Abstract
Background
The LGR5 and CD133 have been identified as cancer stem cells (CSCs) marker and prognostic marker in several cancers including gastric cancer. The purpose of the present study was to determine the association between co-expression of CSCs marker LGR5 and CD133 in patients with gastric cancer and their clinicopathological outcomes; to analyze the efficacy of co-expression of both markers in evaluating the prognosis of gastric cancer.
Methods:
LGR5 and CD133 expression were investigated in a total of 400 patients by using immunohistochemistry. Results were analyzed in association with patient characteristics outcomes. Overall survival was performed using Kaplan-Meier Curve analysis.
Results
LGR5 and CD133 were found positive in 219/400 (54.75%) and 251/400 (62.75%) respectively in gastric cancer tissues. Co-expression of LGR5 and CD 133 were significantly associated with poor clinicopathological outcomes, including lymphatic invasion, vascular invasion, higher pathological T stage, and higher TNM staging (stage IV) (P < .05). The overall survival of patients who were positive for LGR5 and CD133 had shorter than that of LGR5 and CD133-negative gastric cancer, especially in patients who were positive for both markers.
Conclusion
Our finding indicates that co-expression of LGR5 and CD133 could be used as a marker indicating poor prognosis, which can provide information for selected effective treatment and carried out of intensive follow-up in gastric cancer patients.
Keywords: Cancer stem cells, gastric cancer, LGR5, CD133
INTRODUCTION
Gastric cancer is the most common cancer arising from the lining of the stomach, which causes more than 723 000 deaths worldwide annually.1 Approximately 1 million cases are diagnosed with gastric cancer each year around the world, among which 70% occur in developing countries and more than 50% occurring in East Asia, particularly Japan and China.2 The etiological risk factor of gastric cancer were chronic inflammation induced by Helicobacter pylori infection, long-term stomach inflammation, family history of stomach cancer, diets high in salt and smoked foods, a low intake of fruits and vegetables, and smoking.3 Moreover, the alteration of multiple genetic and epigenetic are associated with the multi-process of gastric cancer development.4 Although advances in surgical techniques, chemotherapy, and radiotherapy have made great progress in recent years, the overall survival of patients with gastric cancer remains poor particularly in more advanced stages, with a 5-year survival rate of <30%.5 One of the most important reasons lies in the lack of early diagnostic and prognostic markers to predict patient outcomes in gastric cancer patients. Thus, there is a great need for the identification of prognostic markers, which could be beneficial in improving survival and reducing mortality rates for gastric cancer patients.
Cancer stem cells (CSCs) are a small subpopulation of cancer cells characterized by their ability to self-renewal and multi-lineage differentiation; resulting in the production of a heterogeneous of cells.6 Moreover, they are relatively resistant to conventional chemotherapy because of slow-cycling, lower proliferation, and over-expression of anti-apoptosis and DNA repair genes.7 Accumulated evidence has suggested that CSCs play an important role in the progression and prognosis of several cancers, including gastric cancer.8,9 Despite some of the CSC markers cluster of differentiation (CD) 44, CD133, leucine-rich repeat-containing G protein-coupled receptor 5 (LGR5), octamer-binding transcription factor 4 (OCT4), Nanog, and Sox2 have been described in gastric cancer,9-12 which CSCs marker could be used for predicting the prognosis in gastric cancer remains debated.
Leucine-rich repeat-containing G protein-coupled receptor 5 (LGR5), also known as G-protein coupled receptor 49 (GPR49), is a member of the Wnt signaling pathway. LGR5 promotes tumor initiation, proliferation, and invasion, and LGR5 is considered a better prognostic biomarker in various cancers including gastric cancer.13 Although several studies have described the relationship between expression of LGR5 and prognosis in gastric cancer, however, the association between LGR5 and clinicopathological outcomes of gastric cancer and its prognostic value is relatively unclear. CD133 (Human prominin-1, PROM1), a 5-transmembrane glycoprotein, and is primarily localized in membrane protrusions.14 CD133 alone, or in combination with other markers, has been used extensively as a candidate marker to identify CSCs and predict prognosis from a variety of cancers.15,16 Evidence shows that CD133 expression can be found in over half of human gastric cancers and in both diffuse and intestinal subtypes; additionally, positive CD133 expression is significantly associated with poor prognosis using immunohistochemistry.12 Here, based on current evidence, we conducted an immunohistochemical study to examine the association between co-expression of CSCs marker LGR5 and CD133 in patients with gastric cancer and their clinicopathological outcomes; to analyze the efficacy of combined expression of both markers in evaluating the prognosis of gastric cancer.
MATERIALS AND METHODS
Patients and Tissue Samples
In total, 400 patients with gastric cancer and underwent surgical resection at the Suranaree University of Technology Hospital, Surin Hospital, and Buriram Hospital were enrolled in the study from January 2012 until December 2016. The demographic data of all patients were recorded, and TNM (tumor-node-metastasis) staging was evaluated according to the American Joint Committee on Cancer (7th edition, 2010) for gastric cancer. All patients signed informed consent to participate in the study and the protocol was approved by the Ethics Committee for Research Involving Human Subjects, Suranaree University of Technology (EC-62-03). The study was conducted in accordance with the guidelines of good clinical practice and the Declaration of Helsinki.
Pathological Specimens
Tissues from each patient were fixed in 10% formalin and embedded in paraffin. Sections were cut into 4-µm and stained with hematoxylin and eosin (H&E) for routine histopathological determination, and a representative tissue block was chosen from each patient to perform immunohistochemical staining. The diagnosis, histological type, and grade of all tumor tissues were determined by 3 pathologists.
Immunohistochemistry
An immunohistochemistry study was performed with the avidin-biotin complex method (ABC; ThermoFisher, Rockford, IL, USA) for the detection of LGR5 and CD133 expression. Consecutive sections were deparaffinized using xylene and were rehydrated in a graded series of alcohol (100, 95, 80, and 70%) to water. The sections were unmasked by heating in a microwave oven at 500 watts for 5 min in a 10 mm citrate buffer (pH 6.0) and were incubated with 5% normal serum for 1 h at room temperature to block non-specific background staining. The slides were then incubated with monoclonal mouse LGR5 (1:100, clone DF1485; Santa Cruz Biotechnology, Inc. Dallas, TX, USA) or CD133 antibody (1:100, clone SMP 14; Santa Cruz Biotechnology, Inc.) overnight at 4°C in a moist chamber. Subsequently, the sections were washed 3 times in PBS and were incubated with the biotinylated goat anti-mouse secondary antibody (1 µg/mL) for 30 min, followed by incubation with ABC HRP-conjugated avidin-biotin-complex (ThermoFisher). The color was developed using an aminoethyl carbazole substrate solution (Life Technologies Corp, Carlsbad, CA, USA). Sections were finally counterstained with Mayer’s hematoxylin.
Immunohistochemistry Evaluation
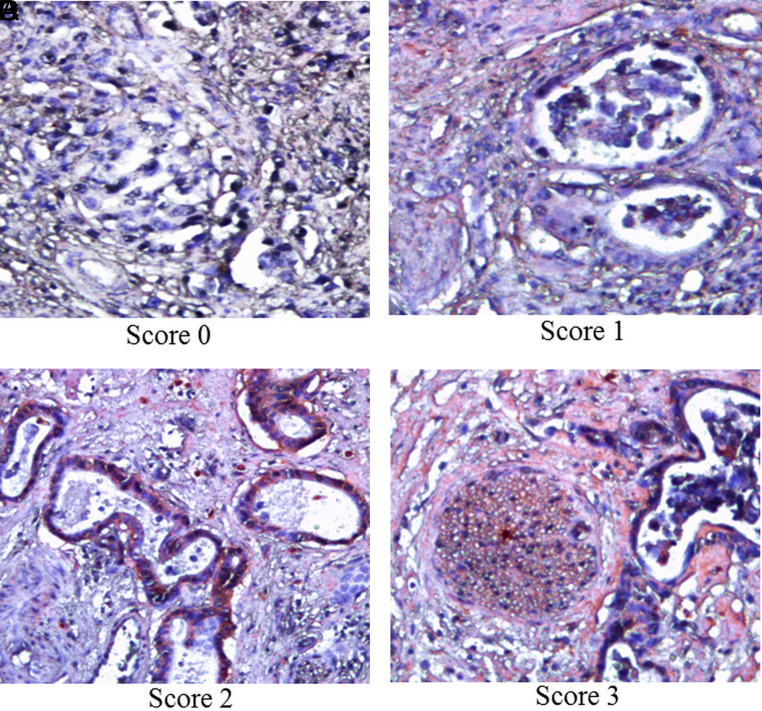
The assessment of LGR5 or CD133 expression was graded based on the percentage of positive cells; 500 cells per field for at least 5 different fields were counted under high magnification (×400). The following criteria were used for score: 0, lack of staining; 1+, 1, 10% staining; 2+, > 10 and ≤ 50% staining; and 3+, > 50% staining. LGR5 and CD133 were considered positive cases when scores were ≥1. Positive cells were graded independently by 3 pathologists, who were blinded to patient clinicopathological outcomes. The discrepancy between the pathologists’ analyses was minimal when it was present and was resolved by consensus.
Statistical Analysis
All statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) version 20 software (IBM Corp.; Armonk, NY, USA). The statistical significance of any associations between LGR5 and CD133 protein expression and the clinicopathological characteristics of gastric cancer were evaluated using χ2 and Fisher’s exact tests. To assess the prognostic index, we first used a univariate logistic regression model analysis. Significant parameters from the univariate analysis were then assessed in the final analysis by using a multivariate logistic regression model with a stepwise forward selection methodology. Overall survival (OS) was performed using the non-parametric Kaplan–Meier method, and comparisons between groups were performed using the log-rank test. A Cox proportional hazards model was used for univariate and multivariate analysis of prognostic values. A P < .05 with 95% CI was considered statistically significant.
RESULTS
Association Between Co-Expression of LGR5 and CD133 and Clinicopathological Outcomes of Gastric Cancer
To examine the clinical relevance of LGR5 and CD133 expression in gastric cancer, we performed immunohistochemical staining of the 400 gastric cancer specimens, 219/400 (54.75%) and 251/400 (62.75 %) of the patients were LGR5 and CD133-positive, respectively. These 2 markers have predominantly expressed both membrane and cytoplasm of the cancer cells (Figures 1 and 2). There were no significant differences between the samples that were positive or negative for LGR5 and CD133 expression for age, gender, or underlying conditions (Table 1). Taking into account the clinicopathological outcomes of TNM stage (American Joint Committee on Cancer), lymphatic invasion, vascular invasion, higher pathological T stage and higher TNM staging (stage IV) were significant associated with risk of patient positive for both LGR5 and CD133 staining (P < .05) (Table 2). However, no association was established between LGR5 and CD133 expression and other clinicopathological outcomes including the location of the tumor, tumor size, and histologic type (P > .05) (Table 2). Any variables that were significantly associated with LGR5 and CD133 expression in the univariate analysis were further analyzed by the multivariate logistic regression model. The results found that lymphatic invasion (OR = 4.92; 95% CI, 1.67-9.62; P = .017), vascular invasion (OR = 3.17; 95% CI, 1.52-7.85; P = .036), higher pathological T stage (OR = 3.79; 95% CI, 1.16-6.94; P = .031), and higher TNM staging (stage IV) (OR = 2.13; 95% CI, 1.24-5.06; P = .029) were significant associated with risk of patient positive for both LGR5 and CD133 staining (Table 3).
Figure 1.
Representative photomicrographs of immunohistochemistry for LGR5 scores of 0, 1, 2, and 3. The red staining indicates the presence of LGR5 protein in gastric cancer tissues (magnification, ×200).
Figure 2.
Representative photomicrographs of immunohistochemistry for CD133 scores of 0, 1, 2, and 3. The red staining indicates the presence of CD133 protein in gastric cancer tissues (magnification, ×200).
Table 1.
Patient’s Demographics Data Among Gastric Cancer Patients and LGR5, CD133 status
| Demographics Data | LGR5 Status | P | CD133 Status | P | ||
|---|---|---|---|---|---|---|
| LGR5 (−) | LGR5 (+) | CD133 (−) | CD133 (+) | |||
| Age (year ± SD) | 55.02 ± 9.42 | 57.12 ± 10.73 | .478 | 51.23 ± 10.32 | 53.22 ± 11.43 | .561 |
| Sex [male (%)] | 95 (52.48) | 102 (46.57) | .136 | 83 (55.70) | 121 (48.20) | .218 |
| Underlying condition [n (%)] | ||||||
| HT | 15 (8.28) | 17 (7.76) | .674 | 19 (12.75) | 22 (8.76) | .086 |
| DM | 16 (8.83) | 12 (5.47) | .248 | 19 (12.75) | 26 (10.35) | .368 |
| Hyperlipidemia | 12 (6.62) | 10 (4.56) | .095 | 10 (6.71) | 21 (8.36) | .089 |
| Smoking | 29 (16.02) | 31 (14.15) | .115 | 24 (16.10) | 35 (13.94) | .197 |
| Alcohol | 15 (8.28) | 15 (6.84) | .664 | 19 (12.75) | 20 (7.96) | .066 |
| Family history of gastric cancer | 9 (4.97) | 8 (3.65) | .865 | 5 (3.35) | 9 (3.58) | .976 |
HT, hypertension; DM, diabetes mellitus. Comparisons between the groups were done by using ANOVA. *P < .05 is considered statistically significant.
Table 2.
The Association of Co-expression of LGR5, CD133 status, and Clinicopathological Outcome of Gastric Cancer
| Clinicopathological Outcome | LGR5 and CD 133 Status (n = 400) | OR (95% CI) | P | |
|---|---|---|---|---|
| −/− or +/−, −/+ (n = 217) | +/+ (n = 183) | |||
| Location of tumor [n (%)] | ||||
| Upper | 28 (12.90) | 32 (17.48) | 0.75 (0.53-0.87) | .519 |
| Middle | 57 (26.27) | 45 (24.59) | 0.66 (0.41-0.84) | .622 |
| Lower | 132 (60.83) | 106 (57.92) | 0.74 (0.52-0.91) | .537 |
| Tumor size | ||||
| <70 mm | 162 (74.65) | 144 (78.68) | 0.64 (0.41-0.82) | .475 |
| ≥70 mm | 55 (25.35) | 39 (21.31) | 0.71 (0.51-0 .93) | .714 |
| Histologic type | ||||
| Differentiated | 169 (77.88) | 128 (69.95) | 0.84 (0.61-0.98) | .692 |
| Undifferentiated | 48 (22.12) | 55 (30.05) | 0.62 (0.49-0.94) | .764 |
| Lymphatic invasion | ||||
| Absent | 194 (89.40) | 69 (37.70) | 0.29 (0.16-0.88) | .026* |
| Present | 23 (10.60) | 114 (62.30) | 5.83 (2.28-8.74) | .015* |
| Vascular invasion | ||||
| Absent | 184 (84.80) | 74 (40.44) | 0.17 (0.18-0.64) | .039* |
| Present | 33 (15.20) | 109 (59.56) | 3.24 (1.54-6.92) | .025* |
| Pathological T stage | ||||
| T1-T2 | 174 (80.18) | 31 (16.94) | 5.85 (2.41-8.77) | .017* |
| T3-T4 | 43 (19.82) | 152 (83.06) | 4.72 (2.27-7.14) | .028* |
| Pathological TNM stage | ||||
| I | 34 (15.67) | 16 (8.74) | 0.95 (0.62-1.17) | .198 |
| II | 67 (30.88) | 29 (15.85) | 0.82 (0.51-1.04) | .136 |
| III | 78 (35.94) | 58 (31.70) | 0.62 (0.41-0.87) | .627 |
| IV | 38 (17.51) | 82 (44.81) | 2.36 (0.51-0.97) | .035* |
Univariate logistic regression model analysis. *P < .05 is considered statistically significant.
Table 3.
The Association of Co-expression of LGR5, CD133 Status, and Clinicopathological Outcome of Gastric Cancer
| Clinicopathological Outcome | LGR5 and CD 133 Status (n = 400) | OR (95% CI) | P | |
|---|---|---|---|---|
| −/− or +/−, −/+ | +/+ | |||
| Lymphatic invasion [n (%)] | ||||
| Absent | 194 (89.40) | 69 (37.70) | 0.31 (0.18-0.76) | .032* |
| Present | 23 (10.60) | 114 (62.30) | 4.92 (1.67-9.62) | .017* |
| Vascular invasion | ||||
| Absent | 184 (84.80) | 74 (40.44) | 0.23 (0.14-0.72) | .027* |
| Present | 33 (15.20) | 109 (59.56) | 3.17 (1.52-7.85) | .036* |
| Pathological T stage | ||||
| T1-T2 | 174 (80.18) | 31 (16.94) | 0.42 (0.26-0.82) | .021* |
| T3-T4 | 43 (19.82) | 152 (83.06) | 3.79 (1.16-6.94) | .031* |
| Pathological TNM stage | ||||
| IV | 38 (17.51) | 82 (44.81) | 2.13 (1.24-5.06) | .029* |
Multivariate logistic regression model analysis. *P < .05 is considered statistically significant.
Association Between LGR5 and CD133 Expression and Overall Survival of Gastric Cancer
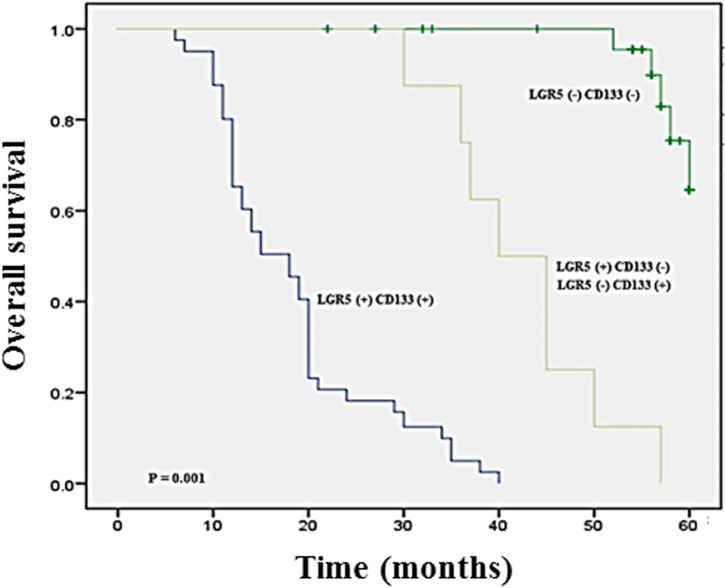
To further determine whether co-expression of LGR5 and CD133 is a prognostic marker for patients with gastric cancer, a survival analysis of overall survival was conducted. The Kaplan–Meier survival curve showed that the overall survival time of gastric cancer patients with LGR5 and CD133-positive expression were shorter than those with LGR5 and CD133-negative expression (median 51.37 months; mean 54.48 months), particularly in patient who has both LGR5 and CD133 expression (median 12.64 months; mean 14.78 months) (P = .001) (Figure 3). Then, we conducted a multivariate logistic regression model to determine the independent value of each variable to predict the overall survival for gastric cancer. The results showed that LGR5 expression (OR = 1.82; 95% CI, 1.26-4.07; P = .031), CD133 expression (OR = 2.36; 95% CI, 1.43-5.58; P = .017), and LGR5/CD133 expression (OR = 5.52; 95% CI, 2.86-8.37; P = .001) were statistically independent predictive prognosis factors for poor prognosis in gastric cancer.
Figure 3.
Kaplan–Meier survival curves of overall survival in patients with gastric cancer according to LGR5, CD133, and LGR5/CD133 expression. Combined expression of LGR5 and CD133 (blue line) showed significantly worse overall survival time than those with negative expression (green line; P = 0.001).
DISCUSSION
Although there has been progress in gastric cancer and the incidence of gastric cancer worldwide reduced, it still remains a substantial life-threatening malignant tumor in humans, characterized by uncontrolled growth and poor prognosis. There are various gastric cancer molecular biomarkers have been identified in recent years. However, the classical tumor markers carbohydrate antigen (CA19-9), carcinoembryonic antigen (CEA), CA72-4, and alpha-fetoprotein (AFP) are routinely used in clinical practice. Despite widely used, they are not ideal markers because the sensitivity and specificity in the diagnosis and prognosis of gastric cancer were low (20 –30%).17 Therefore, it is of great importance to find out more effective biomarkers for gastric cancer in order to have a personalized treatment approach and helpful for predicting and improving outcome in gastric cancer patients. This is the first report to evaluate the association between co-expression of LGR5 and CD133 protein in gastric cancer patients and their clinicopathological outcomes. Our results revealed an association between co-expression of LGR5 and CD133 positivity and clinicopathological outcomes, such as lymphatic invasion, vascular invasion, higher pathological T stage, and higher pathological TMN stage in gastric cancer patients. Furthermore, the CSCs marker expression was found to be associated with overall survival particularly in co-expression of LGR5 and CD133 in gastric cancer.
Over the last decade, CSCs were characterized as the origin of several cancers. CSCs are a subpopulation of cancer cells and are capable of self-renewal, asymmetric division postulated, and differentiation properties that contribute to the cancer initiation, progression, metastasis, disease recurrence, and therapeutic resistance. Evidence has indicated that gastric cancer development is closely related to gastric CSCs (GCSCs).18 LGR5 and CD133 have been identified as CSC surface marker, and could be used as a prognostic marker of multiple types of cancer, including gastric cancer.11-13 In this study, we found 54.75 % (219/400) and 62.75 % (251/400) of cases were positive for LGR5 and CD133, respectively, and stained cells of these markers were frequently founded in a wide sphere of tumor lesions. LGR5 serves as a driving factor in the development and the maintenance of adult stem cells in the gastrointestinal tract. Previously, it was demonstrated that LGR5+ pyloric stem cells are an origin of cells that can promote chemotherapy resistance, cell proliferation, and drive gastric cancer through the Wnt-signaling pathway.13,19,20 Moreover, the number of pseudopodia (lamellipodia and filopodia) of gastric cancer cells was increased by the regulation of LGR5, which resulted in increased cell motility, thereby promoting cell invasion and migration.13 Here, we also found that LGR5 was significantly associated with lymphatic invasion, vascular invasion, higher pathological T stage, and higher pathological TMN stage. This finding supports the role of LGR5 in the progression of tumorigenicity and invasive capabilities, making it may a potential marker of gastric cancer. CD133 has been recognized as a prognostic indicator or predictor of response to therapy in a variety of cancers; most studies indicate that CD133 expression is associated with poor clinical outcome.15,16 Despite there being many studies of the CD133 in several cancers, the associated literature contains conflicting and contradictory results. CD133 is an independent prognostic marker in lung cancer and extrahepatic bile duct and gallbladder cancer.21,22 In contrast, Immervoll et al.23 and Salnikov et al.24 revealed that CD133 expression not correlated with patient survival of non-small cell lung cancer (NSCLC) and pancreatic ductal adenocarcinomas, respectively. In this study, we demonstrated that CD133 expressionwas associated with higher pathological T stage and higher pathological TMN stage. These results are consistent with previous reports that CD133 expression is significantly upregulated in gastric cancer and patients with advanced TMN stage have worse clinical behavior.25 Moreover, we found that CD133-positive was statistically significant with lymphatic invasion and vascular invasion. It has been documented that CD133 interacts with multiple signaling pathways related to invasion and metastasis capabilities of various cancers, which mediate epithelial-to-mesenchymal transition through the activation of Wnt signaling, ERK, and NF-κB (nuclear factor κB).26,27 Furthermore, CD133 is able to induce several MMPs expression, suggesting a role in cancer metastasis.28,29 Taken together, CD133 may be used as a potential marker for predict prognosis in gastric cancer.
Our study demonstrated that co-expression of LGR5 and CD133 could be an important predictive marker for poor prognosis, which is associated with lymphatic invasion, vascular invasion, higher pathological T stage, higher pathological TMN stage in gastric cancer patients. Furthermore, the overall survival of patients who were positive for both LGR5 and CD133 was significantly shorter than those with single LGR5 or CD133 expression. Together, these findings support the idea that combined detection of tumor markers better than an individual, when utilized for screening, diagnosis, and follow-up of gastric cancer.30 Although this study was the multi-center from the 3 hospital centers, however, further studies in more patients are required. In addition, selection bias may occur in the study; most patients present with locally advance and advanced disease because specific amounts of tissues were obtained from surgical resection from 3 hospital centers.
In conclusion, the results of this study show the association between co-expression of LGR5 and CD133 and clinicopathological outcomes, such as lymphatic invasion, vascular invasion, higher pathological T stage, and higher pathological TMN stage in gastric cancer patients. Additionally, co-expression of these markers was found to be associated with overall survival. Our finding indicates that combined detection of LGR5 and CD133 positivity in gastric cancer patients could be more effective to select the patients with a worse prognosis.
Funding Statement
This research was supported by a grant from the Suranaree University of Technology Research and Development Fund.
Footnotes
Ethics Committee Approval: Ethical approval was granted by the Ethics Committee for Research Involving Human Subjects, Suranaree University of Technology (EC-62-03).
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – T.T., W.W.; Design – T.T., W.W., T.S.B.; Supervision – T.T.; Resources – T.T.; Materials – T.T.; Data Collection and/or Processing – T.T., W.W., T.S.B.; Analysis and/or Interpretation – T.T., W.W.; Literature Search – W.W.; Writing Manuscript – W.W., T.T.; Critical Review - T.T., W.W., T.S.B.
Conflict of Interest: The authors have no conflict of interest to declare.
References
- 1. . Yan L. The journey of personalizing gastric cancer treatment. Chin J Cancer. 2016;35(1):84. 10.1186/s40880-016-0149-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. . Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29.. 10.3322/caac.20138) [DOI] [PubMed] [Google Scholar]
- 3. . Ushijima T, Sasako M. Focus on gastric cancer. Cancer Cell. 2004;5(2):121–125.. 10.1016/s1535-6108(04)00033-9) [DOI] [PubMed] [Google Scholar]
- 4. . Fu DG. Epigenetic alterations in gastric cancer (Review). Mol Med Rep. 2015;12(3):3223–3230.. 10.3892/mmr.2015.3816) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. . Thun MJ, DeLancey JO, Center MM, Jemal A, Ward EM. The global burden of cancer: priorities for prevention. Carcinogenesis. 2010;31(1):100–110.. 10.1093/carcin/bgp263) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. . Nguyen LV, Vanner R, Dirks P, Eaves CJ. Cancer stem cells: an evolving concept. Nat Rev Cancer. 2012;12(2):133–143.. 10.1038/nrc3184) [DOI] [PubMed] [Google Scholar]
- 7. . Deonarain MP, Kousparou CA, Epenetos AA. Antibodies targeting cancer stem cells: a new paradigm in immunotherapy? mAbs. 2009;1(1):12–25.. 10.4161/mabs.1.1.7347) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. . Major AG, Pitty LP, Farah CS. Cancer stem cell markers in head and neck squamous cell carcinoma. Stem Cells Int. 2013;2013:319489. 10.1155/2013/319489) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. . Takaishi S, Okumura T, Tu S.et al. Identification of gastric cancer stem cells using the cell surface marker CD44. Stem Cells Dayt Ohio. 2009;27(5):1006–1020.. 10.1002/stem.30) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. . Basati G, Mohammadpour H, Emami Razavi A. Association of high expression levels of SOX2, NANOG, and OCT4 in gastric cancer tumor tissues with progression and poor prognosis. J Gastrointest Cancer. 2020;51(1):41–47.. 10.1007/s12029-018-00200-x) [DOI] [PubMed] [Google Scholar]
- 11. . Xi HQ, Cai AZ, Wu XS.et al. Leucine-richrepeat-containing G-protein coupled receptor 5 is associated with invasion, metastasis, and could be a potential therapeutic target in human gastric cancer. Brit J Cancer. 2014;110(8):2011–2020.. 10.1038/bjc.2014.112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. . Zhao P, Li Y, Lu Y. Aberrant expression of CD133 protein correlates with Ki-67 expression and is a prognostic marker in gastric adenocarcinoma. BMC Cancer. 2010;10:218. 10.1186/1471-2407-10-218) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. . Wang X, Wang X, Liu Y.et al. LGR5 regulates gastric adenocarcinoma cell proliferation and invasion via activating Wnt signaling pathway. Oncogenesis. 2018;7(8):57. 10.1038/s41389-018-0071-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. . Shmelkov SV, St.Clair R, Lyden D, Rafii S. AC133/CD133/Prominin-1. Int J Biochem Cell Biol. 2005;37(4):715–719.. 10.1016/j.biocel.2004.08.010) [DOI] [PubMed] [Google Scholar]
- 15. . Ishigami S, Ueno S, Arigami T.et al. Prognostic impact of CD133 expression in gastric carcinoma. Anticancer Res. 2010;30(6):2453–2457.. [PubMed] [Google Scholar]
- 16. . Sasaki A, Kamiyama T, Yokoo H.et al. Cytoplasmic expression of CD133 is an important risk factor for overall survival in hepatocellular carcinoma. Oncol Rep. 2010;24(2):537–546.. 10.3892/or_00000890) [DOI] [PubMed] [Google Scholar]
- 17. . Liu X, Cai H, Wang Y. Prognostic significance of tumor markers in T4 a gastric cancer. World J Surg Oncol. 2012;10:68. 10.1186/1477-7819-10-68) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. . Li K, Dan Z, Nie YQ. Gastric cancer stem cells in gastric carcinogenesis, progression, prevention and treatment. World J Gastroenterol. 2014;20(18):5420–5426.. 10.3748/wjg.v20.i18.5420) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. . Simon E, Petke D, Böger C.et al. The spatial distribution of LGR5+ cells correlates with gastric cancer progression. PLoS ONE. 2012;7(4):e35486. 10.1371/journal.pone.0035486) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. . Xi HQ, Cui JX, Shen WS.et al. Increased expression of Lgr5 is associated with chemotherapy resistance in human gastric cancer. Oncol Rep. 2014;32(1):181–188.. 10.3892/or.2014.3207) [DOI] [PubMed] [Google Scholar]
- 21. . Woo T, Okudela K, Mitsui H.et al. Prognostic value of CD133 expression in stage I lung adenocarcinomas. Int J Clin Exp Patho. 2010;4(1):32–42.. [PMC free article] [PubMed] [Google Scholar]
- 22. . Mizukami T, Kamachi H, Mitsuhashi T.et al. Cytoplasmic CD133 expression correlates with histologic differentiation and is a significant prognostic factor in extrahepatic bile duct cancer and gallbladder cancer. Oncol Lett. 2018;16(5):6423–6430.. 10.3892/ol.2018.9499) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. . Immervoll H, Hoem D, Sakariassen PØ, Steffensen OJ, Molven A. Expression of the “stem cell marker” CD133 in pancreas and pancreatic ductal adenocarcinomas. BMC Cancer. 2008;8:48. 10.1186/1471-2407-8-48) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. . Salnikov AV, Gladkich J, Moldenhauer G, Volm M, Mattern J, Herr I. CD133 is indicative for a resistance phenotype but does not represent a prognostic marker for survival of non-small cell lung cancer patients. Int J Cancer. 2010;126(4):950–958.. 10.1002/ijc.24822) [DOI] [PubMed] [Google Scholar]
- 25. . Ilhan E, Ureyen O, Meral UM. Ongoing problems concerning 7th TNM staging system and proposals for 8th TNM staging system of gastric cancer. Prz Gastroenterol. 2016;11(4):223–225.. 10.5114/pg.2016.64069) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. . Ding Q, Miyazaki Y, Tsukasa K, Matsubara S, Yoshimitsu M, Takao S. CD133 facilitates epithelial-mesenchymal transition through interaction with the ERK pathway in pancreatic cancer metastasis. Mol Cancer. 2014;13:15. 10.1186/1476-4598-13-15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. . Rappa G, Mercapide J, Anzanello F.et al. Wnt interaction and extracellular release of prominin-1/CD133 in human malignant melanoma cells. Exp Cell Res. 2013;319(6):810–819.. 10.1016/j.yexcr.2013.01.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. . Kohga K, Tatsumi T, Takehara T.et al. Expression of CD133 confers malignant potential by regulating metalloproteinases in human hepatocellular carcinoma. Hepatol. 2010;52(6):872–879.. 10.1016/j.jhep.2009.12.030) [DOI] [PubMed] [Google Scholar]
- 29. . Gao Y, Feng J, Wu L, Zhan S, Sun J. Expression and pathological mechanism of MMP-9 and HIF-2α in CD133(+) lung cancer stem cells. Europe PMC. 2015;95:2607–2611.. [PubMed] [Google Scholar]
- 30. . Chen XZ, Zhang WK, Yang K.et al. Correlation between serum CA724 and gastric cancer: multiple analyses based on Chinese population. Mol Biol Rep. 2012;39(9):9031–9039.. 10.1007/s11033-012-1774-x) [DOI] [PubMed] [Google Scholar]