Abstract
Background:
Acute pancreatitis (AP) was one of the most common disorders of acute hospital admission with significant morbidity and mortality. Some of the patients experienced recurrent attacks of AP, leading to recurrent acute pancreatitis (RAP) and poor clinical outcomes. The association of clinical and laboratory variables with recurrence of AP were analyzed to evaluate the risk of RAP.
Methods:
All patients with AP admitted in the hospital between January 2017 and December 2019 were included in this study. Clinical and laboratory variables were analyzed and risk factors were identified by multivariate logistic regression. The receiver operating characteristic (ROC) analysis for predicting recurrence of AP was performed.
Results:
A total of 834 AP patients, including 671 in the non-RAP group and 167 in the RAP group, were enrolled in the study. There were significant differences in age, sex, body mass index (BMI), history of biliary surgery, cholelithiasis, diabetes, triglyceride (TG), high-density lipoprotein, and cholesterol between the non-RAP group and the RAP group. Two independent variables were identified as risk factors for recurrence of AP: TG (P = .007, odds ratio [OR] = 1.101, 95% CI, 1.025-1.183), and BMI (P = .032, OR = 1.094, 95% CI, 1.009-1.086). The area under the curve of ROC analysis of TG and BMI were 0.702 (95% CI, 0.655-0.749) and 0.593 (95% CI, 0.538-0.647). The best threshold for TG and BMI to anticipate recurrence of AP were 5.9 (sensitivity0.763, specificity 0.595) and 28.24 (sensitivity 0.302, specificity 0.844).
Conclusion:
TG and BMI were identified as independent predictors for recurrence of AP. A TG level of 5.9 mmol/L could be a clinical guide for the target level of lowering TG therapy in AP patients with hypertriglyceridemia.
Keywords: Recurrent acute pancreatitis, triglyceride, body mass index
Main Points
Triglyceride (TG) and body mass index (BMI) are associated with recurrence of acute pancreatitis (AP).
For AP patients with hypertriglyceridemia, a TG level of 5.9mmol/L could be a clinical guidance for the target level of lowering TG therapy.
Losing some weight could be an effective way to avoid recurrence of AP.
Introduction
Acute pancreatitis (AP) was one of the most common disorders of acute hospital admission with significant morbidity and mortality.1 Around 10-40% patients with AP experienced recurrent attack of AP, leading to recurrent acute pancreatitis (RAP) and poor clinical outcomes.2 A research on data from 5 European countries with 1068 patients with AP clarified that 28% had recurrent pancreatitis.3 In China, a multicenter retrospective study with 1471 AP patients showed that the average incidence of RAP was 10.7%, which varied with different etiologies from 5.7% to 20.4%.4
Clinical researches on RAP demonstrated that the incidence of etiologies in RAP varied. In the United States, alcohol accounted for one-third of the cases of RAP,5 the same as in some European countries.6 Cholelithiasis, another common cause of RAP, was responsible for 20-30% of the cases of RAP,7 and recurrence still occurred in 4-8% the patients treated with cholecystectomy or endoscopic sphincterotomy as well.8 Hypertriglyceridemia (HTG) as a metabolic cause of RAP has been reported in nearly one-quarter of the patients in China.4
Previous studies with RAP mainly focused on the analysis of clinical characteristics and outcomes of RAP.2,9-11 Few researches discussed and investigated the association of clinical and laboratory variables with the recurrence of AP. The aim of our study was to comprehensively assess the differences in clinical features between RAP and non-RAP patients in order to achieve clinical guidance for RAP management.
Materials and Methods
Patients
All patients with AP admitted in the hospital between January 2017 and December 2019 were included in this retrospective study. The inclusion criteria were defined as follows: age ≥18 and confirmed diagnosis of AP. The exclusion criteria were defined as follows: death, age<18, chronic pancreatitis, past medical history of AP, malignant tumors, chronic organ dysfunction (kidney, heart, or liver dysfunction), pregnancy, and patients with missing data.
Definition
AP was diagnosed when the clinical, laboratory, and imaging characteristics of patients conformed with at least 2 of the following criteria: (1) abdominal pain relating to AP, (2) serum lipase or amylase increased, with the levels at at least 3 times more than the normal threshold, and (3) abdominal ultrasonic and/or CT scan demonstrating the classic image of AP.12
Recurrent AP (RAP) was defined as follows: (1) confirmed AP, and (2) the interval period between 2 or more separate attacks of AP with complete recovery of at least more than 3 months.13
According to the etiology of AP, 3 common etiological factors including cholelithiasis, alcohol, and hyperlipidemia were contained in this study. Cholelithiasis was confirmed by imaging examination when abdominal ultrasonic and/or CT scan showed calculi or sludge in the gallbladder and/or bile duct significantly visualized. Alcohol was defined as a etiological factor with AP when its consumption had reached at least 80 g/day for more than 5 years or regular alcohol abuse on social or weekends had been continued for at least the same number of years.12 Hypertriglyceridemia associated with AP was defined as follows: the level of triglyceride ≥ 11.3 mmol/L or ≥5.65mmol/L accompanied by milky serum.14
Data Collection
General characteristics including age, sex, smoking, and body mass index (BMI) and etiologies including cholelithiasis, alcohol, and hypertriglyceridemia (HTG) were recorded. Comorbidities including diabetes, hypertension, and coronary heart disease and medical history of biliary surgery were collected. Laboratory variables such as white blood cell counts (WBC), platelets, activated partial thromboplastin time (APTT), fibrinogen (Fbg), blood urea nitrogen (BUN), creatinine, amylase, glycosylated hemoglobin, blood sugar, triglyceride (TG), high-density lipoprotein (HDL), low-density lipoprotein (LDL), and cholesterol were collected while patients were admitted, in 24 hours.
The scores of sequential organ failure assessment (SOFA) and bedside index of severity in acute pancreatitis (BISAP) were performed after admission. Management therapies including cholecystectomy and oral lipid-lowering drugs were recorded. The clinical outcomes were length of stay (LOS) in hospital and ICU admission. The number and time of attacks of AP for each patient were also recorded.
Statistical Analysis
Characteristics were demonstrated as means (standard deviations) or medians (interquartile ranges, IQR) for continuous variables and a percentage or frequency for categorical variables. Continuous variables were compared by the Student’s t-test (normal distribution) or the Mann–Whitney U-test (skewed distribution), while categorical variables were compared by Fisher’s exact test or chi-square analysis. The variables included in multivariate logistic regression were those which were significant in univariate analysis. Finally, the receiver operating characteristic (ROC) analysis for predicting recurrence of AP was performed and the cut-off point, and the summarized area under the curve (AUC) estimates were determined. A P-value of < .05 was defined as statistically significant.
Results
Comparison of General Information of Patients Between the RAP and Non-RAP Groups
Initially, a total of 870 patients with AP were enrolled in our study. According to the exclusion criteria, 46 patients were ruled out, and 834 AP patients including 671 in non-RAP group and 167 in RAP group were analyzed (Figure 1).
Figure 1.
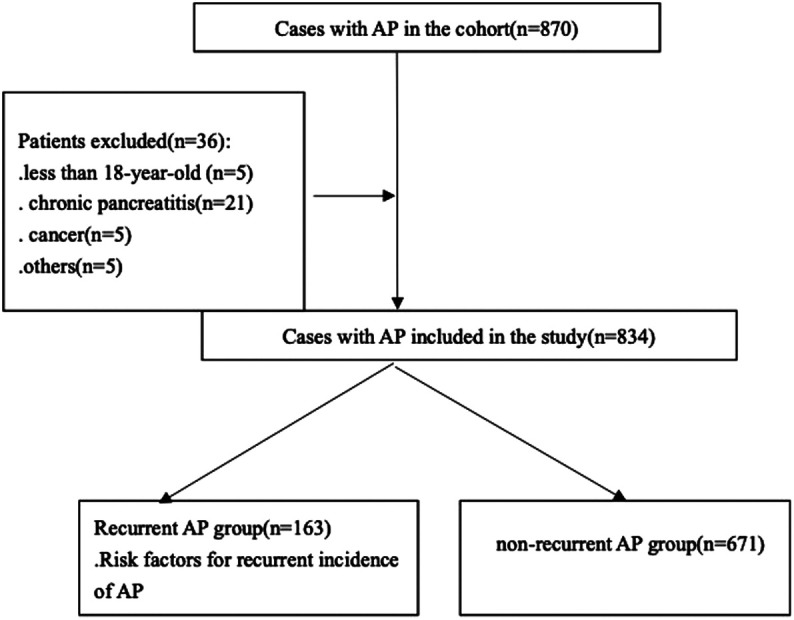
Flow chart for patients’ enrollment and study design.
The general information is demonstrated in Table 1. There were significant differences in age, sex, and BMI between the 2 groups (all P < .001). The patients in the RAP group were younger (40.27 ± 10.00 vs. 48.00 ± 14.96), with a higher BMI (26.33 ± 4.29 vs. 24.68 ± 3.96) compared to the patients in the non-RAP group. The proportion of males in RAP group was 80.98%, while it was 67.06% in the non-RAP group.
Table 1.
Comparison Baseline Characteristics Between RAP and Non-RAP Groups
| Baseline Variables | Non-RAP (n = 671) | RAP (n = 163) | P |
|---|---|---|---|
| Age (years) | 48.00 ± 14.96 | 40.27 ± 10.00 | <.001 |
| Sex | <.001 | ||
| Male (n, %) | 450 (67.06) | 132 (80.98) | |
| Female (n, %) | 221 (32.94) | 31 (19.02) | |
| Smoking (n, %) | 197 (29.35) | 45 (27.6) | .618 |
| BMI (kg/m2) | 24.68 ± 3.96 | 26.33 ± 4.29 | <.001 |
| Etiologies (n, %) | |||
| Cholelithiasis | 168 (25.04) | 9 (5.52) | <.001 |
| Alcohol | 149 (22.21) | 33 (20.25) | .583 |
| Hypertriglyceridemia | 244 (36.36) | 105 (64.42) | <.001 |
| Comorbidities (n, %) | |||
| Diabetes | 97 (14.46) | 37 (22.70) | .010 |
| Hypertension | 124 (18.48) | 26 (15.95) | .451 |
| Coronary heart disease | 27 (4.02) | 3 (1.84) | .179 |
| History of biliary surgery | 64 (9.54) | 1 (0.61) | <.001 |
| Laboratory variables | |||
| White blood cell count (×109/L) | 12.89 (7.38-18.4) | 12.48 (5.61-19.35) | .417 |
| Platelet (×109/L) | 207.8 (151.13-262.3) | 218 (155.1-280.9) | .153 |
| APTT (s) | 28.36 (22.65-34.07) | 27.5 (21.05-33.95) | .220 |
| Fbg (mg/dL) | 3.3 (2.15-4.45) | 3.4 (2.09-4.71) | .355 |
| BUN (mmol/L) | 5.33 (1.9-9.61) | 5.15 (2-11.03) | .219 |
| Creatinine (µmmol/L) | 74.45 (34.75-114.15) | 71.8 (32.6-111) | .743 |
| Amylase (U/L) | 1250 (390-1650) | 1145 (450-1705) | .150 |
| Glycosylated hemoglobin | 7.21 ± 2.38 | 7.43 ± 2.11 | .627 |
| Blood glucose (mmol/L) | 7.8 (6.6-9.9) | 10.15 (7.47-12.83) | .230 |
| TG (mmol/L) | 4.27 (1.39-16) | 15.52 (6-25.18) | <.001 |
| HDL (mmol/L) | 0.98 (0.66-1.33) | 0.69 (0.53-0.87) | <.001 |
| LDL (mmol/L) | 2.17 (1.47-2.94) | 1.85 (1.13-2.84) | .051 |
| Cholesterol (mmol/L) | 5.73 (4.72-8.26) | 7.63 (5.64-10.76) | <.001 |
| Scoring system | |||
| BISAP | 1 (0-1) | 1 (0-2) | .105 |
| SOFA | 0 (0-0.75) | 0 (0-1) | .565 |
| Management (n, %) | |||
| Lipid-lowering therapy | 244 (36.36%) | 105 (64.42%) | <.001 |
| Cholecystectomy | 33 (4.92%) | 7 (4.29%) | .102 |
| Clinical outcomes | |||
| ICU admission (n, %) | 58 (8.64) | 15 (9.2) | .113 |
| LOS in hospital (day) | 6 (5-9) | 7 (5-10) | .004 |
RAP, recurrent acute pancreatitis; BMI, body mass index; APTT, activated partial thromboplastin time; Fbg, fibrinogen; BUN, blood urea nitrogen; TG, triglyceride; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SOFA, sequential organ failure assessment; BISAP, bedside index of severity in acute pancreatitis; LOS, length of stay.
With regard to etiology classification, there were significant differences in cholelithiasis and hypertriglyceridemia between the 2 groups (both P < .001), while there was no significant difference in alcohol consumption. In the RAP group, more patients had hypertriglyceridemia (64.42% vs. 36.63).
Among the comorbidities of diabetes, hypertension, and coronary heart disease, patients with diabetes were likely to be suffering from recurring attack of AP (22.7% vs. 14.46%), while more patients in the non-RAP group had medical history of biliary surgery (9.54% vs. 0.61%). There was no difference in the SOFA and Ranson scores between the 2 groups. With regard to the management, more patients in the RAP group were administered lipid-lowering therapy due to hypertriglyceridemia (P < .001). The median length of stay (LOS) in the hospital was significantly longer (P = .004) for patients in the RAP group.
Different Laboratory Variables in the RAP and Non-RAP Groups
In our study, there were no significant differences between the 2 groups in laboratory variables including WBC, platelets, APTT, Fbg, BUN, creatinine, amylase, glycosylated hemoglobin, blood sugar, and LDL (Table 1). In the RAP group, patients had significantly higher level of TG and cholesterol (both P < .001) and lower level of HDL (P < .001).
Analysis of Recurrence Frequency and Times of AP in RAP Group
Of 163 patients in the RAP group, 110 (67.48%) experienced 2 relapses, 24 (14.72%) had 3 relapses, and 29 (17.79%) had 4 relapses (Figure 2). Comparing the time of first relapse, 43(26.38%) patients had the relapse within 6 months, 74 (45.4%) had recurrence in a year, 32 (19.63%) in 18 months, 7 (4.29%) in 2 years, and 7 (4.29%) in more than 2 years after discharge (Figure 3).
Figure 2.
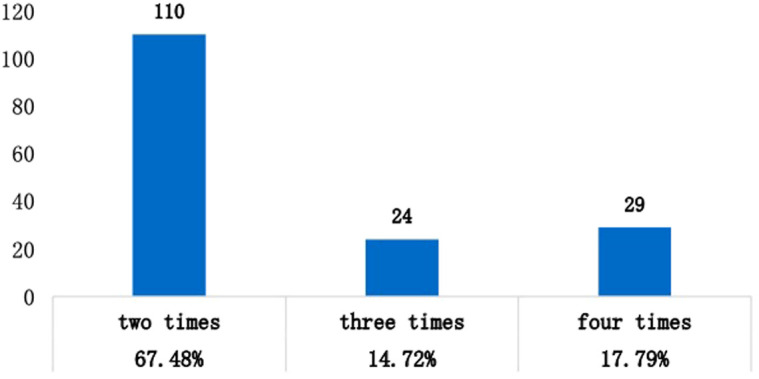
Distribution of number of attacks in 163 patients with RAP.
Figure 3.
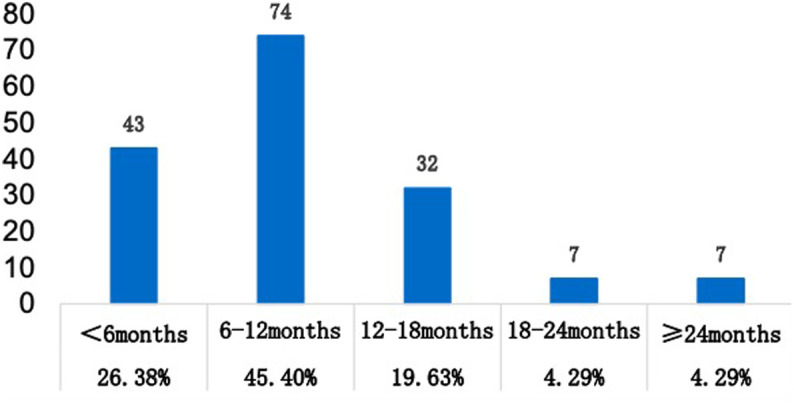
Distribution of different times of recurrent attacks in 163 patients with RAP.
Multivariate Logistic Regression Analysis of Variables Associated with Recurrence of AP
Two independent variables were identified as risk factors for recurrence of AP: TG (P = .007, Odds ratio (OR) = 1.101, 95% CI, 1.025-1.183), and BMI (P = .032, OR = 1.094, 95% CI, 1.009-1.086) (Table 2).
Table 2.
Multivariate Logistic Regression Analysis of Variables Associated with Recurrence of AP
| Variables | B | SE | Wald | P | OR | 95% CI for OR Lower | Upper |
|---|---|---|---|---|---|---|---|
| Sex | −0.803 | 0.418 | 3.705 | .054 | 0.448 | 0.197 | 1.018 |
| Age | −0.001 | 0.017 | 0.003 | .953 | 1.001 | 0.967 | 1.036 |
| BMI | 0.088 | 0.041 | 4.604 | .032 | 1.094 | 1.009 | 1.186 |
| History of biliary surgery | −0.596 | 1.187 | 0.252 | .616 | 0.745 | 0.066 | 8.371 |
| Cholelithiasis | −0.380 | 0.697 | 0.298 | .585 | 0.461 | 0.098 | 2.181 |
| Diabetes | 0.441 | 0.438 | 1.011 | .315 | 1.559 | 0.660 | 3.680 |
| TG | 0.099 | 0.037 | 7.296 | .007 | 1.101 | 1.025 | 1.183 |
| HDL | −0.106 | 0.076 | 1.944 | .163 | 0.477 | 0.120 | 1.904 |
| Cholesterol | −0.73 | 0.700 | 1.088 | .297 | 0.900 | 0.775 | 1.044 |
AP, acute pancreatitis; BMI, body mass index; TG, triglyceride; HDL, high-density lipoprotein; SE, standard error; OR, odds ratio.
ROC Analysis
The AUC of ROC analysis of TG and BMI were 0.702 (95% CI, 0.655-0.749) and 0.593 (95% CI, 0.538-0.647). The best threshold for TG and BMI to anticipate recurrence of AP were 5.9 (sensitivity 0.763, specificity 0.595) and 28.24 (sensitivity 0.302, specificity 0.844) respectively (Table 3 and Figure 4).
Table 3.
Predictive Performance of TG and BMI
| Variables | ROC | 95% CI | Best Threshold | Sensitivity | Specificity |
|---|---|---|---|---|---|
| TG | 0.702 | 0.655-0.749 | 5.9 mmol/L | 0.763 | 0.595 |
| BMI | 0.593 | 0.538-0.647 | 28.24 kg/m2 | 0.302 | 0.844 |
BMI, body mass index; TG, triglyceride; OR, odds ratio; ROC, receiver operating characteristic.
Figure 4.
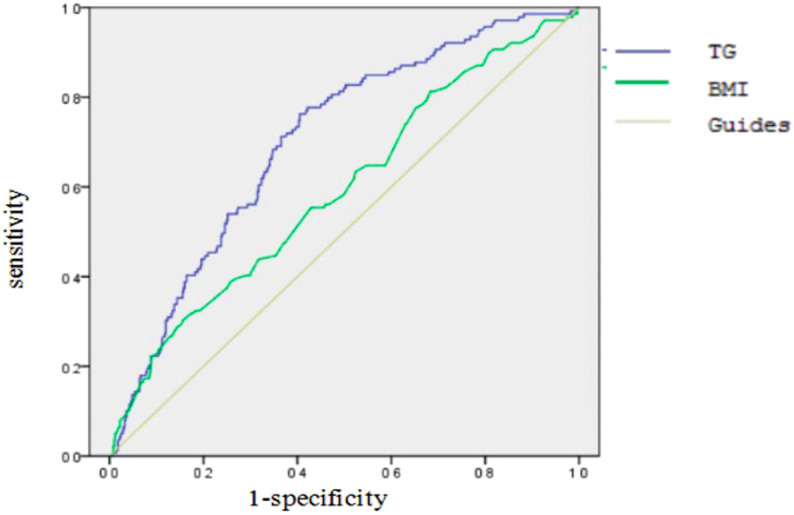
Analysis of the receiver operating characteristic curves of TG and BMI.
TG in Different Subgroups Between the RAP and Non-RAP Cohorts
TG in different subgroups (HTG, alcohol, and cholelithiasis) between RAP and non-RAP cohorts were analyzed in Table 4. The levels of TG showed significant differences between the RAP and non-RAP cohorts in hypertriglyceridemia (P = .031) and alcohol (P = .045) subgroups, and no significant difference in the cholelithiasis group (P = .696).
Table 4.
TG Levels in Different Subgroups Between RAP and Non-RAP Cohorts
| Non-RAP | RAP | P | |
|---|---|---|---|
| HTG (n = 349) | 16.14 (8.01-25.59) | 21.17( 11.63-26.24) | .031 |
| Alcohol (n = 182) | 3.2 (1.67-5.68) | 4.3 (2.33-6.16) | .045 |
| Cholelithiasis (n = 177) | 1.26 (0.87-1.84) | 1.19 (1.04-1.24) | .696 |
HTG, hypertriglyceridemia; TG, triglyceride; RAP, recurrent acute pancreatitis.
TG Levels at Different Times During Hospitalization in Patients with HTG Between the RAP and Non-RAP Groups
All patients with HTG, including 105 in the RAP group and 244 in the non-RAP group were administered antilipidemic treatment (Table 1) after admission, and the TG levels were dynamically checked during hospitalization based on the medical records (Table 5). There was no significant difference in TG levels at different time points (48 hours after admission, 72 hours after admission, and the time before discharge) during hospitalization among patients with hypertriglyceridemia, of both the RAP and the non-RAP groups.
Table 5.
TG Levels at Different Times in Patients with HTG, in the RAP and Non-RAP Groups
| Non-RAP (n = 244) | RAP (n = 105) | P | |
|---|---|---|---|
| 48 hours after admission | 4 (2.26-7.95) | 4.64 (2.76-7.17) | .225 |
| 72 hours after admission | 3.82 (2.47-5.59) | 4.5 (3.42-6.525) | .105 |
| Time before discharge | 1.43 (0.88-2.01) | 1.54 (1.05-2.11) | .114 |
HTG, hypertriglyceridemia; TG, triglyceride; RAP, recurrent acute pancreatitis.
Analysis of Recurrence Frequency and Times of AP in HTG Group and Non-HTG Group
All patients were divided into HTG group (n = 349) and non-HTG group (n = 485) (Table 6). The recurrence of AP demonstrated significant difference between the 2 groups (30.08% vs. 11.96%, P < .001). Of 349 patients in HTG group, 60 (17.19%), 20 (5.73%), and 25 (7.16%) patients experienced 2 relapses, 3 relapses, and 4 relapses, respectively, while the corresponding numbers were 50 (10.13%), 4 (0.82%), and 4 (0,82%) in the non-HTG group, respectively. (all P < .001).
Table 6.
Recurrence Frequency and Times of AP in the HTG Group and Non-HTG Groups
| Non-HTG (n = 485) | HTG (n = 349) | P | |
|---|---|---|---|
| RAP (n, %) | 58 (11.96) | 105 (30.08) | <.001 |
| Twice | 50 (10.3) | 60 (17.19) | <.001 |
| Thrice | 4 (0.82) | 20 (5.73) | <.001 |
| Four-times | 4 (0.82) | 25 (7.16) | <.001 |
HTG, hypertriglyceridemia; RAP, recurrent acute pancreatitis.
Discussion
In our study, RAP was more common in AP patients who were of a younger age, male, and had increased BMI. Moreover, in patients with biliary pancreatitis, there was a significant decrease in recurrence with cholecystectomy. Significant differences of TG levels in the HTG and alcohol subgroups between non-RAP and RAP were found, which demonstrated that in RAP group, patients with HTG or alcohol had higher levels of TG compared those in non-RAP group. Moreover, the HTG group had a higher incidence of RAP and greater number of attacks of AP. Hypertriglyceridemia, as the most common etiology in both groups, was a risk factor for developing RAP. Triglycerides and BMI were identified as independent predictors for recurrence of AP.
Current evidence revealed that RAP was partly associated with younger males. An 8-year retrospective research on AP and RAP showed that males accounted for over 50% of AP patients, while nearly 80% of RAP patients were male. Compared with the average age, RAP patients were significantly younger (34.57 ± 10.65 vs. 39.49 ± 13.46).6 One study with etiology of RAP from Poland revealed that there was significant difference in age between RAP and non-RAP patients (50.1 ± 18.5 vs. 59.4 ± 17.1).10 Anther cohort study which included consecutive patients with AP admitted to the Cleveland Clinic between 2008 and 2011 proved that with the recurrence of attacks increasing, the proportion of males also increased, while the average age of RAP patients with more attacks decreased.15 Although the age and the proportion of males in RAP varied in different studies, the basic conclusion that younger male AP patients were prone to developing RAP was more or less the same. The reason that younger male patients accounted for the majority of cases of RAP could be partly explained by the fact that men a wreilling to attend social events and drink more, and alcohol consumption is one of common causes of RAP.4
BMI, as a risk factor in cardiovascular diseases, was also closely linked to morbidity and outcome of AP with different etiologies.16 One Korean research with 512 928 participants showed that elevated BMI was associated with increasing the risk of AP with or without cholelithiasis, but more strongly for cholelithiasis-related AP.17 A meta-analysis with 5129 AP cases and 1 693 657 participants proved that with per 5 kg/m2 increase in BMI, the relative risk for AP increased by a factor of 1.18.18 BMI >25 was proved to be significantly associated with increased risk of severity and mortality of AP.19 A population-based study of 118 000 individuals concluded that compared to individuals with lower BMI < 25, higher BMI was associated with higher risk of AP, with a multivariable adjusted hazard ratio of 1.4 for BMI of 25-29.9, 2.1 for BMI of 30-34.9, and 2.8 for BMI > 35.20 In China, a retrospective review analyzed a total of 1005 patients with hypertriglyceridemia pancreatitis (HTGP) and found that there was significant difference in BMI between the HTGP group and non-HTGP group (26.91 (25.69-27.49) vs. 24.18 (22.69-25.39)), and higher BMI was one of the independent risk factors for developing severe AP.21 Few research studies have focused on the association of BMI and RAP. In our study, BMI in RAP was significantly higher than that in the non-RAP group, which was identified as a predictor for recurrence of AP. Although BMI had a relatively low ROC, with sensitivity of 0.302 and specificity of 0.844, it also can provide clinical guidance for AP management.
Cholecystectomy was identified as being negatively associated with recurrence of AP in our study. A research with 5079 patients on the long-term effect of cholecystectomy in the management of biliary pancreatitis proved that in 18 months of follow-up, cholecystectomy was the most effective method for preventing recurrence of AP.8 Another meta-analysis also concluded that cholecystectomy significantly reduced the recurrence rate of acute pancreatitis.22
In our study, TG was closely associated with RAP. Moreover, patients with HTG had higher risk of RAP and a greater number of AP attacks. Hypertriglyceridemia was one of risk factors of AP,23 and of cerebral cardiovascular disease and all-cause mortality.14 Excess amounts of triglycerides in circulation are hydrolyzed into high levels of free fatty acids (FFA) by high levels of pancreatic lipase and released into the vascular bed of the pancreas. FFA impairs platelets and vascular endothelium in microcirculation, which results in an increased viscosity, causing impaired blood flow, inflammation, and damage of pancreatic acinar cells.24 However , there has been no consensus yet on a definite threshold TG level which clearly leads to AP and is associated with the severity and outcomes of AP. Recently, many studies have reported an effect of different levels of HTG on the clinical course of AP. On admission, the TG level of ≥2.26 mmol/L was an independent risk factor for predicting the severity of AP, which included local and systemic complications, the hospital length of stay, admission to the ICU, and ICU length of stay.25 A population-based study with 67 269 individuals revealed that the hazard ratio (HR) for AP associated with severe hypertriglyceridemia (≥5.6 mmol/L) was 3.2 (95% CI, 1.99-5.16), which was much higher than the HR of 1.50 (95% CI, 1.14-1.97) associated with moderate hypertriglyceridemia (1.7-5.6 mmol/L).26 AP patients admitted with a TG level ≥ 5.6 mmol/L had much more severe outcomes than the patients without or with mild HTG (<5.65 mmol/L).27 A conclusion from the cohort analysis of 1457 patients with HTG-AP, that HTG demonstrated that elevated TG was independently associated with any organ failure, while with an increased TG level for each 100 mg/dL, the increased risk of persistent renal failure, persistent shock, and persistent multiorgan failure were 6%, 7%, and 6%, respectively.28 Few studies discussed the association of TG with RAP. One research mentioned that AP patients with TG ≥ 2.26 mmol/L faced an increased risk of recurrence.29 In our research, TG with a best threshold of 5.9 mmol/L had a better predictive performance for recurrence of AP, which had a ROC of 0.702, with sensitivity of 0.763 and specificity of 0.595.
The strength of this study concluded that BMI and TG were independent risk factors for recurrence of AP, which enabled physicians to implement early therapeutic management to reduce the incidence of RAP, such as administering more effective therapies to lower the level of BMI and TG in patients with AP. In addition, the best threshold level of TG with 5.9 mmol/L could be a clinical guidance for the target level of lowering TG therapy.
Some limitations should be also clarified. First, the retrospective nature of this study could cause certain selection biases. The hospital in this study was a tertiary hospital in an urban city of China, and the economic development was much better than that in the suburb of city and countryside, which may lead to the higher incidence of AP patients with HTG. It could partly explain why the etiologies and variables linked with RAP in our study were different compared to some other researches. In our study, HTG as the major cause of AP accounted for around 40% in total, and TG was identified as a predictive factor for RAP occurrence. Hence, when applying our results to other centers, the difference of etiologies in AP should be considered. Second, its retrospective design did not allow follow-up of patients and the data were taken from each clinical record; therefore, not all the variables with RAP could be comprehensively analyzed. Third, a further prospective cohort study with a large population should be explored for validating our conclusion, and research on the mechanism of TG with RAP also should be done in the future.
Conclusion
In this study, TG and BMI were identified as independent predictors for recurrence of AP. A TG level of 5.9 mmol/L could be a clinical guidance for the target level of TG-lowering in AP patients with HTG.
Funding Statement
This manuscript was supported by the Sanitation and Health Committee Foundation of Hunan Province, China (No. 20200075).
Footnotes
Availability of Data and Materials: The datasets used and/or analyzed in the present study were availed by the corresponding author on reasonable request.
Ethics Committee Approval: Ethics approval was provided by the Medical Ethics Committee of Changsha Central Hospital.
Informed Consent: Due to the retrospective nature of the study, informed consent was waived.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – K.S., N.D.; Design - K.S., N.D.; Supervision - N.D., C.L.; Resource - K.S., C.G.; Materials - N.D.; Data Collection and/or Processing - K.S., C.G.; Analysis and/or Interpretation - K.S., C.G.; Literature Search - N.D.; Writing - N.D.; Critical Reviews - K.S., C.L., N.D.
Conflict of Interest: The authors have no conflict of interest to declare.
References
- 1. . Lindkvist B, Appelros S, Regnér S, Manjer J. A prospective cohort study on risk of acute pancreatitis related to serum triglycerides, cholesterol and fasting glucose. Pancreatology. 2012;12(4):317–324.. 10.1016/j.pan.2012.05.002) [DOI] [PubMed] [Google Scholar]
- 2. . Fonseca Sepúlveda EV, Guerrero-Lozano R. Acute pancreatitis and recurrent acute pancreatitis: an exploration of clinical and etiologic factors and outcomes. J Pediatr. 2019;95(6):713–719.. 10.1016/j.jped.2018.06.011) [DOI] [PubMed] [Google Scholar]
- 3. . Gullo L, Migliori M, Pezzilli R, et al. An update on recurrent acute pancreatitis: data from five European countries. Am J Gastroenterol. 2002;97(8):1959–1962.. 10.1111/j.1572-0241.2002.05907.x) [DOI] [PubMed] [Google Scholar]
- 4. . Gao YJ, Li YQ, Wang Q, et al. Analysis of the clinical features of recurrent acute pancreatitis in China. J Gastroenterol. 2006;41(7):681–685.. 10.1007/s00535-006-1820-3) [DOI] [PubMed] [Google Scholar]
- 5. . Yang AL, Vadhavkar S, Singh G, Omary MB. Epidemiology of alcohol-related liver and pancreatic disease in the United States. Arch Intern Med. 2008;168(6):649–656.. 10.1001/archinte.168.6.649) [DOI] [PubMed] [Google Scholar]
- 6. . Mallick B, Shrama DJ, Siddappa P, et al. Differences between the outcome of recurrent acute pancreatitis and acute pancreatitis. JGH Open. 2018;2(4):134–138.. 10.1002/jgh3.12060) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. . Machicado JD, Yadav D. Epidemiology of recurrent acute and chronic pancreatitis: similarities and differences. Dig Dis Sci. 2017;62(7):1683–1691.. 10.1007/s10620-017-4510-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. . Mustafa A, Begaj I, Deakin M, et al. Long-term effectiveness of cholecystectomy and endoscopic sphincterotomy in the management of gallstone pancreatitis. Surg Endosc. 2014;28(1):127–133.. 10.1007/s00464-013-3138-6) [DOI] [PubMed] [Google Scholar]
- 9. . Lee PJW, Stevens T. Reply to: Yang et al., Clinical features of recurrent acute pancreatitis experience from a single center. Pancreas. 2017;46(5):e37–e38.. 10.1097/MPA.0000000000000630) [DOI] [PubMed] [Google Scholar]
- 10. . Takuma K, Kamisawa T, Hara S, et al. Etiology of recurrent acute pancreatitis, with special emphasis on pancreaticobiliary malformation. Adv Med Sci. 2012;57(2):244–250.. 10.2478/v10039-012-0041-7) [DOI] [PubMed] [Google Scholar]
- 11. . Zhang W, Shan HC, Gu Y. Recurrent acute pancreatitis and its relative factors. World J Gastroenterol. 2005;11(19):3002–3004.. 10.3748/wjg.v11.i19.3002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. . Guda NM, Muddana V, Whitcomb DC, et al. Recurrent acute pancreatitis: international state-of-the-science conference with recommendations. Pancreas. 2018;47(6):653–666.. 10.1097/MPA.0000000000001053) [DOI] [PubMed] [Google Scholar]
- 13. . Jagannath S, Garg PK. Recurrent acute pancreatitis: current concepts in the diagnosis and management. Curr Treat Options Gastroenterol. 2018;16(4):449–465.. 10.1007/s11938-018-0196-9) [DOI] [PubMed] [Google Scholar]
- 14. . Nordestgaard BG. Triglyceride-rich lipoproteins and atherosclerotic cardiovascular disease new insights from epidemiology, genetics, and biology. Circ Res. 2016;118(4):547–563.. 10.1161/CIRCRESAHA.115.306249) [DOI] [PubMed] [Google Scholar]
- 15. . Lee PJW, Bhatt A, Holmes J, et al. Decreased severity in recurrent versus initial episodes of acute pancreatitis. Pancreas. 2015;44(6):896–900.. 10.1097/MPA.0000000000000354) [DOI] [PubMed] [Google Scholar]
- 16. . Türkoğlu A, Böyük A, Tanrıverdi MH, et al. The potential role of BMI, plasma leptin, nesfatin-1 and ghrelin levels in the early detection of pancreatic necrosis and severe acute pancreatitis: a prospective cohort study. Int J Surg. 2014;12(12):1310–1313.. 10.1016/j.ijsu.2014.10.040) [DOI] [PubMed] [Google Scholar]
- 17. . Choi JS, Yi SW, Park JW, et al. Body mass index and the risk of acute pancreatitis by etiology: a prospective analysis of Korean National Screening Cohort. J Gastroenterol Hepatol. 2019;34(3):603–611.. 10.1111/jgh.14570) [DOI] [PubMed] [Google Scholar]
- 18. . Aune D, Mahamat-Saleh Y, Norat T, Riboli E. High body mass index and central adiposity is associated with increased risk of acute pancreatitis: a meta-analysis. Dig Dis Sci. 2021;66(4):1249–1267.. 10.1007/s10620-020-06275-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. . Dobszai D, Mátrai P, Gyöngyi Z, et al. Body-mass index correlates with severity and mortality in acute pancreatitis: a meta-analysis. World J Gastroenterol. 2019;25(6):729–743.. 10.3748/wjg.v25.i6.729) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. . Hansen SEJ, Madsen CM, Varbo A, Nordestgaard BG. Body mass index, triglycerides, and risk of acute pancreatitis: a population-based study of 118 000 individuals. J Clin Endocrinol Metab. 2020;105(1):163–174.. 10.1210/clinem/dgz059) [DOI] [PubMed] [Google Scholar]
- 21. . Yu S, Wu D, Jin K, et al. Low serum ionized calcium, elevated high-sensitivity C-reactive protein, neutrophil-lymphocyte ratio, and body mass index (BMI) are risk factors for severe acute pancreatitis in patients with hypertriglyceridemia pancreatitis. Med Sci Monit. 2019;25:6097–6103.. 10.12659/MSM.915526) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. . Yuan X, Xu B, Wong M, et al. The safety, feasibility, and cost-effectiveness of early laparoscopic cholecystectomy for patients with mild acute biliary pancreatitis: a meta-analysis. Surgeon. 2020. 10.1016/j.surge.2020.06.014) [DOI] [PubMed] [Google Scholar]
- 23. . Langlois MR, Nordestgaard BG, Langsted A, et al. Quantifying atherogenic lipoproteins for lipid-lowering strategies: consensus-based recommendations from EAS and EFLM. Clin Chem Lab Med. 2020;58(4):496–517.. 10.1515/cclm-2019-1253) [DOI] [PubMed] [Google Scholar]
- 24. . Valdivielso P, Ramírez-Bueno A, Ewald N. Current knowledge of hypertriglyceridemic pancreatitis. Eur J Intern Med. 2014;25(8):689–694.. 10.1016/j.ejim.2014.08.008) [DOI] [PubMed] [Google Scholar]
- 25. . Tariq H, Gaduputi V, Peralta R, et al. Serum triglyceride level: a predictor of complications and outcomes in acute pancreatitis? Can J Gastroenterol Hepatol. 2016;2016:1–8.. 10.1155/2016/8198047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. . Murphy MJ, Sheng X, MacDonald TM, Wei L. Hypertriglyceridemia and acute pancreatitis. JAMA Intern Med. 2013;173(2):162–164.. 10.1001/2013.jamainternmed.477) [DOI] [PubMed] [Google Scholar]
- 27. . Zhang R, Deng L, Jin T, et al. Hypertriglyceridaemia-associated acute pancreatitis: diagnosis and impact on severity. HPB. 2019;21(9):1240–1249.. 10.1016/j.hpb.2019.01.015) [DOI] [PubMed] [Google Scholar]
- 28. . Pascual I, Sanahuja A, García N, et al. Association of elevated serum triglyceride levels with a more severe course of acute pancreatitis: cohort analysis of 1457 patients. Pancreatology. 2019;19(5):623–629.. 10.1016/j.pan.2019.06.006) [DOI] [PubMed] [Google Scholar]
- 29. . Wu BU, Batech M, Dong EY, et al. Influence of ambulatory triglyceride levels on risk of recurrence in patients with hypertriglyceridemic pancreatitis. Dig Dis Sci. 2019;64(3):890–897.. 10.1007/s10620-018-5226-x) [DOI] [PubMed] [Google Scholar]


