.v. administration was 7.0 h. The mean Cmax, Tmax, and AUC0–∞ of SCH 56592 following p.o. administration at 10, 20, 40, 80, and 120 mg/kg as a suspension in MC are shown in Table 2. There was a dose-related increase in both Cmax (up to 80 mg/kg) and AUC0–∞ (up to 120 mg/kg), while the Tmax range was 4 to 6 h.
FIG. 2.
Serum concentration-time profiles of SCH 56592 in rats following the i.v. or p.o. administration of a 20-mg/kg dose of SCH 56592. ▵, i.v. as a solution in HPβCD; ○, p.o. as a solution in HPβCD; ●, p.o. as a suspension in MC. Each vertical bar represents 1 standard deviation from the mean.
The mean serum concentration-time profile of SCH 56592 in fed rabbits following p.o. administration of a 20-mg/kg dose in an MC suspension is shown in Fig. 3, with mean pharmacokinetic parameters presented in Table 1. The t1/2 was approximately 9 h. The high concentrations of SCH 56592 in serum suggest good oral bioavailability.
FIG. 3.
Serum concentration-time profile of SCH 56592 in rabbits following the p.o. administration of a 20-mg/kg dose of SCH 56592 as a suspension in MC. Each vertical bar represents 1 standard deviation from the mean.
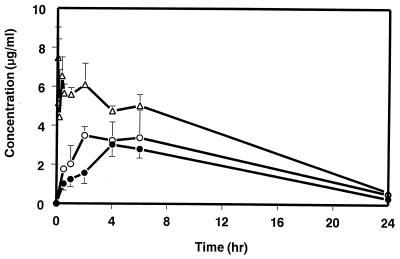
Mean serum concentration-time profiles of SCH 56592 following i.v. and p.o. administration to dogs are illustrated in Fig. 4, with mean pharmacokinetic parameters presented in Table 1. The oral bioavailability values were 72 and 37% for the HPβCD solution and the MC suspension, respectively. Following p.o. administration in HPβCD and MC, the Cmax values were 1.4 and 0.7 μg/ml, and the Tmax values were 2.5 and 15 h, respectively. The t1/2 was 15 h following i.v. administration. Mean pharmacokinetic parameters of SCH 56592 following p.o. administration at 10 mg/kg in fed and fasting dogs are shown in Table 2. Food enhanced the bioavailability of SCH 56592, while the shape of the serum concentration-time profile was not affected by food (data not shown). The data in Table 2 show that both Cmax and AUC0–∞ were increased approximately fourfold in the fed dogs compared to the fasting dogs. Mean pharmacokinetic parameters of SCH 56592 following p.o. administration of doses of 10, 40, 80, and 120 mg/kg to fed dogs are shown in Table 2. As the dose increased, concentrations of SCH 56592 in serum increased as reflected by dose-related increases in both Cmax (up to 80 mg/kg) and AUC0–∞ (up to 120 mg/kg).
FIG. 4.
Serum concentration-time profiles of SCH 56592 in dogs following the i.v. or p.o. administration of a 10-mg/kg dose of SCH 56592. ▵, i.v. as a solution in HPβCD; ○, p.o. as a solution in HPβCD; ●, p.o. as a suspension in MC.
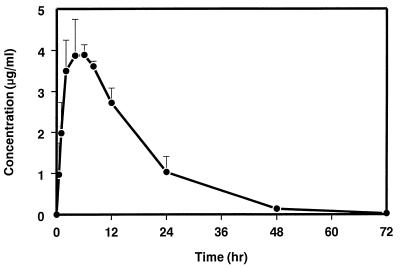
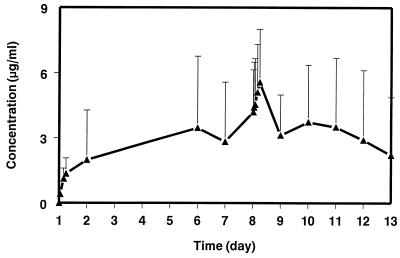
The mean serum concentration-time profile of SCH 56592 in fed dogs on days 1 to 13 during and after daily p.o. administration at 40 mg/kg for eight consecutive days as a suspension in MC is shown in Fig. 5; mean pharmacokinetic parameters on days 1 and 8 are shown in Table 3. Because of the limited number of blood samples taken after the first dose (day 1), the results of the single dose-exposure study at the 40-mg/kg dose level were included in Table 3 to compare single- and multiple-dose data. Concentrations of SHC 56592 in serum appeared to be at steady state by day 8, since concentrations in serum on days 6, 7, and 9 were similar (Fig. 5). Concentrations in serum were higher upon multiple dosing; the accumulation index was approximately 2.6.
FIG. 5.
Serum concentration-time profile of SCH 56592 in dogs during and following daily p.o. administration of 40 mg of SCH 56592 per kg as a suspension in MC for eight consecutive days. Sampling times were at 1, 4, 6, and 8 h on the first day of dosing (day 1), predose on days 2, 6, and 7, and at 0.5, 1, 2, 4, 6, 8, 12, 24, 48, 72, 96, and 120 h after the last dose (administered on day 8). Each vertical bar represents 1 standard deviation from the mean.
TABLE 3.
Single- versus multiple-dose pharmacokinetics of SCH 56592 in fed dogs following daily administration in an MC suspension of 40 mg/kg for 8 consecutive days
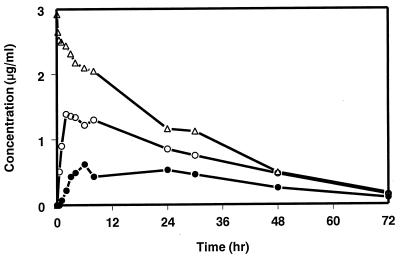
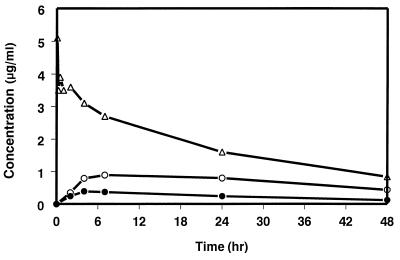
Mean serum concentration-time profiles of SCH 56592 in fasting monkeys following i.v. and p.o. administration at a 10-mg/kg dose level are shown in Fig. 6, with mean pharmacokinetic parameters presented in Table 1. The oral bioavailability values were approximately 52 and 14% from the HPβCD solution and the MC suspension, respectively. Following p.o. administration in HPβCD and MC, the Cmax values were 1.1 and 0.4 μg/ml, respectively. The t1/2 was calculated from the i.v. data to be approximately 23 h.
FIG. 6.
Serum concentration-time profiles of SCH 56592 in cynomolgus monkeys following the i.v. or p.o. administration of a 10-mg/kg dose of SCH 56592. ▵, i.v. as a solution in HPβCD; ○, p.o. as a solution in HPβCD; ●, p.o. as a suspension in MC.
DISCUSSION
SCH 56592 was bioavailable in all five species studied. The oral bioavailability was higher with the HPβCD solution than with the MC suspension, which was not surprising, since the compound is completely in solution following administration in HPβCD. This also suggests either that SCH 56592 did not precipitate out from the HPβCD solution in the gastrointestinal tract or that it was dissolved quickly in the gastrointestinal tract contents after precipitation. The latter explanation is unlikely because the bioavailability from the MC suspension was always lower than that from HPβCD solution.
Oral bioavailability was higher in mice, rats, and dogs than in monkeys. In rabbits, high concentrations in serum suggested good oral bioavailability from the MC suspension. The t1/2 values in mice, rats, and rabbits were 7 to 9 h, while in dogs and monkeys they were 15 and 23 h, respectively. Dose-related increases in serum drug concentrations were observed in mice (dose range, 20 to 160 mg/kg), rats (10 to 120 mg/kg), and fed dogs (10 to 120 mg/kg) following a single p.o. dose in the MC suspension. However, as the dose increased, the increase in the Cmax was less than that in the AUC, suggesting that absorption is slowed at higher doses. Also, concentrations above the MICs and minimal fungicidal concentrations were observed at 24 h following a single p.o. dose in the MC suspension in all five species studied (data not shown), suggesting that a once-daily administration could be the clinical dose regimen.
Studies in dogs showed that the administration of SCH 56592 as a suspension in MC with food increased both the Cmax and the AUC0–∞ approximately fourfold. This increase in bioavailability is likely due to a greater dissolution in the presence of food because of increased residence time in the stomach as well as increased gastrointestinal secretions (4, 6, 13, 14). This is supported by the finding that p.o. administration of the same dose of SCH 56592 as a solution in HPβCD with food increased its bioavailability by only 60% compared to administration of the same solution under fasting conditions (data not shown).
Following daily administration of 40 mg/kg for eight consecutive days in an MC suspension to fed dogs, concentrations in serum were higher than those following a single dose, as indicated by an approximately 2.6-fold increase in the Cmax. However, the AUC0–∞ following a single dose (105 μg · h/ml) was similar to the AUC0–24 following multiple doses (107 μg · h/ml) (Table 3), indicating no untoward accumulation upon multiple dosing in the dog.
In conclusion, SCH 56592 was orally bioavailable in mice, rats, rabbits, dogs, and monkeys. Dose-related increases in concentrations of SCH 56592 in serum were observed in mice, rats, and dogs following administration in an MC suspension. Food improved the oral bioavailability in dogs approximately fourfold. SCH 56592 concentrations in serum increased upon multiple dosing with no untoward accumulation.
REFERENCES
- 1.Armstrong D. Problems in management of opportunistic fungal infections. Rev Infect Dis. 1989;11:S1591. doi: 10.1093/clinids/11.supplement_7.s1591. [DOI] [PubMed] [Google Scholar]
- 2.Emmons C W. Medical mycology. 3rd. ed. Philadelphia, Pa: Lea and Febiger; 1977. [Google Scholar]
- 3.Gilbaldi M, Perrier D. Pharmacokinetics. 2nd ed. New York, N.Y: Marcel Dekker; 1982. pp. 409–417. [Google Scholar]
- 4.Hebbard G S, Sun M W, Bochner F, Horowitz M. Pharmacokinetic considerations in gastrointestinal motor disorders. Clin Pharmacokinet. 1995;28:41–66. doi: 10.2165/00003088-199528010-00005. [DOI] [PubMed] [Google Scholar]
- 5.Lutz J E, Clemons K V, Aristizabal B H, Stevens D A. Activity of the triazole SCH 56592 against disseminated murine coccidioidomycosis. Antimicrob Agents Chemother. 1997;41:1558–1561. doi: 10.1128/aac.41.7.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nomeir A A, Mojavarian P, Kosoglou T, Affrime M B, Nezamis J, Radwanski E, Lin C C, Cayen M N. Influence of food on the oral bioavailability of loratadine and pseudoephedrine from extended-release tablets in healthy volunteers. J Clin Pharm. 1996;36:923–930. doi: 10.1002/j.1552-4604.1996.tb04759.x. [DOI] [PubMed] [Google Scholar]
- 7.Oakley K L, Morrissey G, Denning D W. Efficacy of SCH-56592 in a temporarily neutropenic murine model of invasive aspergillosis with an itraconazole-susceptible and an itraconazole-resistant isolate of Aspergillus fumigatus. Antimicrob Agents Chemother. 1997;41:1504–1507. doi: 10.1128/aac.41.7.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oakley K L, Moore C B, Denning D W. In vitro activity of SCH-56592 and comparison with activities of amphotericin B and itraconazole against Aspergillus spp. Antimicrob Agents Chemother. 1997;41:1124–1126. doi: 10.1128/aac.41.5.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perfect J R, Cox G M, Dodge R K, Schell W A. In vitro and in vivo efficacies of the azole SCH56592 against Cryptococcus neoformans. Antimicrob Agents Chemother. 1996;40:1910–1913. doi: 10.1128/aac.40.8.1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pfaller M A, Messer S, Jones R N. Activity of a new triazole, Sch 56592, compared with those of four other antifungal agents tested against clinical isolates of Candida spp. and Saccharomyces cerevisiae. Antimicrob Agents Chemother. 1997;41:233–235. doi: 10.1128/aac.41.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sugar A M, Liu X-P. In vitro and in vivo activities of SCH 56592 against Blastomyces dermatitidis. Antimicrob Agents Chemother. 1996;40:1314–1316. doi: 10.1128/aac.40.5.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walsh T J, Pizzo P A. Nosocomial fungal infections: a classification for hospital-acquired infections and mycoses arising from endogenous flora and reactivation. Annu Rev Microbiol. 1988;42:517. doi: 10.1146/annurev.mi.42.100188.002505. [DOI] [PubMed] [Google Scholar]
- 13.Welling P G. Pharmacokinetics: process and mathematics. Washington, D.C.: American Chemical Society; 1986. [Google Scholar]
- 14.Welling P G. Influence of food and diet on gastrointestinal drug absorption: a review. J Pharmacokinet Biopharm. 1977;5:291–334. doi: 10.1007/BF01061694. [DOI] [PubMed] [Google Scholar]