Abstract
Background/Aims:
Chronic hepatitis C (CHC) is the only viral infection that can be treated with oral antiviral agents. However, CHC awareness is a major barrier to the World Health Organization’s target of eliminating the hepatitis C virus (HCV) by 2030. Here, CHC awareness trends were analyzed in Hacettepe University Hospital, Turkey, between January 2000 and December 2017.
Methods:
Central laboratory data were retrospectively analyzed for HCV test results (anti-HCV, HCV RNA, HCV genotype). After combining 548 141 anti-HCV test results, 395 103 cases were analyzed. The following 2 parameters were defined for CHC awareness: (1) the presence of HCV RNA results for anti-HCV positives and (2) the presence of a genotype result for HCV RNA positives.
Results:
Anti-HCV positives were older than negatives (mean age-years ± SD, 59.4 ± 19.0 vs. 44.0 ± 18.9), and the positivity rate was higher in women than in men (1.4% vs. 1.0%). Anti-HCV positivity decreased from 3.1% to 0.6% from 2000 to 2015 and subsequently stabilized. The overall percentage of RNA testing among anti-HCV positives was 53.1% (range, 20%-70%), which stabilized at approximately 50% after 2010. The genotyping rate for RNA positives varied between 40% and 70%. The main genotype identified was genotype 1 (85.7%).
Conclusion:
In an ideal CHC awareness state, all anti-HCV positives should undergo RNA testing, and genotyping should be performed when RNA tests are positive. However, even in our referral center, the combined rate of RNA and genotype testing was only approximately 50% during the last 10 years.
Keywords: Viral hepatitis, anti-HCV, hepatitis awareness, chronic hepatitis C, elimination program, screening of HCV
INTRODUCTION
There have been major developments in the understanding of the hepatitis C virus (HCV) since its identification in the 1980s.1-3 The current goal of the World Health Organization (WHO) is to eliminate HCV by 2030.4 If 90% of patients with HCV infection could be diagnosed and a further 80% could be treated by 2030, it would be possible to decrease new HCV infection and related mortality by 80% and 65%, respectively. The global prevalence of HCV viremia is 1.0% (95% uncertainty interval, 0.8-1.1), based on the estimated 71.1 million (62.5-79.4) patients with an active HCV infection.5 However, based on the first Global Hepatitis Report released by the WHO and according to global data for 2015, it was estimated that only 20% (range, 5.7%-36.3%) of patients are diagnosed and 7.4% (range, 2.2%-12.1%) are treated.6 This means that out of 71 million patients with chronic, active HCV infection, only 2.86 million initiated treatment globally in 2015 and 2016. The WHO has a target of increasing the rates of diagnosis and treatment of HCV infection to 90% and 80%, respectively, by 2030.7 Furthermore, in 2017, the number of individuals with HCV infection was greater than the number of individuals who were treated with HCV infection. Therefore, there is an urgent need to improve testing strategies and reduce financial barriers to drug availability to achieve the WHO’s elimination target by 2030. A major barrier to achieving these goals is the lack of awareness among both the public and doctors on chronic hepatitis C (CHC), which is generally asymptomatic.
As viral infections are the leading cause of liver diseases in Turkey, the health ministry agrees with the WHO’s elimination program and has developed a country-specific prevention and control program for viral hepatitis.8 Data from the health ministry for 2014 estimated that 0.7% of the population (317 000-540 000) is infected with HCV, most of whom were undiagnosed. The anti-HCV positivity rates among the at-risk groups are as follows: 3.8% in hemodialysis patients, 1.7% in peritoneal dialysis patients, 1.96% in kidney transplantation patients, and 7.6% in liver transplant patients. The annual number of new CHC patients is 5500, and 4200 patients are treated annually for CHC. CHC is the second leading cause of hepatocellular carcinoma (HCC) and liver transplantation in Turkey. Furthermore, the estimates reveal that from 2013 to 2030, cases of HCV-associated cirrhosis, decompensated cirrhosis, and HCC will rise to 40%, 60%, and 70%, respectively. Akarca et al. evaluated HCV awareness in the Turkish population by retrospectively reviewing 19 627 anti-HCV tests that were requested by surgical clinics to determine whether positive patients were undergoing HCV RNA testing.9 The anti-HCV positivity rate was 0.8%, but only half of the positive patients underwent HCV RNA testing or were informed about the necessity of being tested.
Although Turkey is a low endemic country for HCV infection, difficulty in detecting patients with an active HCV infection is a major barrier to achieving the WHO’s target of HCV elimination by 2030. This study aimed to determine HCV awareness in a tertiary referral center from 2000 to 2017 to estimate trend changes in HCV infection and awareness.
PATIENTS AND METHODS
The data system of the Clinical Laboratory at Hacettepe University Hospital, Turkey, was reviewed in the last 18 years (2000-2017). The study protocol was approved by the Institutional Review Board of Hacettepe University (GO 18/360-05). Retrospective data, including the patient’s name and surname, date of birth, sex, the date on which the test was requested, and the name of the clinic requesting it, as well as HCV tests (anti-HCV, HCV RNA, and HCV genotype), were collected. The patient’s age was calculated by subtracting the date of birth from that of the test date. To avoid the bias caused by wrong entries, if the calculated age was greater than 100 years, it was accepted as a missing value. Preprocessing of the data was applied to clean up, edit, and use it. For this purpose, all tests belonging to the same patient were combined based on the patient’s name, surname, and date of birth. During this merging process, if multiple HCV RNA results were available for the same patient, only the first positive test was included in the analysis. After combining the results of 548 141 anti-HCV tests using this method, 395 103 cases were analyzed, and the trend change in CHC awareness over the study period and between clinics was evaluated. According to this evaluation, 2 parameters defining CHC awareness were created: (1) the HCV RNA screening rate in patients with anti-HCV positivity and (2) the HCV genotyping rate in HCV RNA-positive patients.
Anti-HCV Test
Two different versions of the ARCHITECT anti-HCV test (Abbott Diagnostics, USA) were used to identify anti-HCV antibodies between 2000 and 2014. The anti-HCV antibody test was performed between 2015 and 2016 using the same technology-based ADVIA Centaur anti-HCV test (Siemens Healthineers, Germany), and subsequently, starting from 2017, testing was again performed using the ARCHITECT anti-HCV test.
The ARCHITECT anti-HCV test is an immunological test using chemiluminescent microparticle technology that qualitatively detects immunoglobulin G and immunoglobulin M antibodies against HCV in human serum and plasma. In the first phase of this 2-stage test, recombinant HCV antigens on paramagnetic microparticles are combined with anti-HCV antibodies from the sample. In the second stage, the result is obtained by the addition of a conjugate after washing and then by adding solutions that cause the chemiluminescent reaction. The resultant color change is detected by the device’s optical system.
HCV RNA Test
HCV RNA testing was performed between 2000 and 2004 using the HCV MONITOR test (Roche Diagnostics, Germany) in a COBAS AMPLICOR device (Roche Diagnostics, Germany) with a reverse transcriptase-polymerase chain reaction (RT-PCR) method. In performing this test, PCR mixtures were prepared in tubes according to the manufacturer’s recommendations and were placed in the device, where they underwent reverse transcription, amplification, and hybridization processes. In the first step, biotin-marked primers were attached to the target RNA sequence before cDNA synthesis was performed using a reverse transcriptase enzyme. Subsequently, millions of copies of the cDNA complementary target sequence were generated by PCR amplification. Finally, replicated products were determined quantitatively.
Between 2005 and 2014, HCV RNA testing was performed using the COBAS TaqMan system (Roche Diagnostics, Germany). In this method, PCR mixtures were prepared in tubes according to the manufacturer’s recommendations and were placed in the device, where they underwent reverse transcription, amplification, and hybridization processes. For these processes, the 5′-translational zones of the HCV genome, a well-preserved region for all genotypes, act as the target region. Hydrolysis probes (TaqMan probes) were used to determine the PCR products. As the amount of products produced in each cycle increased, a simultaneous increase in the level of fluorescence was observed, and the results were obtained quantitatively.
Since 2015, the HCV RNA test has been performed using the Abbott RealTime HCV kit on the m2000rt RealTime PCR device (Abbott Diagnostics, USA) using reverse transcription PCR. Following nucleic acid isolation with magnetic microparticles, the device automatically prepared the PCR amplification reaction mixture, which was distributed to the optical reaction plates. The presence of HCV RNA in the sample was quantitatively determined at the end of the RealTime PCR process.
HCV Genotype Testing
HCV genotyping was performed using the AMPLIQUALITY HCV type Plus kit (AB ANALITICA, Italy). This is a strip test based on the application of reverse hybridization to the 5ʹ UTR and “core” regions of the HCV genome following RNA isolation and reverse transcription PCR. As a result of the test, 7 HCV genotypes and several subtypes can be determined according to the positions of the hybrid bands formed on the strips (1, 1a, 1b, 2, 2a, 2b, 2c, 3, 3a, 3b/g/I, 3c, 3f/k, 3h, 4, 4a/b/c/d/f, 4e, 4h/k, 4m, 4n, 4q, 4r, 4v, 5a, 6a/b, 6c, e-v, 7a).
Statistical Analysis
Statistical analysis was performed using the International Business Machines Corporation Statistical Package for the Social Sciences Statistics for Windows.10 Graphics were drawn in R11 using the ggplot2 package.12 Due to the large number of observations (395 103), even small differences were found to be statistically significant. Therefore, only descriptive statistics, with no significance (P-value), were given. Continuous variables were reported with the mean ± standard deviation. Categorical variables were summarized with the frequency and percentage.
RESULTS
The Anti-HCV Test
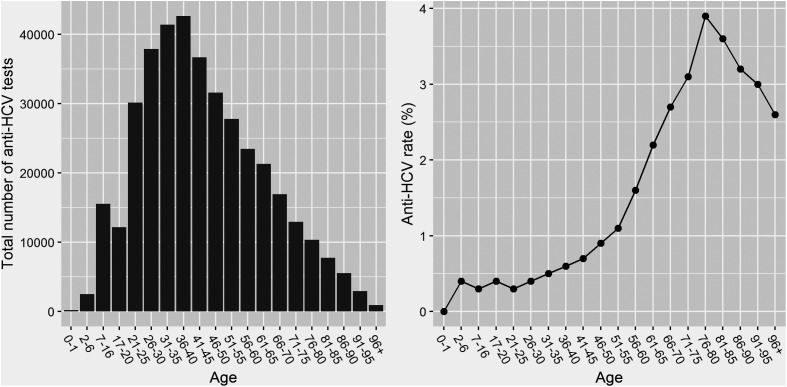
Of the 395 103 patients who underwent anti-HCV testing, 1.2% had a positive result. There were only 42 borderline results for anti-HCV testing, which were considered positive. Although 60.5% of anti-HCV tests were performed in men, anti-HCV positivity was higher in women than that in men (women vs. men, 1.4% vs. 1.0%). Anti-HCV positives were generally older than negatives (age: mean ± SD, 59.4 ± 19.0 vs. 44.0 ± 18.9). The positivity rate consistently increased from age 21 to 80 years, before decreasing (Figure 1).
Figure 1.
The number of anti-HCV tests and the positivity rate according to the patient’s age.
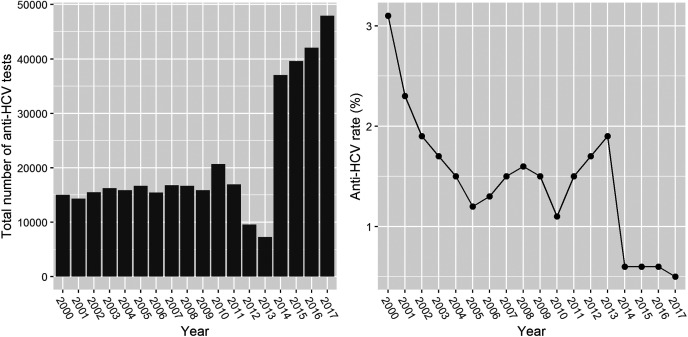
The average number of anti-HCV tests per year increased from 10 000 to 40 000 after 2015. The anti-HCV positivity rate decreased from 3.1% to 0.6% from 2000 to 2014 and subsequently stabilized at approximately 0.6% (Figure 2).
Figure 2.
The number of anti-HCV tests and positivity rate for each year of the study period.
The HCV RNA Test
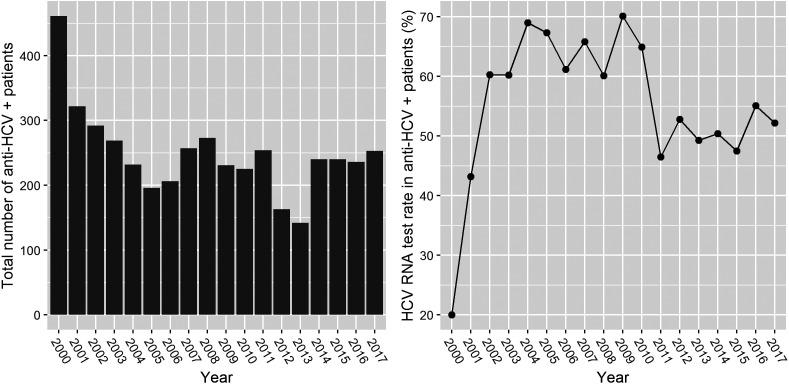
During the study, the HCV RNA testing rates among anti-HCV positives ranged from 20% to 70% (mean, 53.1%). After 2010, the HCV RNA testing rate stabilized at approximately 50% (Figure 3). The rates of HCV RNA positivity in men and women were 31.6% and 35.0%, respectively. The mean age of HCV RNA positives was higher than that of HCV RNA negatives (60.9 ± 17.5 vs. 48.6 ± 19.9).
Figure 3.
The number of HCV RNA tests and the rate of positivity in each year of the study period.
Table 1 summarizes the rates of anti-HCV positivity, HCV RNA testing, and HCV RNA positivity among anti-HCV positives according to the department requesting the tests. The department requesting the anti-HCV test was unknown in 9.3% of cases. The departments that most frequently requested anti-HCV tests were the blood bank (117 193 tests, 29.7%) and surgical departments (74 319 tests, 18.8%), while the dentistry department requested the test only 19 times in 18 years. The number of anti-HCV tests requested by emergency departments was higher than that of intensive care units, probably due to the referral of needle punctures to emergency departments. The anti-HCV positivity rate was highest in the gastroenterology outpatient clinic (8.0%) and lowest in the blood bank (2.0%). As expected, anti-HCV positivity was lower in pediatric than that in adult departments. The rate of HCV RNA testing among anti-HCV-positive patients was very low for all departments at, generally, less than 50%. The highest rate of HCV RNA testing was observed in the gastroenterology outpatient clinic (87.0%), and the lowest rate was observed in the blood bank (0.0%), the latter of which is probably biased due to the referral of individuals to other departments. Additionally, high HCV RNA test positivity was observed among tested individuals (85.9%), which was independent of the requesting department.
Table 1.
Anti-HCV and HCV RNA Positivity Rates According to the Department Requesting the Tests
| Anti-HCV Tests | HCV RNA Tests | |||
|---|---|---|---|---|
| Number of Tests | Positivity Rate (%) | Testing Rate Among Anti-HCV Positives (%) | Positivity Rate (%) | |
| Emergency Departments | ||||
| Pediatrics | 2059 | 0.2 | 25 | 100 |
| Adult | 8702 | 2.3 | 68 | 91 |
| Intensive care units | ||||
| Pediatrics | 997 | 0.4 | 50 | 100 |
| Adult | 931 | 2.1 | 45 | 100 |
| Surgery | 929 | 1.2 | 27 | 100 |
| Coronary | 759 | 1.6 | 17 | 100 |
| Outpatient clinics | ||||
| Pediatrics | 10 690 | 0.9 | 78 | 100 |
| Gastroenterology | 16 500 | 8.0 | 87 | 86 |
| Internal medicine | 52 056 | 1.4 | 53 | 100 |
| Adult other than the internal medicine | 5942 | 0.9 | 26 | 89 |
| Surgery | 74 319 | 0.6 | 20 | 100 |
| Dentistry | 19 | 0.0 | NA | NA |
| Anesthesiology | 671 | 0.9 | 33 | 100 |
| Blood bank | 117 193 | 0.2 | 0 | NA |
| Services | ||||
| Pediatrics | 2553 | 0.2 | 33 | 50 |
| Pediatric surgery | 4216 | 0.2 | 20 | 100 |
| Internal medicine | 5274 | 3.1 | 58 | 100 |
| Adult services other than the internal medicine | 12 600 | 0.8 | 21 | 91 |
| Surgery | 25 156 | 1.3 | 12 | 95 |
| Family medicine | 5868 | 0.3 | 16 | 100 |
| Other campus (Beytepe Campus) | 1867 | 0.4 | 29 | 100 |
| Unknown | 36 742 | 1.7 | 45 | 83 |
NA, not applicable.
The HCV Genotyping Test
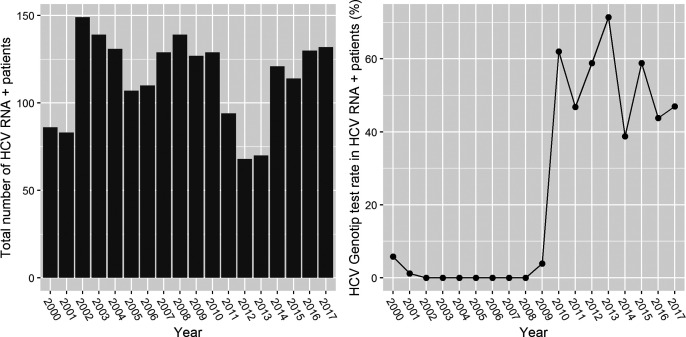
After the exclusion of repetitive tests among 503 HCV genotype results, there were 469 patients who underwent HCV genotype testing. HCV genotype testing did not begin until 2010. After this point, the rate of HCV genotyping among HCV RNA positives ranged from 40% to 70% in different departments, with an average of 53% (Figure 4). The major genotype identified was genotype 1 (85.7%). The percentages for all genotypes were as follows: genotype 1, 2.1%; genotype 1a, 8.4%; genotype 1b, 72.9%; genotype 1a and b, 2.3%; genotype 2, 6.6%; genotype 3, 4.9%; and genotype 4, 2.8%.
Figure 4.
The number of HCV genotyping tests and the rates of positivity for each year of the study period.
DISCUSSION
This is the first study to examine HCV awareness in Turkey over a number of years and across different medical departments. The anti-HCV positivity rate in our tertiary center decreased from 3.1% to 0.6% between 2000 and 2014 and subsequently stabilized at 0.6% from 2014 to 2017. The most frequently identified genotype was genotype 1b (72.9%). Under an ideal CHC awareness state, all anti-HCV positives should undergo RNA testing, followed by genotyping if their RNA result is positive. However, even in our referral center, the combined rate of RNA and genotype testing was only approximately 50% during the last 10 years. Therefore, this study revealed that, Turkey is a low endemic country for HCV infection, however the difficulty in detecting patients with active CHC is the major barrier to achieving WHO’s target for HCV elimination by 2030.
Early detection and treatment of patients with CHC is the best strategy to prevent liver-related morbidity and mortality and to globally eliminate HCV by preventing the transmission of infection. However, according to the 2015 global data from the WHO, only 20% of HCV-infected people worldwide are aware of their infection.4 The National Health and Nutrition Examination Survey (NHANES) revealed a lack of HCV infection awareness among both the public and doctors. The NHANES evaluated the HCV tests of 30 140 participants from 2001 to 2008. They found that 1.3% of participants were anti-HCV positive, and, of these, 43% (170/393) completed a follow-up survey and interview. The awareness of HCV positivity before the NHANES was only 49.7%, of whom only 3.7% were diagnosed by their doctor following the screening of the high-risk groups.13 Therefore, the United States has recommended the improvement of HCV screening and treatment strategies in the following groups of populations: (1) those not engaged in routine health care (through primary care services and high-risk group screening, education, and media campaigns for HCV awareness), (2) those engaged in routine health care with unknown HCV status (through routine screening for patients born between 1945 and 1965, educating patients and clinicians, proactively identifying cases through health records), and (3) those engaged in routine health care with untreated CHC (through enhancing referral networks, improving access through non-specialty-based treatment, and proactively identifying cases through health records).14 Awareness of HCV and hepatitis B virus (HBV) was prospectively evaluated among 1125 patients undergoing elective outpatient endoscopy at a large, urban safety-net hospital.15 Although 66.5% were at a high risk of CHC, only 30.9% had received prior testing. Among those patients who underwent HCV screening, CHC was present in 14.7%, but only 29.3% of these patients were aware of their test results. The authors concluded that improving hepatitis screening and patient awareness of their own test results are particularly important in urban hospitals.
A national seroprevalence survey is neither a feasible method nor a cost-effective one to detect patients with CHC in Turkey, which is a middle-income country with low HCV prevalence. On the contrary, micro-elimination programs targeting those populations at high risk of transmitting HCV, and based on the concept of treatment as prevention, may be effective.16 That is, the successful “treatment” of a CHC patient eliminates the possibility of further transmission, thereby achieving “prevention.” The Turkish Ministry of Health recommends screening for all patients who received blood products or organ transplantation before 1996; were born to an HCV-positive mother; inject drugs; have sexual contact with a man or multiple partners; have a history of frequent transfusions, long-term dialysis, dental treatment, tattoos, piercings, or bulk circumcision; have been incarcerated in a prison, nursery, nursing home, and military ward; have family members with CHC; have an occupational risk, such as health workers, barbers, hairdressers, and sex workers; have coinfection with hepatitis B or human immunodeficiency virus (HIV); and are immunosuppressed.8 Tertiary hospitals are important centers for proactive detection and treatment of CHC in Turkey. Ward et al. proposed the facilitation of HCV testing by reflex RNA testing of specimens that are positive for HCV antibodies to improve the detection of HCV-infected individuals.17 The first HCV awareness parameter for this study was the rate of HCV RNA testing among anti-HCV-positive patients. This has been approximately 50% for the last 5 years and was less than 50% for most medical departments. This persistently low rate of HCV awareness in our center reveals an urgent need for education programs directed at physicians. The primary strategies to achieve HCV elimination in Turkey should be the following: raising the awareness of HCV screening among the public and family physicians, educating doctors on the successful treatments available for HCV, and establishing a referral system to treat detected HCV cases.
McLeod et al. examined the effects of Scotland’s Action Plan on Hepatitis C, which included awareness-raising campaigns of HCV testing for general practitioners (GPs).18 The GP response rate for the surveys in 2007 and 2013 was approximately 60%, but only 25% of responders had undertaken an HCV-related professional development program. Although several national and local activities were conducted for HCV awareness between 2008 and 2011, improvements in testing practice were limited to patients with abnormal liver function tests and a history of medical or dental treatment. The most frequent barriers perceived by GPs for a lack of HCV testing were the inability to identify patients at risk of HCV infection and short consultation times. The study found that an electronic reminder directed at GPs could be used to overcome “poor awareness” and a “limited knowledge of testing protocols.” Therefore, it was suggested that educational activities targeted to GPs and physicians, rather than the public, should be considered to diagnose a greater majority of the HCV-infected population. Similarly, Reipold et al. conducted a “values and preferences according to WHO testing guidelines recommendations” survey in different countries, including Turkey.19 They found that HCV testing programs are inadequate and largely led by doctors rather than lay or unskilled health workers. The survey identified public and healthcare worker education and awareness as future priorities. Since this current study also emphasizes the lack of HCV infection awareness among physicians in Turkey, we believe that the development of education programs and the use of electronic reminders directed at physicians are urgently required.
In 2014, the International Committee on Taxonomy of Viruses validated that HCV has seven genotypes and 67 subtypes, which means it has greater genetic diversity than HBV and HIV.20 Genotypes 1 and 3 are widespread globally (44% and 25%, respectively), whereas genotype 4 (15%) is endemic to the Middle East and Africa, and genotypes 5 and 6 are most commonly reported in South Africa and Southeast Asia, respectively, while the recently described genotype 7 appears to be confined to Central Africa.5 The second HCV awareness parameter of our study was the rate of HCV genotype testing among HCV RNA-positive patients. HCV genotyping was not performed until 2010, after which rates of HCV genotyping among RNA positives ranged from 40% to 70%. According to estimates, the prevalence of HCV viremia in Turkey is 0.6% (0.3%-1.0%), corresponding to 492 000 (271 000-763 000) patients. Previous reports from Turkey have revealed the distribution of genotypes 1, 2, 3, 4, 5, and 6 as 83.3% (1a, 12.9%; 1b, 80.4%; and 1c, 0%), 1.5%, 3.7%, 1.5%, 0%, and 0%, respectively.4 The HCV genotype distribution in this study was consistent with this finding. The major genotype identified was genotype 1 (85.7%), followed by genotype 2 (6.6%), genotype 3 (4.9%), and genotype 4 (2.8%). Quasi-species (genotype 1a and b) were detected in 2.3% of patients, whereas genotypes 5, 6, and 7 were not detected in our center.
The long study period and a large number of the tests allowed us to analyze trend changes in HCV testing habits and the rate of CHC in our tertiary referral hospital, whose results can be generalized to the general Turkish population. However, there were several key limitations to this study. As the data were collected retrospectively, the incidence of missing data and wrong entries was considered a major source of bias. It is possible that some positive patients, that is, those detected at the blood bank, were referred to and treated at other centers. We were also not able to contact the patients to ask questions regarding their awareness of their test results or disease outcome.
This large, retrospective cohort study based on a single center, tertiary hospital data from 2000 to 2017 reveals that the prevalence of HCV infection has decreased (0.6%) in Turkey. However, physician awareness of HCV testing has stabilized at approximately 50% during the last 10 years. Therefore, educational and electronic warning programs for screening that are directed at doctors need to be established to achieve the WHO’s HCV elimination target in Turkey by 2030.
Funding Statement
The authors declare that this study has received no financial support.
Footnotes
Ethics Committee Approval: N/A.
Informed Consent: N/A.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - H.Y.B., A.A., N.T., C.V., M.A.G., T.P., G.H., H.D., B.S., H.Ş.; Design - H.Y.B., A.A., N.T., C.V., M.A.G., T.P., G.H., H.D., B.S., H.Ş.; Supervision - H.Y.B.; Materials - H.Y.B. A.A.; Data Collection and/or Processing - H.Y.B., A.A., N.T., C.V., M.A.G., T.P., G.H., H.D., B.S., H.Ş.; Analysis and/or Interpretation - H.Y.B., O.D.; Literature Search - H.Y.B.; Writing - H.Y.B., O.D.; Critical Reviews - H.Y.B., O.D., N.T., C.V., M.A.G., T.P., G.H., H.D., B.S., H.Ş.
Acknowledgments: All authors sincerely acknowledge and thank the Hacettepe Teknokent Technology Transfer Center, R&D Consultancy Energy Health Environment Communication Industry, and Trade Joint Stock Company for the advanced editing service to this article.
Conflicts of Interest: The authors have no conflict of interest to declare.
References
- 1. . Maugh TH. Where is the hepatitis C virus? Science. 1980;210(4473):999–1000.. 10.1126/science.6776629) [DOI] [PubMed] [Google Scholar]
- 2. . Choo QL, Kuo G, Weiner AJ, Overby LR, Bradley DW, Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989;244(4902):359–362.. 10.1126/science.2523562) [DOI] [PubMed] [Google Scholar]
- 3. . Ray RB, Ray R. Hepatitis C virus manipulates humans as its favorite host for a long-term relationship. Hepatology. 2019;69(2):889–900.. 10.1002/hep.30214) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. . Global hepatitis programme WHO/HIV/2016.06. http://www.who.int/hepatitis. [Google Scholar]
- 5. .Polaris Observatory HCVC. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2017;2(3):161–176.. 10.1016/S2468-1253(16)30181-9) [DOI] [PubMed] [Google Scholar]
- 6. . Cooke GS, Andrieux-Meyer I, Applegate TL.et al. Accelerating the elimination of viral hepatitis: a Lancet Gastroenterology and Hepatology Commission. Lancet Gastroenterol Hepatol. 2019;4(2):135–184.. 10.1016/S2468-1253(18)30270-X) [DOI] [PubMed] [Google Scholar]
- 7. . Thomas DL. Global elimination of chronic hepatitis. N Engl J Med. 2019;380(21):2041–2050.. 10.1056/NEJMra1810477) [DOI] [PubMed] [Google Scholar]
- 8. . Türkiye Viral Hepatit Önleme ve Kontrol Programı (2018-2023); 2018. https://dosyaism.saglik.gov.tr/Eklenti/47672,turkiye-viral-hepatit-onleme-ve-kontrol-programidoc.doc?0. [Google Scholar]
- 9. . Akarca USDN, Altuglu I, Arslan A, Buyruk M, Gunsar F, Zeytinoglu A. Anti-HCV Screening before Surgical Procedures, to Protect the Surgical Team or to Detect New Treatable Patients. Pavia, Italy: 20th ESCV, European Society for Clinical Virology; 2017. [Google Scholar]
- 10. .IBM Corp. IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp. (computer program); 2011. [Google Scholar]
- 11. .R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2016. https://www.r-project.org/. [Google Scholar]
- 12. . Wickham H. ggplot2: Elegant Graphics for Data Analysis. New York: Springer; 2009. [Google Scholar]
- 13. . Denniston MM, Klevens RM, McQuillan GM, Jiles RB. Awareness of infection, knowledge of hepatitis C, and medical follow-up among individuals testing positive for hepatitis C: National Health and Nutrition Examination Survey 2001-2008. Hepatology. 2012;55(6):1652–1661.. 10.1002/hep.25556) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. . Kanwal F, Bacon BR, Beste LA.et al. Hepatitis C virus infection care pathway-a report from the American Gastroenterological Association Institute HCV Care Pathway Work Group. Gastroenterology. 2017;152(6):1588–1598.. 10.1053/j.gastro.2017.03.039) [DOI] [PubMed] [Google Scholar]
- 15. . Wong RJ, Campbell B, Liu B, Baden R, Bhuket T. Sub-optimal testing and awareness of HCV and HBV among high risk individuals at an underserved Safety-Net Hospital. J Commun Health. 2018;43(1):65–69.. 10.1007/s10900-017-0388-6) [DOI] [PubMed] [Google Scholar]
- 16. . Lazarus JV, Safreed-Harmon K, Thursz MR.et al. The micro-elimination approach to eliminating hepatitis C: strategic and operational considerations. Semin Liver Dis. 2018;38(3):181–192.. 10.1055/s-0038-1666841) [DOI] [PubMed] [Google Scholar]
- 17. . Ward JW, Hinman AR. What is needed to eliminate hepatitis B virus and hepatitis C virus as global health threats? Gastroenterology. 2019;156(2):297–310.. 10.1053/j.gastro.2018.10.048) [DOI] [PubMed] [Google Scholar]
- 18. . McLeod A, Cullen BL, Hutchinson SJ.et al. Limited impact of awareness-raising campaigns on hepatitis C testing practices among general practitioners. J Viral Hepat. 2017;24(11):944–954.. 10.1111/jvh.12724) [DOI] [PubMed] [Google Scholar]
- 19. . Reipold EI, Trianni A, Krakower D.et al. Values, preferences and current hepatitis B and C testing practices in low- and middle-income countries: results of a survey of end users and implementers. BMC Infect Dis. 2017;17(Suppl 1):702. 10.1186/s12879-017-2769-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. . Smith DB, Bukh J, Kuiken C.et al. Expanded classification of hepatitis C virus into 7 genotypes and 67 subtypes: updated criteria and genotype assignment web resource. Hepatology. 2014;59(1):318–327.. 10.1002/hep.26744) [DOI] [PMC free article] [PubMed] [Google Scholar]