Background
Bile acid metabolism is a contributing factor that promotes cholelithiasis. Recent studies have suggested novel roles of leptin in the formation of gallbladder stones (GS); however, no evidence confirmed the function of leptin in the formation of primary intrahepatic bile duct stones (PIBDS) . In the current study, the liver tissues of patients with GS and PIBDS were collected to check the mRNA and protein expression levels of BSEP.Methods: L02 cells stimulated with leptin were served for the expression of OB-Rb, AMPKα2, and BSEP by quantitative-polymerase chain reaction (q-PCR), Western blot, and immunohistochemistry, respectively.Results: The results showed that the level of serum leptin was higher in the GS group than in the control and PIBDS groups. Compared with the control group, the expression levels of OB-Rb, p-AMPKa2, and BSEP decreased significantly in the GS and PIBDS groups. In vitro, compared with the control cells, the protein levels of OB-Rb, p-AMPKa2, and BSEP increased in the L02 cells cultured with leptin. However, these enhancements disappeared when the cells were co-cultured with leptin plus Compound C.Conclusion: The present results suggest that cholelithiasis, especially the formation of PIBDS, was connected with leptin, which could regulate bile acid metabolism through the OB-Rb/AMPKa2/BSEP signaling pathway.
Keywords: Leptin, bile acid, BSEP, cholelithiasis
INTRODUCTION
Cholelithiasis, which refers to the formation of gallstones (GS), primary intrahepatic bile duct stones (PIBDS), and extrahepatic bile duct stones (EBDS), is highly prevalent. The primary mechanisms of GS formation are biliary cholesterol supersaturation and gallbladder hypomotility.1 GS—mostly cholesterol stones—are related to lipid metabolism disorder. Pigment stones used to be the main PIBDS, but now mixed or even cholesterol stones are gradually increasing. The mechanisms underlying the formation of PIBDS are not clearly understood. Disordered bile acid metabolism is a key factor that triggers the precipitation of cholesterol microcrystals from supersaturated lithogenic bile.2 Hepatic hypersecretion and the decreased secretion of bile acid salts may result in a bile composition change and therefore trigger the pathogenesis of PIBDS.3 But few researches have examined the relationship between energy metabolism and PIBDS.
The bile salt export pump (BSEP) is a canalicular-specific exporter that is mostly expressed in the cholesterol-rich apical membranes of hepatocytes.4 BSEP facilitates the secretion of bile salts, lipids, cholesterol, and phosphatidylcholine from hepatocytes into bile canaliculi.5,6 BSEP dysfunction can induce the prevention of bile acid flow into bile canaliculi—a crucial event that leads to cholelithiasis.7 BSEP expression is ATP-dependent and can be enhanced by hepatocyte-specific AMP-activated protein kinase (AMPK), which was identified in a screen, thus promoting bile acid secretion.8 AMPK activation can act as a therapeutic agent to treat various metabolic diseases, such as vascular calcification and diabetes.9-11 However, AMPK activity is altered by numerous physiological factors, such as hormones, cytokines, and dietary nutrients.12
Leptin, a class I helical cytokine encoded by the obese gene (ob), regulates a multitude of physiological processes, including appetite, lipid metabolism, growth, reproduction, stress, and immune function through its interaction with the leptin receptor.13 The long-form leptin receptor (OB-Rb) is essential for multiple intracellular signal transduction pathways.14 Growing evidence shows that leptin may play an important role as a glucoregulatory hormone in the regulation of bile acid, via AMPK activation.15,16 However, the function of leptin has been less studied in cholelithiasis.
The present study aimed to investigate the relationship between leptin and PIBDS formation in terms of energy metabolism.
MATERIALS AND METHODS
The present study was a cross-sectional study conducted between February 2013 and December 2017 at the hospital. The hospital’s ethics committee approved the study protocol (ky2013027). All the study participants provided signed informed consent.
Reagents and Antibodies
Recombinant human leptin (P6969) and β-actin (AF0003) were purchased from Beyotime. AMPK inhibitor—Compound C—was purchased from CALBIOCHEM (171260). The rabbit polyclonal antibody of the leptin receptor was obtained from Abcam (ab5593). The antibody of AMPKα2 (CST2757) and Phospho-AMPKα2 (CST50081) was purchased from Cell Signaling Technology. The goat polyclonal antibody to BSEP was obtained from Santa Cruz (sc-17292).
Samples
Liver tissues and blood specimens were harvested intraoperatively from 68 patients with PIBDS and 89 patients with GS. Another set of 72 normal liver tissues and blood specimens, as the control group, were harvested from patients with hepatic hemangioma or traumatic liver rupture. Liver specimens were placed in liquid nitrogen after collection and were preserved at −80°C until use.
Inclusion and Exclusion Criteria
The inclusion criteria for the patients were as follows: a) without liver cirrhosis, atrophy, or cancer on preoperative ultrasound or magnetic resonance imaging (MRI) evaluation; b) without current episode of acute cholecystitis, cholangitis, or pancreatitis; and c) without diabetes or hyperthyroidism. Cases with postoperative diagnosis (e.g. cirrhosis, atrophy, or cancer) inconsistent with preoperative diagnosis were excluded.
Cell Culture and Treatment
Hepatocytes L02 were provided as a gift from the central laboratory of the Affiliated Hospital of Southwest Medical University. To find the best stimulatory conditions, L02 cells were incubated with a series of concentrations of leptin (10−8 mol/L, 10−7 mol/L, 10−6 mol/L). Subsequently, cells were collected at 24 h, 48 h, and 72 h to detect the protein expression of BSEP by Western blot. When the appropriate concentration of leptin was determined, L02 cells were incubated with PBS, leptin, or leptin plus Compound C for 0 h, 12 h, 24 h, 48 h, and 72 h (PBS is a phosphate-buffered saline solution, which is a solvent to dissolve the leptin and Compound C).
Human Serum and Supernatant Analysis
All plasma samples were harvested early morning from the participants on an empty stomach, and the sera were immediately separated by centrifugation and stored at −80°C until use. Supernatants were harvested for each time point after the cells were cultured with leptin. The leptin levels were measured using a commercial enzyme-linked immunoassay kit. The plasma leptin level was assessed by radioimmunoassay (RIA), using the kit for human leptin (XL-85K Multi-Species Leptin RIA, Millipore, USA).
The plasma total bile acids (TBA), total bilirubin (TBIL), alanine aminotransferase (ALT), and aspartate aminotransferase (AST) levels were assessed with an automatic biochemical analyzer.
L02 Intracellular Proteins and Medium Bile Acid Quantification
The L02 cell lysate and medium were separately collected. The content of TBA in the supernatant and medium was measured by a TBA kit (Nanjing SenBeiJia Biological Technology Co, Ltd., Nanjing, China) through an enzyme colorimetric method.
RNA Isolation and q-PCR
Total RNA was isolated from cells and tissues using the RNA kit (CW0597; cwbiotech, China). Following the protocols of the PrimeScript™ RT Reagent Kit (RR047A; Takara Biotechnology, Japan), the RNA samples were reverse transcribed for amplification. The ΔΔCt formula was used to count the expression levels of the target genes after normalization. The primers of target genes were as follows:
OB-Rb 5′ Forward, GCCTTCATAAGCCTACCAATGTAGA;
OB-Rb 5′ Reverse, GGAAACAAGGGACCTTCCCACTA;
AMPKα2 5′ Forward, CAGGAGATGCAGTCCAGCACAA;
AMPKα2 5′ Reverse, GCCCACATCTACCTATCACATTCAG;
BSEP 5′ Forward, TCGCTTGTCCACCATCCAGAA;
BSEP 5′ Reverse, GGGGATCCAGTGGTGACTAGTT.
Western Blotting
Based on the instructions described in the protein extraction kits, total protein from 3 groups of hepatocytes was extracted and stored in the fridge at −80°C. For immunoblotting, proteins were loaded at 50 µg on each polyacrylamide gel and electrotransferred to PVDF membranes. 5% skimmed milk powder/TBST buffer was used to block the PVDF membranes for 1 h and incubated with relevant antibodies specific for leptin, OB-Rb, AMPKa2, p-AMPKa2, and BSEP overnight at 4°C. The next day, the PVDF membranes were incubated with the corresponding secondary antibody at 37°C for 1 h. Finally, the immunoreactive bands were capture by Bio-rad gel imaging system, and the gray value of each band was measured using Image J software.
Statistical Analysis
All experiments were repeated at least 3 times. Data were expressed as x– ± s and analyzed with the Statistical Package for the Social Sciences (SPSS) 19.0 (IBM Corp.; Armonk, NY, USA). Differences among groups were determined by conducting multiple comparison tests, and relationship of the different variances of the groups with each other was analyzed with Pearson’s product-moment correlation coefficient. P < .05 or less indicated that the difference was statistically significant.
RESULTS
Leptin Associated with Abnormal Liver Function in Patients with Cholelithiasis
We collected data on patients with PIBDS, GS, and non-cholelithiasis for the study. The numbers of females in PIBDS and GS groups were more than males. The average age PIBDS group members was significantly higher than that of GS and control groups (Table 1). Serum leptin was determined by RIA in each group, and we found leptin in the GS group was higher than that in the PIBDS group and control group. Subsequently, indicators related to bile acid—including body mass index (BMI), AST, ALT, TBA, TBIL, direct bilirubin (DBIL), total cholesterol (TC), triglyceride (TG), cholecystokinin (CCK), and gamma-glutamyl transpeptidase (GGT)—were also determined, and there were certain differences between the groups. BMI and leptin of the GS group were significantly higher than those of the control group and the PIBDS group. Neither TC nor TG expression shows no significant difference among those three groups. CCK and GGT in the PIBDS group decreased compared with those in the control group and the GS group, respectively. When comparing AST, ALT, TBA, TBIL, and DBIL, which are associated with liver function, results showed that these indexes in the PIBDS group were significantly higher than those in the control group and the GS group. Analysis with Pearson’s product-moment correlation coefficient revealed that leptin positively correlated with BMI and CCK, and negatively with TBA in the GS group; there was a positive correlation between leptin and TBA, ALT, AST, TBIL, DBIL, in the PIBDS group (Table 2). It indicated leptin is significantly correlated with the liver function of patients with both forms of cholelithiasis, leptin disfunction may contribute to cholelithiasis.
Table 1.
Values Measured in the Control, GS, and PIBDS Groups
| Parameter | Control | GS | PIBDS | F | P |
|---|---|---|---|---|---|
| Cases | 72 | 89 | 68 | ||
| Male/Female | 35/37 | 39/50 | 27/41* | –Δ | .56 |
| Age (years) | 46.46 ± 8.58 | 51.6 ± 14.92 | 55.9 ± 13.01* | 2.40 | .36 |
| BMI (kg/m2) | 22.75 ± 3.69 | 23.12 ± 3.51* | 20.12 ± 2.36# | 10.43 | .04 |
| Leptin (μg/L) | 5.15 ± 0.33 | 8.78 ± 0.38* | 4.78 ± 0.33# | 41.28 | .01 |
| ALT (U/L) | 21.48 ± 1.05 | 28.88 ± 1.07 | 43.01 ± 4.93*# | 5.05 | .00 |
| AST (U/L) | 19.32 ± 1.04 | 29.81 ± 1.24 | 50.21 ± 5.99*# | 6.33 | .00 |
| TBA (μmol/L) | 3.90 ± 0.23 | 5.00 ± 0.34 | 10.37 ± 1.75*# | 2.62 | .09 |
| TBIL (μmol/L) | 12.54 ± 0.57 | 13.76 ± 0.73 | 22.36 ± 2.35*# | 3.13 | .00 |
| DBIL (μmol/L) | 3.81 ± 0.21 | 5.03 ± 0.35 | 11.53 ± 1.79*# | 3.42 | .00 |
| TC (mmol/L) | 4.35 ± 0.84 | 4.74 ± 1.12 | 4.5 ± 1.73 | 0.48 | .73 |
| TG (mmol/L) | 1.51 ± 1.2 | 1.96 ± 1.84 | 1.37 ± 0.88 | 1.34 | .89 |
| CCK (pg/mL) | 4.06 ± 1.77 | 4.76 ± 2.35 | 3.45 ± 1.78# | 2.06 | .04 |
| GGT (U/L) | 17.32 ± 13.58 | 137.41 ± 173.69 | 205 ± 192.37* | 5.28 | .00 |
*P < .05 vs. control group; # P < .05 vs. GS group; ΔChi-square test used, χ2 = 1.13.
BMI, body mass index; ALT, alanine aminotransferase; AST, aspartate aminotransferase; TBIL, total bilirubin; DBIL, direct reacting bilirubin; TC, total cholesterol; TG, triglycerides; CCK, cholecystokinin; GGT, gamma-glutamyl transpeptidase.
Table 2.
Relationship Between Leptin and Other Components Measured in the GS and PIBDS Groups
| Variables | Leptin (GS) | Leptin (PIBDS) | ||
|---|---|---|---|---|
| γ | P | γ | P | |
| BMI | 0.299 | .02 | 0.275 | .09 |
| TBA | −0.053 | .03 | 0.302 | .04 |
| ALT | 0.131 | .14 | 0.413 | .02 |
| AST | 0.014 | .21 | 0.299 | .01 |
| TBIL | −0.104 | .47 | 0.358 | .03 |
| DBIL | −0.101 | .32 | 0.355 | .03 |
| TC | 0.128 | .03 | 0.002 | .15 |
| TG | 0.087 | .06 | 0.445 | .08 |
| CCK | 0.253 | .04 | 0.053 | .25 |
| GGT | 0.036 | .08 | −0.015 | .13 |
The relationship between leptin and components measured in the GS group and PIBDS group was analyzed by Pearson’s product-moment correlation coefficient.
γ, Pearson correlation; P, significance.
Insufficiency of Leptin Associated with the Decline of BSEP
Leptin is an important regulator of energy metabolism. It was lower in the PIBDS group than in the control and GS groups (Table 1). Thus, we aimed to verify whether leptin was connected to engendering cholelithiasis. Neither TC nor TG expression shows no significant difference among those three groups.
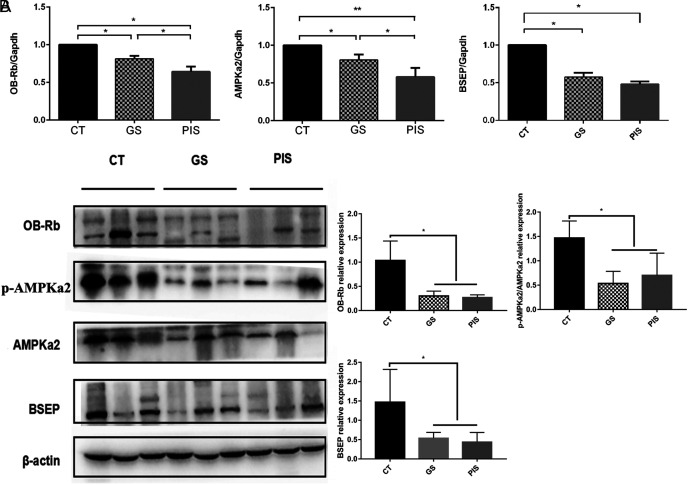
To further confirm the effect of leptin on cholelithiasis, qRT-PCR and Western blot were used to determine the mRNA and protein expression of OB-Rb, p-AMPKα2/AMPKα2 among three groups. The mRNA and protein levels of BSEP were lower in the GS and PIBDS groups than those in the control group (Figure 1A and B). Specifically, the mRNA level of BSEP in the PIBDS group showed a dramatic decline compared with that in the control group (Figure 1A).
Figure 1.
The mRNA and protein expression of OB-Rb, AMPKa2, p-AMPKa2, and BSEP in the 3 groups of patients. (A) The mRNA of OB-Rb, AMPKa2, and BSEP in CT group (n = 72) was higher than that in the other groups (GS, n = 89; PIBDS, n = 68). (B) The protein expression of OB-Rb, p-AMPKa2/AMPKa2, and BSEP in CT group was higher than that in the other groups. *P < .05.
We assume that leptin can regulate the expression of BSEP through OB-Rb, p-AMPKa2/AMPKa2 signaling pathway. To test this hypothesis, mRNA and protein expression levels of OB-Rb, p-AMPKa2/AMPKa2 in the liver of the 3 groups of patients were determined. As expected, the mRNA and protein expression levels of OB-Rb, p-AMPKa2/AMPKa2 also declined in the GS and PIBDS groups (Figure 2A and B), indicating that OB-Rb, p-AMPKa2/AMPKa2 expression was correlated with BSEP expression. In summary,inhibition of leptin function resulted in decreased p-AMPKa2/AMPKa2 and BSEP in the livers of the GS and PIS patients.
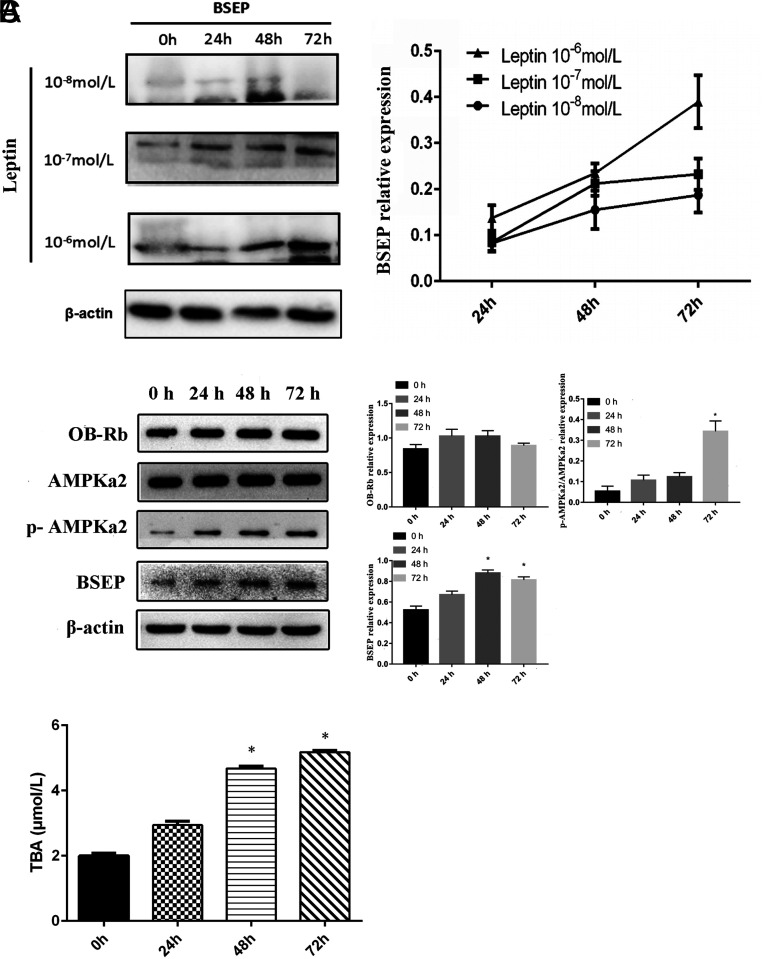
Figure 2.
Leptin promotes the production of TBA in L02 cells. (A) The best concentration of leptin was chosen to promote BSEP protein expression in L02 cells. (B and C) Leptin promoted the secretion of TBA by L02 cells through the OB-Rb/AMPKa2/BSEP signaling pathway. *P < .05.
The Expression of BSEP in L02 Cells Promoted by Leptin
To verify that leptin regulates hepatic bile acid metabolism through OB-Rb/p-AMPKa2/AMPKa2/BSEP signaling pathways, hepatocytes L02 were stimulated with leptin to observe the changes of these proteins. To determine the best concentration of leptin for the expression of BSEP in L02 cells, cells were co-cultured with different concentrations of leptin (10−8 mol/L, 10−7 mol/L, 10−6 mol/L) for 24 h, 48 h, and 72 h, respectively. Then, the cells were collected at each time point to detect BSEP expression by Western blot. Finally, we determined that a leptin concentration of 10−6 mol/L was appropriate to promote BSEP protein expression (Figure 2A). Afterward, the L02 cells were cultured with 10−6 mol/L leptin for 0 h, 24 h, 48 h, and 72 h and were harvested to detect the protein levels of OB-Rb, AMPKa2, p-AMPKa2, and BSEP. We found that the levels of these proteins increased gradually in a time-dependent manner and that most reached maximum values at 72 h, except for BSEP, which reached its maximum at 48 h (Figure 2B). Meanwhile, supernatants were collected to measure the TBA concentration at each time point. The TBA began to increase after leptin stimulation and reached a maximum at 72 h (Figure 2C). These results suggest that leptin promotes bile acid secretion in hepatocytes.
AMPKa2: A Key Protein in the Regulation of Leptin-Stimulated BSEP Expression
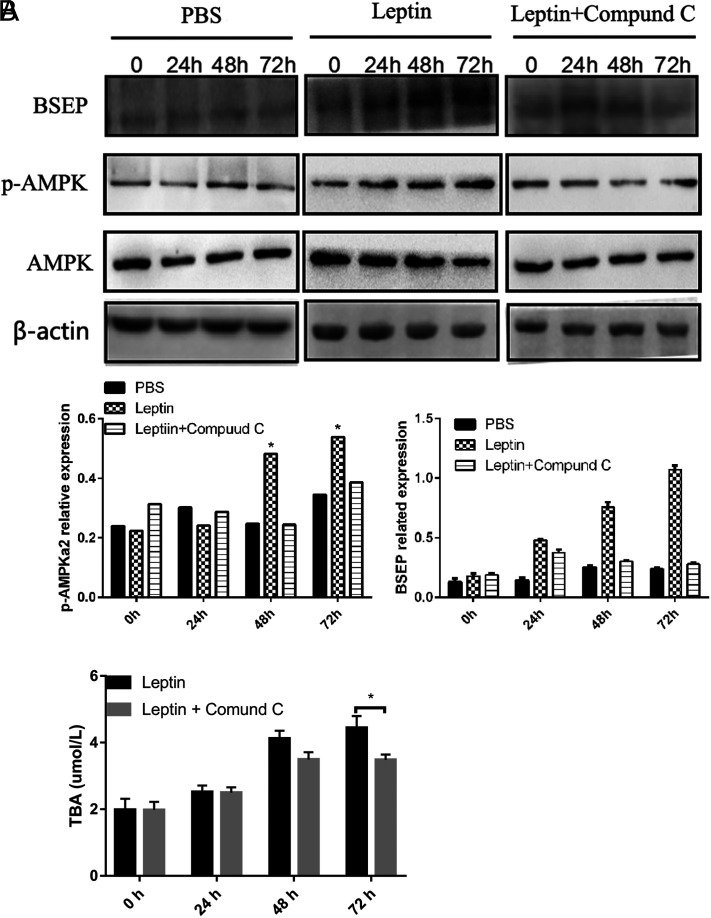
To assess whether the role of AMPKa2 was sufficient to trigger BSEP in L02 cells, Compound C, an inhibitor of AMPKa2, was added to the L02 co-culture. No change in BSEP expression was evident in the culture with PBS (Figure 3A). However, the promoting effect of leptin toward BSEP expression was significantly shut down when L02 cells were co-cultured with leptin and Compound C (Figure 3A). Moreover, BSEP expression in the leptin+ Compound C group also increased slightly at the early time point but began to decrease thereafter, especially at 72 h (Figure 3A). At the same time, the TBA content in the supernatant was detected, and it was found that the content of TBA in the leptin+ Compound C group was significantly lower than that in the leptin group (Figure 3B). By adding Compound C, it was confirmed that leptin indeed regulated bile acid excretion through the AMPKa2/BSEP signaling pathway.
Figure 3.
Compound C inhibits the leptin-mediated effect on BSEP. (A) BSEP expression in L02 cells cultured with leptin significantly increased at 48 h and 72 h, but not in cells cultured with leptin+ Compound C. (B). The content of TBA in L02 cells cultured with leptin was significantly increased. *P < .05.
DISCUSSION
Bile acids are synthesized in the liver and constitute the most important component of bile. Impaired bile acid flow leads to bile disproportionality that is characterized by the separating out of cholesterol and by cholestasis.17 The sedimentation of cholesterol or calcium bilirubinate, introduced by various causes of bile compositional change, is the main factor involved in cholelithiasis.18 Although the causes of cholelithiasis, especially of GS, have been extensively studied, whether bile acid dysfunction initiates PIBDS remains unknown.
Leptin and its long form receptor, OB-Rb, are related to the generation and development of multiple metabolic diseases, especially obesity and diabetes, and also cardiovascular disease.19,20 On measuring the serum leptin concentrations of the cholelithiasis patients, the leptin concentration was found to be significantly high in the GS group, but not in the PIBDS group, compared with the control group. The high expression of leptin was related to the occurrence of GS; however, whether the low expression of leptin was related to the occurrence of PIBDS is unknown. There was a negative correlation between leptin and TBA, ALT, AST, TBIL, and DBIL in the PIBDS group, but not in the GS group. These results suggested that though both caused by Leptin dysfunction, the pathogenesis of GS and PIS are different.
OB-Rb in the liver was observed to have dropped in the GS group and PIBDS group. In the absence of OB-Rb, no matter how much leptin was expressed, effective biological function of leptin could not be analyzed. It is mentionable that elevated circulating leptin levels and hypothalamic leptin resistance are associated with obesity.21 Leptin signaling is blocked by chronic overstimulation of the leptin receptor or hypothalamic pro-inflammatory responses, which, due to elevated levels of saturated fatty acids, can lead to leptin resistance by activating negative feedback pathways.22 These can explain that the mismatch between high leptin and the low amount of OB-Rb expression in the GS patients resembled the dynamic for leptin resistance in obesity. Regardless of the leptin level, the biological function of leptin cannot be fully understood due to the low expression of OB-Rb. As a deficiency of leptin induces both adiposity- and obesity-related tissue damage,23 leptin may participate in bile acid secretion. Thus, we hypothesized that leptin could affect BSEP—a key factor in the secretion of bile salts—to regulate the progress of cholelithiasis through interactions with OB-Rb. In our study, BSEP expression was found to be significantly decreased, while serum bile acids increased in the PIBDS group compared with the control group. BSEP expression and the supernatant bile acid concentration significantly increased in L02 cells by leptin stimulation. Therefore, we identified a connection between leptin and BSEP, and this connection may provide a new explanation of how leptin affects bile acid.
The exact molecular mechanism underlying the leptin regulation of BSEP in the liver is not clear.24 Because BSEP belongs to the ABC (ATP-binding cassette transporters) super-gene family, whose transporting functions are completely dependent on ATP, we speculated that leptin regulated BSEP through an ATP-related protein. Porat-Shliom N has found that the loss of LKB1 or AMPK impairs apical BSEP trafficking and bile canalicular formation, resulting in cholestasis.25
The hepatic AMPK-SRC-2 axis regulates bile acid secretion into the gut, and the insufficient bile secretion caused by this axis can be completely rescued by replenishing intestinal BA or by genetically restoring the levels of BSEP.8 On measuring AMPK expression in the patients with cholelithiasis, we discovered that the levels had declined in both the GS and PIBDS groups compared to those in the control group. To examine more precisely the role of AMPKa2 in leptin-regulated BSEP expression, we treated cells with Compound C, an inhibitor of AMPKa2, to confirm whether AMPKa2 played an important role in BSEP expression when L02 cells were stimulated with leptin. The enhanced effect of leptin on BSEP was blocked by Compound C. Thus, we have directly demonstrated that AMPKa2 is a key player in the leptin regulation of BSEP expression.
The present study reveals that leptin can regulate bile acid metabolism through the OB-Rb/AMPKa2/BSEP axis. Abnormal leptin expression, including elevation or reduction, can lead to cholelithiasis. These findings are relevant to the pathogenesis of cholelithiasis, especially with regard to PIBDS, which inhibits the leptin pathway in the liver.
Funding Statement
This study was supported by a grant from the Youth Fund Projectsof Luzhou medical university (No.2014QN-063). Doctoral Research Fund of The Affiliated Hospital of Southwest Medical University (NO.19072).
Footnotes
Ethics Committee Approval: The Ethics committee approval was received for this study from the Ethics Committee of Affiliated Hospital of Luzhou Medical College, NO.ky2013027.
Informed Consent: Written informed consent was obtained from the patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - M.Y.; Design - W.F.; Supervision - Z.L.; Resource - J.W.; Materials - J.H.; Data Collection and/or Processing - J.H., Y.J.; Analysis and/or Interpretation - J.W., M.Y.; Literature Search - J.W., J.H.; Writing - J.W., W.F.; Critical Reviews - M.Y., W.F.
Acknowledgments: We thank the staff of Academician(Expert) Work Station of Sichuan Province, The Affiliated Hospital of Southwest Medical University, Luzhou, China.
Conflict of Interest: The authors have no conflict of interest to declare.
References
- 1. . Tharp KM, Khalifeh-Soltani A, Park HM.et al. Prevention of gallbladder hypomotility via FATP2 inhibition protects from lithogenic diet-induced cholelithiasis. Am J Physiol Gastrointest Liver Physiol. 2016;310(10):G855–G864.. 10.1152/ajpgi.00316.2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. . Pasternak A, Gil K, Matyja A. Telocytes: new players in gallstone disease. Adv Exp Med Biol. 2016;913:7 7–1. 03. 10.1007/978-981-10-1061-3_5) [DOI] [PubMed] [Google Scholar]
- 3. . Di Ciaula A, Garruti G, Wang DQ, Portincasa P. Cholecystectomy and risk of metabolic syndrome. Eur J Intern Med. 2018;53:3–1. 1. 10.1016/j.ejim.2018.04.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. . Kim SR, Saito Y, Itoda M.et al. Genetic variations of the ABC transporter gene ABCB11 encoding the human bile salt export pump (BSEP) in a Japanese population. Drug Metab Pharmacokinet. 2009;24(3):27 7–2. 81. 10.2133/dmpk.24.277) [DOI] [PubMed] [Google Scholar]
- 5. . Cheng X, Buckley D, Klaassen CD. Regulation of hepatic bile acid transporters Ntcp and Bsep expression. Biochem Pharmacol. 2007;74(11):166 5–1. 676. 10.1016/j.bcp.2007.08.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. . Keogh JP. Membrane transporters in drug development. Adv Pharmacol. 2012;63:1–4. 2. 10.1016/B978-0-12-398339-8.00001-X) [DOI] [PubMed] [Google Scholar]
- 7. . Kong J, Liu BB, Wu SD, Wang Y, Jiang QQ, Guo EL. Enhancement of interaction of BSEP and HAX-1 on the canalicular membrane of hepatocytes in a mouse model of cholesterol cholelithiasis. Int J Clin Exp Pathol. 2014;7(4):164 4–1. 650. [PMC free article] [PubMed] [Google Scholar]
- 8. . Chopra AR, Kommagani R, Saha P.et al. Cellular energy depletion resets whole-body energy by promoting coactivator-mediated dietary fuel absorption. Cell Metab. 2011;13(1):3 5–4. 3. 10.1016/j.cmet.2010.12.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. . Kume S, Kondo M, Maeda S.et al. Hypothalamic AMP-activated protein kinase regulates biphasic insulin secretion from pancreatic β cells during fasting and in Type 2 diabetes. EBiomedicine. 2016;13:16 8–1. 80. 10.1016/j.ebiom.2016.10.038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. . Xu M, Liu L, Song C, Chen W, Gui S. Ghrelin improves vascular autophagy in rats with vascular calcification. Life Sci. 2017;179:2 3–2. 9. 10.1016/j.lfs.2016.11.025) [DOI] [PubMed] [Google Scholar]
- 11. . Zhang F, Zhao S, Yan W.et al. Branched chain amino acids cause liver injury in obese/diabetic mice by promoting adipocyte lipolysis and inhibiting hepatic autophagy. EBiomedicine. 2016;13:15 7–1. 67. 10.1016/j.ebiom.2016.10.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. . Viollet B, Horman S, Leclerc J.et al. AMPK inhibition in health and disease. Crit Rev Biochem Mol Biol. 2010;45(4):27 6–2. 95. 10.3109/10409238.2010.488215) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. . Deck CA, Honeycutt JL, Cheung E, Reynolds HM, Borski RJ. Assessing the functional role of leptin in energy homeostasis and the stress response in vertebrates. Front Endocrinol. 2017;8:63. 10.3389/fendo.2017.00063) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. . Burguera B, Couce ME, Long J.et al. The long form of the leptin receptor (OB-Rb) is widely expressed in the human brain. Neuroendocrinology. 2000;71(3):18 7–1. 95. 10.1159/000054536) [DOI] [PubMed] [Google Scholar]
- 15. . Mullur R, Liu YY, Brent GA. Thyroid hormone regulation of metabolism. Physiol Rev. 2014;94(2):35 5–3. 82. 10.1152/physrev.00030.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. . Bhuiyan MJ, Do HV, Mun S.et al. Hypocholesterolemic and hypoglycemic effects of enzymatically modified carbohydrates from rice in high-fat-fed C57BL/6J mice. Mol Nutr Food Res. 2011;55(Suppl 2):S214–S226.. 10.1002/mnfr.201100121) [DOI] [PubMed] [Google Scholar]
- 17. . Cai SY, Boyer JL. Studies on the mechanisms of bile acid initiated hepatic inflammation in cholestatic liver injury. Inflamm Cell Signal. 2017;4(2):2e1561. [PMC free article] [PubMed] [Google Scholar]
- 18. . Jungst D, Del Pozo R, Christoph S.et al. Sedimentation of biliary sludge: effect on composition of gallbladder bile from patients with cholesterol, mixed, or pigment stones. Scand J Gastroenterol. 1996;31(3):27 3–2. 78. 10.3109/00365529609004878) [DOI] [PubMed] [Google Scholar]
- 19. . Ramos-Lobo AM, Donato Jr J. The role of leptin in health and disease. Temperature. 2017;4(3):258–291.. 10.1080/23328940.2017.1327003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. . Ekmen N, Helvaci A, Gunaldi M, Sasani H, Yildirmak ST. Leptin as an important link between obesity and cardiovascular risk factors in men with acute myocardial infarction. Indian Heart J. 2016;68(2):13 2–1. 37. 10.1016/j.ihj.2015.07.032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. . Hubert A, Bochenek ML, Schütz E, Gogiraju R, Münzel T, Schäfer K. Selective deletion of leptin signaling in endothelial cells enhances neointima formation and phenocopies the vascular effects of diet-induced obesity in mice. Arterioscler Thromb Vasc Biol. 2017;37(9):168 3–1. 697. 10.1161/ATVBAHA.117.309798) [DOI] [PubMed] [Google Scholar]
- 22. . Balland E, Cowley MA. New insights in leptin resistance mechanisms in mice. Front Neuroendocrinol. 2015;39:5 9–6. 5. 10.1016/j.yfrne.2015.09.004) [DOI] [PubMed] [Google Scholar]
- 23. . Thon M, Hosoi T, Chea C, Ozawa K. Loss of stearoyl-CoA desaturase-1 activity induced leptin resistance in neuronal cells. Biol Pharm Bull. 2017;40(8):116 1–1. 164. 10.1248/bpb.b17-00311) [DOI] [PubMed] [Google Scholar]
- 24. . Chen YS, Liu HM, Lee TY. Ursodeoxycholic acid regulates hepatic energy homeostasis and white adipose tissue macrophages polarization in leptin-deficiency obese mice. Cells. 2019;8(3):253. 10.3390/cells8030253) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. . Porat-Shliom N, Tietgens AJ, Van Itallie CM.et al. Liver kinase B1 regulates hepatocellular tight junction distribution and function in vivo. Hepatology. 2016;64(4):131 7–1. 329. 10.1002/hep.28724) [DOI] [PMC free article] [PubMed] [Google Scholar]