Abstract
Background:
Alpha-fetoprotein (AFP) has been widely used as a tumor marker in the treatment of hepatocellular carcinoma (HCC) in patients with cirrhosis. However, a large number of HCC patients are diagnosed without cirrhosis, and the prognostic capability of AFP was unclear in HCC patients without cirrhosis. Our purpose was to investigate the prognostic efficiency of AFP in patients with non-cirrhosis, single, and small HCC who were treated with surgical resection.
Methods:
Among the 111 374 liver cancer patients included in the Surveillance, Epidemiology, and End Results database, we selected 224 patients without cirrhosis with a single HCC ≤3 cm in diameter who were identified at diagnosis and treated with surgical resection. The AFP test results were recorded as AFP-positive and AFP-negative levels.
Results:
Kaplan–Meier method showed that there was no significant survival difference between the AFP-positive and AFP-negative groups (P = .566). The same results were found in the subgroups of patients with tumor size ≤2 cm and 2-3 cm (P = .710 and .687, respectively). Receiver operating characteristic (ROC) curve analysis showed that AFP had inadequate accuracy to discriminate survivors and deceased patients in subgroups of patients with tumor size ≤3 cm, 2-3 cm, or ≤2 cm (area under the ROC curve = 0.449, 0.458, 0.443; 95% confidence interval = 0.366-0.533, 0.346-0.571, 0.317-0.569, respectively).
Conclusion:
AFP levels have no predictive value in well-compensated non-cirrhosis patients with single, small HCC (≤3 cm) treated with surgical resection for curative intent.
Keywords: Alpha-Fetoprotein, non-cirrhosis, hepatocellular carcinoma, surgery, survival
INTRODUCTION
Worldwide, liver cancer is the fifth common cancer but the third leading cause of cancer-related mortality,1 and hepatocellular carcinoma (HCC) is the most common pathological type of liver cancer. The incidence of HCC in Asia and parts of Africa is high, and although the incidence is low in the Western world, it is gradually increasing.1-5 The American Association for the Study of Liver Diseases (AASLD) and the Barcelona Clinic Liver Cancer (BCLC) guidelines are commonly used for the management of HCC. These systems assume the coexistence of cirrhosis; however, a large portion of HCC patients present with absence of cirrhosis.6,7 Furthermore, HCC patients presenting without cirrhosis may show different characteristics than patients with cirrhosis.
Early detection of HCC is the main goal of screening and monitoring programs.8 With an increase in these screening and surveillance programs, more early stage small HCC cases will be identified.
In the classification of BCLC, single and small HCC in patients with compensated cirrhosis, and optimal performance status are categorized as very early (Grade 0) and early (Grade A) HCC, and these patients are usually curable.9 Therefore, these patients with single, small HCC with non-cirrhosis and better optimal performance status should be particularly amenable to curative treatment that will help improve their prognosis.
Alpha-fetoprotein (AFP) is a tumor marker that has been applied as a prognostic factor for HCC in patients with cirrhosis. AFP assessment was recently dropped from the AASLD guidelines of HCC treatment and diagnosis because the early diagnosis of HCC has poor sensitivity and lacks specificity.1 However, whether AFP is effective as a prognostic factor when HCC is diagnosed in the most favorable setting, such as in patients with single, small HCC with non-cirrhosis and optimal performance status, has not been sufficiently investigated. Some studies suggested that the prognostic role of AFP in HCC patients may be affected by the number and size of the HCC nodules, although the conclusions have been inconsistent.10-13
In this research, we investigated the predictive value of AFP in patients with non-cirrhosis, optimal performance status, and single, small HCC (≤3 cm) identified at diagnosis and who were cured with surgical resection per treatment intent. Our purpose was to assess whether AFP levels may have any prognostic power in this specific setting.
MATERIALS AND METHODS
Data Source
The study cohort was assembled using data associated with HCC from the Surveillance, Epidemiology, and End Results (SEER) program (from 1988 through 2015). The SEER database is maintained by the US National Cancer Institute and provides information on cancer incidence and survival.14 These data were publicly available and participant consent was not necessary. A total of 111 374 patients who matched for liver cancer were initially identified in SEER. The SEER database classifies fibrosis according to Ishak scores defined by the American Joint Committee on Cancer that range from 0 to 4 (undetectable to moderate fibrosis), designated “F0,” and 5 to 6 (severe fibrosis or cirrhosis), designated “F1.”15 In the SEER database, cases in which the serum level of AFP was elevated were recorded as AFP-positive and cases with normal AFP serum levels were recorded as AFP-negative.
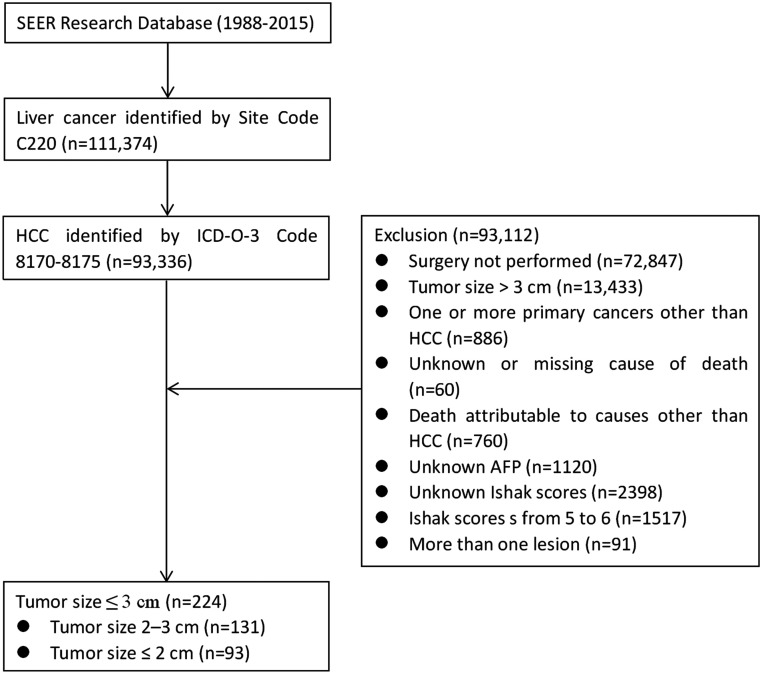
Histological codes were limited to HCC (8170, 8171, 8172, 8173, 8174, 8175). We excluded patients who did not undergo surgical resection of HCC; patients who had more than one primary cancer and HCC was not the first; patients with more than one lesion (CS extension was limited to codes: 100, 150, 200, 250, 270, 350, 370, 380, 510, 520, 530, 550); patients died from causes other than HCC; patients with Ishak scores from 5 to 6; patients with an unknown or missing cause of death; and patients with unknown AFP level, degree of fibrosis or survival length. As shown in Figure 1, the final patient group included 224 patients.
Figure 1.
CONSORT diagram. SEER, Surveillance, Epidemiology, and End Results; HCC, hepatocellular carcinoma; AFP, alpha-fetoprotein.
Statistical Analysis
The baseline demographic and tumor characteristics of patients in the AFP-positive and AFP-negative groups were compared using the chi-square test. The Kaplan–Meier method was used to compare the HCC death rate between groups. Multivariate Cox regression models were used to identify predictive factors for survival outcomes. Variables with P < .10 in univariate analysis were included in the final multivariate model. Moreover, the receiver operating characteristic (ROC) curve was used to identify the overall accuracy of AFP levels for discriminating between survivors and deceased. P values < .05 were considered statistically significant. All statistical analyses were performed using SPSS 22.0 software (IBM Corp., Armonk, NY, USA).
RESULTS
Baseline Patient Characteristics According to Tumor Size
We obtained data on a total of 224 patients who had HCC tumor size ≤3 cm, 131 patients who had HCC tumor size 2-3 cm, and 93 patients who had HCC tumor size ≤2 cm.
Table 1 shows the main demographic and tumor characteristics of patients in the AFP-positive and AFP-negative groups subdivided according to tumor size. There were no obvious differences between AFP-positive and AFP-negative groups among the evaluated parameters, including race, age, sex, pathological grade, tumor, nodes, and metastases (TNM) stage, and SEER stage. However, we found a significantly higher proportion of females among AFP-positive patients than AFP-negative patients with a tumor size ≤3 cm (25.0% vs. 12.5%, respectively; P = .020).
Table 1.
Baseline Demographic and Tumor Characteristics of HCC Patients with AFP-Positive and AFP-Negative Serum Levels
| Characteristic | AFP (Tumor Size ≤3 cm) | P | AFP (Tumor Size 2-3 cm) | P | AFP (Tumor Size ≤2 cm) | P | |||
|---|---|---|---|---|---|---|---|---|---|
| Positive (N = 128) | Negative (N = 96) | Positive (N = 69) | Negative (N = 62) | Positive (N = 59) | Negative (N = 34) | ||||
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | ||||
| Sex | .020 | .059 | .160 | ||||||
| Male | 96 (75.0) | 84 (87.5) | 51 (73.9) | 54 (87.1) | 45 (76.3) | 30 (88.2) | |||
| Female | 32 (25.0) | 12 (12.5) | 18 (26.1) | 8 (12.9) | 14 (23.7) | 4 (11.8) | |||
| Age | .296 | .241 | .848 | ||||||
| ≤60 | 73 (57.0) | 48 (50.0) | 36 (52.2) | 26 (41.9) | 37 (62.7) | 22 (64.7) | |||
| >60 | 55 (43.0) | 48 (50.0) | 33 (47.8) | 36 (58.1) | 22 (37.3) | 12 (35.3) | |||
| Race | .215 | .699 | .200 | ||||||
| White | 64 (50.0) | 54 (56.3) | 35 (50.7) | 35 (56.5) | 29 (49.2) | 19 (55.9) | |||
| Black | 13 (10.2) | 14 (14.6) | 8 (11.6) | 8 (12.9) | 5 (8.5) | 6 (17.6) | |||
| Other* | 51 (39.8) | 28 (29.2) | 26 (37.7) | 19 (30.6) | 25 (42.4) | 9 (26.5) | |||
| Pathological grade | .380 | .263 | .710 | ||||||
| Well/Moderate | 76 (59.4) | 61 (63.5) | 39 (56.5) | 37 (59.7) | 37 (62.7) | 24 (70.6) | |||
| Poor/Anaplastic | 15 (11.7) | 6 (6.3) | 12 (17.4) | 5 (8.1) | 3 (5.1) | 1 (2.9) | |||
| Unknown | 37 (28.9) | 29 (30.2) | 18 (26.1) | 20 (32.3) | 19 (32.2) | 9 (26.5) | |||
| TNM stage | .059 | .231 | .209 | ||||||
| I | 66 (51.6) | 59 (61.5) | 38 (55.1) | 39 (62.9) | 28 (47.5) | 20 (58.8) | |||
| II | 4 (3.1) | 7 (7.3) | 2 (2.9) | 4 (6.5) | 2 (3.4) | 3 (8.8) | |||
| III | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||
| IV | 0 (0.0) | 1 (1.0) | 0 (0.0) | 1 (1.6) | 0 (0.0) | 0 (0.0) | |||
| Unknown | 58 (45.3) | 29 (30.2) | 29 (42.0) | 18 (29.0) | 29 (49.2) | 11 (32.4) | |||
| SEER stage | .135 | .211 | .731 | ||||||
| Localized | 123 (96.1) | 92 (95.8) | 66 (95.7) | 58 (93.5) | 57 (96.6) | 34 (100.0) | |||
| Regional | 5 (3.9) | 2 (2.1) | 3 (4.3) | 2 (3.2) | 2 (3.4) | 0 (0.0) | |||
| Distant | 0 (0.0) | 2 (2.1) | 0 (0.0) | 2 (3.2) | 0 (0.0) | 0 (0.0) | |||
*Other includes American Indian/Alaska native, Asian/Pacific Islander, and unknown. #Non-married includes widowed, never married, divorced, separated, unmarried, and domestic partner.
HCC, hepatocellular carcinoma; AFP, alpha-fetoprotein; SEER, Surveillance, Epidemiology, and End Results.
Subgroup Analysis of the Relation Between AFP and Survival According to Tumor Size
During follow-up, in the subgroup with tumor size ≤3 cm, 62 patients (27.7%) died, and there was no obvious difference in the proportion of deaths between AFP-positive and AFP-negative groups (31.3% vs. 12.5%; P = .168). In the subgroup with tumor size 2-3 cm, 34 patients (26.0%) died, and there was no obvious difference in the proportion of deaths between AFP-positive and AFP-negative groups (29.0% vs. 22.6%, P = .404). In the subgroup with tumor size ≤2 cm, 28 patients (30.1%) died, and there was no obvious difference in the proportion of deaths between AFP-positive and AFP-negative groups (33.9% vs. 23.5%, P = .294).
Survival curves for hepatocellular carcinoma-specific survival (HCSS) of AFP-positive and AFP-negative patients were generated according to tumor size. There was no statistically significant difference in postoperative survival between AFP-positive and AFP-negative groups with a tumor size ≤3 cm (10-year HCSS: 52.5% vs. 51.8%, log rank χ2 = 0.330, P = .566; Table 2, Figure 2A).
Table 2.
Kaplan–Meier Analysis of the Association of AFP-Negative and AFP-Positive Serum Levels on Survival
| Variable | Total | 1-year HCSS | 3-year HCSS | 5-year HCSS | 8-year HCSS | 10-year HCSS | Log rank χ2 test | P |
|---|---|---|---|---|---|---|---|---|
| AFP (≤3 cm in diameter) | 224 | 0.330 | .566 | |||||
| Positive | 128 | 94.9% | 83.5% | 68.1% | 52.5% | 52.5% | ||
| Negative | 96 | 97.9% | 86.4% | 72.5% | 58.2% | 51.8% | ||
| AFP (2-3 cm in diameter) | 131 | 0.163 | .687 | |||||
| Positive | 69 | 93.7% | 84.1% | 68.3% | 53.2% | 53.2% | ||
| Negative | 62 | 98.4% | 85.0% | 73.3% | 54.7% | - | ||
| AFP (≤2 cm in diameter) | 93 | 0.138 | .710 | |||||
| Positive | 59 | 96.4% | 82.9% | 68.4% | 52.1% | 52.1% | ||
| Negative | 34 | 97.0% | 88.7% | 70.4% | 61.6% | 49.3% |
AFP, alpha-fetoprotein; HCSS, hepatocellular carcinoma-specific survival.
Figure 2.
Survival curves based on Kaplan–Meier analysis comparing the association of AFP-positive and AFP-negative status with HCC cause-specific survival according to tumor size. (A) Tumor size ≤3 cm, (B) tumor size 2-3 cm, and (C) tumor size ≤2 cm.
Repeat analysis was performed on the subgroup of 131 patients with HCC tumor size of 2-3 cm and the subgroup of 92 patients with HCC tumor size ≤2 cm; similarly, there was no significant survival difference between AFP-positive and AFP-negative groups (HCC 2-3 cm: 8-year HCSS: 53.2% vs. 54.7%, log rank χ2 = 0.163, P = .687; Table 2, Figure 2B, HCC ≤2 cm: 10-year HCSS: 52.1% vs. 49.3%, log rank χ2 = 0.138, P = .710; Table 2, Figure 2C).
Subgroup Analysis of the ROC Curve According to Tumor Size
In the subgroup with tumor size ≤3 cm, the ROC curve showed that AFP had insufficient accuracy in distinguishing survivors and deceased patients (area under the ROC curve = 0.449, 95% CI = 0.366-0.533). The AFP cutoff value identified by ROC curve analysis had unacceptably low sensitivity (35.5%) and specificity (54.3%).
In the subgroup with tumor size 2-3 cm, the ROC curve also showed that AFP had inadequate accuracy to discriminate survivors and deceased patients (area under the ROC curve = 0.458, 95% CI = 0.346-0.571). An ROC curve-identified AFP cutoff value also had unacceptably low sensitivity (41.2%) and specificity (50.5%).
In the subgroup with tumor size ≤2 cm, the ROC curve also showed that AFP had inadequate accuracy to discriminate survivors and deceased patients (area under the ROC curve = 0.443, 95% CI = 0.317-0.569). An ROC curve-identified AFP cutoff value also had unacceptably low sensitivity (28.6%) and specificity (60.0%).
Finally, we also assessed the predictors of death in this very homogenous population of non-cirrhotic patients with small HCC after surgical resection and found that age was the only predictor of survival (Table 3). However, there was no significant survival difference associated with sex, race, pathological grade, TNM stage, marital status, SEER stage, AFP level (positive or negative) or tumor size (≤2 or 2-3 cm).
Table 3.
Univariate and Multivariable Survival Analysis to Identify Predictors of Postoperative-Specific Survival of HCC Patients with Tumor Size ≤3 cm
| Total (n = 224) | 5-year HCSS | 8-year HCSS | Univariate Analysis | Multivariable Analysis | |||
|---|---|---|---|---|---|---|---|
| Log rank χ2 test | P | HR (95% CI) | P | ||||
| Sex | 0.032 | .859 | NI | ||||
| Male | 180 | 68.2 | 56.7 | ||||
| Female | 44 | 76.0 | 47.5 | ||||
| Age | 6.322 | .012 | .029 | ||||
| ≤60 | 121 | 74.2 | 65.2 | Reference | |||
| >60 | 103 | 63.3 | 34.3 | 1.777 (1.059-2.981) | |||
| Race | 2.981 | .225 | NI | ||||
| White | 118 | 68.3 | 46.8 | ||||
| Black | 27 | 69.2 | 69.2 | ||||
| Other* | 79 | 67.3 | 63.3 | ||||
| Pathological grade | 1.680 | .432 | NI | ||||
| Well/Moderate | 137 | 70.5 | 59.0 | ||||
| Poor/Anaplastic | 21 | 85.0 | 51.0 | ||||
| Unknown | 66 | 64.6 | 48.3 | ||||
| TNM stage | 7.644 | .054 | .258 | ||||
| I | 125 | 68.7 | - | Reference | |||
| II | 11 | 44.4 | - | 1.966 (0.584-6.615) | .275 | ||
| III | 0 | - | - | - | - | ||
| IV | 1 | - | - | 5.011 (0.658-38.145) | .120 | ||
| Unknown | 87 | 73.1 | 57.4 | 0.887 (0.493-1.599) | .691 | ||
| SEER stage | 3.163 | .206 | NI | ||||
| Localized | 215 | 71.0 | 54.8 | ||||
| Regional | 7 | 33.3 | 33.3 | ||||
| Distant | 2 | 50.0 | 50.0 | ||||
| Tumor size | 0.085 | .771 | NI | ||||
| ≤2 cm | 93 | 68.9 | 55.2 | ||||
| 2-3 cm | 131 | 70.4 | 53.8 | ||||
| AFP | 0.330 | .566 | NI | ||||
| Positive | 128 | 68.1 | 52.5 | ||||
| Negative | 96 | 72.5 | 58.2 | ||||
*Other includes American Indian/Alaska native, Asian/Pacific Islander, and unknown. #Non-married includes widowed, never married, divorced, separated, unmarried and domestic partner.
HCC, hepatocellular carcinoma; AFP, alpha-fetoprotein; SEER, Surveillance, Epidemiology, and End Results;
NI, not included in the multivariable survival analysis.
DISCUSSION
AFP was removed as a monitoring equipment for HCC in patients with cirrhosis in the latest AASLD HCC diagnostic and management guidelines of HCC, although this decision was controversial.1,16,17 Indeed, surveillance programs using a combination of AFP and ultrasound for the detection of early HCC increases the sensitivity of ultrasound alone by a mere 6%-8%, resulting in doubling in the rate of false alarm rate and growing at an unacceptable rate (by 84%) in surveillance-related costs.18,19 Thus, AFP does not provide an extra advantage over ultrasound.
A staging system using AFP levels showed that AFP was positively correlated with the degree of HCC pathological grade and tumor progression.4 A retrospective study in a large cohort demonstrated that the AFP level at diagnosis was an independent risk predictor associated with pathological grade, TNM stage, tumor size, and survival.20 Two studies reported that a high preoperative AFP level was significantly associated with a worse survival in HCC patients with non-cirrhotic livers.21,22 However, most studies assessed the prognostic ability of AFP involving heterogeneous cohorts of patients, and thus their effectiveness as a prognostic tool in well-defined patients cannot be properly assessed.10,23 HCC patients either with or without cirrhosis should be separated into subsets for separate analysis. Moreover, since previous studies showed that the prognostic ability of AFP in HCC patients may be affected by the number and size of the HCC nodules, a subanalysis in patients subdivided by size and number of the HCC nodules should be performed.10-13
Several reports have revealed that a significant proportion of HCC patients do not show cirrhosis.6,7 One study identified 433 patients undergoing liver resection for HCC with complete information on fibrosis scores using SEER data from 2004 to 2013.6 Of these patients, 55% (783/1433) had undetectable to moderate fibrosis (0-4 Ishak scores). In a study in Kentucky, a significantly higher proportion of HCC patients (60.2%, 62/103) presented without cirrhosis or hepatitis.7 The present study collected data from 1988 through 2015 in SEER from 16 618 HCC patients with Ishak score information; among these patients, 3208 (19.3%) had undetectable to moderate fibrosis and among patients with tumor size ≤3 cm (5825 cases), 756 (13.0%) had undetectable to moderate fibrosis.
In this study, we defined small HCC using a cut-off of 3 cm, as reported by several studies demonstrating a remarkable result after effective treatment in these cases and as this threshold was accepted for treatment by the Asian Pacific Association for the Study of the Liver.24-26 One report revealed that AFP showed no prognostic ability in well-compensated cirrhosis patients with single, small (tumor size ≤3 cm) HCC treated with curative intent.27 The present study also showed that AFP had no prognostic role in non-cirrhosis patients with single, small HCC treated with surgical resection. In addition, we did not detect any correlation between AFP and pathological grade and TNM stage in all subgroups with tumors ≤3 cm, 2-3 cm, or ≤2 cm.
The poor prognostic performance of AFP observed in our study may be due to several reasons. First, AFP has a significantly positive correlation with the size of HCC,20,28 and therefore for small HCC, the mean AFP level may be lower, which may lead to diluted efficiency of the prognostic ability of AFP. Second, as reported by previous studies,27,29,30 our findings also suggested that increased AFP levels were associated with female gender, and no significant relationship was detected between AFP and other characteristics. In fact, the prognostic capability of AFP seems to be highly dependent on tumor size and curative effect because of different treatment modalities; this capability was more evident in patients with higher stage HCC and in those who received palliative care, and less apparent in those with small tumor and in those who received curative treatment (for example, surgical resection).10-13,31,32 In fact, when patients with advanced liver disease and/or terminal HCC were excluded from the analysis, the prognostic ability of AFP was greatly impaired. These conclusions are also in favor of our findings that there is no “therapeutic disparity” in our series, and mortality and cause of death are evenly distributed among patients with negative and positive AFP levels, which may exclude the presence of other possible prognostic confounding factors. Thus, combined with our findings, we propose that some determinants such as degree of fibrosis, tumor size, and different treatment strategies should be defined within a narrow range as inclusion criteria according to the study.
This study has some limitations. First, the SEER database does not provide information of tumor recurrence after surgery, and thus the influence of AFP levels on recurrence endpoints could not be evaluated in this study. Second, the etiologies of cirrhosis in the SEER database are not available and could not be included in the study. Third, the precise AFP test results were unavailable, as the SEER database only divided AFP results into AFP-positive and AFP-negative levels; therefore, the ROC curve could not be performed using actual AFP test values, and univariate and multivariable survival analyses could not be performed with more specific levels. Fourth, fibrosis was divided into only two stages, as Ishak scores 0-4 (undetectable to moderate fibrosis) and Ishak scores 5-6 (severe fibrosis or cirrhosis); thus, it was not possible to perform subanalysis according to individual fibrosis stage. Finally, the selection criteria for this study were very rigorous, and so the study population was limited to 224 cases.
CONCLUSION
We found that AFP has no effect on the prognosis of patients with a single, small HCC treated with surgical resection and without cirrhosis. These findings highlight the ineffectiveness of serum AFP assays in the clinical setting, where monitoring of HCC may provide its maximal benefit in terms of amenability to curative treatment. Therefore, there is a need to identify new markers that have a highly accurate predictive ability for the prognosis of patients with early HCC.
Funding Statement
This study was partially supported by grants from the Scientific Research Subject of Jiangsu Province Health Department (No. H201661) and the Project of Invigorating Health Care through Science, Technology and Education: Jiangsu Provincial Medical Youth Talent (QNRC2016331).
Footnotes
Ethics Committee Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This article does not contain any studies with human participants or animals performed by any of the authors. It has been permitted to obtain the data from SEER database (Reference Number 10778-Nov2018).
Informed Consent: As this study is based on a publicly available database without identifying patient information, informed consent was not needed.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – J.G.Q., G.T.M., B.D.S.; Supervision – J.G.Q., G.T.M., B.D.S.; Resource – Q.J.J., C.P., J.S.J.; Materials – J.G.Q., Q.J.J., C.P., J.S.J.; Data Collection and/or Processing – G.T.M., B.D.S., Q.J.J., C.P., J.S.J.; Analysis and/or Interpretation - G.T.M., B.D.S., Q.J.J., C.P., J.S.J.; Literature Search – G.T.M., B.D.S., Q.J.J., C.P., J.S.J.; Writing: - G.T.M., B.D.S., J.G.Q.; Critical Reviews – J.G.Q.
Conflict of Interest: The authors have no conflict of interest to declare.
References
- 1. . Bruix J, Sherman M.American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020–1022.. 10.1002/hep.24199) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. . Taylor-Robinson SD, Foster GR, Arora S, Hargreaves S, Thomas HC. Increase in primary liver cancer in the UK, 1979-1994. Lancet. 1997;350(9085):1142–1143.. 10.1016/S0140-6736(05)63789-0) [DOI] [PubMed] [Google Scholar]
- 3. . Deuffic S, Poynard T, Buffat L, Valleron AJ. Trends in primary liver cancer. Lancet. 1998;351(9097):214–215.. 10.1016/S0140-6736(05)78179-4) [DOI] [PubMed] [Google Scholar]
- 4. . El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340(10):745–750.. 10.1056/NEJM199903113401001) [DOI] [PubMed] [Google Scholar]
- 5. . Witjes CDM, Karim-Kos HE, Visser O.et al. Hepatocellular carcinoma in a low endemic area: rising incidence and improved survival. Eur J Gastroenterol Hepatol. 2012;24(4):450–457.. 10.1097/MEG.0b013e32835030ce) [DOI] [PubMed] [Google Scholar]
- 6. . Kamarajah SK. Fibrosis score impacts survival following resection for hepatocellular carcinoma (HCC): a surveillance, end results and epidemiology (SEER) database analysis. Asian J Surg. 2018;41(6):551–561.. 10.1016/j.asjsur.2018.01.001) [DOI] [PubMed] [Google Scholar]
- 7. . Martin RC, Loehle J, Scoggins CR, McMasters KM. Kentucky hepatoma: epidemiologic variant or same problem in a different region? Arch Surg. 2007;142(5):431–436.; discussion 436–437.. 10.1001/archsurg.142.5.431) [DOI] [PubMed] [Google Scholar]
- 8. . Sherman M. Hepatocellular carcinoma: screening and staging. Clin Liver Dis. 2011;15(2):323–33. 4, vii. 10.1016/j.cld.2011.03.003) [DOI] [PubMed] [Google Scholar]
- 9. . Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19(3):329–338.. 10.1055/s-2007-1007122) [DOI] [PubMed] [Google Scholar]
- 10. . Tandon P, Garcia-Tsao G. Prognostic indicators in hepatocellular carcinoma: a systematic review of 72 studies. Liver Int. 2009;29(4):502–510.. 10.1111/j.1478-3231.2008.01957.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. . Pompili M, Rapaccini GL, Covino M.et al. Prognostic factors for survival in patients with compensated cirrhosis and small hepatocellular carcinoma after percutaneous ethanol injection therapy. Cancer. 2001;92(1):126–135.. [DOI] [PubMed] [Google Scholar]
- 12. . Huo TI, Huang YH, Lui WY.et al. Selective prognostic impact of serum alpha-fetoprotein level in patients with hepatocellular carcinoma: analysis of 543 patients in a single center. Oncol Rep. 2004;11(2):543–550.. [PubMed] [Google Scholar]
- 13. . Kim HS, Park JW, Jang JS.et al. Prognostic values of a-fetoprotein and protein induced by vitamin K absence or antagonist-II in hepatitis B virus-related hepatocellular carcinoma. A prospective study. J Clin Gastroenterol. 2009;43(5):482–488.. 10.1097/MCG.0b013e318182015a) [DOI] [PubMed] [Google Scholar]
- 14. . Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence – SEER 9 Regs Research Data, Nov 2017 Sub (1973-2015) <Katrina/Rita Population Adjustment> – Linked To County Attributes – Total U.S., 1969-2016 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2018, based on the November 2017 submission. [Google Scholar]
- 15. . Ishak K, Baptista A, Bianchi L.et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22(6):696–699.. 10.1016/0168-8278(95)80226-6) [DOI] [PubMed] [Google Scholar]
- 16. . Marrero JA, El-Serag HB. Alpha-fetoprotein should be included in the hepatocellular carcinoma surveillance guidelines of the American Association for the Study of Liver Diseases. Hepatology. 2011;53(3):1060–1061.. 10.1002/hep.24033) [DOI] [PubMed] [Google Scholar]
- 17. . Giannini EG, Farinati F, Trevisani F. Alpha-fetoprotein in hepatocellular carcinoma surveillance: wake not the dead. Hepatology. 2011;54(1):376–377.. 10.1002/hep.24196) [DOI] [PubMed] [Google Scholar]
- 1 8. . Zhang B, Yang B. Combined alpha fetoprotein testing and ultrasonography as a screening test for primary liver cancer. J Med Screen. 1999;6(2):108–110.. 10.1136/jms.6.2.108) [DOI] [PubMed] [Google Scholar]
- 19. . Singal A, Volk ML, Waljee A.et al. Meta-analysis: surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment Pharmacol Ther. 2009;30(1):37–47.. 10.1111/j.1365-2036.2009.04014.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. . Bai DS, Zhang C, Chen P, Jin SJ, Jiang GQ. The prognostic correlation of AFP level at diagnosis with pathological grade, progression, and survival of patients with hepatocellular carcinoma. Sci Rep. 2017;7(1):12870. 10.1038/s41598-017-12834-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. . Witjes CD, Polak WG, Verhoef C.et al. Increased alpha-fetoprotein serum level is predictive for survival and recurrence of hepatocellular carcinoma in non-cirrhotic livers. Dig Surg. 2012;29(6):522–528.. 10.1159/000348669) [DOI] [PubMed] [Google Scholar]
- 22. . Burnett NP, Dunki-Jacobs EM, Callender GG.et al. Evaluation of alpha-fetoprotein staging system for hepatocellular carcinoma in noncirrhotic patients. Am Surg. 2013;79(7):716–722.. 10.1177/000313481307900717) [DOI] [PubMed] [Google Scholar]
- 23. . Huo TI, Lee SD. Role of the model for end-stage liver disease and serum alpha-fetoprotein as predictors for hepatocellular carcinoma. Liver Int. 2006;26(10):1300–1301.. 10.1111/j.1478-3231.2006.01379.x) [DOI] [PubMed] [Google Scholar]
- 24. . Omata M, Lesmana LA, Tateishi R.et al. Asian Pacific Association for the Study of the liver consensus recommendations on hepatocellular carcinoma. Hepatol Int. 2010;4(2):439–474.. 10.1007/s12072-010-9165-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. . Lencioni RA, Allgaier HP, Cioni D.et al. Small hepatocellular carcinoma in cirrhosis: randomized comparison of radio-frequency thermal ablation versus percutaneous ethanol injection. Radiology. 2003;228(1):235–240.. 10.1148/radiol.2281020718) [DOI] [PubMed] [Google Scholar]
- 26. . Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Difference in tumor invasiveness in cirrhotic patients with hepatocellular carcinoma fulfilling the Milan criteria treated by resection and transplantation: impact on long-term survival. Ann Surg. 2007;245(1):51–58.. 10.1097/01.sla.0000225255.01668.65) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. . Giannini EG, Marenco S, Borgonovo G.et al. Alpha-fetoprotein has no prognostic role in small hepatocellular carcinoma identified during surveillance in compensated cirrhosis. Hepatology. 2012;56(4):1371–1379.. 10.1002/hep.25814) [DOI] [PubMed] [Google Scholar]
- 28. . Abbasi A, Bhutto AR, Butt N, Munir SM. Correlation of serum alpha fetoprotein and tumor size in hepatocellular carcinoma. J Pak Med Assoc. 2012;62(1):33–36.. [PubMed] [Google Scholar]
- 29. . Di Bisceglie AM, Sterling RK, Chung RT.et al. Serum alpha-fetoprotein levels in patients with advanced hepatitis C: results from the HALT-C Trial. J Hepatol. 2005;43(3):434–441.. 10.1016/j.jhep.2005.03.019) [DOI] [PubMed] [Google Scholar]
- 30. . Chen TM, Huang PT, Tsai MH.et al. Predictors of alpha-fetoprotein elevation in patients with chronic hepatitis C, but not hepatocellular carcinoma, and its normalization after pegylated interferon alfa 2a-ribavirin combination therapy. J Gastroenterol Hepatol. 2007;22(5):669–675.. 10.1111/j.1440-1746.2007.04898.x) [DOI] [PubMed] [Google Scholar]
- 31. . Nomura F, Ohnishi K, Tanabe Y. Clinical features and prognosis of hepatocellular carcinoma with reference to serum alpha-fetoprotein levels . Analysis of 606 patients. Cancer. 1989;64(8):1700–1707.. [DOI] [PubMed] [Google Scholar]
- 32. . Riaz A, Ryu RK, Kulik LM.et al. Alpha-fetoprotein response after locoregional therapy for hepatocellular carcinoma: oncologic marker of radiologic response, progression, and survival. J Clin Oncol. 2009;27(34):5734–5742.. 10.1200/JCO.2009.23.1282) [DOI] [PubMed] [Google Scholar]