Abstract
Background
: The quality of sleep in people with irritable bowel syndrome (IBS) is reduced by increased oxidative stress and clinical problems. Assessing the effects of ellagic acid (EA) on sleep quality and gastrointestinal symptoms in patients with IBS was the aim of this study.
Methods
: In this research that was conducted as a randomized, double-blind, placebo-controlled clinical trial, 44 patients with IBS were enlisted. Individuals approved by the project clinical counselor were divided into two intervention groups to receive 180 mg of EA per day (n = 22) and a placebo group (n = 22) for 2 months. Petersburg’s Sleep Quality (PSQI) questionnaire and IBS severity score system (IBSSS) were assessed at the beginning and end of the study. Statistical analysis was performed using SPSS software.
Results
: At the end of the study, changes in mean PSQI and scores related to sleep subgroups were significant between the two groups (P < .05). Also, the significant changes were not seen in sleep and sleep subgroups scores in the placebo group at the end of the study (P > .05). EA consumption reduced IBSSS score and IBS symptoms in the intervention group after 2 months (P < .05).
Discussion
: The results arisen from this study indicated that receiving EA had a beneficial effect on sleep quality and gastrointestinal symptoms in IBS patients. The antioxidant and anti-inflammatory properties of EA may be responsible for these beneficial effects.
Keywords: Ellagic acid, sleep quality, gastrointestinal symptoms, irritable bowel syndrome.
Main Points
Sleep problems are related to inflammation and oxidative stress in patients with inflammatory bowel syndrome.
Ellagic acid is an antioxidant and belongs to the group of natural polyphenolic acids found in many foods such as fruits, nuts, and plant extracts.
Supplementation with ellagic acid improved the quality of sleep in IBS patients.
Introduction
Sleep is one of the natural functions of the body that forms about one-third of human life.1 Poor sleep affects the body’s physiological functions such as the endocrine system, metabolism, and nervous system. The sleep disorder can occur in many forms, such as inadequate sleep, alertness, or abnormal movements during sleep. In the United States, about 70 million people have insomnia symptoms.2
Recent studies suggest that sleep disorders can directly cause hypersensitivity of the gastrointestinal tract, and also sleep deprivation can increase the production of proinflammatory cytokines. Several inflammatory cytokines such as interleukin 1 (IL1), interleukin 6 (IL6), and tumor necrosis factor α (TNFα) play important roles in the sleep and wake cycle, and these cytokines are elevated in some inflammatory diseases such as irritable bowel syndrome (IBS) and rheumatoid arthritis.
Irritable bowel syndrome is a common gastrointestinal disease characterized by abdominal discomfort or unacceptable pain. There are both mental and physical symptoms of this disease. Mental symptoms include depression and anxiety and physical symptoms such as bloating, gas, diarrhea, and constipation.3 The prevalence of IBS is approximately 7% to 20% all over the world. The incidence of IBS is more common in the third and fourth decades of life, which is higher in women than in men.4 Scientific reports show that women with IBS who have sleep disorders, develop abdominal pain, anxiety, and fatigue the next day.5
Recent scientific investigations have shown that in patients with IBS, sleep disturbance is associated with gastrointestinal function. The results of a study that examined sleep quality in people with IBS showed that severe symptoms of IBS and sleep disorder are related.6 The conclusion of another study was that people with IBS during the day have longer sleep but less comfortable sleep and that they also experience more waking periods.5 It has been shown that microscopic inflammation of the colon may play a key role in the pathology of IBS. After IBS inflammation, the number of lymphocyte cells increases. Sleep deprivation leads to increased microscopic inflammation in the intestine, and people with IBS or small intestinal bacterial overgrowth appear to be more likely to experience sleep disturbance.7 In previous studies, the association between inflammation, oxidative stress, and antioxidants intake in sleep quality and sleep duration was determined, and results indicated that poor sleep increased C-reactive protein levels, but in men, this relationship was not established.8
The antioxidants decrease proinflammatory cytokines and can improve the symptoms of IBS and then improve the quality of sleep in these patients. One of the strong antioxidants is ellagic acid (EA). Fruits such as pomegranate and some nuts are rich sources of this polyphenol and have this formula (4,4,5,5,6,6-hexahydroxidifenic acid 2,6,2,6-dilactone).9 The results of the studies showed several properties of EA such as the fight against oxidants and reduce inflammation and apoptosis.10 In one study, EA showed beneficial and effective effects against oxidative stress in diabetic rats. Recent studies of EA administration in animal models have reported protective properties against Crohn’s disease due to a decrease in inflammatory proteins such as mitogen-activated protein kinase and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB).11,12 Assessing the EA oral supplement on sleep quality and gastrointestinal symptoms in patients with IBS was the aim of this study.
Materials and Methods
Study Participants
This randomized, double-blinded, placebo-controlled clinical trial was done on 44 subjects, aged 19-60 years old. A total of 44 patients with IBS include both genders with normal body mass index (BMI; 19-25 kg/m2) after a clinical examination and gastroenterologist confirmation were enrolled in the study. The patient’s selection in this study was based on the diagnostic criteria of Rome III.13 for digestive functional disorders and with sleep problems based on Pittsburg Sleep Quality Index (PSQI).14 referring to a specialized hospital. The inclusion criterion in this study was having a score of 5 or higher in this questionnaire by the patient. Several studies conducted in Iran have examined the validity and reliability of this questionnaire. Patients with a history of abdominal surgery, gastrointestinal diseases such as celiac disease, as well as pregnant and lactating women, and those who have been taking supplements in the last 3 months were not included in the study. Also having underlying illnesses like diabetes, severe psychiatric, and behavioral disorders and usage of aspirin, warfarin, heparin, anti-inflammatory drugs (including non-steroid, steroids, antihistamines, and mast cell stabilizers), and using sleep medications have been other exclusion criteria.
Randomization and Blinding
Individuals were randomly assigned to the intervention group (n = 22) receiving EA supplements (180 mg) or placebo recipients (n = 22), who were admitted to the study according to the inclusion criteria. The appearance of the placebo capsule was like a supplement capsule and it had starch powder (180 mg). The duration of this study was 8 weeks. During the study, people were excluded from the study if they changed a diet or physical activity pattern. The supplement was purchased from Supplement Spot and the placebo was made by the School of Pharmacy. The allocation sequence was blinded by using the table of random numbers. The patients were divided into two groups by randomized block allocation according to BMI. In this study, the patient, researcher, and specialist physician were blind to supplements and placebo. Capsules prepared by someone else outside the study in A and B groups that were placed in the same package so that the investigator would be unaware of the contents of the capsules.
The questionnaire that is used to assess the quality of sleep and identify possible sleep disorders is called PSQI. This questionnaire, with nine questions and seven sections, has a score of 0 to 21, usually examines the past month, and scores above 5 indicate the presence of sleep disorders.15 Also, the IBS severity score system (IBSSS) was used for evaluating the IBS severity. IBS severity score system consists of five parts, that covers five clinically relevant items; (1) severity of abdominal pain, (2) frequency of abdominal pain, (3) severity of abdominal distention or tightness, (4) dissatisfaction with bowel habits, and (5) interference of IBS with life in general. These items were assessed with 100-mm visual analog scale, where 0 indicated no symptoms and 100 indicated the worst symptoms.16 These mentioned questionnaires were presented to the patients and completed at the base and at the end of the study. Questionnaires were introduced and explained by a specialist researcher for patients. Anthropometric measurements, clinical history, demographic data of each individual were evaluated. Anthropometric indicators including weight and height were measured using digital scales and a stadiometer. Three-day food recalls were used to assess dietary intake, and the Nutritionist IV program (San Bruno, CA) modified for Iranian food composition was used for estimating the dietary intake of participants. Also, to evaluate the physical activity, we used the International Physical Activity Questionnaire (IPAQ). Data from the IPAQ were converted to metabolic equivalent minutes/week using existing guidelines.17
Sample Size Calculation
We used the MDA factor before and after the intervention to determine the sample size, which was used in Hosseini B et al. study (18). Therefore, if the mean and standard deviation of the MDA before and after the supplementation was 3/3 ± 1 and 2/1 ± 0/7, for each group of 18 people was calculated. Considering the dropout in participants during the study, 22 people were considered for each group.
Statistical Analysis
Statistical analyses were conducted using SPSS version 20. All data were presented as mean ± SD and were checked for normality by the Kolmogorov–Smirnov test. Due to the normal distribution of variables, the paired sample t-test and the independent sample t-test were applied to analyze differences in variables within and between groups, respectively. The P < .05 was considered statistically significant.
Results
A total of 44 patients with IBS were recruited for the study. Of the participants, one withdrew from the placebo group for personal reasons, and finally, 43 patients completed the study (Figure 1). Patient compliance in this study was 97.72%. The final analysis was done on the subjects who finished the study. Table 1 presents the patient’s demographic and baseline characteristics. Participants’ mean age was 34.91 ± 5.34 years. There was no significant difference in age between the two intervention and placebo groups (34.55 ± 4.93 vs. 35.29 ± 5.84 years). Also, there was no significant difference between the intervention and the placebo groups in weight (63.67 ± 7.91 vs. 63.48 ± 9.14), BMI (23.81 ± 1.34 vs. 23.75 ± 1.42 kg/m2), and physical activity (37.53 ± 2.76 vs. 36.21 ± 2.91) factors at the beginning of the study (P > .05; Table 1). Also at the end of the study, no significant changes in BMI, weight, and physical activity between the patients’ weight were observed (Table 1). We did not receive any adverse effect reports from patients about EA consumption in this study. Table 2 shows the results of the survey of energy macronutrients and some micronutrients intake in the subjects. There was no significant difference between the groups in terms of nutritional factors (P > .05). The data related to sleep score and sleep subgroups score were summarized in Table 3. At baseline, there was no significant difference between the groups in terms of overall sleep quality score and sleep subgroups score (PSQI (score): 9.01 ± 2.35 vs 9.78 ± 2.21 in placebo and intervention groups, respectively, P > .05). After the EA supplementation, the changes made in the sleep score (PSQI), sleep duration, sleep latency, sleep efficiency, sleep disturbances, use of sleep medications, daytime dysfunction, and the subjective sleep quality factors between the two groups were significant (P < .05). According to Intergroup comparisons, the changes in sleep and sleep subgroups scores in the placebo group at the end of the study were not significant (P > .05). The effect of EA supplementation on IBS severity score has been summarized in Table 4. Ellagic acid consumption reduced abdominal pain and distention, dissatisfaction with bowel habits, flatulence, and rumbling, and finally, the symptoms in the intervention group after 2 months. Intake of EA decreased IBSSS score in the intervention group and these changes were significant in comparison with placebo group change (P < .05).
Figure 1.
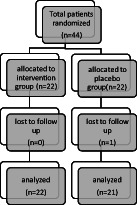
Trial Profile and Design.
Table 1.
The Comparison of Baseline Characteristics of the Participants
| Characteristics | Mean ± SD Placebo (n = 21) | Mean ± SD Ellagic acid (n = 22) | P1 |
|---|---|---|---|
| Age (year) | 35.29 ± 5.84 | 34.55 ± 4.93 | 0.611 |
| Weight (kg) | |||
| Baseline | 63.48 ± 9.14 | 63.67 ± 7.91 | 0.941 |
| 2 months Change | 63.03 ± 8.45 | 63.66 ± 7.81 | 0.799 |
| P2 | 0.843 | 0.903 | |
| BMI (kg/m2) | |||
| Baseline | 23.75 ± 1.42 | 23.81 ± 1.34 | 0.827 |
| 2 months Change | 23.61 ± 1.36 | 23.81 ± 1.39 | 0.69 |
| P2 | 0.813 | 0.89 | |
| Physical activity (met h/week) | |||
| Baseline | 36.21 ± 2.91 | 37.53 ± 2.76 | 0.136 |
| 2 months change | 36.72 ± 3.03 | 38.20 ± 2.80 | 0.104 |
| P2 | 0.603 | 0.189 |
Data are expressed as means ± SD.
P1, Comparison of the mean of baseline characteristics between the two groups of ellagic acid and placebo (independent samples t-test); P2, Comparison of mean of baseline characteristics in each group at baseline and end of study (Paired samples t-test).
Table 2.
The Comparison of the Dietary Intake at the Baseline and the End of the Study in Patients With IBS
| Variables | Mean ± SD Placebo (n = 21) | Mean ± SD Ellagic Acid (n = 22) | P1 |
|---|---|---|---|
| Energy (kcal) | |||
| Baseline | 1908.27 ± 380.67 | 1961.31 ± 367.27 | 0.307 |
| End | 1890.49 ± 401.19 | 1973.20 ± 360.09 | 0.299 |
| P2 | 0.503 | 0.499 | |
| Protein (g) | |||
| Baseline | 74.34 ± 17.14 | 76.41 ± 19.02 | 0.314 |
| End | 73.63 ± 16.97 | 76.88 ± 21.1 | 0.3 |
| P2 | 0.407 | 0.48 | |
| Carbohydrate (g) | |||
| Baseline | 248.83 ± 41.93 | 254.67 ± 42.97 | 0.54 |
| End | 245.52 ± 41.53 | 256.21 ± 43.1 | 0.492 |
| P2 | 0.607 | 0.643 | |
| Fat (gr) | |||
| Baseline | 71.47 ± 16.22 | 72.14 ± 20.48 | 0.716 |
| End | 68.55 ± 37.01 | 71.31 ± 24.11 | 0.695 |
| P2 | 0.291 | 0.382 | |
| Saturated fatty acids (g) | |||
| Baseline | 19.27 ± 5 | 19.45 ± 5.72 | 0.705 |
| End | 18.17 ± 6.4 | 20.08 ± 3.23 | 0.612 |
| P2 | 0.415 | 0.541 | |
| Monounsaturated Fatty acid (g) | |||
| Baseline | 27.19 ± 7.09 | 28.02 ± 6.08 | 0.749 |
| End | 26.11 ± 5.03 | 28.91 ± 6.11 | 0.546 |
| P2 | 0.617 | 0.719 | |
| Polyunsaturated Fatty acid (gr) | |||
| Baseline | 22.09 ± 8.13 | 22.85 ± 7.29 | 0.78 |
| End | 21.19 ± 6.12 | 21.01 ± 4.18 | 0.803 |
| P2 | 0.307 | 0.243 | |
| Fiber (g) | |||
| Baseline | 6.39 ± 0.91 | 6.48 ± 1.04 | 0.407 |
| End | 6.03 ± 2.24 | 6.71 ± 2.17 | 0.354 |
| P2 | 0.207 | 0.21 | |
| Vitamin C (mg) | |||
| Baseline | 68.09 ± 17.09 | 69.47 ± 13.27 | 0.704 |
| End | 66.28 ± 47.11 | 68.27 ± 9.67 | 0.501 |
| P2 | 0.57 | 0.609 | |
| Vitamin E (IU) | |||
| Baseline | 6.39 ± 0.11 | 6.79 ± 0.27 | 0.405 |
| End | 5.83 ± 0.91 | 6.8 ± 0.4 | 0.102 |
| P2 | 0.311 | 0.821 | |
| Selenium | |||
| Baseline | 119.47 ± 29.12 | 120.08 ± 21.14 | 0.457 |
| End | 118.6 ± 27.13 | 119.13 ± 23.04 | 0.263 |
| P2 | 0.302 | 0.451 |
Data are expressed as means ± SD.
P1, Comparison of the mean of dietary intake between the two groups of ellagic acid and placebo (independent samples t-test); P2, Comparison of mean of baseline characteristics in each group at baseline and end of study (Paired samples t-test)
Table 3.
The Comparison of the Sleep Score and Sleep Subgroups Score Changes in the Study Groups at the Beginning and the End of the Study
| Variables | Mean ± SD Placebo (n = 21) | Mean ± SD Ellagic Acid (n = 22) | P1 |
|---|---|---|---|
| PSQI (score) | |||
| Baseline | 9.01 ± 2.35 | 9.78 ± 2.21 | 0.145 |
| End | 8.69 ± 2.91 | 6.04 ± 1.83 | 0.036 |
| P2 | 0.109 | 0.002 | |
| Sleep duration (hour) | |||
| Baseline | 5.29 ± 1.36 | 5.78 ± 1.09 | 0.319 |
| End | 5.98 ± 2.04 | 7.03 ± 2.01 | 0.003 |
| P2 | 0.206 | 0.017 | |
| Sleep latency (minute) | |||
| Baseline | 49.88 ± 38.99 | 51.09 ± 18.27 | 0.491 |
| End | 45.39 ± 15.17 | 30.09 ± 12.06 | 0.021 |
| P2 | 0.317 | 0.002 | |
| Sleep efficiency (%) | |||
| Baseline | 80.68 ± 19.07 | 81.27 ± 14.37 | 0.267 |
| End | 84.06 ± 14.29 | 89.41 ± 17.39 | 0.031 |
| P2 | 0.108 | 0.017 | |
| Sleep disturbances (score) | |||
| Baseline | 1.95 ± 0.31 | 1.99 ± 0.42 | 0.33 |
| End | 1.65 ± 0.8 | 0.98 ± 0.2 | 0.027 |
| P2 | 0.163 | 0.001 | |
| Use of sleep medications (time per week) | |||
| Baseline | 2.65 ± 0.84 | 2.36 ± 0.9 | 0.142 |
| End | 2.01 ± 0.19 | 0.94 ± 0.06 | 0.013 |
| P2 | 0.186 | 0.01 | |
| Day time dysfunction (score) | |||
| Baseline | 1.95 ± 0.41 | 1.87 ± 0.27 | 0.44 |
| End | 1.37 ± 0.26 | 0.61 ± 0.11 | 0.002 |
| P2 | 0.19 | 0.001 | |
| Subjective sleep quality (score) | |||
| Baseline | 1.89 ± 0.39 | 2.03 ± 0.69 | 0.32 |
| End | 1.73 ± 0.21 | 1.19 ± 0.43 | 0.039 |
| P2 | 0.249 | 0.024 |
Data are expressed as means ± SD.
P1, Comparison of the mean of sleep score and sleep subgroups score between the two groups of ellagic acid and placebo (independent samples t-test); P2, Comparison of mean of baseline sleep score and sleep subgroups score changes in each group at baseline and end of study (paired samples t-test)
Table 4.
The Comparison of the Irritable Bowel Syndrome Severity Score from Baseline to Endpoint Measures in Two Groups
| Characteristics | Mean ± SD Placebo (n = 21) | Mean ± SD Ellagic (n = 22) | P1 |
|---|---|---|---|
| Abdominal pain | |||
| Baseline | 47.09 ± 9.04 | 45.63 ± 9.21 | 0.509 |
| After 2 months | 45.69 ± 6.19 | 30.07 ± 6.29 | 0.001 |
| P2 | 0.412 | 0.017 | |
| Abdominal distention | |||
| Baseline | 44.23 ± 10.05 | 45.61 ± 9.42 | 0.625 |
| After 2 months | 41.03 ± 11.12 | 28.54 ± 7.71 | 0.001 |
| P2 | 0.394 | 0.014 | |
| Dissatisfaction with bowel habits | |||
| Baseline | 39.47 ± 11.9 | 37.93 ± 10.32 | 0.401 |
| After 2 months | 37.02 ± 8.35 | 25.19 ± 8.4 | 0.001 |
| P2 | 0.213 | 0.012 | |
| Flatulence | |||
| Baseline | 42.61 ± 7.07 | 41.75 ± 5.91 | 0.42 |
| After 2 months | 41.03 ± 9.19 | 25.5 ± 5.72 | 0.001 |
| P2 | 0.362 | 0.029 | |
| Rumbling | |||
| Baseline | 41.91 ± 6.84 | 39.07 ± 7.12 | 0.325 |
| After 2 months | 40.64 ± 7.46 | 23.58 ± 5.04 | 0.001 |
| P2 | 0.31 | 0.027 | |
| Overall | |||
| Baseline | 40.062 ± 8.98 | 41.998 ± 8.396 | 0.425 |
| After 2 months | 39.082 ± 8.46 | 26.576 ± 6.632 | 0.001 |
| P2 | 0.309 | 0.001 |
P1, Comparison of the mean of irritable bowel syndrome symptoms between two groups (independent samples t test); P2, Comparison of the mean of irritable bowel syndrome symptoms in each group at the baseline and end of the study (paired samples t-test).
Discussion
Irritable bowel syndrome is a common and costly complication that can cause gastrointestinal disorders and symptoms such as abdominal pain, altered bowel function, and periods of constipation or diarrhea.16 Physiological studies show that the disease is associated with physiological factors such as depression and anxiety. In some scientific studies, the association of physiological problems with IBS has been estimated at up to 60%. In addition to the physiological problems caused by IBS, they also experience some degree of impairment of quality of life. Scientific reports indicate that poor sleep quality with gastrointestinal diseases is one of the major complaints of people with diabetes IBS.3 About 26% to 55% of people with IBS also have some degree of sleep disorder.7 Also, the results of scientific investigations showed the role of stress oxidative in sleep quality as well as the beneficial effects of different antioxidants intake in patients with sleep problems.19
The effects of EA on sleep quality in people with IBS were investigated in this scientific project. In this study, 180 mg of EA was received as a supplement in the intervention group for 60 days. The supplement of EA significantly increased the quality of sleep at the end of the study. The high scores obtained at the beginning of the study by the PSQI decreased significantly at the end of the study. There are currently no comprehensive studies on the effect of EA on sleep quality in patients with IBS. However, several scientific projects have evaluated the antioxidant functions on quality of sleep, including a case study alleviating IBS symptoms with a high dose of vitamin D (3000 IU / day)20 and also in an intervention study, the use of Pearl vitamin D 50 000 IU for 8 weeks reduced the delay in falling asleep and PSQI.21 In an animal study by Alzoubi et al.,22 sleep-deprived rats received vitamin E as an important and potent antioxidant. After receiving the supplement in the amount of 100 mg/kg, the antioxidant factors such as glutathione (GSH), oxidized glutathione (GSSG), and GSH/GSSG ratio, glutathione peroxidase (GPx), and superoxide dismutase (SOD) were evaluated. The results of this study showed the positive effect of this antioxidant in increasing sleep quality and reducing insomnia, which occurred mainly through increasing the activity of antioxidant enzymes. In a clinical study conducted by Mohammad Shahi et al.21 on vitamin D supplementation on sleep quality in people aged 20 to 50 years with sleep disorders, it was found that receiving this antioxidant vitamin increases sleep quality and reduces sleep disorders, especially sleep latency. In this interventional study, the supplement dose was 50 000-unit, which was 1 weekly for 2 months. The researchers of this study reported a significant decrease in the score of the questionnaire in the intervention group compared to the placebo group. However, the study on resveratrol supplementation and its effect on sleep quality had the opposite effect. In this clinical study, no significant changes in sleep quality were reported in people receiving antioxidants in grapes. One of the weaknesses of the resveratrol study was the recruitment of healthy individuals who may have participated in the study without sleep disorders.23
One of the scientific points of interest for researchers in the field of sleep disorders is to investigate the role of increasing inflammatory factors and indicators related to oxidative stress in these patients.15 According to the results, disturbing the hormonal balance caused by sleep disorders can increase inflammatory factors such as interleukins and TNFα, the induction of which will also be due to increased cortisol and insulin.19 On the other hand, the proper sleep cycle is reduced to less oxygen due to the increase in the reactive oxygen species (ROS), which increases inflammation in the central nervous system can aggravate the condition of this defective cycle. Increasing ROS causes the body to stress oxidative more heavily than the body’s antioxidants, in other words, the body’s antioxidants such as melatonin, which play an essential role in promoting sleep and improving sleep quality, decrease.24 This disturbance can cause damage to lipids, proteins, and DNA.8 In the waking state due to high metabolism in the brain system, the production of ROS is increased, however, if there is also a lack of antioxidants, it can impair brain function and can lead to sleep disorders or impaired sleep quality. Taking antioxidant supplements like EA can improve sleep quality by improving oxidative stress and neutralizing ROS.25 Malondialdehyde (MDA) is also one of the harmful metabolites that can be reduced by this polyphenol and shift the balance toward antioxidants. The inverse relationship between the amounts of this metabolite produced due to the oxidation of cell membrane fats in the nervous system with sleep quality has been reported.26 According to the results of scientific projects, inflammation is increased in people with sleep disorders, which increases systemic inflammation, leading to decreased immune function. Finally, the relationship between the increase of inflammatory factors and metabolites due to fatty acid oxidation has been identified in scientific articles and it has been proven that receiving antioxidants such as EA reduces the gene expression of proinflammatory cytokines in the gastrointestinal tract and prevents disorders related to this organ, balances oxidative stress and antioxidants, and thus improves sleep quality.27 The results of our study indicated that supplementation with EA, decreased the IBS severity score, so that clinical symptoms, such as abdominal pain, were significantly lower in these subjects than before the study. In Abbasnezhad et al.20 study, supplementation with vitamin D (50 000 IU) leads to improve quality of life and symptoms of IBS disease. Considering the results of Mohammad Shahi et al.21 and Abbasnezhad et al.20 studies, it can be concluded that this vitamin with antioxidant and anti-inflammatory properties, helping to improve the digestive symptoms in these people and reduce oxidative stress and increase sleep quality.
Strengths and Limitations
One of the strengths of this study is that for the first time the effect of the pure supplement of EA was investigated in patients with IBS for assessing effects of it on quality of sleep. Also, the design of this study was a double-blind randomized clinical trial that had parallel groups, making the results of this study remarkable. However, the present study, like other clinical trials, had limitations such as single dose, budget deficit, and lower factor measurements. For obtaining a perfect picture of EA treatment, it would be necessary to execute a randomized clinical trial with a greater number of participants and different doses of it. In conclusion, the results of our study indicated that supplementation with EA, 180 mg/day for 8 weeks, in IBS patients, improved the quality of sleep, and also decreased the IBSSS. These results provide evidence to support the view that EA can play an important role in enhancing the quality of sleep and life among IBS patients. Nevertheless, further studies are needed to provide additional evidence.
Funding Statement
This work was financially supported by a Grant (Number: IR.QUMS.REC.1397.201) from Vice-Chancellor for Research Affairs of Qazvin University of Medical Sciences, Qazvin, Iran. This investigation was extracted from the Master of Science thesis of Zahra Mirzaie, a student of Qazvin University of Medical Sciences.
Footnotes
Ethics Committee Approval: The protocol of the study after approving with the ethic committee of Qazvin University of Medical Sciences, Qazvin, Iran, with Grant number: IR.QUMS.REC.1397.201, was registered in the Iranian Registry of Clinical Trials website by the IRCT20141025019669N11code.
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: All persons who met authorship criteria are listed as authors. Z Mirzaie, M Kavianpour, and H Khadem Haghighian contributed significantly to the work’s conception, participated in the writing and critical revision of the manuscript in a manner sufficient to establish the ownership of the intellectual content. S Hesami and E Pouryousefi contributed significantly to the work’s conception. H Khadem Haghighian analyzed and interpreted data. A Bastani and M Kavianpour were involved in the design of the work. All authors approved the final version of the manuscript to be published.
Acknowledgments: The authors would like to thank all of the participants who completed the study protocol. This work was financially supported by a Grant (Number: IR.QUMS.REC.1397.201) from Vice-Chancellor for Research Affairs of Qazvin University of Medical Sciences, Qazvin, Iran.
Conflict of Interest: The authors have no conflict of interest to declare.
References
- 1. . Xu D, Chen VL, Steiner CA.et al. Efficacy of fecal microbiota transplantation in irritable bowel syndrome: a systematic review and meta-analysis. Am J Gastroenterol. 2019;114(7):1043–1050.. 10.14309/ajg.0000000000000198) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. . Kamp KJ, Weaver KR, Sherwin LB.et al. Effects of a comprehensive self-management intervention on extraintestinal symptoms among patients with IBS. J Psychosom Res. 2019;126:109821. 10.1016/j.jpsychores.2019.109821) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. . Fournier A, Mondillon L, Dantzer C.et al. Emotional overactivity in patients with irritable bowel syndrome. Neurogastroenterol Motil. 2018;30(10):e13387. 10.1111/nmo.13387) [DOI] [PubMed] [Google Scholar]
- 4. . Rostamkhani M, Khosravi S, Rezaie K, Forozan A, Rafiei F. The effect of ginger on the symptoms of irritable bowel syndrome: a randomized clinical trial. J Med Plants. 2018;4(68):112–121.. [Google Scholar]
- 5. . Patel A, Hasak S, Cassell B.et al. Effects of disturbed sleep on gastrointestinal and somatic pain symptoms in irritable bowel syndrome. Aliment Pharmacol Ther. 2016;44(3):246–258.. 10.1111/apt.13677) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. . Bellini M, Gemignani A, Gambaccini D.et al. Evaluation of latent links between irritable bowel syndrome and sleep quality. World J Gastroenterol. 2011;17(46):5089-5096. 10.3748/wjg.v17.i46.5089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. . Khanijow V, Prakash P, Emsellem HA, Borum ML, Doman DB. Sleep dysfunction and gastrointestinal diseases. Gastroenterol Hepatol (N Y). 2015;11(12):817-825. [PMC free article] [PubMed] [Google Scholar]
- 8. . Kanagasabai T, Ardern CI. Inflammation, oxidative stress, and antioxidants contribute to selected sleep quality and cardiometabolic health relationships: a cross-sectional study. Mediators Inflamm. 2015;2015:824589. 10.1155/2015/824589) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. . Ascacio-Valdés JA, Aguilera-Carbó A, Martínez-Hernández JL, Rodríguez-Herrera R, Aguilar CN. Euphorbia antisyphilitica residues as a new source of ellagic acid. Chem Pap. 2010;64(4):528–532.. 10.2478/s11696-010-0034-6) [DOI] [Google Scholar]
- 10. . García-Niño WR, Zazueta C. Ellagic acid: pharmacological activities and molecular mechanisms involved in liver protection. Pharmacol Res. 2015;97:84–103.. 10.1016/j.phrs.2015.04.008) [DOI] [PubMed] [Google Scholar]
- 11. . Panchal SK, Ward L, Brown L. Ellagic acid attenuates high-carbohydrate, high-fat diet-induced metabolic syndrome in rats. Eur J Nutr. 2013;52(2):559–568.. 10.1007/s00394-012-0358-9) [DOI] [PubMed] [Google Scholar]
- 12. . Uzar E, Alp H, Cevik MU.et al. Ellagic acid attenuates oxidative stress on brain and sciatic nerve and improves histopathology of brain in streptozotocin-induced diabetic rats. Neurol Sci. 2012;33(3):567–574.. 10.1007/s10072-011-0775-1) [DOI] [PubMed] [Google Scholar]
- 13. . Toghiani A, Maleki I, Afshar H, Kazemian A. Translation and validation of the Farsi version of Rome III diagnostic questionnaire for the adult functional gastrointestinal disorders. J Res Med Sci Off J Isfahan Univ Med Sci. 2016;21:103. 10.4103/1735-1995.193175) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. . Mollayeva T, Thurairajah P, Burton K.et al. The pittsburgh sleep quality Index as a screening tool for sleep dysfunction in clinical and non-clinical samples: a systematic review and meta-analysis. Sleep Med Rev. 2016;25:52–73.. 10.1016/j.smrv.2015.01.009) [DOI] [PubMed] [Google Scholar]
- 15. . Mosarrezaii A, Ghasemzadeh N, Rahimi-Golkhandan A.et al. Sleep quality in patients with multiple sclerosis. J Sleep Sci. 2018;3(1-2):17–20.. [Google Scholar]
- 16. . Mobilio N, Iovino P, Bruno V, Catapano S. Severity of irritable bowel syndrome in patients with temporomandibular disorders: a case-control study. J Clin Exp Dent. 2019;11(9):e802-e806. 10.4317/jced.55649) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. . Craig C, Marshall A, Sjostrom M.et al. International Physical Activity Questionnaire-Short Form; 2017. [Google Scholar]
- 18. . Hosseini B, Saedisomeolia A, Wood LG, Yaseri M, Tavasoli S. Effects of pomegranate extract supplementation on inflammation in overweight and obese individuals: a randomized controlled clinical trial. Complement Ther Clin Pract. 2016;22:44–50.. 10.1016/j.ctcp.2015.12.003) [DOI] [PubMed] [Google Scholar]
- 19. . Zhou L, Chen P, Peng Y, Ouyang R. Role of oxidative stress in the neurocognitive dysfunction of obstructive sleep apnea syndrome. Oxid Med Cell Longev. 2016;2016:9626831. 10.1155/2016/9626831) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. . Abbasnezhad A, Amani R, Hajiani E.et al. Effect of vitamin D on gastrointestinal symptoms and health-related quality of life in irritable bowel syndrome patients: a randomized double-blind clinical trial. Neurogastroenterol Motil. 2016;28(10):1533–1544.. 10.1111/nmo.12851) [DOI] [PubMed] [Google Scholar]
- 21. . Majid MS, Ahmad HS, Bizhan H, Hosein HZM, Mohammad A. The effect of vitamin D supplement on the score and quality of sleep in 20-50 year-old people with sleep disorders compared with control group. Nutr Neurosci. 2018;21(7):511–519.. 10.1080/1028415X.2017.1317395) [DOI] [PubMed] [Google Scholar]
- 22. . Alzoubi KH, Khabour OF, Rashid BA, Damaj IM, Salah HA. The neuroprotective effect of vitamin E on chronic sleep deprivation-induced memory impairment: the role of oxidative stress. Behav Brain Res. 2012;226(1):205–210.. 10.1016/j.bbr.2011.09.017) [DOI] [PubMed] [Google Scholar]
- 23. . Wightman EL, Haskell-Ramsay CF, Reay JL.et al. The effects of chronic trans-resveratrol supplementation on aspects of cognitive function, mood, sleep, health and cerebral blood flow in healthy, young humans. Br J Nutr. 2015;114(9):1427–1437.. 10.1017/S0007114515003037) [DOI] [PubMed] [Google Scholar]
- 24. . Blunden SL, Beebe DW. The contribution of intermittent hypoxia, sleep debt and sleep disruption to daytime performance deficits in children: consideration of respiratory and non-respiratory sleep disorders. Sleep Med Rev. 2006;10(2):109–118.. 10.1016/j.smrv.2005.11.003) [DOI] [PubMed] [Google Scholar]
- 25. . Kang HH, Kim IK, Lee SH. Total oxidant and antioxidant status in patients with obstructive sleep apnea and the effect of continuous positive airway pressure. Chronobiol Med. 2019;1(3):126–129.. 10.33069/cim.2019.0019) [DOI] [Google Scholar]
- 26. . Lungato L, Marques MS, Pereira VG.et al. Sleep deprivation alters gene expression and antioxidant enzyme activity in mice splenocytes. Scand J Immunol. 2013;77(3):195–199.. 10.1111/sji.12029) [DOI] [PubMed] [Google Scholar]
- 27. . Rizk NI, Rizk MS, Mohamed AS, Naguib YM. Attenuation of sleep deprivation dependent deterioration in male fertility parameters by vitamin C. Reprod Biol Endocrinol. 2020;18(1):2. 10.1186/s12958-020-0563-y) [DOI] [PMC free article] [PubMed] [Google Scholar]


