Abstract
Objective:
To evaluate the presence of anti-compensatory saccades (AcS) using the video head impulse test (vHIT) in the healthy inner ear in patients with vestibular neuritis (VN) during the acute and subacute stages of VN.
Methods:
We retrospectively considered a chart review of 2420 patients evaluated for acute vestibular syndrome from 2016 to 2020 in the Cassino (Italy) clinic. Nine hundred fifty-four patients with acute onset of vestibular syndrome who received an instrumental otoneurological assessment within 24 hours from the onset of the symptoms, evaluated by simultaneously using a combination of vHIT, ocular vestibular-evoked myogenic potential (VEMP), and cervical VEMP, were included in the study.
Results:
Thirty-two patients with superior VN that showed an altered horizontal canal function when tested with vHIT and quick AcS on the healthy side were enrolled. We found that all patients with VN, evaluated in the first 24 hours from the onset of the symptoms, showed AcS when their head was abruptly and passively turned toward the healthy inner ear side. At follow-up within 8 weeks from the first evaluation, 29 out of 32 patients did not show increased AcS.
Conclusions:
Our findings support the hypothesis that the AcS on the healthy side are a clinical sign of vestibular canal hypofunction or paresis in patients affected by the acute stage of VN.
Keywords: Vestibular, vestibular neuritis, anti-compensatory saccades, head impulse test, video head impulse test
Introduction
The video head impulse test (vHIT) constitutes an important clinical tool to evaluate horizontal and vertical vestibulo-ocular reflex (VOR).1-3
Individuals undergoing this test are instructed to maintain their eyes on a target placed in front of them, in solidarity with the earth, during short, passive, unpredictable, horizontal head turns. This is now called the head impulse test paradigm (HIMP).
The decreased canal function does not allow the patient to maintain fixation on the target during the vHIT, and to respond in the best way to the fixation task, the patient needs a series of corrective eye saccadic movements during or at the end of the head rotation.4 The first 100 ms of the eye movement does not depend on other sources of vision control, and the earliest part of the eye movement responds only to a vestibular stimulus.5,6 The head impulse test or HIMP assesses the functional state of each semicircular canal.7
Over the past few years, a new test paradigm has been proposed to evaluate the vestibular function.7-10 During abrupt, unpredictable head turns, the subjects are asked to look at a target that moves with their head rather than the fixed target used in HIMP. This new paradigm is called SHIMP, suppression head impulses. In fact, at the end of each head turn, a corrective saccade (the so-called “SHIMPs” saccade) appears in normal subjects, whereas it does not appear in patients in the acute stage of peripheral diseases such as neuritis, or iatrogenic vestibular diseases such as intratympanic gentamicin or neurectomy.7 The SHIMPs paradigm is not affected by covert saccades and could give more information on slow-phase velocity of the VOR, compared to HIMPs. Recently we have demonstrated that the VOR gain value of the affected side in patients with vestibular neuritis (VN), might be different at the time of the attack (within 72 hours) rather than in the subacute or chronic stage (from 72 hours to 6 weeks after the onset of symptoms), as well as the related impact on patients’ quality of life.11 Furthermore, we found that when the operator turns the patient’s head toward the unaffected side, a series of quick anti-compensatory saccades (AcS) is generated. This group of eye movements, at a first glance, seems related to the quick phase of spontaneous nystagmus. The latter could be reduced at the time of the attack of an acute vestibular syndrome but not completely suppressed by fixation. To confirm this, it should be emphasized how the vHIT test is typically performed in a well-lit room.12
Whereas the study of VOR gain through the vHIT is entirely focused on the affected side, our clinical hypothesis is that in the very early stage of the vestibular neuritis, the healthy side can also yield important clinical signs to guide the physician in the diagnosis of acute VN. Therefore, this study aims to retrospectively investigate the presence of AcS in the healthy side in patients with VN during the acute and subacute phases.
Materials and Methods
Study Design
The aim of this study was to explore the presence of anti-compensatory saccades (AcS) in the healthy side during the acute and subacute phase in VN patients. Ethical standards and guidelines on human experimentation were observed during all procedures of this work. Guidelines for reporting observational studies (Strengthening the Reporting of Observational Studies in Epidemiology) were followed. All participants gave their written informed consent for participation in the study. This study was approved by the IRB of the ENT MSA Academy Center.
Setting
Medical records of patients who were admitted to the ENT medical Center from 2016 to 2020 with a diagnosis of VN evaluated within 24 hours from the onset (T0) and after 8 weeks (T1) were included.
Participants
The inclusion criteria were diagnosis of VN evaluated within 24 hours from the onset of the symptoms), and patients who had not received therapy for VN. We excluded patients with a diagnosis of Meniere’s disease, benign paroxysmal positional vertigo bilateral, vestibular migraine, the presence of somatic or psychiatric disorders, and neurological diseases. The vestibular evaluation included the vHIT, a self-assessment dizziness handicap inventory (DHI), bone-conducted vibration and air-conducted sound cervical and ocular vestibular-evoked myogenic potentials (VEMPs). The VN patients were diagnosed has having acute vestibular syndrome (AVS) characterized by rotatory vertigo, postural imbalance, and neurovegetative symptoms such as nausea and vomiting; abnormal results of the horizontal semicircular canal function;1 an asymmetry ratio (AR) greater than 40% of n10 to oVEMPs, an AR greater than 30% of p13-n23 to cVEMPs, absence of neurological signs, and the presence of HINTS peripheral pattern or the presence of horizontal spontaneous nystagmus without evidence of a central vestibular lesion; and absence of cerebral lesions at MRI of the brain evaluation performed 72 hours after the AVS onset.9
Dizziness Handicap Inventory
The quality of life was assessed by the self-assessment inventory DHI. With 25 questions, the DHI evaluates self-perceived activity limitation and restriction resulting from dizziness.10
Vestibular-Evoked Myogenic Potentials
The oVEMP n10 is a small (5-10 µV) negative (excitatory), crossed, VEMP of the stretched inferior oblique eye muscles13,14 recorded by surface EMG electrodes on the skin beneath the eyes, in response to stimulation by bone-conducted vibration (BCV) delivered to the midline of the forehead at the hairline (Fz) and to air-conducted stimuli (ACS), 500 Hz short-tone burst. Based on evidence of utriculo-ocular projections15 and neural evidence of the preferential activation by BCV and ACS of irregular otolithic afferent neurons,16-21 n10 to oVEMP mainly reflects the utricular macula activity for these stimuli.
In contrast, BCV and ACS p13-n23 to cVEMP is a positive (inhibitory) uncrossed potential recorded by surface EMG electrodes over the tensed sternocleidomastoid muscle and reflects mainly the saccular macula function.6,13,22
Statistical Analysis
Data were analyzed using the Statistical Package for the Social Sciences version 23 (IBM Corp., Armonk, NY, USA). The independent samples t-test was used to analyze the improvement in VOR gain, and the Mann–Whitney U-test was applied to compare DHI data within subjects. P-values <.05 were considered significant.
Results
Thirty-two VN patients (13 females and 29 males), mean age 59.19 ± 16.23, met the inclusion criteria and were included in the study (participants’ demographic characteristics are reported in Table 1). All patients showed an altered ipsilesional VOR gain at T0 (0.40 ± 0.16) and a physiological contralesional VOR gain (0.91 ± 0.10). The enrolled patients had an AR greater than 40% to oVEMP in response to BCV and ACS stimulus, with the n10 component being markedly reduced beneath the eye opposite the affected side. In contrast, the ACS and BCV AR p13-n23 to cVEMP was symmetrical. Significant differences were found in the within-subjects analysis at T0 and T1 in DHI scores (P = .000). Affected hVOR gain values showed significant differences at T1 with respect to T0 (P = .02), whereas no differences were found in the contralesional VOR gain values (P = .11).
Table 1.
Clinical Characteristics
| Age (Years) ± SD | Sex, n (%), F-M | DHI | Ipsilesional hVOR Gain | Contralesional hVOR Gain | AcS Healthy Side, n (%) | |
| T0 (n=32) | 59.19 ± 16.23 | 13 (41)-19 (59) | 76.81 ± 5.79 | 0.40 ± 0.16 | 0.91 ± 0.10 | 32 (100) |
| T1 (n=32) | 59.19 ± 16.23 | 13 (41)-19 (59) | 20.30 ± 4.14 | 0.57 ± 0.25 | 0.89 ± 0.11 | 3 (9) |
| P-value | .000* | .02* | .11 |
T0, evaluation within 24 hours from the onset; T1, evaluation within 60 days from T0, Mean ± standard deviation; DHI, Dizziness Handicap Inventory; hVOR, horizontal canal vestibulo-ocular reflex; AcS, anti-compensatory saccades; *significant for P < .05.
All patients showed AcS on the healthy side at T0. Twenty-nine out of 32 patients evaluated at T1 did not show increased AcS.
Discussion
This study aimed to explore the presence of AcS in the healthy side in patients with VN during the acute and subacute phases. We found that in all patients evaluated during the acute phase, AcS were present during the rotation toward the healthy side. The presence of AcS can be related to the VOR destabilization process that occurs during the acute stages of VN.23 In particular, this can also be emphasized by passive head rotations toward the healthy side at the time of the attack and seems to disappear in all T1 patients (Figure 1A-C). Essentially, AcS can be regarded as a sign of altered VOR function, similar to what happens on the affected side or the desired realization of the reflex, “eyes on target,” at the end of the head turning. These rapid eye movements always temporally follow the movements of the head, the anti-compensatory saccades, but unlike the rotation toward the affected side, they have the same direction. Other authors have speculated that these quick eye movements are an indicator of peripheral injury.23
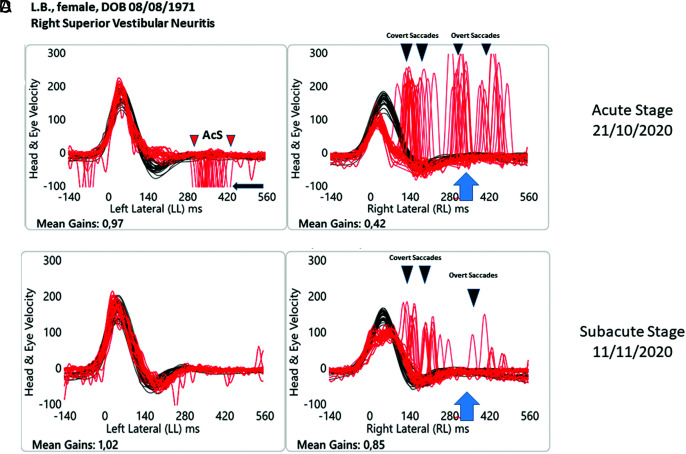
Figure 1.
Objective measures of the VOR for horizontal semicircular canals tested on 2 occasions for a patient of our cohort with acute unilateral (right) superior vestibular neuritis. Evaluation number 1(A-B), “early vHIT” measurements less than 24 hours from the symptom onset (October 21, 2020) and at a succeeding time thereafter (C-D), the time we define as the “subacute stage” approximately 20 days later (November 11, 2020). Please note that for a better understanding of the tracks, the signs of head velocity (black traces) for leftward impulses and of eye velocity (red traces) for rightward impulses have been inverted to allow for easier comparison and vice versa. In this way, each panel shows a superimposed time-series of head velocity and the corresponding eye velocity for the tests of horizontal canal dynamic function using vHIT. Normal horizontal VOR gains are approximately 0.7-1.0.1 At the time of the attack (A and B). When the patient’s head is turned to the affected side, the eye slow-phase velocity was clearly less than the corresponding head velocity during the head turn, so the vestibulo-ocular reflex (VOR) was significantly less than toward the healthy side. The value of VOR gain in this case was 0.42, while for unpredictable rotation to the unaffected side, there was an improvement to 0.97 of the VOR gain. It can be appreciated how there are covert (during the head turns) + overt (at the end of the head turns) saccades (black arrow). When the patient’s head is abruptly and unpredictably turned to the left, the healthy side, anti-compensatory saccades (AcS), in the same direction of the head rotation, can be observed (black arrow). The latter are clearly overt AcS. Subacute stage (C and D) At the time of the second evaluation, approximately 20 days later, when the examiner (LM) turned the patient’s head to the affected side, it can be appreciated how the slow-phase eye velocity has improved and the corrective saccades are reduced in terms of number and velocity. VOR gain was improved to 0.85. When the examiner turned the patient’s head to the unaffected side, the VOR slow-phase eye velocity retained the same value, while AcS disappeared.
For this reason, considering our large sample of subjects evaluated in the first 24 hours––practically in the time that we like to define as “the time of the attack”––it is possible to relate this phenomenon to the acute stage of the peripheral nerve injury or a sign of the very early stages of the pathophysiological insult that underlies VN.
However, the reason why AcS are present only in “early” vHIT evaluation is still under debate, and our aim is certainly to shed new light on the matter.
Indeed, related features of kinematics share both rapid phases and saccades, which are rapid eye movements. Furthermore, the genesis of horizontal eye movements recognizes 2 distinct neural circuits. The first of these circuits is undoubtedly generated by the trineuronal arch for the VOR slow phase.
The second is a separate neural circuit which is located in the brainstem, responsible for the genesis of the quick phase represented by a double network: (a) burst neurons and (b) pause neurons.24
The latter circuit, activated by vestibular inputs, generates both vestibular nystagmus rapid phase during horizontal angular acceleration and voluntary saccades triggered by descending axons from the superior colliculus. This is a first possibility for the genesis of saccades, the other is represented by contribution or trigger from neck afferents. There is also evidence that this rapid process of the neural circuit may also be triggered by these neck inputs.25
In our sample, we found a very low VOR gain within 24 hours from the onset of the symptoms, suggesting that the acute insult to the superior component of the vestibular nerve had disabled the horizontal slow-phase mechanism (Fig. 1B).
In any case, during the vHIT toward the unaffected side, similar to what happens when the head is turned toward the affected side, the quick phase mechanism is not compromised, and right in the first hours of illness, AcS are generated similar to the quick phase of the spontaneous nystagmus, directed away from the affected side. Essentially, these quick eye movements seem to be due to the presence of spontaneous nystagmus that does not seem to be fully suppressed by fixation. Indeed, the vHIT test is usually conducted in a normally lit room. We recognize some limitations in the present study.
First, there are limitations to the interpretation of our findings, because this was a retrospective study. Second, the timing of follow-up is heterogeneous, and future studies could better identify the timing of re-evaluation to verify how long-lasting this clinical sign is, to better understand the early stage of the vestibular compensation process.
Conclusion
Our findings suggest that the presence of anti-compensatory overt saccades, measured using the vHIT while turning the head to the healthy side in a patient affected by VN, is a new instrumental clinical sign of acute VOR impairment, and a marker for recognizing the test execution timing.
Funding Statement
The authors declared that this study has received no financial support.
Footnotes
Ethics Committee Approval: This study was approved by IRB of MSA ENT Academy Center.
Informed Consent: All patients gave their written consent.
Peer Review: Externally peer-reviewed.
Author Contributions: Concept – L.M.; Design - M.T., L.M.; Data Collection and/or Processing - L.M., M.T.; Analysis and/or Interpretation - L.M., M.T.; Writing - M.T., L.M.
Conflict of Interest: The authors have no conflict of interest to declare.
References
- 1. MacDougall HG, Weber KP, McGarvie LA, Halmagyi GM, Curthoys IS. The video head impulse test: diagnostic accuracy in peripheral vestibulopathy. Neurology. 2009;73(14):1134–1141.. 10.1212/WNL.0b013e3181bacf85) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. MacDougall HG, McGarvie LA, Halmagyi GM, Curthoys IS, Weber KP. Application of the video head impulse test to detect vertical semicircular canal dysfunction. Otol Neurotol. 2013;34(6):974–979.. 10.1097/MAO.0b013e31828d676d) [DOI] [PubMed] [Google Scholar]
- 3. Macdougall HG, McGarvie LA, Halmagyi GM, Curthoys IS, Weber KP. The Video Head Impulse Test (vHIT) detects vertical semicircular canal dysfunction. PLOS ONE. 2013;8(4):e61488. 10.1371/journal.pone.0061488) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Halmagyi GM, Chen L, MacDougall HG.et al. The video head impulse test. Front Neurol. 2017;8:258. 10.3389/fneur.2017.00258) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Curthoys IS. A critical review of the neurophysiological evidence underlying clinical vestibular testing using sound, vibration and galvanic stimuli. Clin Neurophysiol. 2010;121(2):132–144.. 10.1016/j.clinph.2009.09.027) [DOI] [PubMed] [Google Scholar]
- 6. Curthoys IS. The interpretation of clinical tests of peripheral vestibular function. Laryngoscope. 2012;122(6):1342–1352.. 10.1002/lary.23258) [DOI] [PubMed] [Google Scholar]
- 7. Manzari L, Tramontano M. Suppression Head Impulse Paradigm (SHIMP) in evaluating the vestibulo-saccadic interaction in patients with vestibular neuritis. Eur Arch Otorhinolaryngol. 2020;277(11):3205–3212.. 10.1007/s00405-020-06085-6) [DOI] [PubMed] [Google Scholar]
- 8. Shen Q, Magnani C, Sterkers O.et al. Saccadic velocity in the new suppression head impulse test: a new indicator of horizontal vestibular canal paresis and of vestibular compensation. Front Neurol. 2016;7:160. 10.3389/fneur.2016.00160) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Park JS, Lee JY, Nam W.et al. Comparing the suppression head impulse paradigm and the head impulse paradigm in vestibular neuritis. Otol Neurotol. 2020;41(1):e76–e82.. 10.1097/MAO.0000000000002453) [DOI] [PubMed] [Google Scholar]
- 10. de Waele C, Shen Q, Magnani C, Curthoys IS. A novel saccadic strategy revealed by suppression head impulse testing of patients with bilateral vestibular loss. Front Neurol. 2017;8:419. 10.3389/fneur.2017.00419) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Manzari L, Graziano D, Tramontano M. The Different Stages of Vestibular Neuritis from the Point of View of the Video Head Impulse Test. Audiol Res. 2020. 24;10(2):31–38.. 10.4081/audiores.2020.248) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. MacDougall HG, McGarvie LA, Halmagyi GM.et al. A new saccadic indicator of peripheral vestibular function based on the video head impulse test. Neurology. 2016;87(4):410–418.. 10.1212/WNL.0000000000002827) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Manzari L, Koch G, Tramontano M. Selective Asymmetry of Ocular Vestibular-Evoked Myogenic Potential in Patients with Acute Utricular Macula Loss. J Int Adv Otol. 2021;17(1):58–63.. 10.1097/WCO.0b013e32835c5ef3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Weber KP, Rosengren SM, Michels R.et al. Single motor unit activity in human extraocular muscles during the vestibulo-ocular reflex. J Physiol. 2012;590(13):3091–3101.. 10.1113/jphysiol.2011.226225) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Suzuki JI, Tokumasu K, Goto K. Eye movements from single utricular nerve stimulation in the cat. Acta Otolaryngol. 1969;68(4):350–362.. 10.3109/00016486909121573) [DOI] [PubMed] [Google Scholar]
- 16. McCue MP, Guinan JJ. Acoustically responsive fibers in the vestibular nerve of the cat. J Neurosci. 1994;14(10):6058–6070.. 10.1523/JNEUROSCI.14-10-06058.1994) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McCue MP, Guinan JJ. Sound-evoked activity in primary afferent neurons of a mammalian vestibular system. Am J Otol. 1997;18(3):355–360.. [PubMed] [Google Scholar]
- 18. Curthoys IS, Kim J, McPhedran SK, Camp AJ. Bone conducted vibration selectively activates irregular primary otolithic vestibular neurons in the guinea pig. Exp Brain Res. 2006;175(2):256–267.. 10.1007/s00221-006-0544-1) [DOI] [PubMed] [Google Scholar]
- 19. Manzari L, Tedesco AR, Burgess AM, Curthoys IS. Ocular and cervical vestibular-evoked myogenic potentials to bone conducted vibration in Ménière's disease during quiescence vs during acute attacks. Clin Neurophysiol. 2010;121(7):1092–1101.. 10.1016/j.clinph.2010.02.003) [DOI] [PubMed] [Google Scholar]
- 20. Curthoys IS, Vulovic V. Vestibular primary afferent responses to sound and vibration in the guinea pig. Exp Brain Res. 2011;210(3-4):347–352.. 10.1007/s00221-010-2499-5) [DOI] [PubMed] [Google Scholar]
- 21. Curthoys IS, Vulovic V, Burgess AM.et al. The basis for using bone-conducted vibration or air-conducted sound to test otolithic function. Ann N Y Acad Sci. 2011;1233:231–241.. 10.1111/j.1749-6632.2011.06147.x) [DOI] [PubMed] [Google Scholar]
- 22. Rosengren SM, Welgampola MS, Colebatch JG. Vestibular evoked myogenic potentials: past, present and future. Clin Neurophysiol. 2010;121(5):636–651.. 10.1016/j.clinph.2009.10.016) [DOI] [PubMed] [Google Scholar]
- 23. Luis L, Lehnen N, Muñoz E.et al. Anticompensatory quick eye Movements After head impulses: A peripheral vestibular sign in spontaneous nystagmus. J Vestib Res. 2016;25(5-6):267–271.. 10.3233/VES-160566) [DOI] [PubMed] [Google Scholar]
- 24. Curthoys IS. Generation of the quick phase of horizontal vestibular nystagmus. Exp Brain Res. 2002;143(4):397–405.. 10.1007/s00221-002-1022-z) [DOI] [PubMed] [Google Scholar]
- 25. Barmack NH, Errico P, Ferraresi A, Pettorossi VE. Interactions of cervico-ocular and vestibulo-ocular fast-phase signals in the control of eye position in rabbits. J Physiol. 1989;410:213–225.. 10.1113/jphysiol.1989.sp017529) [DOI] [PMC free article] [PubMed] [Google Scholar]



 Content of this journal is licensed under a
Content of this journal is licensed under a