Abstract
BACKGROUND
To characterize the workflow during transcanal totally endoscopic tympanoplasty by recording the time and instrumentation used for different steps in the procedure. This analysis aims to identify aspects of instrumentation and surgical technique that could be modified to improve surgical efficiency.
METHODS
Thirty-one endoscopic tympanoplasty procedures were observed at a single academic center. Patient age ranged from 2.7 to 17.8 years. The procedure was separated into distinct steps. The duration in minutes and the instruments used were recorded by an independent observer.
RESULTS
Raising the tympanomeatal flap (median 9.82 minutes) and positioning the graft and replacing the flap (median 9.13 minutes) took significantly longer than all other steps (P > .05, Wilcoxon method). Teaching a trainee significantly increased step duration by a total of 32.8 minutes (P > .05, Wilcoxon method). There was no correlation between age of the patient, side of the ear, surgical technique, or graft type, and duration of surgery. Suction instruments with a functional tip (dissector or knife tip) were most commonly used to dissect and maneuver soft tissue while maintaining the surgical field clear of blood.
CONCLUSION
As elevation of the tympanomeatal flap and graft placement are the most time-consuming steps in endoscopic tympanoplasty, especially for surgical trainees, surgical efficiency could most dramatically be enhanced by modification of instrumentation or technique to facilitate these steps. Modification of simpler steps such as hair trimming and ear canal packing have less potential for shortening surgical duration.
Keywords: Endoscopic ear surgery, tympanoplasty, instrumentation, time flow, workflow
Introduction
Although the choice of optimal surgical technique for repair of tympanic membrane perforation focuses primarily on the likelihood of successful perforation closure, minimization of hearing loss, post-operative morbidity, and consumption of health care resources are other important factors to consider, particularly for techniques that lead to equivalent closure rates. Many surgical techniques are utilized, ranging from simple patching of the perforation through the ear canal (referred to in this manuscript as “push-through myringoplasty”) to more invasive time-consuming approaches involving elevation of a tympanomeatal flap for graft placement (referred to as “tympanoplasty”), for example, through a post-auricular incision, sometimes with addition of a cortical mastoidectomy. Push-through myringoplasty has the advantage of being quick and of negligible morbidity for the patient, yet has significantly lower success at closing the perforation than a more time-consuming tympanoplasty performed through a post-auricular approach.1 Recent studies have shown that transcanal endoscopic ear surgery (TEES) achieves equivalent perforation closure rates to a microscope-guided post-auricular approach, but with significantly less post-operative morbidity.1-4 Complications such as keloid, dehiscence, infection, and hyperalgesia are avoided especially if non-autologous graft material is used, and pain is reduced.1,2,5,6 Available data suggest that TEES is also quicker than post-auricular microscope-guided surgery.4,7-9 Yet, any tympanoplasty approach is likely to take more time and so consume more operative resources than the simple, albeit less efficacious, push-through myringoplasty.
Although TEES is utilized increasingly around the world,10 it is recognized that the challenges associated with 1-handed surgery and surgical instrument design may not have been fully overcome yet.11,12 We hypothesize that areas of difficulty in endoscopic tympanoplasty could be quantified by time flow analysis, and that data arising could be used to identify measures that could improve surgical efficiency and so reduce the time needed for effective tympanic membrane perforation closure. In this paper, we study workflow in endoscopic tympanoplasty by recording the time and instrumentation used for different steps in the procedure. The primary outcome measure is timing of endoscopic tympanoplasty with non-autogenous graft material as completed by an experienced surgeon in a tertiary care practice. Limited data on the effect of trainees and variations in technique are included. By revealing and quantifying more time-consuming maneuvers, data will be provided that can be used to quantify the magnitude of improvements in operating room efficiency, which could potentially be realized by the introduction of new instrumentation or altered techniques.
Methods
Observations during endoscopic tympanoplasty were recorded pertaining to the surgical steps, time taken, and instruments used. Analysis included the effect of patient’s age and side of the ear, graft type, graft placement technique, and the effect of teaching trainee surgeons, on step duration.
Study Design
Approval for this study was granted by the Research Ethics Board at the Hospital for Sick Children (REB#1000055626). This was a single-center, single staff-surgeon observational study on 31 pediatric patients undergoing endoscopic tympanoplasty. No formal sample size calculation was performed; participant inclusion during the study period (2017-2021) was based upon availability of 1 of 2 independent observers to record timing, and parental consent.
For the time flow analysis, the tympanoplasty procedure was segmented into a series of separate sequential steps: (a) debriding the ear canal; (b) injecting local anesthesia in the ear canal; (c) trimming the hairs; (d) excising the edges of the perforation; (e) raising the tympanomeatal flap; (f) preparing the graft; (g) placing and positioning the graft; (h) replacing the flap; and (i) packing the ear canal. In practice, it was found that the graft was often re-adjusted after replacing the flap, requiring subsequent repositioning of the flap, therefore these 2 steps were amalgamated into 1 for analysis. The duration of these 9 steps was recorded using a standard stopwatch by an independent observer behind the surgeon so that the surgeon was unaware of whether timing was being measured. The observer noted whether teaching of a resident or fellow was occurring during each step. During 10 surgeries, 1 observer also recorded which surgical instruments were used during these steps, to gain an appreciation of the functional requirements of instruments during ear surgery and the design advantages of different instruments for specific maneuvers. Details of grafting technique were recorded.
Surgical Technique
Surgery was performed under hypotensive general anesthesia on children with tympanic membrane perforations without cholesteatoma. All were completed endoscopically via a transcanal approach without use of an operating microscope and without an external skin incision. A 3 mm 0 degree 14 cm rigid endoscope (Karl Storz SE & Co. KG, Tuttlingen, Germany) was used, connected to a high-definition camera system, with a conventional middle instrument set supplemented by Panetti suction–dissection instruments (Spiggle &Theis Medizintechnik GmbH, Overath, Germany) and the Thomassin dissector (Karl Storz SE & Co. KG, Tuttlingen, Germany). Debridement of the external auditory canal and trimming of meatal hairs was performed to avoid dirtying the endoscope lens during surgery. Injection of Marcaine (0.25%) with epinephrine (1 : 200 000) in the ear canal was used for local anesthesia and vasoconstriction for hemostasis. A posteriorly placed meatal skin incision was made and the meatal skin flap elevated in all cases. Non-autogenous material derived from porcine submucosa (Biodesign, Cook Medical, Bloomington IN, USA) or autogenous tragal cartilage were used as grafts. Grafts were placed with the simple underlay, interlay technique or lateral grafting technique, as described previously.1 Underlay tympanoplasty was used for more posterior perforations. Elevation of the tympanomeatal flap and graft placement were relatively straightforward for this approach, and so it was expected to be faster than the other approaches. Interlay tympanoplasty, in which the squamous layer is dissected off the fibrous layer, was used for central perforations in which a significant proportion of the fibrous layer of the drum remained to support the graft. Lateral graft tympanoplasty was only performed for perforations involving the anterior pars tensa, including subtotal perforations. This approach requires elevation of a large meatal flap to elevate the entire annulus.
Data Analysis
The data consisted of durations in minutes of each step. The results of a Shapiro–Wilk test concluded that the data did not fit a normal distribution, and therefore, median values of the data are presented and nonparametric statistical tests used for analysis. The Wilcoxon rank-sums test, also known as the Mann–Whitney U-test, was used to perform pairwise comparisons of the duration of each step. Tests resulting in a P < .05 were considered to indicate statistical significance. Statistical analysis was performed using JMP statistical analysis software (JMP Version 14.0; SAS Institute, Cary, NC, USA).
Results
The average age of the study participants was 11.8 years (2.7-17.8 years). For the study, 14 left ears and 17 right ears were included. All surgeons were right-handed. Surgical trainees, including some with no prior otologic experience, were involved in 20 of the 31 cases (65%). Non-autogenous graft material was used in 26 cases (84%) and tragal cartilage in 4 cases (13%). One case was an exploratory tympanotomy for congenital hearing loss in which no edge freshening or grafting was required, but the other steps were completed as in tympanoplasty. Placement of the graft was by lateral graft technique in 18 ears (58%), interlay technique in 4 ears (13%), and simple underlay in 7 ears (23%). One case was a scutumplasty using a cartilage graft.
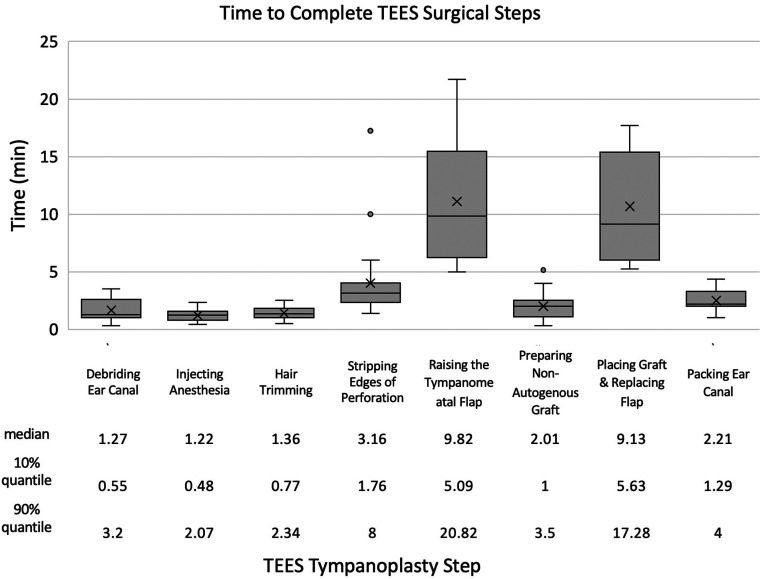
The median total of all surgical steps, performed only by the staff surgeon, was 32 minutes. There was no correlation between age of the patient and duration of surgery (R 2 = 4E-05) or side of the ear and duration of surgery (median difference in total surgery time between right and left side = −10.2 min, P = .321). As shown in Figure 1, raising the tympanomeatal skin flap (median 9.82 minutes) and placing the graft and replacing the flap (median 9.13 minutes) took significantly longer than all other steps (P < .05). Simpler steps, including infiltration and hair trimming, took less than 2.5 minutes each. The combination of flap elevation, graft positioning, and flap replacement (median 18.95 minutes) comprised 60% of the median total time for surgery.
Figure 1.
Time taken by staff surgeon to complete surgical steps during endoscopic tympanoplasty.
Grafting Technique
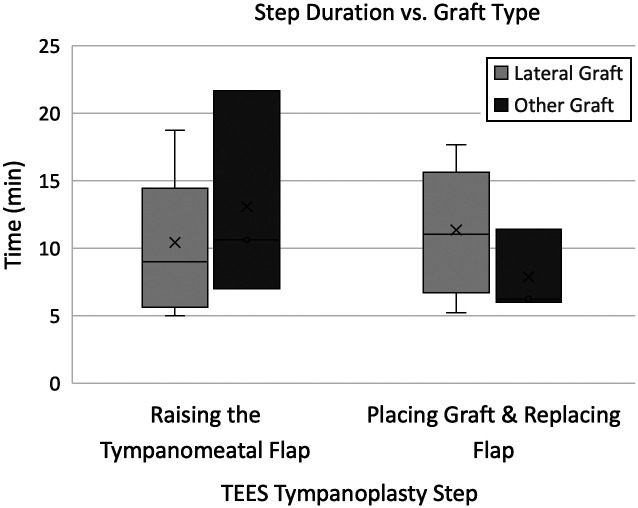
Figure 2 shows that the time required to raise the tympanomeatal flap was shorter with the lateral graft technique (median 9 minutes, n = 9 performed by staff surgeon) than using other graft techniques (median 10.6 minutes, n = 3 performed by staff surgeon), but this was not statistically significant (P = .5175). Although more time was taken to place the graft and replace the flap with the lateral graft technique (median 11.1 minutes, n = 12, performed by staff surgeon) than other graft placement techniques (median 6.3 minutes, n = 3, performed by staff surgeon), this was not statistically significant with this small sample size (P = .3477).
Figure 2.
Box plots demonstrating the time taken for tympanomeatal flap elevation and graft placement and flap replacement while using a lateral graft placement technique or other graft placement technique.
Surgical Training
The median total of all surgical steps performed by trainees was 64.8 minutes. This is in the context of a 2.5-hour OR time slot for tympanoplasty, calculated to allow adequate time for induction of pediatric general anesthesia, training of surgical and other trainees, wake-up and room turn-around. The median difference in duration between cases with and without trainee involvement was 32.8 minutes (P < .0001). In a straightforward case without a surgical trainee, the shortest duration of all tympanoplasty steps was 22 minutes in this series. Surgical trainees typically took longer to complete each step of the surgery as shown in Table 1. The contribution of a trainee to each step was determined by their level of experience, the complexity of the case, and the amount of OR time available. Often they would contribute to a part and not necessarily to the completion of selected steps.
Table 1.
Influence of Surgical Training on Duration of Surgical Steps in Endoscopic Tympanoplasty
| Surgical Step | Number of Cases with Teaching | Teaching Median (min) | No Teaching Median (min) | Median Teaching-Median no Teaching (min) | P (Wilcoxon) |
| Debriding ear canal | 1 | 6.2 | 1.3 | 5 | 0.0971 |
| Injecting anesthesia | 14 | 3.2 | 1.2 | 2 | <.0001 |
| Hair trimming | 15 | 4.5 | 1.4 | 3.2 | <.0001 |
| Stripping edges of perforation | 4 | 8.0 | 3.2 | 4.9 | .0058 |
| Raising the tympanomeatal skin flap | 19 | 27.3 | 9.8 | 17.5 | <.0001 |
| Preparing non-autogenous graft | 0 | N/A | 2.0 | N/A | .0253 |
| Placing graft & replacing flap | 15 | 22.1 | 9.1 | 13 | <.0001 |
| Packing ear canal | 7 | 4.6 | 2.2 | 2.3 | .0018 |
| Total surgical time | 20 | 64.8 | 32.0 | 32.8 | <.0001 |
Instrumentation
Table 2 shows how commonly different instruments were used in each of the surgical steps of the tympanoplasty procedure. Some simple steps, such as debridement, hair trimming, and meatal skin incision could be completed with the use of only 1 or occasionally 2 instruments. Many different instruments were required to complete the more invasive surgical steps. In comparison with microscope-guided surgery, in which 2 surgical instruments are used together in a complementary fashion, each instrument is used in solitary fashion in endoscopic surgery. While stripping the edges of the perforation, there were frequent switches between suction and different pairs of cupped-forceps or suction and scissors. While raising the tympanomeatal flap, there were common switches between Panetti suction dissector (PSD) and epinephrine-soaked cotton ball, PSD and Thomassin dissector or PSD and forceps. While placing the graft, there were common switches between Rosen Needle and PSD, Rosen Needle and Derlacki mobilizer, Thomassin dissector and epinephrine-soaked cotton ball, or PSD and forceps. A suction instrument (straight cannula, curved tip PSD or Panetti suction round knife) was used during every surgical step to dissect and maneuver soft tissue while clearing blood from the surgical field.
Table 2.
Instruments Used During the Different Surgical Steps in Endoscopic Tympanoplasty
| Step/Instrument | Straight Suction (%) | Round Knife (%) | Wax Curette (%) | Scissors (%) | Sickle Knife (%) | Rosen Needle (%) | Forceps (%) | Panetti Round Knife (%) | Panetti Suction Dissector (%) | Adrenaline Ball (%) | Thomassin Dissector (%) | Derlacki (%) |
| Debriding ear canal | 100 | 1-25 | 1-25 | 1-25 | ||||||||
| Hair trimming | 1-25 | 100 | ||||||||||
| Stripping edges of perforation | 75-100 | 1-25 | 1-25 | 75-100 | 100 | 75-100 | 25-50 | |||||
| Raising the tympanomeatal skin flap | 1-25 | 1-25 | 1-25 | 50-75 | 100 | 100 | 75-100 | 50-75 | ||||
| Placing the graft | 1-25 | 1-25 | 100 | 75-100 | 50-75 | 1-25 | 25-50 | 25-50 | ||||
| Replacing the flap | 50-75 | 1-25 | 1-25 | 1-25 | 25-50 | 1-25 | 25-50 | 50-75 |
The frequency with which each tool was used for each step is indicated as: 0% (unused), 1-25%, 25-50%, 50-75%, 75-100% and 100% (used every time this step is performed). Instruments with similar tip functionalities are grouped as follows: straight suction includes 18- and 19-gauge cannulae; forceps include alligator, cup, left- and right-biting types; scissors include iris and middle ear scissors.
Discussion
In this study, we measured the duration of surgical steps that comprise endoscopic tympanoplasty, and found that the majority of the surgical time is required for manipulation of the tympanomeatal flap and the graft, while around 1 quarter of the time is spent on smaller steps such as preparing and packing the ear canal. Teaching had a significant effect on the duration of surgical steps, but there was no relationship found between step duration and patient age or side of ear. There was no significant effect of surgical technique or graft type on surgical step duration, probably because of the small number of cases in these subgroup analyses. We also noted the instrumentation used to facilitate each step and found that tools that integrate a functional tip with suction were most commonly used to facilitate the endoscopic 1-handed surgical technique.
Surgical Technique
From our data, opportunities to improve operating room efficiency by shortening the time required for endoscopic tympanoplasty could be achieved by changes to surgical technique. As any surgeon would expect, the more complex steps of raising a tympanomeatal flap and positioning a graft took most of the time required for tympanoplasty and had the most variable data. Lea and Mijovic also noted that raising the tympanomeatal flap is the most challenging and bloodiest step of surgery.13 Completion of perforation repair without elevation of a flap would offer a considerable time saving, but our experience of the simple “push-through” myringoplasty without a tympanomeatal flap is that is significantly less reliable method of closing the perforation.1 In contrast, Saliba and Woods achieved equivalent results using a fat plug with esterified hyaluronic acid lamina that would be well-suited to a totally endoscopic transcanal approach.14
The interlay graft technique was introduced to endoscopic practice, having been recommended by Dr David Pothier (personal communication) as ideally suited for endoscopic graft placement. While graft placement is certainly easier, and closure rates comparable,15 we did not find an appreciable time saving from the technique in this study: elevation of the squamous layer from the fibrous layer can be difficult to complete without trapping squamous cells under the graft, and in the few cases included this study we found it to be more time-consuming than the more extensive complete meatal skin flap elevation required for lateral graft tympanoplasty.15 The additional time requirement for graft harvest, wound morbidity, and limitation of the smaller size of the pediatric tragus are reasons why cartilage grafting is usually reserved for ear drums associated with a risk of retraction, in our practice.
Teaching During Endoscopic Tympanoplasty
All tympanoplasty steps took significantly longer when teaching. In comparison with other otologic literature, 1 study found that teaching residents increased the duration of cochlear implantation, whereas another found no increase when teaching.16,17 A large multicenter study found implant surgery was completed more quickly by experienced surgeons.18 Inevitably, teaching styles, experience of the trainee, and availability of OR time will all influence impact of training on duration of surgical steps. A safe stepwise approach to training in endoscopic ear surgery has been recommended.19 In our program, trainees start with low-risk steps and move progressively toward more complex maneuvers as competence is demonstrated (e.g., hair trimming, then raising lateral part of meatal flap, finally manipulating graft in middle-ear space). Completion of even simple steps can initially be time-consuming for new trainees.
Instrumentation to Facilitate Single-handed Surgery
Suction–dissection instruments were used extensively in this series for meatal skin incision and flap elevation, and to a lesser extent for flap replacement. This dual-functionality was found to be transformative in facilitating the adoption of TEES into an otological practice, allowing the operative field to be cleared of blood while dissecting with 1 hand.1 Despite the availability of these instruments, evidence that additional functionality is required was provided by the use of multiple other instruments during these complex steps. Considering that each transition between instruments interrupts efficient surgical progress, the availability of instruments with enhanced functionality that obviate the need to switch between instruments of more limited functionality can be seen as an opportunity to enhance surgical efficiency and save time in the operating room. The biggest opportunities to improve efficiency will lie in the more complex steps that take longer and require the largest number of conventional instruments for completion.
Less substantial time savings could be achieved by improving efficiencies of simpler steps. Surgical instruments that provide adequate control of bleeding, perhaps, for example, with cauterization ability added to suction–dissection, might obviate the need for infiltration. Coupled with an alternative strategy for removing hairs from the field20 and avoidance of ear packing,21 around 5-10 minutes could be saved without adversely affecting outcome. Independently from this study, we informally evaluated a commercially available 1-handed sterilizable ear hair trimmer, but found that the hairs were not trimmed sufficiently short and the time saved was negligible.22 Completion of these steps in the immediate pre-operative period would help to reduce the duration of surgery, but logistical considerations and ability of children to cooperate may prevent this in some settings. In isolation, small changes to these simple steps cannot be expected to make a meaningful difference to OR efficiency. However, in conjunction with other gains in surgical efficiency for endoscopic tympanoplasty, it is conceivable that enough time could be saved in a day’s operating list of similar cases to make enough time for an additional procedure.
Limitations
The principal limitation of this study when considering the wider applicability of the results is that workflow data are available from only 1 staff surgeon, and therefore may not be representative of other surgical practices. Availability of different specialist endoscopic instrumentation, such as the PSD, would influence instrument selection and possibly step- duration. Even with 1 surgeon, the variance in time flow between cases was high, based on patient-specific factors such as extent of bleeding, ear canal morphology, and complexity of perforation repair. Data were collected primarily for the endoscopic lateral graft technique; numbers completed with other techniques were too small to allow reliable comparison. Data are available only from children, but there was no difference in timing between young and old children, and it is not considered that adult ear canal morphology would significantly alter workflow from that of teenagers. Another limitation is that workflow data were only collected for tympanoplasty and not for cholesteatoma. Variability in the extent and location of cholesteatoma is so considerable that averaged data for duration of resection would not be useful for assessing the limits of instrumentation or other aspects of surgical efficiency. We have instead explored the limited reach of instruments for endoscopic cholesteatoma dissection using different methodology and printed temporal bone models.23 Despite these limitations, this study provides a more accurate and objective assessment of surgical workflow than anecdotal surgeon’s recall.
Conclusion
Raising the tympanomeatal flap, positioning the graft, and replacing the flap are the most time-consuming steps in endoscopic tympanoplasty. Suction instruments with a functional tip (dissector or knife tip) are favored for dissection and maneuvering of soft tissue while maintaining the surgical field clear of blood.
Funding Statement
A.S. was supported by a grant from Perioperative Services at the Hospital for Sick children and a Director’s Innovation Award, Institute of Biomaterials and Biomedical Engineering, University of Toronto.
Footnotes
Ethics Committee Approval: Ethical committee approval for this study was granted by the Research Ethics Board at the Hospital for Sick Children (REB#1000055626).
Informed Consent: Written informed consent was obtained from the patients (or their parents) who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – A.S., A.J.; Design - A.S., A.J.; Supervision - A.J.; Resource - A.S., A.J.; Data Collection and/or Processing - A.S., N.C.; Analysis and/or Interpretation - A.S., N.C., K.E., A.J.; Literature Search - A.S., N.C.; Writing - A.S., N.C., K.E., A.J; Critical Reviews - N.C., K.E., A.J.
Conflict of Interest: The authors have no conflict of interest to declare.
References
- 1. James AL. Endoscope or microscope-guided pediatric tympanoplasty? Comparison of grafting technique and outcome. Laryngoscope. 2017;127(11):2659–2664.. [DOI] [PubMed] [Google Scholar]
- 2. James AL, Papsin BC. Ten top considerations in pediatric tympanoplasty. Otolaryngol Head Neck Surg. 2012;147(6):992–998.. [DOI] [PubMed] [Google Scholar]
- 3. Han SY, Lee DY, Chung J, Kim YH. Comparison of endoscopic and microscopic ear surgery in pediatric patients: a meta-analysis. Laryngoscope. 2019;129(6):1444–1452.. [DOI] [PubMed] [Google Scholar]
- 4. Lee SY, Lee DY, Seo Y, Kim YH. Can endoscopic tympanoplasty be a good alternative to microscopic tympanoplasty? A systematic review and meta-analysis. Clin Exp Otorhinolaryngol. 2019;12(2):145–155.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kwinter A, Purcell PL, Leonard CL, James AL. Comparing transcanal endoscopic surgery to post-auricular microscope-guided surgery in pediatric ossiculoplasty: hearing outcomes and post-operative pain. Otol Neurotol. 2021. e-publication ahead of print. [DOI] [PubMed] [Google Scholar]
- 6. Kakehata S, Furukawa T, Ito T, et al. Comparison of postoperative pain in patients following transcanal endoscopic versus microscopic ear surgery. Otol Neurotol. 2018;39(7):847–853.. [DOI] [PubMed] [Google Scholar]
- 7. Patel N, Mohammadi A, Jufas N. Direct cost comparison of totally endoscopic versus open ear surgery. J Laryngol Otol. 2018;132(2):122–128.. [DOI] [PubMed] [Google Scholar]
- 8. Hsu YC, Kuo CL, Huang TC. A retrospective comparative study of endoscopic and microscopic tympanoplasty. J Otolaryngol Head Neck Surg. 2018;47(1):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Manna S, Kaul VF, Gray ML, Wanna GB. Endoscopic Versus microscopic middle ear surgery: a meta-analysis of outcomes following tympanoplasty and stapes surgery. Otol Neurotol. 2019;40(8):983–993.. [DOI] [PubMed] [Google Scholar]
- 10. Kapadiya M, Tarabichi M. An overview of endoscopic ear surgery in 2018. Laryngoscope Investig Otolaryngol. 2019;4(3):365–373.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yong M, Mijovic T, Lea J. Endoscopic ear surgery in Canada : a cross-sectional study. J Otolaryngol Head Neck Surg. 2016;45:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Swarup A, le Nobel GJ, Andrysek J, James AL. The current limitations and future direction of instrument design for totally endoscopic ear surgery: a Needs Analysis Survey. Otol Neurotol. 2018;39(6):778–784.. [DOI] [PubMed] [Google Scholar]
- 13. Mijovic T, Lea J. Training and education in endoscopic ear surgery. Curr Otorhinolaryngol Rep. 2015;3(4):193–199.. [Google Scholar]
- 14. Saliba I, Woods O. Hyaluronic acid fat graft myringoplasty: a minimally invasive technique. Laryngoscope. 2011;121(2):375–380.. [DOI] [PubMed] [Google Scholar]
- 15. Ranguis SC, Leonard CG, James AL. Prospective comparison of pediatric endoscopic lateral graft and interlay tympanoplasty. Otol Neurotol. 2021;42(6):867–875.. [DOI] [PubMed] [Google Scholar]
- 16. Semaan MT, Fredman ET, Shah JR, et al. Surgical duration of cochlear implantation in an academic university-based practice. Am J Otolaryngol. 2013;34(5):382–387.. [DOI] [PubMed] [Google Scholar]
- 17. Puram S V., Kozin ED, Sethi RKV, et al. Influence of trainee participation on operative times for adult and pediatric cochlear implantation. Cochlear Implants Int. 2015;16(3):175–179.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Majdani O, Schuman TA, Haynes DS, et al. Time of cochlear implant surgery in academic settings. Otolaryngol Head Neck Surg. 2010;142(2):254–259.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Anschuetz L, Bonali M, Ghirelli M, et al. An ovine model for exclusive endoscopic ear surgery. JAMA Otolaryngol Head Neck Surg. 2017;143(3):247–252.. [DOI] [PubMed] [Google Scholar]
- 20. Kim Y, Kang JM, Song HY, et al. Self-expandable retainer for endoscopic visualization in the external auditory canal: proof of concept in human cadavers. Appl Sci. 2020;10(5) . [Google Scholar]
- 21. Borgstein J, De Zwart G, Bruce IA. Ear packing after ear surgery: is it really necessary? J Laryngol Otol. 2008;122(3):253–254.. [DOI] [PubMed] [Google Scholar]
- 22. James A, Chayaopas N, Swarup A. How can we improve endoscopic ear instrumentation? In: World Congress on Endoscopic Ear Surgery 3.0; 2019. [Google Scholar]
- 23. Chayaopas N, Swarup A, Eastwood KW, et al. A novel instrument for endoscopic ear surgery with a steerable flexible tip: a pediatric anatomical validation study. Otol Neurotol. 2021 In press. [DOI] [PubMed] [Google Scholar]



 Content of this journal is licensed under a
Content of this journal is licensed under a