Abstract
Background:
The initial treatment for fecal incontinence (FI) includes supportive treatment and medical treatment. If the initial treatment fails, biofeedback therapy (BFT) is recommended. However, there are limited and conflicting results in the literature supporting the beneficial effect of BFT for FI. The aim of the study is to analyze the efficacy of BFT in 126 patients who have FI due to several causes.
Methods:
The data of 126 patients (88 females (69.8%) and 38 males (30.2%)) were collected retrospectively. Colonoscopy, anorectal manometry (ARM), and 3D-Endoanal ultrasonography (EAUS) were performed for all patients before applying BFT. In addition, all patients received toilet training instruction and training in Kegel and other pelvic floor strengthening exercises from an experienced nurse, before BFT.
Results:
The median age of participants was 54 years (range 18-75 years). While 80 patients (63.5%) had clinical and manometric benefit from BFT, 46 patients (36.5%) did not respond to BFT. According to the EAUS and ARM findings, BFT was beneficial in patients who had partial external sphincter failure, and was unsuccessful in patients who had both internal and external sphincter failure, both internal and external sphincter tears, and external sphincter tear rates of more than 25%. After BFT, significant increases in squeeze pressures were observed, with this increase being higher in the positive-response group.
Conclusion:
The results suggest that BFT is effective in the treatment of FI for specific patient populations.
Keywords: Fecal incontinence, biofeedback therapy, anorectal diseases
Main Points
BFT is effective in the treatment of FI, especially in those without spinal cord injury and those with normal external sphincter functions, and in those without tears in the external sphincter or with less than 25% tears.
BFT has a high response rate, with no side effects.
BFT should be planned as a second-line treatment method in appropriate patients who do not respond to conservative treatments.
Introduction
Functional anorectal disorders are common throughout different populations and are characterized by specific symptoms. The 3 major functional anorectal diseases defined according to the Roma IV criteria are defecation disorders (DD), fecal incontinence (FI), and anorectal pain disorders.1,2 FI describes involuntary solid or liquid fecal loss, while anal incontinence describes involuntary solid or liquid fecal or flatus loss. The degree of incontinence may vary from flatus to full discharge of the intestinal contents.3
Generally, FI has significant social and economic burdens and significantly decreases the quality of life.4-6 FI may affect people of any age, with prevalence ranging from 1.6% to 15%, depending on age and the presence of medical comorbidities. Bor et al. have shown that the frequency of FI in individuals between 55 and 74 years of age in Turkey is 5.2%, and it is 13.2% in individuals over the age of 75. The prevalence can be up to 50% for residents of elderly care homes. Although the true prevalence of FI in the general population is high, it is suggested that FI is generally underreported by patients.1,3,5
Approximately 80% of patients have more than 1 underlying pathologic abnormality. It is more frequently caused due to complex sensory and motor defects of the sphincter, rectum, and pelvic floor.7 Damage to the perineum during vaginal deliveries, disruption of the anal sphincter structure after anal dilation, and anorectal surgeries like hemorrhoidectomy, sphincterotomy, or fistulectomy are the main known causes of FI.7,8
The initial treatment for FI includes supportive treatment and medical treatment.2,3 If initial treatment fails, biofeedback therapy (BFT) is recommended, after researching the functional and structural abnormalities causing the FI.1,9,10 However, there are limited and conflicting results in the literature that support the beneficial effect of BFT for FI.
This study aimed to analyze the efficacy of BFT in 126 patients who have FI due to several causes.
Materials and Methods
Patients
Although BFT was initially planned for 149 patients with FI, 23 or those patients were excluded from the study because they did not continue the BFT sessions. The data from 126 patients with FI who underwent BFT from January 2013 to January 2018 (88 females (69.8%) and 38 males (30.2%)) were collected retrospectively. The data were retrieved from medical records in the digital database of the motility laboratory in the gastroenterology department of the hospital where the study was performed.
None of the patients in the study population responded to conservative treatment modalities,1-4 including (a) Avoiding the intake of foods which worsen symptoms, like incompletely digested sugars (e.g., fructose, lactose); (b) Maintaining a food and symptom diary to identify factors that cause diarrhea and incontinence; (c) Receiving a dietary supplement with a bulking agent to improve stool consistency; and (d) Obtaining a regular defecation program. Anorectal examination, colonoscopy, anorectal manometry (ARM), and 3D-Endoanal ultrasonography (EAUS) were performed for all patients before applying BFT. The degrees of tear rates of internal or external sphincters tears were determined via EAUS. The exclusion criteria were age less than 18 years, chronic diarrhea, duration of complaints less than 6 months, non-compliance with BFT procedures, pregnancy, external sphincter tear rates of more than 50% on EAUS, active intestinal disease, and dyssynergic defecation disorder accompanied by constipation and FI. Dyssynergic defecation disorder (impaired coordination of the abdominal and pelvic floor muscles during the discharge of feces) is seen in most patients with constipation accompanied by FI. The main problem in these patients is disruption of the stool discharge, resulting in distal stool accumulation in the rectum. This situation causes FI (it causes overflow incontinence and rectal sensation disturbances in the patient). These patients were treated with a different method of BFT, which is why they were not included in the present study.
Patients with active ulcerative colitis, perianal disease, or bacterial or parasitic infections were excluded while the disease was active. Patients were included in the study if their incontinence persisted after their active illness passed.
According to weekly incontinence counts, patients were divided into 3 groups: those with incontinence 2 or fewer times per week, those with incontinence 3 to 5 times per week, and those with incontinence more than 5 times per week or daily.
Patients were also grouped according to the degree of tear rates of the external sphincter on EAUS, as 10-25%, and more than 25%.
Anorectal Physiological Tests
Conventional ARM was performed using an 8-channel (Dentsleeve International, Mui Scientific) water perfusion system. The catheter was connected to calibrated pressure transducers, and the data coming from the pressure transducers were recorded digitally.11,12 All standard procedures were performed by an experienced nurse. Anal resting pressure, anal maximum squeezing pressure, anal pressure while coughing, and the recto-anal inhibitor reflex were recorded. Rectal sensation was evaluated by inflating a rectal balloon and measuring the first sensation, desire to defecate, and maximum tolerable volumes.
With conventional ARM, the average resting pressure is measured with sensors inside the rectum. The main source of the resting pressure is the internal sphincter. Therefore, resting pressure provides information about internal sphincter function. Mean anal squeeze pressure is measured with sensors in the anal canal (by asking the patient to squeeze the anal canal). The anal squeeze pressure provides information about external sphincter function.
BFT
Before undergoing BFT, all patients received toilet training. In order to prevent urge or overflow incontinence, an attempt was made to encourage regular defecation habits 2 to 3 times per day (usually at the same time of day) for all patients in the study. The patients were instructed by a nurse who was experienced in Kegel and other exercises to strengthen the pelvic floor muscles, and were also given visual and written documents to allow them perform these exercises at home.
In this study, an electromyography (EMG)-BFT technique was applied. While the patient was lying in the left lateral decubitus position, surface EMG probes were adhered to the skin on the bilateral anal canal. The patients watched the manometric tracings on a computer monitor from surface EMG probes around the bilateral anal canal. Patients with BFT tried to control the sphincter and pelvic floor muscles with visual feedback on the monitor, and were taught to fulfill the commands given by the nurse. The patients received at least 6 sessions of BFT, applied under the supervision of a motility nurse. BFT was discontinued in patients who showed no reduction in the number of daily or weekly incontinence episodes by the end of their sixth session. The treatment was extended to 10 to 15 sessions for patients who responded to the first 6 sessions. Each BFT session was conducted for 30 to 45 minutes. After the final BFT session, clinical and manometric reevaluations were performed. FT was considered to be successful in patients who became asymptomatic and in whom the anorectal manometric findings were ameliorated.
Ethical approval for this study was obtained from by the local ethics committee (Study No. 29620911/929) of Turkey Yuksek Ihtisas Training and Research Hospital, Ankara, Turkey.
Statistical Analysis
Statistical analyses were performed with the SPSS 20.0 (SPSS, Chicago, Illinois, USA) statistical package for Windows. Continuous variables were compared using the Student’s t-test or the Mann–Whitney U-test, depending on the normality of distribution. The categorical variables were compared by the Pearson’s or Fisher’s exact chi-square tests. The Wilcoxon signed-rank test and paired samples test were used, depending on normality of distribution, for comparing 2 related samples to assess whether their population mean ranks differed. A receiver operator characteristic (ROC) curve was built to determine the predictive value of anal squeeze pressures before BFT, for treatment response. Binary logistic regression analysis was performed to detect independent predictive factors for successful BFT. Any value of P < .05 was considered statistically significant.
Results
The study included 88 females (69.8%) and 38 males (30.2%). The median age was 54 years (with a range of 18-75 years). The initial demographic and clinical features, the ARM and EAUS findings, and the BFT results of the patients are shown in Table 1.
Table 1.
Outcomes of Biofeedback Therapy
| Value, n (%) | Responders, n (%) | Non-responders, n (%) | P | |
|---|---|---|---|---|
| Demographics | ||||
| Age, year, median (range) | 54 (18-75) | 54.5 (18-75) | 53.5 (19-74) | .36 |
| Male | 38 (30.2) | 25 (65.8) | 13 (34.2) | .72 |
| Female | 88 (69.8) | 55 (62.5) | 33 (37.5) | |
| İncontinence history | ||||
| Duration of fecal incontinence, month, median (range) | 36 (6-444) | 60.79 | 68.22 | .27 |
| Grading of incontinence | ||||
| Less than 2 per week | 32 (25.4%) | 19 (59.4) | 13 (40.6) | .1 |
| 3 to 5 per week | 39 (31%) | 30 (76.9) | 9 (23.1) | |
| More than 5 per week or everyday | 55 (43.6) | 31 (54.4) | 24 (43.6) | |
| Medical history | ||||
| Diabetes mellitus | 26 | 18 (69.2) | 8 (30.8) | .49 |
| Previous incomplete spinal cord injury | 13 | 3 (23.1) | 10 (76.9) | .02 |
| History of previous anorectal surgery | ||||
| Previous distal rectal surgery | 12 | 6 (50) | 6 (50) | .3 |
| Hemorrhoidectomy | 8 | 7 (87.5) | 1 (12.5) | |
| Obstetric history | ||||
| Number of women who had a vaginal delivery | 74 | 49 (66.2) | 25 (33.8) | .44 |
| Number of women who had a history of difficult vaginal delivery | 23 | 15 (65.2) | 8 (34.8) | .84 |
| Number of women who had a history of pelvic trauma during vaginal delivery | 15 | 8 (53.3) | 7 (46.7) | .38 |
| Anal tone in physical examination | ||||
| Normal | 38 (30.2%) | 28 (73.6) | 10 (26.4) | .89 |
| Decreased | 88 (69.8%) | 52 (59.1) | 36 (40.9) | |
| Endoanal USG findings | ||||
| Normal | 42 | 32 (76.2) | 10 (23.8) | .36 |
| Internal sphincter tears | 3 | 2 (66.7) | 1 (33.3) | .90 |
| Atrophy in the internal sphincter | 9 | 8 (88.9) | 1 (11.1) | .10 |
| External sphincter tears | 26 | 17 (65.4) | 9 (34.6) | .82 |
| Atrophy in the external sphincter | 6 | 2 (33.3) | 4 (66.7) | .11 |
| Defect in both internal and external sphincter | 20 | 7 (35) | 13 (65) | .004 |
| External sphincter tear rate (%) | ||||
| 10-25 | 25 | 19 (76) | 6 (24) | .001 |
| > 25 | 21 | 5 (23.8) | 16 (76.2) | |
| İnternal sphincter tear rate (%) | ||||
| 10-25 | 12 | 7 (58.3) | 5 (41.7) | .06 |
| < 25 | 11 | 2 (18.2) | 9 (81.8) | |
| Biofeedback number of sessions, median (range) | 12 (12-15) | 8 (7-15) | ||
| Anorectal manometry findings | ||||
| Normal | 11 | 9 (81.8) | 2 (18.2) | .18 |
| İnternal sphincter failure | 3 | 2 (66.7) | 1 (33.3) | .90 |
| External sphincter failure | 26 | 12 (46.2) | 14 (53.8) | .39 |
| Partial external sphincter failure | 47 | 38 (80.9) | 9 (19.1) | .02 |
| Internal and external sphincter failure | 29 | 13 (44.8) | 16 (55.2) | .017 |
| Partial external sphincter and internal sphincter failure | 10 | 6 (60) | 4 (40) | .81 |
| Rectal capacity | ||||
| Normal | 62(49.2%) | 43 (69.4) | 19 (30.6) | .36 |
| Decreased | 11(8.7%) | 7 (63.6) | 4(36.4) | |
| Increased | 53(42.1%) | 30 (56.6) | 23(43.4) |
P < .05.
The patients had a history of incontinence for a median of 36 (min-max: 6-444) months. Of those, 43.6% experienced incontinence more than 5 times per week or daily, while 31% experienced incontinence 3 to 5 times per week, and 25.4% experienced incontinence 2 or fewer times per week. Of the patients, 26 had diabetes mellitus, and 13 had spinal cord injury history. Twenty-three patients had undergone previous anorectal surgery. Of these patients, 12 had undergone operations due to distal rectal carcinoma. Of the female patients, 74 had given birth by the normal vaginal route. Twenty-three female patients had difficult deliveries, and fifteen had perineum tears during delivery.
According to the ARM findings, 47 patients had partial external sphincter failure (squeeze pressure >80 mmHg, but duration of anal canal contraction <3 s), 29 patients had both external and internal sphincter failure, 26 patients had external sphincter failure, 10 patients had partial external sphincter failure and internal sphincter failure, 3 patients had internal sphincter failure, and 11 patients were normal.
When the rectal capacity was evaluated by inflating a rectal balloon, it was found that 62 patients had normal rectal capacity, 53 patients had rectal hypersensitivity, and 11 patients had rectal hyposensitivity.
Outcomes of BFT
While 80 patients (63.5%) had clinical and manometric benefit from BFT, 46 patients (36.5%) did not respond to BFT. Those who benefited from BFT had an average of 12 sessions of BFT, while those who did not benefit had an average of 8.5 sessions.
According to the EAUS and ARM findings, BFT was beneficial in patients who had partial external sphincter failure, and was unsuccessful in patients who had both internal and external sphincter failure, both internal and external sphincter tears, and an external sphincter tear rate of more than 25%.
After BFT, significant increases in resting and squeeze pressures were observed, with this increase being higher in the success group (Table 2).The difference in squeeze pressure with BFT was significantly higher in the BFT-positive-response group, while the difference in resting pressure was comparable between BFT responders and non-responders (Table 3).
Table 2.
Comparison of Anorectal Manometry Findings Between the Responders and Non-responders, and the Results Before and After Training
| Anal Squeeze Pressure (mmHg) | Anal Resting Pressure (mmHg) | Duration of Anal Canal Contraction (s) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Before Training | After Training | P | Before Training | After Training | P | Before Training | After Training | P | |
| Responders | 98.45 ± 39.62 | 132.3 ± 45.09 | <.001 | 47.95 ± 19.06 | 52.32 ± 17.9 | .031 | 1.56 ± 0.5 | 3.36 ± 0.78 | <.001 |
| Non-responders | 68.8 ± 33.98 | 79.43 ± 38.23 | <.001 | 41.08 ± 22.26 | 42.65 ± 23.32 | .113 | 1.38 ± 0.5 | 1.92 ± 0.64 | .008 |
Table 3.
Comparison of Median Anal Sphincter Pressure Changes According to Biofeedback Therapy Response
| Non-responders | Responders | P | |
|---|---|---|---|
| Δ Resting Pressure mmHg, (median, min-max) | 0.0 (−40 to 27) | 0.0 (−40 to 70) | .634 |
| Δ Squeeze Pressure, mmHg, (median, min-max) | 5.5 (−55 to 70) | 25 (−45 to 182) | <.001 |
Binary logistic regression analysis revealed that squeeze pressure before BFT was an independent factor for BFT success (Table 4).
Table 4.
Binary Logistic Regression Analysis to Predict the Independent Factors for BFT Response
| Beta | Standard Error | OR | P | |
|---|---|---|---|---|
| Presence of previous spinal cord injury | 2.579 | 1.166 | 13.179 | .02 |
| Defect both in the internal and external sphincter | 1.548 | 1.147 | 4.701 | .177 |
| Resting pressure | 0.025 | 0.026 | 1.025 | .342 |
| Squeezing pressure | 1.118 | 0.546 | 3.059 | .041 |
| Duration of anal canal contractions | 0.737 | 0.727 | 2.089 | .311 |
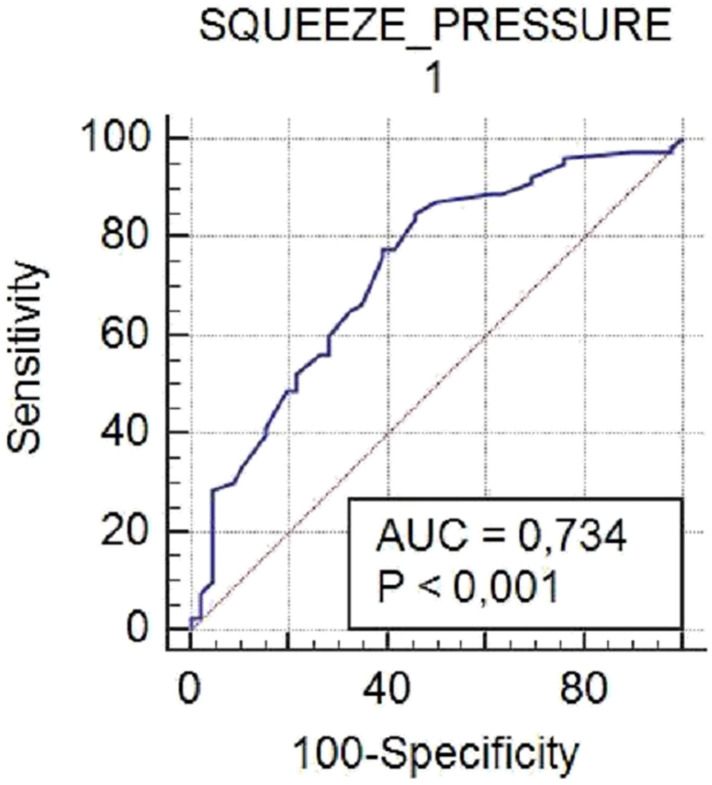
The ROC curve was analyzed to identify the cut-off value of squeeze pressures before biofeedback for those with successful BFT, and found to be 60 mmHg, with a sensitivity of 85% and specificity of 55% (95% CI: 39.0-69.1) (Figure 1).
Figure 1.
Receiver-operating Characteristic (ROC) Curve Analysis to Show the Effectiveness of Squeeze Pressures Before Biofeedback Therapy on Treatment Success.
Discussion
This study documented that BFT is beneficial in FI patients with high initial squeeze pressure on ARM, and in those with partial external sphincter failure. In addition, the results revealed that patients with spinal cord trauma history, concomitant internal and external sphincter failure on ARM, and anal sphincter tears (IAS+EAS) in EAUS were less responsive to BFT.
BFT is a non-invasive, non-drug and low-cost therapeutic approach for FI. There are reports with treatment success of 38-100% for FI patients.13-15 One study found that BFT was successful for 53% of patients,15 while another found full response in 41% of patients and partial response in 35% of patients.11 In this study’s patient group, the BFT response rate was higher (63.5%) than most of the data reported in the current literature. This variability in treatment success may be due to differences in the BFT methods applied to patients, number of treatment sessions, and differences between the patient groups. For instance, only hospital-based BFT was applied, in combination with pelvic floor exercises and Kegel exercises performed at home. In the study of Heymen et al.,11 the investigators applied both hospital and home-based BFT in patients with FI, while Sun et al.13 used only hospital-based BFT in patients who had FI after anorectal surgery. In several studies, the number of BFT sessions, and the mode of application of BFT therapy––as home-based or hospital-based, and alone or combined with pelvic floor muscle exercises, Kegel exercises, or sacral nerve stimulation––have been shown to be predictive factors for the treatment success in patients with FI.12,16 Norton et al.15 reported that BFT was not superior to conventional treatment for FI patients. In contrast, Heymen et al.11 found that BFT was superior to conservative treatment and pelvic floor muscle exercises.
Previously, it has been suggested that BFT may provide improvements in resting anal sphincter tonus, the voluntary contraction of the anal sphincter and puborectalis muscle, abdominopelvic coordination during excretion, and rectal capacity functions in patients.1,3,9 A study by Sun et al.13 identified increases in resting and squeeze pressures of patients after BFT. This increase was shown to be higher in those who responded to BFT. At the same time, those with high anal squeeze pressure provided better response to BFT, and similarly, those without good sphincter functions provided less response.17,18 Similarly, this study documented that both anal resting and anal squeeze pressures increased in the BFT-positive-response group. The rate of response to BFT was significantly higher in patients with anal squeeze pressure above 60 mmHg. In contrast, the BFT response rate was significantly lower in patients with internal and external sphincter failure.
The duration of anal canal contraction is one of the factors affecting incontinence. If individuals with sufficient external sphincter squeeze pressure do not have sufficient squeeze duration, it may cause anal incontinence.19 Marcello et al.20 found that the mean squeeze duration was 3.2 seconds for anal continence. This present study found that there were significant increases in squeeze duration in BFT responders. It was also shown that incontinence continued in the majority of FI patients without improvement of squeeze durations. Another study similarly found a significant increase in squeeze durations after BFT, related with treatment response.13
The prevalence of FI usually increases with age, and it is also high in middle-aged women. In the present study, the mean age of participants was 54 years and nearly 70% were female.21-25 However, the difference in distribution of age or gender in terms of treatment response to BFT was not identified. It is reported that the prevalence of FI increases due to trauma to the perineum through the vaginal tract (especially third- or fourth-degree vaginal tears), which is one of the main causes of FI in women. However, while childbirth may cause FI without sphincter tears, every tear occurring during delivery does not cause FI.26,27 Of the participants in this study, 74 of 88 women (85%) had history of vaginal delivery. However, no significant difference between those with vaginal delivery and those without in terms of BFT treatment success was identified.
The degree of external sphincter tear affects the efficacy of BFT treatment. In particular, patients with third- or fourth-degree tears respond less favorably to BFT.26-29 Correspondingly, as the tear rate increased, less benefit from BFT was seen.
Sphincter dysfunction occurring after anorectal surgery disrupted rectal sensation and anal stenosis, and the disrupted sensorial and motor functions in the anal region may cause FI. The FI rates vary from 30% to 56% after anorectal surgery.30 Of the patients in this study, 23 had a history of anorectal surgery. While 14 of these patients (60.8%) responded to BFT, 9 patients did not. However, the BFT response rates did not differ between the patients who had anorectal surgery and those who did not. The study by Sun et al.13 found 30 of 55 patients with anorectal surgery history responded to BFT. In another study, a clear improvement in the FI score was observed with BFT after anorectal surgery.9 However, in both studies, in contrast to this study, there was no comparison with a non-surgical group.
The American Neurogastroenterology and Motility Society and the European Society of Neurogastroenterology and Motility both recommend 6 BFT sessions of at least 1 hour per week for FI patients.10 BFT may require many sessions varying from a few weeks to months.31,32 The present study planned to include at least 7 sessions for those who did not see any benefit from BFT, with an average of 12 sessions for those who benefited. The sessions in this study lasted for 30 to 45 minutes, rather than 1 hour. However, it is believed that the face-to-face interviews at the hospital and the increased number of sessions motivated the increased positive responses in patients, affecting the treatment success rate.
The limitations of this study include the retrospective design of the study and the lack of homogeneity in the patient groups. Moreover, no information was obtained on whether patients also simultaneously experienced urinary incontinence, and the FI score and incontinence type (urge or passive incontinence) were not identified either. A further limitation of this study is the lack of a control group. These limitations can be attributed to the retrospective nature of the study. However, we believe that conducting regular follow-up of the patients at the motility department, updating patient files regularly, and following the patients one-by-one during the BFT sessions, as well as the sufficient number of patients in the study, have addressed these deficiencies.
Conclusion
BFT is a non-invasive and inexpensive therapy. It has a high response rate and has no side effects. BFT is effective in the treatment of FI, especially in specific patient populations. BFT should be planned as a second-line treatment method in appropriate patients who do not respond to conservative treatments. In addition, BFT should not only be performed in tertiary treatment centers, but also in other centers, because it is an easy-to-apply treatment method.
Funding Statement
The authors declared that this study has received no financial support.
Footnotes
Ethics Committee Approval: The study was approved by the Ethics Committee of Türkiye Yüksek İhtisas Training and Research Hospital.
Informed Consent: Written informed consent was obtained from the patients who participated in this study.
Peer review: Externally peer-reviewed.
Author Contributions: Concept – Ö.O., Y.O.; Design - Ö.O., Y.O.; Supervision - Y.O., Z.M.Y.; Resource - Ö.O., Y.O., Ö.A.; Materials - Ö.O., Y.O.; Data Collection and/or Processing - F.B., İ.T., D.A., V.G., M.Y.; Analysis and/or Interpretation - Ö.O., Y.O., Ö.A., Z.M.Y.; Literature Search - Ö.O., I.T., F.B., M.Y.; Writing - Ö.O., İ.T., D.A.; Critical Reviews - Ö.O., Y.O., Z.M.Y.
Conflict of Interest: The authors have no conflicts of interest to declare.
References
- 1. . Narayanan SP, Bharucha AE. A practical guide to biofeedback therapy for pelvic floor disorders. Curr Gastroenterol Rep. 2019;21(5):21. 10.1007/s11894-019-0688-3) [DOI] [PubMed] [Google Scholar]
- 2. . Rao SS, Bharucha AE, Chiarioni G. et al. Functional anorectal disorders. Gastroenterology. 2016;150:1430–1442.. 10.1053/j.gastro.2016.02.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. . Guillaume A, Salem AE, Garcia P, Chander Roland BC. Pathophysiology and therapeutic options for fecal incontinence. J Clin Gastroenterol. 2017;51(4):324–330.. 10.1097/MCG.0000000000000797) [DOI] [PubMed] [Google Scholar]
- 4. . Nelson R, Norton N, Cautley E, Furner S. Community-based prevalence of anal incontinence. JAMA. 1995;274(7):559-561. [PubMed] [Google Scholar]
- 5. . Tokay Tarhan S, Atuğ Ö, Giral A, İmeryüz N. Effect of gender on the etiology of fecal incontinence: Retrospective analysis of a tertiary referral center in Turkey. Turk J Gastroenterol. 2019;30(9):782-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. . Rothbarth J, Bemelman WA, Meijerink WJ. et al. What is the impact of fecal incontinence on quality of life? Dis Colon Rectum. 2001;44(1):67-71. 10.1007/BF02234823) [DOI] [PubMed] [Google Scholar]
- 7. . Bajwa A, Emmanuel A. The physiology of continence and evacuation. Best Pract Res Clin Gastroenterol. 2009;23(4):477–485.. 10.1016/j.bpg.2009.06.002) [DOI] [PubMed] [Google Scholar]
- 8. . Madoff RD, Parker SC, Varma MG, Lowry AC. Faecal incontinence in adults. Lancet. 2004;364(9434):621–632.. 10.1016/S0140-6736(04)16856-6) [DOI] [PubMed] [Google Scholar]
- 9. . Kim KH, Yu CS, Yoon YS. et al. Effectiveness of biofeedback therapy in the treatment of anterior resection syndrome after rectal cancer surgery. Dis Colon Rectum. 2011;54(9):1107–1113.. 10.1097/DCR.0b013e318221a934) [DOI] [PubMed] [Google Scholar]
- 10. . Rao SS, Benninga MA, Bharucha AE. et al. ANMS-ESNM position paper and consensus guidelines on biofeedback therapy for anorectal disorders. Neurogastroenterol Motil. 2015;27(5):594–609.. 10.1111/nmo.12520) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. . Heymen S, Scarlett Y, Jones K. et al. Randomized controlled trial shows biofeedback to be superior to pelvic floor exercises for fecal incontinence. Dis Colon Rectum. 2009;52(10):1730–1737.. 10.1007/DCR.0b013e3181b55455) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. . Tjandra JJ, Chan MK, Yeh CH, Murray-Green C. Sacral nerve stimulation is more effective than optimal medical therapy for severe fecal incontinence: a randomized, controlled study. Dis Colon Rectum. 2008;51(5):494–502.. 10.1007/s10350-007-9103-5) [DOI] [PubMed] [Google Scholar]
- 13. . Sun XB, Zhang L, Li YH, Li JL, Chen YL. The effects of biofeedback training of pelvic floor muscles on fecal incontinence. J Pediatr Surg. 2009;44(12):2384–2387.. 10.1016/j.jpedsurg.2009.07.062) [DOI] [PubMed] [Google Scholar]
- 14. . Ozturk R, Niazi S, Stessman M, Rao SS. Long-term outcome and objective changes of anorectal function after biofeedback therapy for faecal incontinence. Aliment Pharmacol Ther. 2004;20(6):667–674.. 10.1111/j.1365-2036.2004.02125.x) [DOI] [PubMed] [Google Scholar]
- 15. . Norton C, Chelvanayagam S, Wilson-Barnett J, Redfern S, Kamm MA. Randomized controlled trial of biofeedback for fecal incontinence. Gastroenterology. 2003;125(5):1320-1329. 10.1016/j.gastro.2003.09.039) [DOI] [PubMed] [Google Scholar]
- 16. . Boyle DJ, Knowles CH, Lunniss PJ. et al. Efficacy of sacral nerve stimulation for fecal incontinence in patients with anal sphincter defects. Dis Colon Rectum. 2009;52(7):1234–1239.. 10.1007/DCR.0b013e31819f7400) [DOI] [PubMed] [Google Scholar]
- 17. . Iwai N, Nagashima M, Shimotake T, Iwata G. Biofeedback therapy for fecal incontinence after surgery for anorectal malformations: preliminary results. J Pediatr Surg. 1993;28(6):863–866.. 10.1016/0022-3468(93)90347-n) [DOI] [PubMed] [Google Scholar]
- 18. . Tan EK, Vaizey C, Cornish J, Darzi A, Tekkis PP. Surgical strategies for faecal incontinence: a decision analysis between dynamic graciloplasty, artificial bowel sphincter and end stoma. Colorectal Dis. 2008;10(6):577–586.. 10.1111/j.1463-1318.2007.01418.x) [DOI] [PubMed] [Google Scholar]
- 19. . Norton C, Chelvanayagam S. Conservative management of faecal incontinence in adults. In: Norton C, Chelvanayagam S.eds. Bowel Continence Nursing. Beaconsfield, UK: Beaconsfield Publishers Ltd; 2004:114 -131. [Google Scholar]
- 20. . Marcello PW, Barrett RC, Coller JA. et al. Fatigue rate index as a new measurement of external sphincter function. Dis Colon Rectum. 1998;41(3):336–343.. 10.1007/BF02237488) [DOI] [PubMed] [Google Scholar]
- 21. . Quander CR, Morris MC, Melson J, Bienias JL, Evans DA. Prevalance of and factors associated with fecal incontinence in a large community study of older individuals. Am J Gastroenterol. 2005;100(4):905–909.. 10.1111/j.1572-0241.2005.30511.x) [DOI] [PubMed] [Google Scholar]
- 22. . Norton C, Whitehead WE, Bliss DZ. et al. Management of fecal incontinence in adults. Neurourol Urodyn. 2010;29(1):199–206.. 10.1002/nau.20803) [DOI] [PubMed] [Google Scholar]
- 23. . Whitehead WE, Borrud L, Goode PS. et al. Fecal incontinence in US adults: epidemiology and risk factors. Gastroenterology. 2009;137(2):512–7, 517.e1.. 10.1053/j.gastro.2009.04.054) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. . Ng KS, Sivakumaran Y, Nassar N, Gladman MA. Fecal incontinence: community prevalence and associated factors: a systematic review. Dis Colon Rectum. 2015;58(12):1194-1209. 10.1097/DCR.0000000000000514) [DOI] [PubMed] [Google Scholar]
- 25. . Tokay Tarhan ST, Atuğ Ö, Giral A, İmeryüz N. Effect of gender on the etiology of fecal incontinence: retrospective analysis of a tertiary referral center in Turkey. Turk J Gastroenterol. 2019;30(9):782–788.. 10.5152/tjg.2019.18923) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. . Oberwalder M, Connor J, Wexner SD. Meta-analysis to determine the incidence of obstetric anal sphincter damage. Br J Surg. 2003;90(11):1333-1337. 10.1002/bjs.4369) [DOI] [PubMed] [Google Scholar]
- 27. . Bharucha AE, Zinsmeister AR, Locke GR. et al. Risk factors for fecal incontinence: a population-based study in women. Am J Gastroenterol. 2006;101(6):1305–1312.. 10.1111/j.1572-0241.2006.00553.x) [DOI] [PubMed] [Google Scholar]
- 28. . Falk PM, Blatchford GJ, Cali RL, Christensen MA, Thorson AG. Transanal ultrasound and manometry in the evaluation of fecal incontinence. Dis Colon Rectum. 1994;37(5):468–472.. 10.1007/BF02076193) [DOI] [PubMed] [Google Scholar]
- 29. . Wheeler TL II, Richter HE. Delivery method, anal sphincter tears and fecal incontinence: new information on a persistent problem. Curr Opin Obstet Gynecol. 2007;19(5):474–479.. 10.1097/GCO.0b013e3282ef4142) [DOI] [PubMed] [Google Scholar]
- 30. . Levitt MA, Penã A. Outcomes from the correction of anorectal malformations. Curr Opin Pediatr. 2005;17(3):394–401.. 10.1097/01.mop.0000163665.36798.ac) [DOI] [PubMed] [Google Scholar]
- 31. . Jodorkovsky D, Dunbar KB, Gearhart SL, Stein EM, Clarke JO. Biofeedback therapy for defecatory dysfunction: “real life” experience. J Clin Gastroenterol. 2013;47(3):252–255.. 10.1097/MCG.0b013e318266f43a) [DOI] [PubMed] [Google Scholar]
- 32. . Rey E, Choung RS, Schleck CD. et al. Onset and risk factors for fecal incontinence in a US community. Am J Gastroenterol. 2010;105(2):412–419.. 10.1038/ajg.2009.594) [DOI] [PMC free article] [PubMed] [Google Scholar]