Abstract
Background
Acute pancreatitis (AP) is a widespread disease resulting from the inflammation of acinar cells in the pancreas. β-hydroxybutyrate (BHB) is a water-soluble main ketone body synthesized in the human liver. The purpose of this study was to examine the possible therapeutic effects of BHB in the experimentally-induced AP model in rats.
Methods
In our study, male rats were randomly allotted into 6 groups, as control (0.9% saline i.p.), BHB1 (200 mg/kg BHB i.p.), BHB2 (2 doses of 200 mg/kg BHB i.p.), AP (4 doses of 50 µg/kg cerulein i.p., 4 doses at 1 h intervals), AP+BHB1 and AP+BHB2 groups. In pancreatic tissue sections, immunohistochemistry staining and western blot analysis for the inflammasome complex (caspase-1, ASC, and NLRP3) and inflammation-associated proteins (TNF-α and NF-κB) and a histopathological examination were performed. The levels of lipase, amylase, interleukin (IL)-18 and IL-1β in serum were measured.
Results
Several pathological degenerations, including edema, inflammatory cell infiltration, acinus necrosis, and bleeding were observed in the AP group, while the histological architecture of the control and the sham BHB1 and BHB2 groups were regular. The AP-induced pathological changes were considerably alleviated in the AP+BHB1 and AP+BHB2 groups. In the AP group, a conspicuous increase in caspase-1, ASC, NLRP3, TNF-α, and NF-κB proteins, and in the levels of amylase, lipase, IL-18, and IL-1β were detected. BHB treatments after AP induction decreased those proteins to the level of control.
Conclusions
We demonstrated that BHB has the potential to cure AP by suppressing the NLRP3 inflammasome and can be used in the treatment of many diseases which progress through the NLRP3 inflammasome.
Keywords: Acute pancreatitis, NLRP3 inflammasome, β-hydroxybutyrate, cerulein
Introduction
Acute pancreatitis is an acute inflammation of the pancreas and is based on the inflammation occurring in the acinar cells.1 Pancreatic damage exacerbates as the inflammation progresses. In a couple of hours after the onset of pancreatic damage, there is a surge in the serum concentrations of pancreatic amylase and lipase enzymes, which assist the digestion of nutrients. Since lipase remains at higher levels in the blood than amylase does, it gives more accurate information in the diagnosis of AP.2 In the etiology of AP, there are many causes such as gallstones blocking the pancreatic duct and obscuring fluid drainage, alcohol, hypertriglyceridemia, pancreatic injury during endoscopic retrograde cholangio-pancreatography, hypercalcemia, abdominal trauma, various infections, carcinomas of pancreas head, autoimmune causes, and ischemia of the pancreas, along with hereditary causes.3 AP is initiated via the conversion of trypsinogen into active trypsin in the acinar cells. The trypsin activates several destructive enzymes (amylase, lipase, elastase, phospholipase A2, etc.), leading to the degradation of biomolecules and the internal digestion of cellular components. The homeostatic imbalance in the tissue with disrupted and impaired cells elevates inflammation, by increasing proinflammatory cytokine synthesis by the local macrophages IL-1, IL-6, IL-8, and tumor necrosis factor alpha (TNF-α), and the subsequent diapedesis of immunomodulatory cells such as macrophages, neutrophils, and lymphocytes to the inflammatory site.4 Recently, the importance of the NOD-like receptor family pyrin domain-containing-3 (NLRP3) inflammasome, which is an intracellular multiprotein complex, has been emphasized in the pathogenesis of AP.5
Inflammation can also be caused by ischemia–reperfusion, trauma, and chemical agents, apart from microorganisms, which is defined as sterile inflammation. Sterile inflammation underlies many diseases, including chronic obstructive pulmonary disease, silicosis, obesity, gout, pseudogout, arthritis, Type 2 diabetes, myocardial infarction, Alzheimer’s disease, and AP.6 There are receptors in the immune system cells for recognizing microorganisms and the molecules of damaged cells. Among them are Toll-like Receptors, nucleotide-binding domain leucine-rich repeat-containing receptors (NLRs), RIG-I-like RNA helicases, and C-type lectin receptors.7 The stimulation of any of these receptors triggers the formation of the inflammasome complex, from which sterile inflammation originates. NLRs are the receptors located in cytoplasm. In the molecular structure of the NLR protein family, there is an N-terminal effector domain, a central nucleotide-binding domain, and a variable number of C-terminal leucine-rich repeats. The NLRP3 inflammasome complex forms with the stimulation of NLRP3, a member of the NLR family. The NLRP3 inflammasome consists of NLRP3, ASC, and pro-caspase 1 proteins. With NLRP3 activation, pro-caspase 1 converts into caspase 1, which leads to the release of proinflammatory cytokines like IL-1α, IL-1β and IL-18, causing hyperinflammation.8
β-Hydroxybutyrate (3-hydroxybutanoic acid, BHB) is a water-soluble main ketone body found in humans. It is formed by the oxidation of fatty acids in the mitochondria of hepatocytes in the liver and is released into the blood. It is an alternative source of energy for the brain, heart, and skeletal muscles in low-carbohydrate diets and nutritional deficiencies.9 An increase in BHB levels causes the cellular glycolysis rate to diminish and the mitochondria to produce less ROS through complex II.10 BHB, the NLRP3 inflammation inhibitor, reduces the activity of caspase-1 in bone marrow-derived macrophages treated with NLRP3 activators. In addition, BHB also inhibits the maturation process of IL-1β.11
The purpose of this study is to examine the effects of BHB, which has been shown to experimentally inhibit sterile inflammation through the NLRP3 inflammasome, in the experimental model of AP, which is a widespread disease and becomes chronic if not treated, progresses over the NLRP3 inflammasome, and eventually leads to multiple organ failure and death.
Materials and Methods
Animals and Experimental Design
In general, acute pancreatitis affects males more often than females. Moreover, since we did not want our study to be affected by the hormonal changes in females, this study was performed in male (8- to 10-week-old, 280-300 g Wistar albino rats). The rats were kept in Eskisehir Osmangazi University, Medical and Surgical Experimental Application and Research Center, Turkey. All experimental procedures were carried out in accordance with the guidelines issued by the Local Animal Ethics Committee, of Eskisehir Osmangazi University, Turkey (approval date/number: November 12, 2017/627-2). All rats were housed under a 12-hour light/12-hour dark cycle in a temperature- (21-24°C) and humidity- (50-60%) controlled room. The rats were adapted to the environment for 2 weeks before the experiment. They were fed with standard diet and water adlibitum. They were randomly divided into 6 groups (n = 7) as follows;
Control group
They were fed with standard diet and drinking water. Only 0.9% saline solution was given.
Sham group 1 (BHB1)
A single dose of BHB (200 mg/kg) dissolved in phosphate buffer (PBS) was administered intraperitoneally (i.p.).12 After 12 hours, the rats were euthanized.
Sham group 2 (BHB2)
Two doses of BHB (200 mg/kg) were administered intraperitoneally at 12-hour intervals. The rats were euthanized 12 hours after the last administration of BHB.
AP group
For AP induction, the rats were fasted for 12 hours, and cerulein (50 µg/kg) dissolved in 0.9% saline was administered as 4 doses at 1-hour intervals i.p.13 The rats were euthanized 12 hours after the last administration of cerulein.
AP+BHB1
After AP induction, a single dose of BHB (200 mg/kg) dissolved in PBS was administered. After 12 hours, rats were euthanized.
AP+BHB2
After AP induction, 2 doses of BHB (200 mg/kg) dissolved in PBS were administered at 12-hour intervals. The rats were euthanized 12 hours after the last administration of BHB.
At the end of the experiment, all groups were anesthetized with a mixture of 2.5% ketamine hydrochloride (Ketalar, New York, USA) and 2% xylazine (Rompun, Bayer, Leverkusen, Germany), then euthanized. Blood samples were centrifuged at 1000 rpm for 15 minutes. Serum samples were stored at −80°C in tubes until analysis day. The abdominal area of the rats was opened along the midline and the pancreases were excised and photographed with a digital camera. Each pancreas sample was cut into transverse pieces. One part of the pancreas was stored at −80°C for western blot studies and the other part was placed in 10% neutral formaldehyde for histochemical studies.
Tissue Preparation and Histopathological Scoring
Tissue pieces excised for light microscopic examination were fixed for 24 hours in 10% neutral formaldehyde solution. They were then subjected to a routine histological process to form paraffin blocks. Further, 5-µm thick sections taken from prepared paraffin blocks were stained with hematoxylin–eosin (H&E).
The prepared samples were examined by 2 histologists (blinded) in terms of edema, inflammatory cell infiltration, and acinus necrosis with a photomicroscope (Olympus DP70 with digital camera) according to the scores provided in Table 1.14
Table 1.
Histological Scoring for Acute Pancreatitis
| Condition | Score | Indication |
|---|---|---|
| Edema | 0 | Absent |
| 1 | Focally increased between lobules | |
| 2 | Dıffusely increased between lobules | |
| 3 | Acini disrupted and separated | |
| Inflammatory cell infiltrate | 0 | Absent |
| 1 | In ducts (around ductal margins) | |
| 2 | In the parenchyma (in <50% of the lobules) | |
| 3 | In the parenchyma (in >50% of the lobules) | |
| Acinar necrosis | 0 | Absent |
| 1 | Periductal necrosis (≤ 5%)a | |
| 2 | Focal necrosis (5-20%) | |
| 3 | Diffuse parenchymal necrosis (20-50%) |
aReferring to the approximate percentage of cells involved per examined field.
Immunohistochemical Examination
Routine histological procedures (fixation, washing, tissue processing, and blocking) were applied to the 4-μm thick sections cut from paraffin blocks. For deparaffinization, the sections were put in 2 different xylene solutions for 10 minutes each. After that, sections were placed in ethyl alcohol at a descending series of 100%, 96%, and 80% for 5 minutes each. Next, the sections were put in distilled water for 5 minutes. The antigen retrieval procedure was done in 10% citrate buffer for 5 minutes by using a pressure cooker. To block the endogenous peroxidase activity in the tissues, the slides were kept in 3% hydrogen peroxide for 10 minutes. After the blocking step, the slides were incubated with NLRP3, caspase 1, ASC, NF-KB, and TNF-α (sc-66846, sc-56036, sc-514414, sc-8414 and sc-52746, Santa Cruz, USA) antibodies at +4°C overnight. The following day, the slides were washed with PBS, and biotinylated secondary antibody (Thermo Scientific, TP-125-HL, USA) was added to the slides for 10 minutes. After washing with PBS, the slides were kept in a streptavidin–peroxidase enzyme solution for 10 minutes. They were re-washed with PBS and treated with chromogen amino ethyl-carbazole (Thermo Scientific, TA-060-SA, USA) to make the immune reaction visible. The sections stained with Harris hematoxylin were mounted with water-based medium. The mounted slides were evaluated and photographed with the Olympus BX 51 computer-aided imaging system.
Determination of Serum Amylase, Lipase, IL-18, and IL-1β Levels
The amylase and lipase levels of the rats were measured by the enzymatic–colorimetric method (Cobas c 501 automated analyzer, Roche, USA) in the Medical Biochemistry Laboratory of the Medical Faculty of Eskisehir Osmangazi University. Serum IL-18 and IL-1β levels were evaluated with a commercially available ELISA kit (YL Biont, Jiading District, Shanghai, China, YLC0074RA and YLC0220RA), according to the manufacturer’s instructions.
Western Blot Method
Proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes by the semi-dry method. Non-specific binding in the membranes was blocked by 5% bovine serum albumin. Then, the membranes were incubated with NLRP3, caspase 1, ASC, NF-KB, and TNF-α primary antibodies (sc-66846, sc-56036, sc-514414, sc-8414 and sc-52746, Santa Cruz, US) overnight at 4°C.The membranes were then washed in tris-buffered saline 0.05% Tween (TBST) and incubated with HRP-conjugated secondary antibody for 1 hour at room temperature.
The membranes were washed 3 times for 5 minutes and then developed with the chemiluminescent substrate (LI-COR, Lincoln, NE, USA). The results were then analyzed with a western blot scanner (C-DiGit, LI-COR, Lincoln, NE, USA). Beta (β)-actin was used as a loading control. The normalization of groups was done with its own β-actin band. The fold change of the control group was accepted as 1 and the values for the other groups were calculated through the control group.
Statistical Analysis
Statistical studies were performed with the Statistical Package for the Social Sciences (SPSS). A value of P < .05 was considered significant in all studies. The suitability of the data for normal distribution was examined by the Shapiro–Wilk test. One-way ANOVA, followed by Tukey’s multiple comparison test, were performed on normally distributed data.
Results
Morphological Results
At the end of the experiment, the pancreases excised from anesthetized rats were examined macroscopically and pictures were captured. In the control, BHB1, BHB2, AP+BHB1, and AP+BHB2 groups, the pancreases were macroscopically alike. In the AP group, the pancreases were swollen and edematous (Figure 1).
Figure 1.
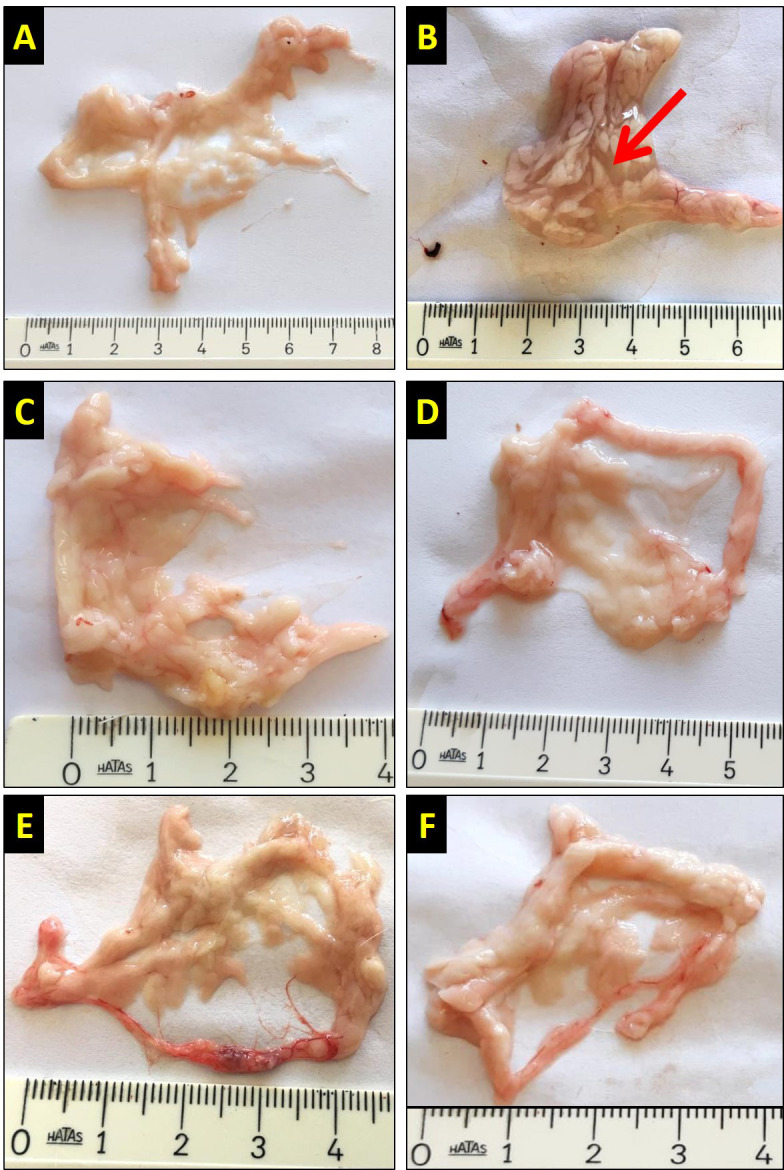
Macroscopic images of the pancreas in the different groups. The abdominal area of the rats was opened along the midline and the pancreases were excised and photographed with a digital camera. In the control (A), BHB1 (E), BHB2 (F), AP+BHB1(C) and AP+BHB2 (D) groups, pancreatic tissue was similar, while in the AP group (B), the pancreas was swollen and edematous (red arrow). The rulers in the figure are at centimeter scale.
Histopathological Examination and Scoring Results
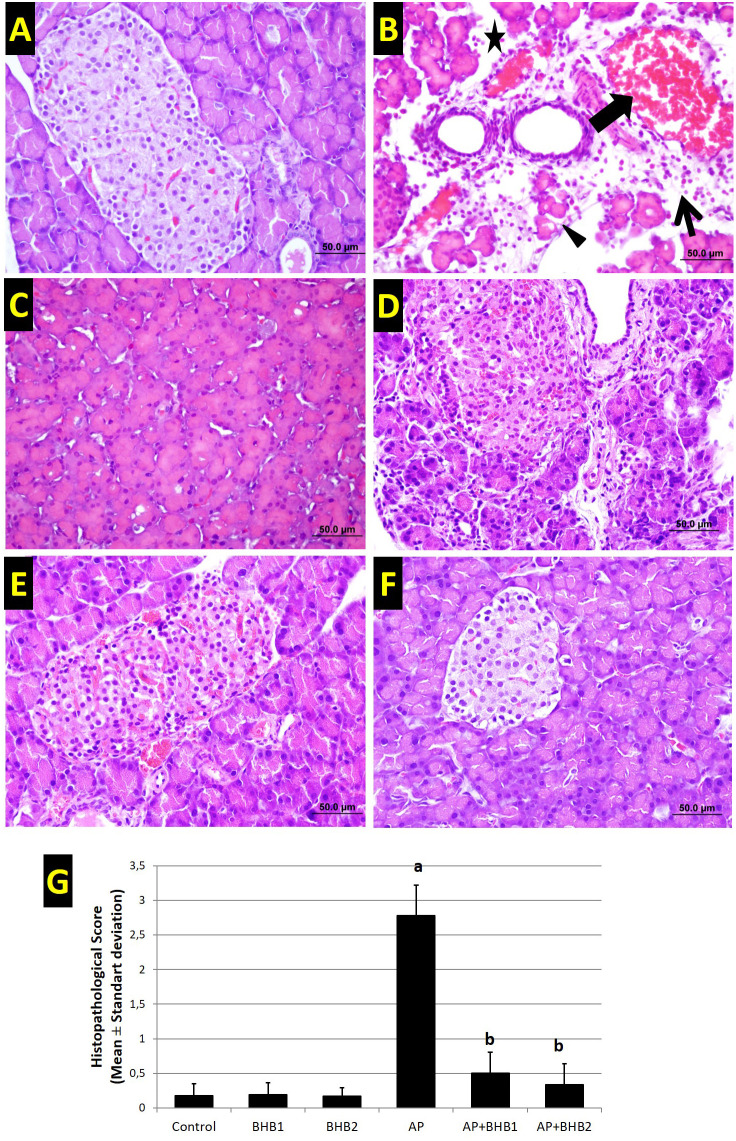
According to H&E staining, normal histological structure was observed in the control, and in the groups with BHB1 and BHB2 alone. However, severe pathological alterations, including intense edema, diffuse inflammatory cell infiltration, acinar necrosis, and hemorrhage were observed in the lobes, lobules, and acini of the AP group. These pathological alterations in the AP group were significantly ameliorated in the AP+BHB1 and AP+BHB2 groups. This amelioration was more apparent in AP+BHB2 group than that in AP+BHB1 group. A statistically significant difference was detected in histopathological scoring. The AP group had the highest mean of the histological score among all groups (P < .05). In the AP+BHB1 and AP+BHB2 groups, the histopathological score was significantly lower than that in the AP group (both P < .05). There was no significant difference among control, BHB1, BHB2, AP + BHB1, and AP + BHB2 groups (P > .05) (Figure 2).
Figure 2.
Hematoxylin–eosin staining and histopathological scoring of groups. Typical pancreatic histological structure is seen in the control (A), BHB1 (E), and BHB2 (F) groups. However, severe pathological alterations, including intense edema (star), diffuse inflammatory cell infiltration (thin arrow), acinar necrosis (arrowhead), and hemorrhage (thick arrow) were observed in the lobes, lobules, and acini of the AP group (B). These pathological alterations in the AP group were significantly ameliorated in the AP+BHB1 (C) and AP+BHB2 (D) groups. This amelioration was more apparent in the AP+BHB2 group than in the AP+BHB1 group. All bars are 50 µm. G: Scoring table. The scores of the groups are shown as mean ± standard deviation. a: AP group is significantly higher than all other groups (P < .05). b: It is different from the AP group (P < .05).
Immunohistochemical Results
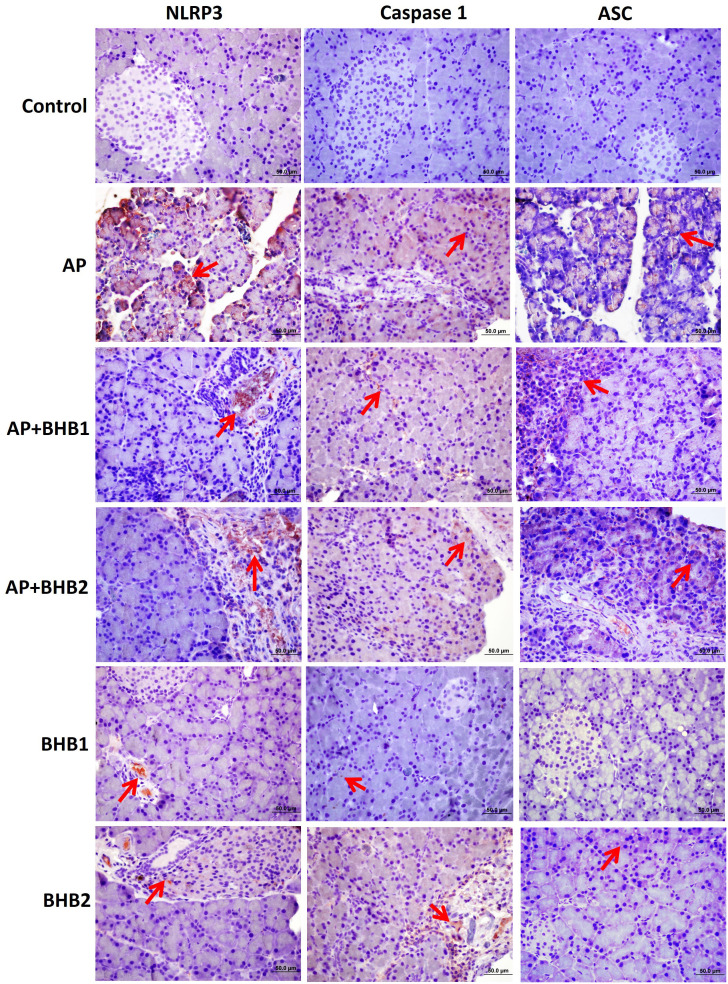
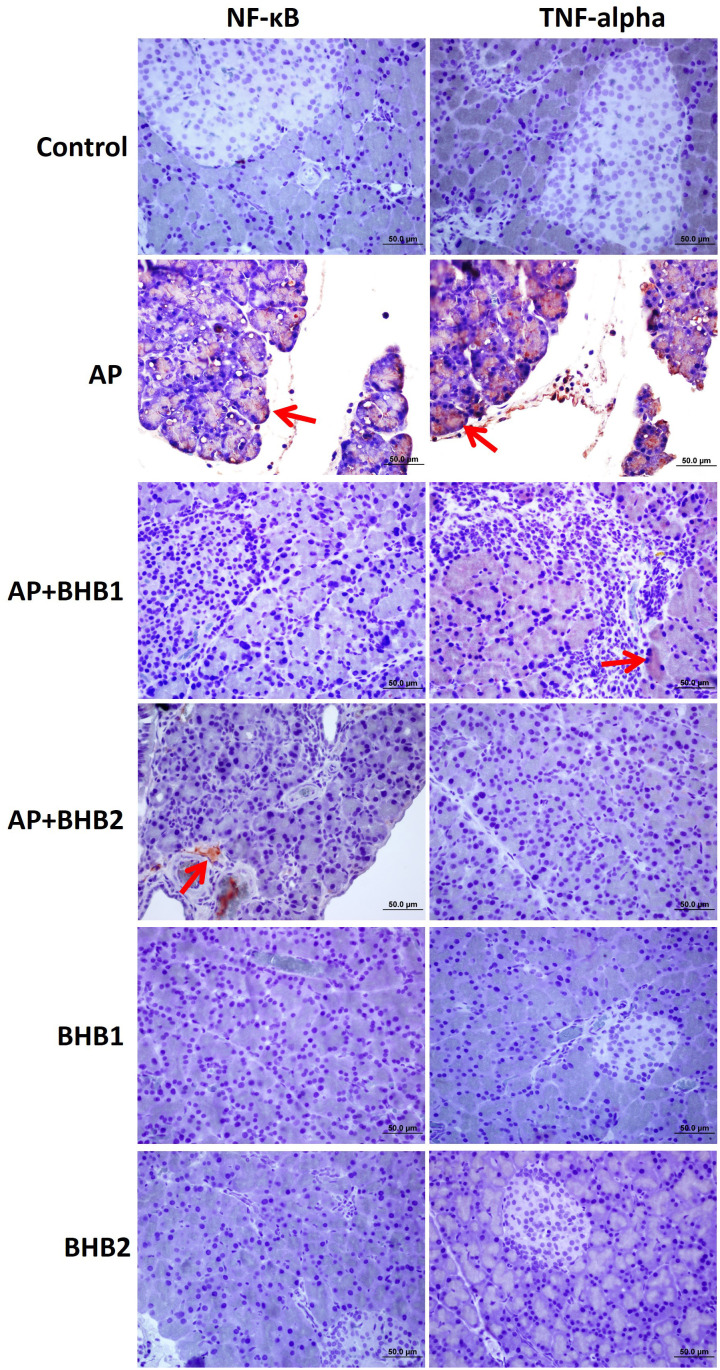
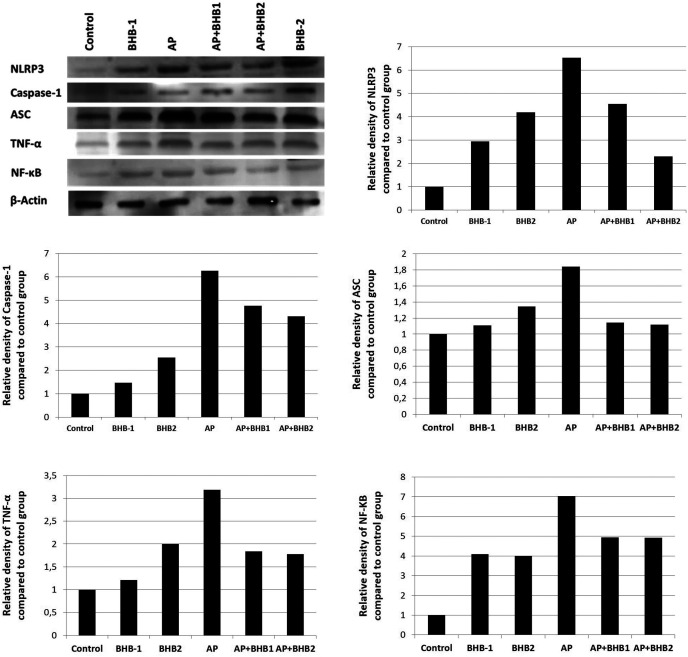
NLRP3, caspase 1, ASC, NF-KB, and TNF-α staining was performed by the indirect immunohistochemical method. The BHB1 and BHB2 groups were poorly stained, while the control group showed negative reaction for all antibodies. The most intensive staining for all antibodies was detected in the AP group. It was determined that the immunoreaction for all antibodies was decreased in the AP + BHB1 and AP + BHB2 groups, when compared to the AP group (Figure 3 and 4).
Figure 3.
NLRP3, caspase 1 and ASC staining of pancreas sections. The BHB1 and BHB2 groups were poorly stained while the control group showed negative reaction for all antibodies. The most intense staining (red arrow) for all antibodies was detected in the AP group. It was determined that the immunoreaction for all antibodies was decreased in the AP + BHB1 and AP + BHB2 groups when compared to the AP group.
Figure 4.
NF-KB and TNF-α staining of pancreas sections. The BHB1 and BHB2 groups were poorly stained while the control group showed negative reaction for all antibodies. The most intense staining (red arrow) for all antibodies was detected in the AP group. It was determined that the immunoreaction for all antibodies was decreased in the AP + BHB1 and AP + BHB2 groups, when compared to the AP group.
Amylase, Lipase, IL-1β, and IL-18 Results
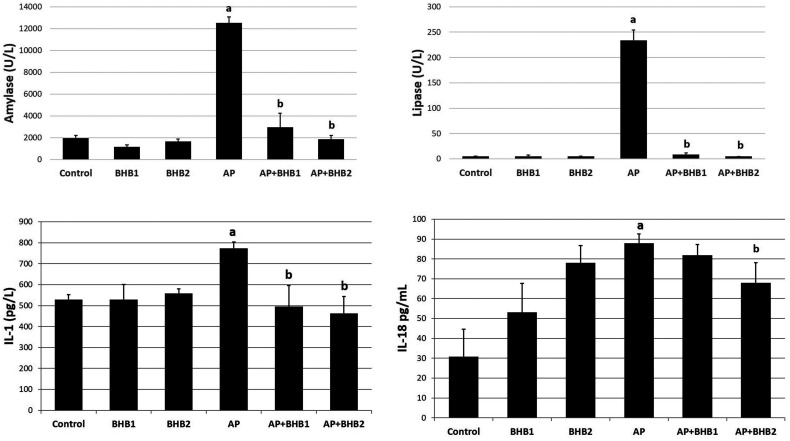
According to the amylase, lipase, and IL-1β results, no significant difference was detected among control, BHB1, BHB2, AP+BHB1, and AP+BHB2 groups (P > .05). The AP group showed a statistically significant difference when compared to each group (for all P < .05) and had the highest amylase and lipase levels. In addition, the amylase and lipase levels were decreased significantly in the AP + BHB1 and AP + BHB2 groups (P < .05). According to the IL-18 results, the lowest IL-18 levels were detected in the control group (P < .05 vs all groups). The BHB1 and BHB2 groups had significantly higher IL-18 levels than the control group (both P < .05). In the AP group, IL-18 levels were the highest (P < .05 vs control). Although IL-18 levels decreased in the AP + BHB1 group, it was not statistically significant when compared to the AP group (P > .05). Yet, there was a significant decrease in the IL-18 levels of the AP + BHB2 group when compared to the AP group (P < .05) (Figure 5).
Figure 5.
ELISA analysis of amylase, lipase, IL-1β, and IL-18 in serum samples. The highest amylase, lipase, IL-1β, and IL-18 expression was found in the AP group; and the amylase, lipase, IL-1β, and IL-18 expression decreased in the BHB-treated groups. a: AP group is significantly higher than all other groups (P < .05). b: It is different from the AP group (P < .05).
Western Blot Results
The levels of NLRP3, caspase 1, ASC, NF-KB, and TNF-α proteins were markedly higher in the AP group when compared to the control group. BHB treatments decreased the expressions of these proteins. The detailed band density and protein fold changes among the groups are shown in Figure 6.
Figure 6.
Western blot analysis of NLRP3, caspase 1, ASC, NF-KB, and TNF-α in pancreas tissues. Western blot results are similar to the immunohistochemistry results. The highest NLRP3, caspase 1, ASC, NF-KB, and TNF-α expressions were found in the AP group and these protein expressions decreased in the BHB-treated groups.
Discussion
In the present study, BHB was shown to produce a therapeutic effect on experimentally-induced acute pancreatitis by suppressing the NLRP3 inflammasome complex.
AP is a rapidly-progressing pancreatic disease that can lead to systemic inflammation and multiple organ failure.15 It can be easily modeled in many experimental animals. In our study, we used cerulein to generate AP in Wistar albino rats. AP can be induced by applying various doses of cerulein.16,17 In our preliminary studies, we determined that 4 doses (50 µg/kg) of cerulein were sufficient to induce AP.
In humans, AP has been diagnosed by measuring the levels of blood amylase and lipase, since 1916. These enzymes increase by at least 3-4 times the normal levels, during AP. 18
In this study, 12 hours after the last cerulein injection, the blood amylase and lipase levels were found to be increased by 6.3- (AP group/Control group, 12508.3/1962.7 U/L) and 48.75- fold (AP group/Control group, 234/4.8 U/L), respectively, when compared to the control. At the end of the experiment, it was observed that the pancreases in the AP group were swollen and had intense edema between the lobes. This result showed similarity with the results of Mayerle et al.19 BHB reduced swelling and edema in the AP groups.
This is the first study in which BHB was used as a therapeutic agent in AP. In this respect, it is an original study. When we look at the histopathological evaluation of the groups, dense and diffuse edema, inflammatory cell infiltration, acinar necrosis, and hemorrhage were observed between the lobes and acini, in the AP group. These findings are similar to the previous results.20 In the BHB1 and BHB2 groups, the histological structure was similar to the control group. In the AP+BHB1 and AP+BHB2 groups, the pathological findings were significantly reduced compared to those of the AP group. When we look at a study on pancreas and BHB, BHB was shown to reduce the oncogenic signal and cellular movement in pancreatic cancer.21 In another study, BHB administration was shown to increase the proliferation of pancreatic β cells, and the number of mitochondria and mitochondrial ATP production.22 There are few studies related with both pancreas and BHB.
When we look at the mechanism of AP, we see that it stems from inflammation. One of the factors involved in the etiology of AP is the conversion of trypsinogen into active trypsin in the acinar cells.
Trypsin activates many destructive enzymes (such as elastase, phospholipase A2, amylase, and lipase) and causes internal degradation of the cell and biomolecules. Infiltrated macrophages in the region of degraded cells increase the synthesis of proinflammatory cytokines (IL-1, IL-6, IL-8, and TNF-α). Then, the diapedesis of neutrophils, macrophages, and lymphocytes into the region accelerates, and finally inflammation begins.4
Inflammation is a complex process that involves many intracellular pathways. One of these pathways is the NLRP3 pathway. The most important event in the NLRP3 pathway is the binding of NLRP3, ASC, and pro-caspase 1 to form the NLRP3 inflammasome. By activated NLRP3, pro-caspase 1 converts into active caspase 1. Active caspase 1 leads to the release of proinflammatory cytokines such as IL-1α, IL-1β, IL-18, and TNF-α. The release of these cytokines exacerbates the inflammation (hyperinflammation) in the cell.8 Recent studies showed that the NLRP3 pathway is active in the pathophysiology of AP.15,23 Fu et al. studied the importance of NLRP3 in NLRP3−/− and NLRP3+/+ mice. After cerulein and lipopolysaccharide injections, AP could not be induced sufficiently in NLRP3−/− mice, but AP was easily induced in the NLRP3+/+ mice. In addition, lung infection developed in AP-induced NLRP3+/+ mice. In the same study, injection of an NLRP3 inhibitor, INF39, to AP-induced NLRP3+/+ mice quenched the severity of AP.24 In our study, we studied the effects of BHB, which is the main ketone body and is synthesized by fatty acid oxidation in hepatocyte mitochondria, on AP, through the NLRP3 pathway. We showed that the expressions of NLRP3, ASC, caspase 1, IL-1β, and IL-18 proteins increased in the AP group, and that 200 mg/kg BHB injection reduced these protein expressions.
Many studies have shown that BHB inhibits NLRP3 inflammasomes.25,26 Trotta et al. showed that BHB (25, 50, and 100 mg/kg) reduced diabetic retinal damage by inhibiting endoplasmic reticulum stress and NLRP3 inflammasomes in diabetic mice, dose-dependently.27 In our study, we had 2 BHB sham control groups, as BHB1 and BHB2. The pancreatic macroscopy and histopathology, and IL-1β, amylase and lipase levels of these groups were similar to those of the control group. However, in these 2 groups, unexpectedly, the BHB doses (200 mg/kg) used, which had been determined as beneficial doses in accordance with the literature,12 increased NLRP3 inflammasome proteins dose-dependently, although they decreased the levels in the AP+BHB groups. For example, the value of IL-18 increased significantly in the groups treated with BHB alone. IL-1β and IL-18 arise as a result of NLRP3 activation. When we examine our results, while IL-1β is similar to the control in the only-BHB-treated groups, it is thought-provoking that the IL-18 value is higher. In similar NLRP3 inflammasome and BHB studies, it has been reported that BHB has a lowering effect on the amount of IL-1β and IL-18 in lower and long-term treatments.27,28 We think that this change may be related to the IL-18 kit we used, or there may be an explicit relationship between the dose we used and the production of IL-18. Another study that may clarify this issue showed that BHB acutely increases the level of NLRP3 and related proteins.29 Based on this study, there may be an acute increase in the IL-18 levels in our study. From another perspective, it can be speculated that since some amount of BHB was bodily utilized against AP, rest of the BHB did not surpass the toxic threshold of BHB; however, it partly induced NLRP3 inflammasomes due to nonconsumption of BHB in the BHB alone groups.
BHB significantly reduced the amylase and lipase levels after AP induction in a dose-dependent manner. This is the first study showing the effect of BHB on amylase and lipase levels. Shang et al. showed that 1, 10, and 25 mM of BHB had anticancer activities and curbed cell migration and NLRP3 inflammasome activation in C6 glioma cells.30 Yet, Deora et al. showed that BHB did not inhibit NLRP3 inflammasomes in the synuclein-stimulated microglia cells.31 Yamanashi et al. reported that BHB had antidepressant and anti-inflammatory effects via the NLRP3 pathway.32 In this study, a single dose of 250 mg/kg has been the highest reported BHB dose in the literature until now. BHB can use many pathways when inhibiting the NLRP3 inflammasomes. For instance, BHB can inhibit NLRP3 inflammasomes via the pathway of endoplasmic reticulum stress.12
In conclusion, in this study, we demonstrated that BHB, an endogenous ketone body, can be used to treat AP due to its suppressing effect on the NLRP3 inflammasomes. For future studies, we suggest that effective BHB doses be investigated and BHB be tested on other NLRP3 inflammasome-related diseases. In addition, as a result of our investigations, studies up to now have investigated only the relationship between NLRP3 inflammasomes and BHB. There are no publications investigating the relationship of BHB with other NLRPs. The effect of BHB on other inflammasomes belonging to other NLR families (NLRP1, NLRP6, NLRC4 etc.) should also be investigated.
Funding Statement
This work was financed by a grant from the Eskisehir Osmangazi University. The authors are grateful to Eskisehir Osmangazi University, Commission of Scientific Research Projects for financial support to Project 201811020.
Footnotes
Ethics Committee Approval: Ethics committee approval for this study was received from the Local Animal Ethics Committee of Eskisehir Osmangazi University, Turkey (approval date/number: November 12, 2017/627-2).
Informed Consent: N/A.
Peer review: Externally peer-reviewed.
Author contributions: Concept, Design and Supervision – E.S.; Resource, Materials, Data Collection and Processing, Analysis and Interpretation – E.S., E.B.A., S.K., R.B. and V.S.; Literature Search and Writing – E.S.; Critical Reviews - E.S., E.B.A., S.K., R.B. and V.S.
Acknowledgment: The authors thank the Eskisehir Osmangazi University.
Conflict of interest: The authors have declared that no conflicts of interest exist.
References
- 1. . Sah RP, Dawra RK, Saluja AK. New insights into the pathogenesis of pancreatitis. Curr Opin Gastroenterol. 2013;29(5):523–530.. 10.1097/MOG.0b013e328363e399) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. . Basnayake C, Ratnam D. Blood tests for acute pancreatitis. Aust Prescr. 2015;38(4):128–130.. 10.18773/austprescr.2015.043) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. . Cappell MS. Acute pancreatitis: etiology, clinical presentation, diagnosis, and therapy. Med Clin North Am. 2008;92(4):889–923, ix.. 10.1016/j.mcna.2008.04.013) [DOI] [PubMed] [Google Scholar]
- 4. . Frossard JL, Steer ML, Pastor CM. Acute pancreatitis. Lancet. 2008;371(9607):143–152.. 10.1016/S0140-6736(08)60107-5) [DOI] [PubMed] [Google Scholar]
- 5. . Ren JD, Ma J, Hou J, et al. Hydrogen-rich saline inhibits NLRP3 inflammasome activation and attenuates experimental acute pancreatitis in mice. Mediators Inflammm. 2014;2014:930894. 10.1155/2014/930894) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. . Lukens JR, Gross JM, Kanneganti TD. IL-1 family cytokines trigger sterile inflammatory disease. Front Immunol. 2012;3:315. 10.3389/fimmu.2012.00315) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. . Zhu G, Xu Y, Cen X, et al. Targeting pattern-recognition receptors to discover new small molecule immune modulators. Eur J Med Chem. 2018;144:82–92.. 10.1016/j.ejmech.2017.12.026) [DOI] [PubMed] [Google Scholar]
- 8. . Hoque R, Farooq A, Mehal WZ. Sterile inflammation in the liver and pancreas. J Gastroenterol Hepatol. 2013;28(suppl 1):61–67.. 10.1111/jgh.12018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. . Klos M, Morgenstern S, Hicks K, Suresh S, Devaney EJ. The effects of the ketone body β-hydroxybutyrate on isolated rat ventricular myocyte excitation-contraction coupling. Arch Biochem Biophys. 2019;662:143–150.. 10.1016/j.abb.2018.11.027) [DOI] [PubMed] [Google Scholar]
- 10. . Greco T, Glenn TC, Hovda DA, Prins ML. Ketogenic diet decreases oxidative stress and improves mitochondrial respiratory complex activity. J Cereb Blood Flow Metab. 2016;36(9):1603–1613.. 10.1177/0271678X15610584) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. . Achanta LB, Rae CD. β-hydroxybutyrate in the brain: one molecule, multiple mechanisms. Neurochem Res. 2017;42(1):35–49.. 10.1007/s11064-016-2099-2) [DOI] [PubMed] [Google Scholar]
- 12. . Bae HR, Kim DH, Park MH, et al. β-hydroxybutyrate suppresses inflammasome formation by ameliorating endoplasmic reticulum stress via AMPK activation. Oncotarget. 2016;7(41):66444–66454.. 10.18632/oncotarget.12119) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. . Qi W, Tan DX, Reiter RJ, et al. Melatonin reduces lipid peroxidation and tissue edema in cerulein-induced acute pancreatitis in rats. Dig Dis Sci. 1999;44(11):2257–2262.. 10.1023/a:1026656720868) [DOI] [PubMed] [Google Scholar]
- 14. . Van Laethem JL, Marchant A, Delvaux A, et al. Interleukin 10 prevents necrosis in murine experimental acute pancreatitis. Gastroenterology. 1995;108(6):1917–1922.. 10.1016/0016-5085(95)90158-2) [DOI] [PubMed] [Google Scholar]
- 15. . Kim MJ, Bae GS, Jo IJ, et al. Fraxinellone inhibits inflammatory cell infiltration during acute pancreatitis by suppressing inflammasome activation. Int Immunopharmacol. 2019;69:169–177.. 10.1016/j.intimp.2019.01.043) [DOI] [PubMed] [Google Scholar]
- 16. . Jaworek J, Szklarczyk J, Kot M, et al. Chemerin alleviates acute pancreatitis in the rat thorough modulation of NF-κB signal. Pancreatology. 2019;19(3):401–408.. 10.1016/j.pan.2019.02.005) [DOI] [PubMed] [Google Scholar]
- 17. . Lau HY, Wong FL, Bhatia M. A key role of neurokinin 1 receptors in acute pancreatitis and associated lung injury. Biochem Biophys Res Commun. 2005;327(2):509–515.. 10.1016/j.bbrc.2004.12.030) [DOI] [PubMed] [Google Scholar]
- 18. . Pekmezci S. Akut Pankreatitte Yaklaşım ve tedavi. İÜ Cerrahpaşa Tıp Fakültesi Sürekli Tıp Eğitimi Etkinlikleri Hepato-Bilier Sistem ve Pankreas Hastalıkları Sempozyum Dizisi. 2002;28:239–262.. [Google Scholar]
- 19. . Mayerle J, Sendler M, Lerch MM. Secretagogue (Caerulein) induced pancreatitis in rodents . Pancreapedia: the Exocrine Pancreas Knowledge Base. 2013. [Google Scholar]
- 20. . Zhang J, Rouse RL. Histopathology and pathogenesis of caerulein-, duct ligation-, and arginine-induced acute pancreatitis in Sprague-Dawley rats and C57BL6 mice. Histol Histopathol. 2014;29(9):1135–1152.. 10.14670/HH-29.1135) [DOI] [PubMed] [Google Scholar]
- 21. . Shukla SK, Chaika NV, Singh PK. Beta-Hydroxybutyrate Inhibits Oncogenic Signaling and Cellular Motility in Pancreatic Cancer Cells. AACR; 2018. [Google Scholar]
- 22. . Sampson M, Lathen D, Dallon W, et al. β-Hydroxybutyrate improves β-cell mitochondrial function and survival. J Insul Resist. 2017;2(1):1–8.. (doi: 10.4102/jir.v2i1.25) [Google Scholar]
- 23. . Zhang Q, Tao X, Xia S, et al. Emodin attenuated severe acute pancreatitis via the P2X ligand‑gated ion channel 7/NOD‑like receptor protein 3 signaling pathway. Oncol Rep. 2019;41(1):270–278.. 10.3892/or.2018.6844) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. . Fu Q, Zhai Z, Wang Y, et al. NLRP3 deficiency alleviates severe acute pancreatitis and pancreatitis-associated lung injury in a mouse model. BioMed Res Int. 2018;2018:1294951. 10.1155/2018/1294951) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. . Youm YH, Nguyen KY, Grant RW, et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21(3):263–269.. 10.1038/nm.3804) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. . Goldberg EL, Asher JL, Molony RD, et al. Beta-hydroxybutyrate deactivates neutrophil NLRP3 inflammasome to relieve gout flares. Cell Rep. 2017;18(9):2077–2087.. 10.1016/j.celrep.2017.02.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. . Trotta MC, Maisto R, Guida F, et al. The activation of retinal HCA2 receptors by systemic beta-hydroxybutyrate inhibits diabetic retinal damage through reduction of endoplasmic reticulum stress and the NLRP3 inflammasome. PLOS ONE. 2019;14(1):e0211005. 10.1371/journal.pone.0211005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. . Deng Y, Xie M, Li Q, et al. Targeting mitochondria-Inflammation Circuit by β-hydroxybutyrate Mitigates HFpEF. Circ Res. 2021;128(2):232–245.. 10.1161/CIRCRESAHA.120.317933) [DOI] [PubMed] [Google Scholar]
- 29. . Neudorf H, Durrer C, Myette-Cote E, et al. Oral ketone supplementation acutely increases markers of NLRP3 inflammasome activation in human monocytes. Mol Nutr Food Res. 2019;63(11):e1801171. 10.1002/mnfr.201801171) [DOI] [PubMed] [Google Scholar]
- 30. . Shang S, Wang L, Zhang Y, Lu H, Lu X. The beta-hydroxybutyrate suppresses the migration of glioma cells by inhibition of NLRP3 inflammasome. Cell Mol Neurobiol. 2018;38(8):1479–1489.. 10.1007/s10571-018-0617-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. . Deora V, Albornoz EA, Zhu K, Woodruff TM, Gordon R. The ketone body β-hydroxybutyrate does not inhibit synuclein mediated inflammasome activation in microglia. J Neuroimmune Pharmacol. 2017;12(4):568–574.. 10.1007/s11481-017-9754-5) [DOI] [PubMed] [Google Scholar]
- 32. . Yamanashi T, Iwata M, Kamiya N, et al. Beta-hydroxybutyrate, an endogenic NLRP3 inflammasome inhibitor, attenuates stress-induced behavioral and inflammatory responses. Sci Rep. 2017;7. 10.1038/s41598-017-08055-1) [DOI] [PMC free article] [PubMed] [Google Scholar]