Background:
COVID-19 pneumonia is a global viral disease and has been classified as a pandemic by the World Health Organization. The coronavirus that causes it can remain in the stool of some infected patients for a short period, even after recovery from COVID-19 pneumonia. Studies have increasingly reported the involvement of other organs, including the gastrointestinal system, in addition to the respiratory system. Ulcerative colitis is an inflammatory bowel disease with an unknown cause. Emerging data suggest that the gas trointestinal system may be influenced by COVID-19 via the expression of angiotensin-converting enzyme-2 (ACE-2), but data on the association between COVID-19 and inflammatory bowel diseases, including ulcerative colitis, are lacking. In this report, we describe a case of ulcerative colitis diagnosed in a 50-year-old male patient who presented with complaints of bloody diarrhea and abdominal pain following the completion of treatment for COVID-19 pneumonia. It is possible for the novel coronavirus to trigger ulcerative colitis. Hence, patients presenting with gastrointestinal com plaints should also be evaluated for COVID-19.
Keywords: COVID-19, ulcerative colitis, gastroenterology
Introduction
In December 2019, cases of a novel coronavirus leading to pneumonia and death were first reported in Wuhan province, China, and rapidly spread around the world.1 Coronavirus are enveloped, single-strand RNA viruses that can infect a wide range of hosts, including birds, wild and domestic mammalian species, and humans. It is known that coronaviruses can rapidly mutate, change tissue tropism, pass through species barriers, and adapt to different epidemiological conditions.2 These viruses are zoonotic pathogens that can manifest with clinical features across a wide spectrum from asymptomatic to requiring intensive care.3 COVID-19 symptoms, including fever, cough, and dyspnea, are more severe in patients with comorbidities, and chronic obstructive pulmonary disease is among the risk factors for COVID-19.4 However, the processes of COVID-19 are highly dynamic, and new symptoms and complications are reported almost every day. There is currently no drug specifically for COVID-19, but, in Turkey, early initiation of medical treatment with existing malaria drugs has produced successful results in the fight against the outbreak.
In a recent study conducted at the Wuhan Inflammatory Bowel Disease (IBD) Center on the measures taken to prevent the spread of the virus, An et al.5 reported that COVID-19 was not detected in 318 registered patients with IBD. Diarrhea was a rare presentation in the first series (3-5%),6 but clinicians have begun to question the prevalence of IBD as a symptom of COVID-19.7 In a study by Wei et al.,8 diarrhea was found in 26 (31%) out of 84 patients with COVID-19 pneumonia. In this report, we present a case of ulcerative colitis diagnosed following the treatment of COVID-19 pneumonia.
CASE PRESENTATION
A 50-year-old male patient presented to the internal diseases outpatient clinic on April 16, 2020, with complaints of fever and shortness of breath lasting for 3 days. In the physical examination, the fever was 37.8°C, respiratory rate was 24/min, and heart rate was 105/min. There was no organomegaly, and the patient was a non-smoker.
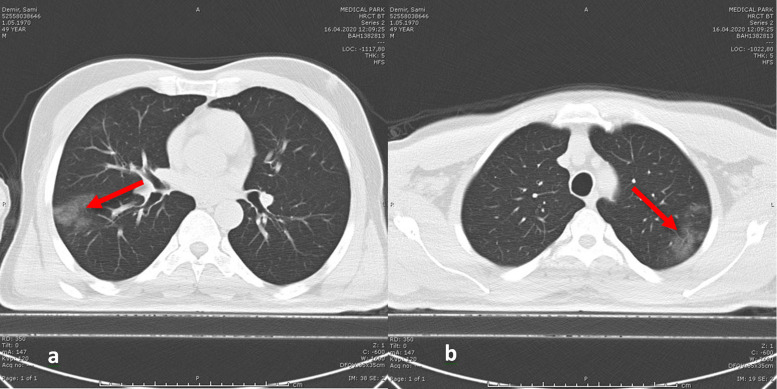
In the initial investigation, WBC was 6.4 × 109/L, C-reactive protein (CRP) was 4.6 mg/L (0-5 mg/L), ferritin was 162 ng/mL (22-274 ng/mL), D-dimer was 842 ng/mL (0-630 ng/mL), and the nasopharyngeal swab polymerase chain reaction (PCR) test was negative. However, diffuse ground-glass opacities consistent with viral pneumonia were observed on the chest computed tomography (CT), and PCR tests of the patient’s wife and 2 children were positive, so the patient was diagnosed with COVID-19, and a 7-day therapy with hydroxychloroquine and azithromycin was initiated (Figure 1).
Figure 1.
(A, B) Initial chest CT of the patient shows diffuse ground-glass opacities (red arrows).
The patient was clinically stabilized and taken to ambulatory follow-up. However, the patient revisited the hospital due to bloody diarrhea, which began 2 weeks after the completion of COVID-19 treatment. In the stool examination, 10-12 erythrocytes and 5-6 leukocytes were found. Tests for amoebas and Clostridium difficile toxins A+B were negative, while complete blood count and CRP were within the normal ranges. The patient was prescribed ciprofloxacin, metronidazole, and probiotics and was followed up. However, despite a week of treatment, his complaints did not improve, and the stool calprotectin level was reported as 1800 µg/g (normal range: 0-50 µg/g).
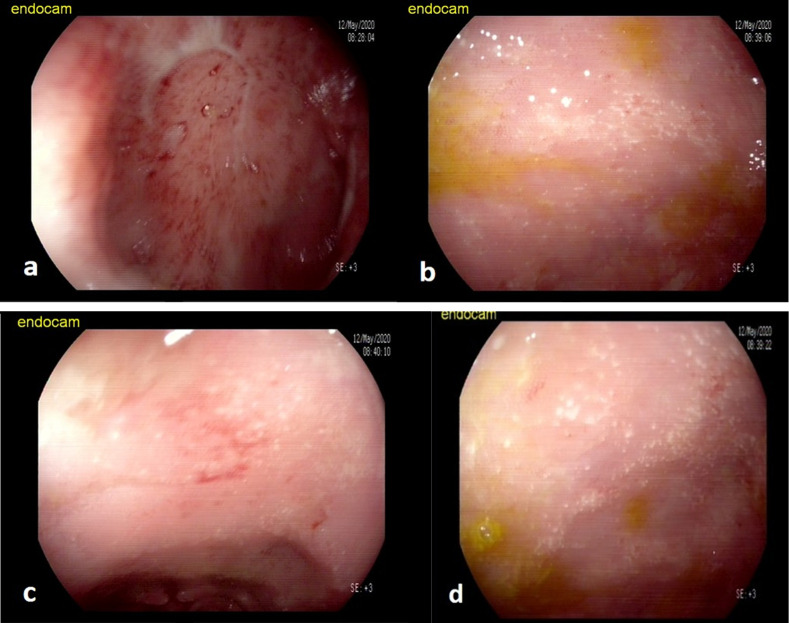
In a colonoscopic examination, the rectum and sigmoid colon had a diffuse, microulcerated, granulated appearance, and the submucosal vascularization was distorted (Figure 2).
Figure 2.
(A) Microulcerated, granulated, bloody mucosa; (B, C, and D) microulcerated, granulated mucosa on colonoscopic images. The submucosal vascular network cannot be distinguished.
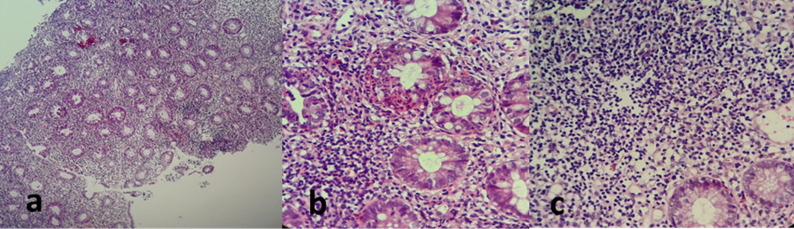
Biopsies were taken with presumed diagnoses of infectious colitis and ulcerative colitis. During the pathological examination, mucin loss and distortion in the colonic glands were found, and polymorphonuclear leukocytes (PMNL) and plasma cell infiltration were observed. Cryptitis and a crypt abscess were observed between the glands, while no granulomatous or specific microorganisms were detected. The patient was determined to have ulcerative colitis triggered by COVID-19, and 5-aminosalicylic acid (5-ASA) therapy was initiated orally and by enema. Bloody diarrhea stopped, and his complaints disappeared 3 days after initiation of this new drug therapy. Anti-SARS-CoV-2 antibodies were found as IgG positive and IgM weak positive. On the repeat control chest CT, a significant regression from the initial findings and a sequela lesion were observed. Written informed consent was obtained from the patient for this case report. Histopathological images of the patient are given in Figure 3.
Figure 3.
(A) Disrupted crypt structure and increased lymphoplasmacytic cells in the propria; (B) loss of goblet cells and a crypt abscess; (C) basal plasmacytosis.
Discussion
COVID-19 pneumonia is a global, viral disease that has been classified as a pandemic by the World Health Organization. It is understandable that the first reports from China focused only on the respiratory symptoms associated with COVID-19, as the disease caused respiratory failure and mortality in the first series. Therefore, these reports did not highlight diarrhea or other gastrointestinal system complaints, which might have led to the under-recognition of these symptoms. The disease most commonly manifests with upper and lower respiratory tract complaints, but there are studies reporting the involvement of other organs and diarrhea.9,10 It has also been shown that COVID-19 RNA can be detected by PCR tests in the stool after respiratory samples become negative in some infected patients,11 but there are no data showing how long the COVID-19 virus can remain viable in the stool.
In a case report of a 19-year-old female non-smoker patient from Italy, the patient presented to the hospital with fever, vomiting, bloody diarrhea, and loss of taste and smell. The PCR test was positive, but the CT did not show pneumonia, and contrast enhancement was detected in the ileum and colon of the patient. All symptoms disappeared following hydroxychloroquine therapy, and a PCR test then returned negative. In the small bowel ultrasonography performed on the 16th day, the thickness of the bowel walls and blood flow revascularization had increased along the entire colon. In the biopsy, ulcerations, crypt distortion, and an active crypt abscess were observed. The patient, who was negative for COVID-19 in stool samples, was diagnosed with ulcerative colitis.12
Similarly, in our patient, gastrointestinal system complaints developed after the completion of COVID-19 treatment. To our knowledge, ours is the second case of COVID-19/ulcerative colitis in the literature, together with the patient reported from Italy. Despite the negative PCR test, our patient’s clinical complaints and positive PCR tests in all family members suggested COVID-19, and the chest CT revealed ground-glass opacities. In COVID-19, ground-glass opacities, with or without consolidation, have been reported as the most common tomographic findings.13 The patient’s complaints disappeared following treatment with hydroxychloroquine and azithromycin, and a significant improvement was observed on repeat tomography.
Carvalho et al.14 similarly reported a case of hemorrhagic colitis associated with COVID-19. In their case, lung involvement and intestinal involvement started at the same time, appearing as patchy areas and focal erythematous areas on an endoscopic view, but, in our case, the appearance was compatible with ulcerative colitis that continued uninterrupted from the dentate line to the sigmoid colon.
Ischemic colitis should also be considered in the differential diagnosis of antibiotic-induced colitis. It is mostly seen in the elderly and people with comorbid diseases and is observed more commonly in the sigmoid corner and splenic flexure. There were advanced forms of a demarcation line and necrosis, and the clinical picture was more severe, but rectal involvement was not expected in this patient, who was not older and had no comorbidity. His clinical picture was neither severe nor compatible with ischemic colitis. He took zidovudine (AZT), and although AZT-associated colitis is not certain, it is not a common antibiotic among classical drugs. Late-onset antibiotic-induced colitis can occur on rare occasions, and our patient had a completely normal period of 2 weeks following the antibiotics, with amoeba and C. difficile toxins negative in stool tests. Although he received ornidazole treatment, his complaints did not improve. The endoscopic view was consistent with ulcerative colitis with uninterrupted involvement, and the patient’s clinical picture improved very quickly with 5-ASA oral and enema treatments. Consideration of all the parameters of the patient’s clinic, absence of a toxic condition other than bloody diarrhea (such as ischemia or necrosis), endoscopic appearance, pathological findings, very rapid response to 5-ASA treatment for ulcerative colitis, and the onset of complaints after recovery from COVID-19 suggest that an immune response triggered by COVID-19 might have been the causal mechanism, and our opinion here is a diagnosis of ulcerative colitis induced by COVID-19.
Ulcerative colitis is an IBD with an unknown cause, and inflammation triggered for any reason may lead to the disease. There had been no previous Gastrointestinal System (GIS) complaints in our patients, and bloody diarrhea and abdominal pain developing shortly after the beginning of COVID-19 symptoms, together with colonoscopy and pathologic examinations compatible with ulcerative colitis, might suggest that the disease could be triggered by COVID-19. Human intestines express high levels of angiotensin-converting enzyme-2 (ACE-2) and transmembrane serine protease, which are needed for the COVID-19 virus to gain entry into cells. Emerging data suggest that the GIS and liver may also be influenced by COVID-19, based on hepatic cells expressing ACE-2, which is a major receptor for gastrointestinal epithelial cells and COVID-19.15 Although research on COVID-19 and IBD is limited, the International Organization for the Study of Inflammatory Bowel Disease (IOIBD) has recently recommended reducing corticosteroid therapy and maintaining thiopurines and biologics.16
So far, most triage has been based on the presence of respiratory symptoms in COVID-19 cases. However, since the disease has a dynamic course and the number of publications reporting gastrointestinal—and other—symptoms are increasing, there is an urgent need to properly determine the clinical features of COVID-19. Our limitation, in this case, was the lack of PCR investigations in the stool or tissue samples; however, 2 PCR tests were already negative, the IgG was positive in the post-treatment viral screening, the clinical and CT findings were compatible with COVID-19, all family members had positive PCR tests, and full recovery was achieved following treatment, suggesting that the patient did indeed experience COVID-19.
Conclusion
We present this case to show that COVID-19 can appear with other organ pathologies, in addition to upper and lower respiratory tract complaints. We believe that ulcerative colitis in our patient was triggered by COVID-19. Patients presenting with gastrointestinal complaints should also be evaluated for COVID-19, and further studies are needed to investigate the association between COVID-19 and IBDs, especially ulcerative colitis.
Funding Statement
The authors declared that this study has received no financial support.
Footnotes
Informed Consent: Informed consent form was obtained from the patient for this case report.
Peer Review: Externally peer-reviewed.
Author Contributions: MFA execute the case, analyzed and interpreted the results and wrote the report. HT executed the case, interpreted the results.
Conflict of Interest: The authors have no conflict of interest to declare.
References
- 1. . Ahn DG, Shin HJ, Kim MH, et al. Current status of epidemiology, diagnosis, therapeutics, and vaccines for novel coronavirus Disease 2019 (COVID-19). J Microbiol Biotechnol. 2020;30(3):313–324.. 10.4014/jmb.2003.03011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. . Decaro N, Mari V, Elia G, et al. Recombinant canine coronaviruses in dogs, Europe. Emerg Infect Dis. 2010;16(1):41–47.. 10.3201/eid1601.090726) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. . Yurdaisik I. Effectiveness of computed tomography in the diagnosis of novel coronavirus 2019. Cureus. 2020;12(5):e8134. 10.7759/cureus.8134) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. . Zhang JJ, Dong X, Cao YY, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75(7):1730–1741.. 10.1111/all.14238) [DOI] [PubMed] [Google Scholar]
- 5. . An P, Ji M, Ren H, et al. Protection of 318 Inflammatory Bowel Disease Patients from the Outbreak and Rapid Spread of COVID-19 Infection in Wuhan, China. SSRN Electronic Journal . 2020. 10.2139/ssrn.3543590) [DOI] [Google Scholar]
- 6. . Huang CW, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506.. 10.1016/S0140-6736(20)30183-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. . Liang W, Feng Z, Rao S, et al. Diarrhoea may be underestimated: a missing link in 2019 novel coronavirus. Gut. 2020;69(6):1141–1143.. 10.1136/gutjnl-2020-320832) [DOI] [PubMed] [Google Scholar]
- 8. . Wei XS, Wang X, Niu YR, et al. Diarrhea is associated with prolonged symptoms and viral carriage in corona virus disease 2019. Clin Gastroenterol Hepatol. 2020;18(8):1753–1759.e2.. 10.1016/j.cgh.2020.04.030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. . Lin L, Jiang X, Zhang Z, et al. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut. 2020;69(6):997–1001.. 10.1136/gutjnl-2020-321013) [DOI] [PubMed] [Google Scholar]
- 10. . Song Y, Liu P, Shi XL, et al. SARS-CoV-2 induced diarrhoea as onset symptom in patient with COVID-19. Gut. 2020;69(6):1143–1144.. 10.1136/gutjnl-2020-320891) [DOI] [PubMed] [Google Scholar]
- 11. . Yeo C, Kaushal S, Yeo D. Enteric involvement of coronaviruses: is faecal-oral transmission of SARS-CoV-2 possible? Lancet Gastroenterol Hepatol. 2020;5(4):335–337.. 10.1016/S2468-1253(20)30048-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. . Calabrese E, Zorzi F, Monteleone G, Del Vecchio Blanco G. Onset of ulcerative colitis during SARS-CoV-2 infection. Dig Liver Dis. 2020;52(11):1228–1229.. 10.1016/j.dld.2020.06.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. . Yurdaisik I, Nurili F. (May 23, 2020) Evaluation of Chest CT Findings in 50 Coronavirus Disease 2019 (COVID-19) Patients Treated in Turkey. Cureus. 12(5):e8252. 10.14309/ajg.0000000000000667) [DOI] [Google Scholar]
- 14. . Carvalho A, Alqusairi R, Adams A, et al. SARS-CoV-2 gastrointestinal infection causing hemorrhagic colitis: implications for detection and transmission of COVID-19 disease. Am J Gastroenterol. 2020;115(6):942–946.. 10.14309/ajg.0000000000000667) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. . Qi F, Qian S, Zhang S, Zhang Z. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem Biophys Res Commun. 2020;526(1):135–140.. 10.1016/j.bbrc.2020.03.044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. . IOIBD. IOIBD update on COVID-19 for patients with Crohn’s disease and ulcerative colitis. 2020. (Available at: https://www.ioibd.org/Ioibd-Update-On-covid19-for-Patients-with-Crohns-Disease-and-Ulcerative-Colitis/), Accessed: 15 June 2020. [Google Scholar]