Abstract
Background:
Hepatocellular carcinoma (HCC) recurrence is still threatening patient survival after liver transplantation (LT). The efficacy and safety of sorafenib in the setting of post-LT recurrence are still equivocal. This study aims to disclose the efficacy and safety profile of sorafenib in treating post-LT HCC recurrence.
Materials and Methods:
Electronic databases were searched to retrieve relevant publications suitable for inclusion. Data from 23 studies containing 411 patients were analyzed. The primary outcome of interest was 1-year survival rate after sorafenib treatment, and the secondary endpoints included median overall survival (OS), time to progression (TTP), treatment response, and adverse events.
Results:
Patients with HCC recurrence after LT treated with sorafenib achieved a 1-year survival rate of 56.8%, with a median OS of 12.8 months and a median TTP of 6.0 months. Univariate logistic regression analysis showed that male gender (P = .048), TTP (P = .021), median duration of sorafenib (P = .021), diarrhea (P = .027), fatigue (P = .044), and partial response (P = .026) were associated with a better 1-year survival rate. In addition, sorafenib exerted a significant superior effect on OS compared with best supportive care in the setting of untreatable post-LT HCC recurrence.
Conclusions:
Based on the results of this meta-analysis, sorafenib therapy seems to be safe and feasible and exhibits survival benefit in patients with post-LT HCC recurrence. However, prospective randomized controlled trials with larger sample sizes and more rigorous study design are required to confirm the efficacy of sorafenib.
Keywords: hepatocellular carcinoma, recurrence, sorafenib, liver transplantation
INTRODUCTION
Hepatocellular carcinoma (HCC) is the sixth most prevalent cancer and the third most common cause of cancer-related death worldwide.1,2 Liver transplantation (LT) is considered as the most effective therapy in certain patients with HCC.3 In patients within the Milan criteria, LT has shown to provide 5-year recurrence-free survival and overall survival (OS) rates of 83 and 75%, respectively.4 Despite the stringent selection of transplant candidates, post-transplant HCC recurrence occurs in 8-20% of the recipients and leads to death in approximately 50% of cases.5,6 Moreover, with the expansion of criteria for LT candidates, the recurrence rate is likely to increase in the future. It is urgent to seek effective treatment strategies for this life-threatening disease.
In some patients, surgical resection, radiofrequency ablation, and transarterial chemoembolization are considered as a curative treatment for HCC recurrence after LT.7 However, patients presenting with untreatable presentation/progression (UP) are not amenable to surgical, ablative, or any locoregional treatment. For these patients, best supportive care (BSC) remained to be the only choice until 2007. Recent data support the efficacy of sorafenib, a multiple tyrosine kinase inhibitor, in the treatment of post-transplant HCC recurrence in the UP stage.8 Several studies suggest a potential survival benefit of sorafenib over BSC in treating post-LT HCC recurrence.9-12 However, some are concerned about the safety of sorafenib.8
Up until now, there is no consensus on the efficacy and safety of sorafenib in the setting of post-LT HCC recurrence. Moreover, a single study cannot conclusively confirm the usefulness of sorafenib for patients with HCC recurrence after LT. Therefore, a systematic review and meta-analysis are needed. The present meta-analysis was performed with the aim of estimating the efficacy and safety profile of sorafenib in the treatment of post-LT HCC recurrence, and identifying factors that affect OS. Also, a comparison between sorafenib and BSC was conducted to further disclose the survival benefit of sorafenib treatment.
MATERIALS AND METHODS
Search Strategy
This meta-analysis was conducted in accordance with the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement.13 Relevant studies published from January 1, 2007 to January 31, 2020 were searched and identified through electronic databases PubMed, Embase, ScienceDirect, and Web of Science, with the following subject headings: HCC, recurrence, LT, and sorafenib. The searches were not limited by date, but exclusive to English publications. All of the titles and abstracts retrieved from the initial search were screened by two reviewers, and the potentially eligible studies were further reviewed as full-text. Manual search for reference lists of the eligible studies and reviews were also performed to find additional studies. Discrepancies between the reviewers were resolved by discussion.
Inclusion and Exclusion Criteria
Studies that assessed the efficacy and safety of sorafenib treatment for HCC recurrence in post-LT setting were within the field of interest in this meta-analysis. All of the eligible studies included patients with post-LT HCC recurrence, with regard to the use of sorafenib for the treatment of HCC recurrence after LT, and provided at least 1-year survival outcome from the time of sorafenib start. Studies that did not meet the aforementioned criteria, review articles, case reports, ongoing trials, and studies that applied sorafenib for the prevention of HCC recurrence after LT were excluded. For multiple reports that included the same cohort of patients, only the latest publication or the one with the most complete data was included.
Data Abstraction and Quality Assessment
Two investigators independently reviewed the retrieved articles and extracted relevant data using a predefined standardized form. Information concerning study design, patients characteristics, treatment option, target outcomes, and other variables that affected patient outcomes were extracted. Disagreements were resolved through discussion or the opinion of the third reviewer. The methodological quality of the included non-randomized studies was estimated using the Newcastle–Ottawa scale (NOS).14 The NOS is based on a star awarded system (range 0-9 stars), with a higher score representing better methodological quality.
Statistical Analysis
A random-effects meta-analysis of proportions model using the Dersimonian and Laird method was run to calculate an overall pooled 1-year survival rate of the included studies. Statistical heterogeneity among the studies was measured using chi-squared Q test and I 2 statistics. Univariate meta-regression analysis was also conducted to determine if variance could affect the heterogeneity and the overall result. The differences on time to recurrence (TTR), time to progression (TTP), and median survival after recurrence between sorafenib and BSC treatment were also calculated and expressed using weighted mean differences (WMD) with 95% CI. A random-effects model was adopted for analysis when heterogeneity existed (P <.05 or I 2 > 50%). Otherwise, a fixed-effects model was used. Hazard ratios (HRs) with 95% CIs were calculated to assess the correlation of sorafenib treatment with OS for patients with HCC recurrence after LT. A HR > 1 indicated that the patients in the comparator group had poor prognosis. On the contrary, a HR < 1 meant that the patients in the comparator group had better prognosis. Sensitivity analysis was performed by removing one study result at a time to determine if a certain study altered the overall effect or heterogeneity. Publication bias related to the 1-year survival rates was calculated with Deeks funnel plot and Egger’s asymmetry testing. The existence of publication bias was confirmed when P <.05. Statistical analysis was performed using Stata version 15.0 (Stata Corporation, College Station, TX, USA) and R-2.15.2 (the Comprehensive R Archive Network) software. A P-value <.05 was considered statistically significant.
RESULTS
Literature Search
By applying the abovementioned search terms and all the possible combinations, the databases identified a total of 1526 articles for initial review. Based on titles and abstracts, 1468 studies were excluded. The remaining 58 publications were full-text reviews, and another 35 reports not satisfying our inclusion criteria were further excluded. No additional study was retrieved from the cross-checking of reference lists. Eventually, 23 studies were enrolled in the meta-analysis. Figure 1 presents the flow chart of the literature selection process.
Figure 1.
PRISMA flow diagram of literature search and study selection. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Study Description
All of the included studies were retrospective cohort studies. Sample sizes ranged from 7 to 54, with a total of 411 patients receiving sorafenib treatment.9-12,15-33 The median patient age ranged from 46.3 to 61 years. The percentage of male patients ranged from 45.5 to 100%. Eighteen studies reported the number of patients within the Milan criteria before LT, with a percentage range from 10 to 100%. The percentage of patients with microvascular invasion on the explant pathology was reported in 17 studies and ranged from 8.3 to 100%. Thirteen studies reported the median follow-up duration (range 3.7-87 months), and the overall follow-up time was 19.9 months (95% CI, 9.3-30.5). TTR was reported in 20 studies (range 6.5-38.1 months) and the overall TTR was 15.6 months (95% CI, 11.9-19.2). Detailed information on each study is presented in Table 1.
Table 1.
Basic Characteristics of the Included Studies
| Author | Year | Regions | Sample (Sorafenib) | Age | Male (%) | Milan In (%) | MIV (%) | Down Staging Before LT (%) | Median Follow-up (months) | Median TTR (months) |
|---|---|---|---|---|---|---|---|---|---|---|
| de’Angelis et al. | 2016 | France | 15 | 58.33 ± 7.10 | 80 | 73.3 | 53.3 | - | - | 17.8 |
| Desimone et al. | 2014 | Italy | 7 | 53 ± 9 | 100 | 71.4 | 100 | 71.4 | 6.5 | 9 |
| Gomez-martin et al. | 2011 | Spain | 31 | 53.6 ± 1.6 | 87.1 | 93.1 | - | - | - | 22.6 |
| Invernizzi et al. | 2019 | Italy | 50 | 57 (41-75) | 80 | 44 | 50 | - | 14 | 16 |
| Jung et al. | 2018 | Korea | 54 | 54.1 ± 6.2 | 88.9 | 44.4 | 63 | - | - | 18.9 |
| Kang et al. | 2018 | Korea | 45 | 55 (49-61) | 91.1 | 24.4 | 55.6 | - | 12.1 | 6.5 |
| Kim et al. | 2010 | USA | 9 | 59 (46-77) | 100 | 44.4 | 55.6 | - | 3.7 | 12.4 |
| López Ortega et al. | 2020 | Spain | 12 | 58 ± 14 | - | 33.3 | 58.3 | - | 13 | 21.5 |
| Martin et al. | 2016 | USA | 21 | 59 (31-72) | 76.2 | - | - | - | 43.6 | - |
| Na et al. | 2016 | Korea | 12 | 55.3 ± 9.1 | 75 | 29.6 | - | - | 8.5 | 6.5 |
| Nagai et al. | 2015 | USA | 15 | 61 (57-63) | - | - | - | - | - | 16.8 |
| Pfeiffenberger et al. | 2013 | Germany | 8 | 55 (50-70) | 87.5 | 100 | 62.5 | 80 | - | 16 |
| Pfiffer et al. | 2011 | Germany | 8 | - | - | - | - | - | - | - |
| Piñero et al. | 2016 | Argentina | 10 | 61 (34-69) | - | - | 35.5 | - | 16.5 | 10.5 |
| Sotiropoulos et al. | 2012 | Germany | 14 | 57 | 64.3 | 64.3 | 35.7 | 43 | 12.2 | 8 |
| Sposito et al. | 2013 | Italy | 15 | 57 (17-71) | 100 | 93.3 | 26.7 | 100 | 87 | 38.1 |
| Staufer et al. | 2012 | Germany | 13 | 60 (44-68) | 92.3 | 38.5 | 61.5 | - | - | 13.6 |
| Tan et al. | 2010 | China | 10 | 46.30 ± 5.95 | 100 | - | - | - | - | - |
| Vitale et al. | 2012 | Italy | 10 | 61 | 80 | 10 | 100 | 100 | 15 | 7 |
| Waghray et al. | 2013 | USA | 17 | 57.8 ± 1.5 | 94.1 | 52.9 | 58.8 | 47 | 19.7 | 22.2 |
| Weinmann et al. | 2012 | Germany | 11 | 51.1 (33.6-68.9) | 45.5 | 45.5 | 8.3 | 100 | - | 37.5 |
| Yoon et al. | 2010 | Korea | 13 | 50 (37-59) | 84.6 | 69.2 | 23 | - | 3.7 | 12.3 |
| Zavaglia et al. | 2013 | Italy | 11 | 57 ± 9 | 81.8 | 36.4 | 45.4 | - | - | 12 |
MIV, microvascular invasion; TTR, time to recurrence.
The methodological quality NOS scores ranged from 4 to 8 stars with a median score of 5.8 (maximum 9), indicating that most of the included studies had low to moderate quality. Only one study with a score of 8 was considered as high quality.9 The total distribution for each study is presented in Supplemental Table 1.
Sorafenib Regimen and Safety
The time of onset of sorafenib treatment after the diagnosis of post-LT HCC recurrence was reported in 14 studies, and the overall time to start of sorafenib therapy was 5.6 months (95% CI, 3.9-7.4). Most of the studies applied an initial dose of 400 mg/b.i.d., and adjusted doses according to the patient’s tolerability and safety. In two studies, sorafenib was started at a dose of 200 mg/b.i.d. and increased subsequently to 400 mg/b.i.d. if the patient demonstrated good tolerance. The overall rate of sorafenib dose reduction due to adverse events from 16 studies was 47.8% (95% CI, 25.0-70.5), while the overall rate of temporary sorafenib discontinuation was 43.3% (95% CI, 25.5-61.1). The median duration of sorafenib treatment was recorded in 13 studies, ranging from 1.7 to 13 months. The overall treatment duration was 5.5 months (95% CI, 3.5-7.5). Ten studies reported the median TTP, and the overall TTP was 6.0 months (95% CI, 4.9-7.1).
The most reported Grade 3-4 adverse event was hand–foot skin reaction, which ranged from 0 to 30% with an overall incidence rate of 11.3% (95% CI, 4.2-18.3) from 10 studies. Diarrhea and fatigue were the second and third most reported Grade 3-4 adverse events, ranging from 0-50% to 0-30%, with overall incidence rates of 23.9% (95% CI, 14.6-33.3) and 24.7% (95% CI, 15.5-34.0), respectively. The detailed characteristics of sorafenib treatment in each study are summarized in Table 2.
Table 2.
Characteristics of Sorafenib Treatment of the Included Studies
| Author | Median Onset of Sorafenib (months) | Initial Dose (b.i.d.) | Median Duration (month) | Reduction (%) | Discontinuation (%) | Hand–Foot Skin Reaction (%) | Grade 3-4 (%) | Diarrhea (%) | Grade 3-4 (%) | Nausea and Vomiting (%) | Grade 3-4 (%) | Fatigue (%) | Grade 3-4 (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| de’Angelis et al. | 15.9 | 400 | - | 53.3 | 13.3 | 40 | 3.2 | 46.7 | - | 20 | - | 66.7 | - |
| Desimone et al. | 11 | 400 | - | 42.9 | 28.6 | 71.4 | - | 28.6 | - | - | - | - | - |
| Gomez-martin et al. | 1.3 | 400 | - | 19.4 | 3.2 | 54.8 | 3.2 | 77.4 | 12.9 | 6.5 | - | 16.1 | 16.1 |
| Invernizzi et al. | 2.7 | 400 | 7.3 | 62 | 84 | 62 | 2 | 48 | 24 | 30 | - | 42 | 30 |
| Jung et al. | - | - | 7 | - | 77.8 | - | - | - | - | - | - | - | - |
| Kang et al. | - | 400 | - | - | 86.7 | 8.9 | - | 13.3 | - | 2.2 | - | - | - |
| Kim et al. | 4.3 | 200 | - | 66.7 | 55.6 | 55.6 | 22.2 | 33.3 | 11.1 | - | - | - | - |
| López Ortega et al. | 12.3 | 400 | 13 | 33.3 | - | 16.7 | - | 75 | - | - | - | - | - |
| Martin et al. | 26 | 400 | 5.5 | 100 | 28.6 | 23.8 | - | 9.5 | - | - | - | 4.8 | - |
| Na et al. | 6 | 400 | - | 25 | - | 25 | - | 66.7 | 25 | - | - | - | - |
| Nagai et al. | - | 400 | - | 13.3 | 6.7 | 13.3 | - | - | - | - | - | - | - |
| Pfeiffenberger et al. | 1.5 | 400 | 3.7 | 66.7 | - | 55.6 | 0 | - | - | - | - | - | 0 |
| Pfiffer et al. | - | - | - | - | - | 25 | 25 | - | - | - | - | - | - |
| Piñero et al. | 2.1 | 400 | 3 | - | 90 | 10 | - | 30 | - | - | - | 40 | - |
| Sotiropoulos et al. | - | 400 | 6.5 | 14.3 | 28.6 | - | - | - | - | - | - | - | - |
| Sposito et al. | 6.9 | 400 | - | 53.3 | 66.7 | 60 | - | 40 | - | - | - | 26.7 | - |
| Staufer et al. | 2.3 | 200 | 1.7 | - | 76.9 | 23.1 | 10 | 61.5 | 30.8 | 15.4 | 7.7 | 15.4 | 0 |
| Tan et al. | - | 400 | - | 10 | 10 | 50 | - | 30 | - | - | - | - | 0 |
| Vitale et al. | - | 200 | 10 | 40 | 30 | 30 | 30 | 50 | 50 | - | - | 20 | 20 |
| Waghray et al. | 1.9 | 200 | 10.4 | - | 5.9 | 5.9 | - | 17.6 | - | 5.9 | - | 11.8 | 11.8 |
| Weinmann et al. | 3.9 | 400 | 8.9 | 72.7 | 63.6 | 54.5 | 27.3 | 81.8 | 45.5 | 63.6 | 18.2 | 72.7 | 18.2 |
| Yoon et al. | - | 400 | 2.4 | - | 30.8 | 61.5 | 23.1 | 30.8 | - | - | - | - | - |
| Zavaglia et al. | - | 400 | 2.3 | 90.9 | 36.4 | 45.5 | - | 18.2 | - | 27.3 | - | 54.5 | 18.2 |
Outcome of Sorafenib Therapy
Median OS from the start of sorafenib treatment was reported in 19 studies, ranging from 5 to 23.5 months, with an overall OS of 12.8 months (95% CI, 10.6-15.1). Treatment response was recorded in 13 studies using the Response Evaluation Criteria in Solid Tumors (RECIST 1.1) criteria. Only two studies, Kim et al. and Waghray et al.20,31 reported complete response after sorafenib therapy. The percentage of patients achieving a complete response was 11.1 and 11.8%, respectively. Partial response was reported in 10 studies, ranging from 0 to 26.7%, with an overall rate of 7.2% (95% CI, 2.7-11.6). Stable disease was reported in 13 studies, ranging from 0 to 73.3%, with an overall rate of 38.3% (95% CI, 25.4-51.3). Progressive disease was reported in 13 studies, ranging from 11.1 to 100%, with an overall rate of 35.5% (95% CI, 28.4-42.5). The detailed treatment outcomes in each study were presented in Table 3.
Table 3.
Outcomes of Sorafenib Therapy
| Author | Median OS (months) | Median TTP (months) | CR (%) | PR (%) | SD (%) | PD (%) | 1-Year Survival Rate (%) |
|---|---|---|---|---|---|---|---|
| de’Angelis et al. | 23.5 | 15.9 | 0 | 13.3 | 46.7 | 26.7 | 60 |
| Desimone et al. | 5 | 3.5 | 0 | 0 | 0 | 100 | 71.4 |
| Gomez-martin et al. | 19.3 | 6.8 | 0 | 3.8 | 50 | 38.5 | 61.3 |
| Invernizzi et al. | - | 6 | - | - | - | - | - |
| Jung et al. | - | - | - | - | - | - | - |
| Kang et al. | 14.2 | 7 | - | - | - | - | - |
| Kim et al. | - | - | 11.1 | 0 | 44.4 | 33.3 | - |
| López Ortega et al. | 7.5 | - | 0 | 0 | 41.7 | 58.3 | - |
| Martin et al. | 9.5 | - | - | - | - | - | - |
| Na et al. | 17.2 | - | - | - | - | - | 41.7 |
| Nagai et al. | - | - | - | - | - | - | - |
| Pfeiffenberger et al. | 9 | 4.5 | - | - | - | - | 20 |
| Pfiffer et al. | 6.7 | - | - | - | - | - | - |
| Piñero et al. | 16.5 | - | - | - | - | - | - |
| Sotiropoulos et al. | 12 | - | - | - | - | - | - |
| Sposito et al. | 21.3 | 9.1 | 0 | 26.7 | 73.3 | 0 | 93.3 |
| Staufer et al. | 19.4 | - | 0 | 7.7 | 30.8 | 53.8 | 69.2 |
| Tan et al. | 14 | - | 0 | 10 | 70 | 30 | 60 |
| Vitale et al. | 18 | 8 | 0 | 20 | 60 | 20 | 80 |
| Waghray et al. | 7 | - | 11.8 | 5.9 | 11.8 | 29.4 | 64.7 |
| Weinmann et al. | 20.1 | 4.1 | 0 | 0 | 36.4 | 63.6 | 54.5 |
| Yoon et al. | 5.4 | 2.9 | 0 | 0 | 46.2 | 30.8 | 38.5 |
| Zavaglia et al. | 5 | - | 0 | 18.2 | 9.1 | 54.5 | 18.2 |
OS, overall survival; TTP, time to progression; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
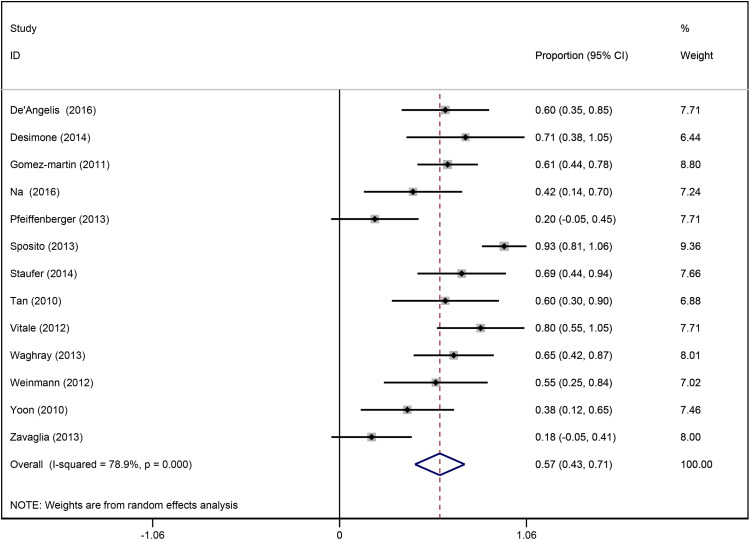
Thirteen studies reported 1-year survival rate after sorafenib therapy, ranging from 18.2 to 93.3%. The overall 1-year survival rate was 56.8% (95% CI, 42.8-70.9; I 2 = 78.9%) (Figure 2). Since significant heterogeneity was detected among studies, univariate meta-regression analysis was conducted to identify the potential sources of heterogeneity and variates associated with 1-year survival rate. Results of univariate logistic regression analysis showed that the following variables were associated with a better 1-year survival rate: male gender (P = .048), TTP (P = .021), median duration of sorafenib (P = .021), diarrhea (P = .027), fatigue (P = .044), and partial response (P = .026) (Table 4).
Figure 2.
Forest plot of 1-year survival rates of patients treated with sorafenib for hepatocellular carcinoma recurrence after liver transplantation in 13 studies.
Table 4.
Results of Univariate Logistic Regression Analysis of 1-Year Survival Rate.
| Subgroup | No. of Studies | No. of Patients | Beta | SE | P |
|---|---|---|---|---|---|
| Region | 13 | 175 | 0.15 | 0.34 | .167 |
| Age | 13 | 175 | 0.16 | 0.29 | .076 |
| Male | 13 | 175 | 0.21 | 0.29 | .048 |
| Milan in (%) | 12 | 165 | 0.04 | 0.32 | .165 |
| Microvascular invasion (%) | 10 | 122 | 0.17 | 0.38 | .155 |
| Down staging before LT (%) | 6 | 70 | 0.51 | 0.44 | .133 |
| mTOR-I (%) | 11 | 152 | −0.27 | 0.22 | .377 |
| Time to recurrence (months) | 12 | 165 | 0.13 | 0.31 | .107 |
| Time to progression (months) | 8 | 112 | 0.57 | 0.29 | .021 |
| Time of onset of sorafenib after recurrence (months) | 9 | 131 | 0.26 | 0.30 | .076 |
| Initiated dosage (mg/b.i.d.) | 13 | 175 | −0.42 | 0.32 | .892 |
| Median duration of sorafenib (months) | 7 | 85 | 0.74 | 0.38 | .021 |
| Reduction (%) | 10 | 132 | −0.41 | 0.35 | .89 |
| Discontinuation (%) | 11 | 152 | −0.32 | 0.27 | .809 |
| Hand–foot skin reaction (%) | 13 | 175 | −0.01 | 0.29 | .178 |
| Diarrhea (%) | 13 | 175 | 0.29 | 0.28 | .027 |
| Nausea and vomiting (%) | 13 | 175 | −0.03 | 0.29 | .198 |
| Fatigue (%) | 13 | 175 | 0.24 | 0.28 | .044 |
| Grade 3-4 adverse effects (%) | 13 | 175 | 0.13 | 0.30 | .079 |
| Partial response (%) | 13 | 175 | 0.35 | 0.28 | .026 |
| Stable disease (%) | 11 | 152 | 0.04 | 0.35 | .239 |
| Progression disease (%) | 11 | 152 | −0.17 | 0.34 | .489 |
LT, liver transplantation; SE, standard error.
Treatment Effect between Sorafenib and Best Supportive Care
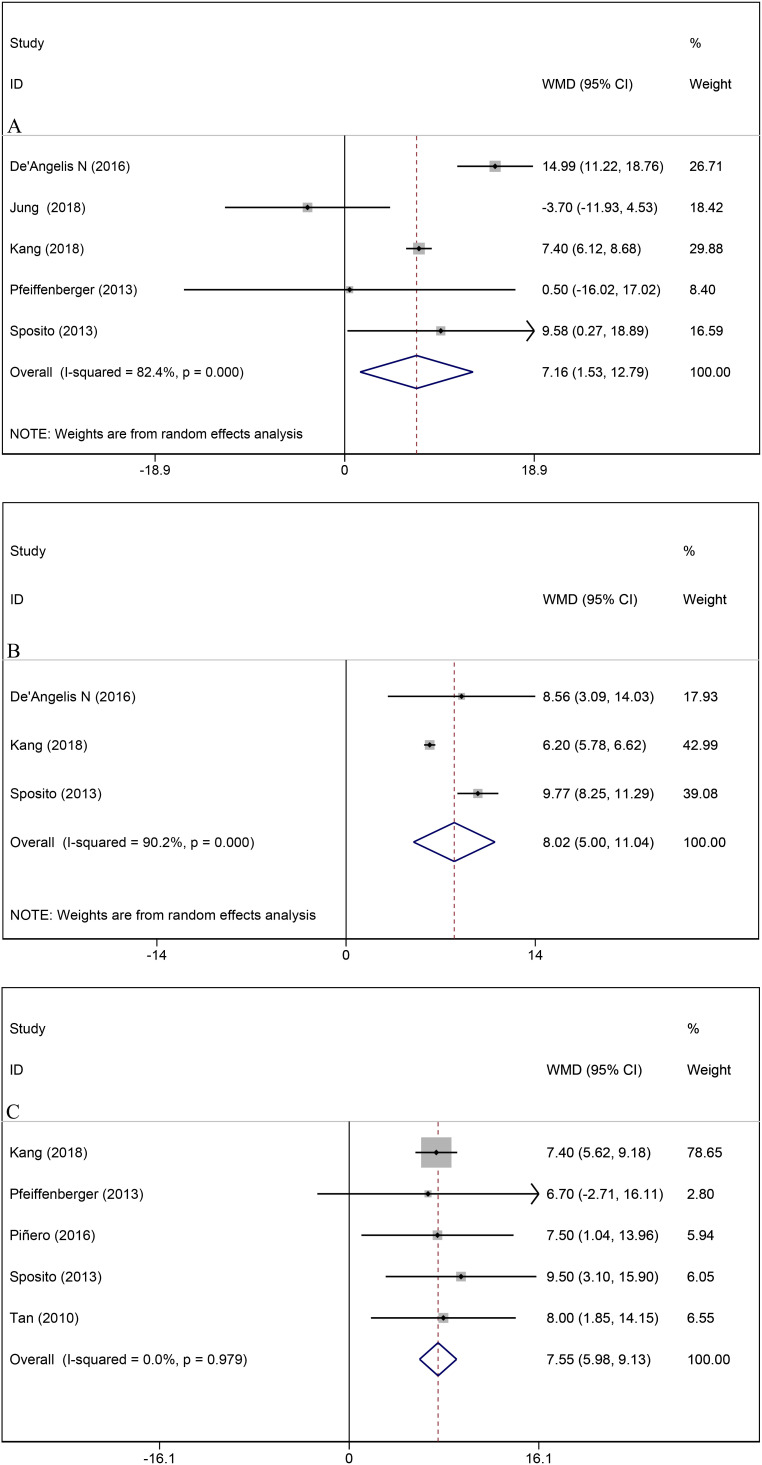
Pooled result from five studies suggested that patients receiving sorafenib therapy had longer TTR compared with that of BSC (WMD = 7.2, 95% CI, 1.5-12.8, I2 = 82.4%, P = .013) (Figure 3A). However, no significant difference in TTR was found between groups in sensitivity analysis when removing the outcome of de’Angelis et al., Kang et al., and Sposito et al., respectively.9,11,19 Pooled outcome from three studies showed longer TTP in the sorafenib group than in the BSC group (WMD = 8.0, 95% CI, 5.0-11.0, I 2 = 90.2%, P < .001) (Figure 3B). The significant difference in TTP remained in sensitivity analysis when removing each study outcome. Median survival after recurrence was recorded in five studies, and patients with sorafenib treatment showed to have a significantly longer median survival compared with patients who only received BSC after HCC recurrence (WMD = 7.6, 95% CI, 6.0-9.1, I 2 = 0%, P < .001) (Figure 3C).
Figure 3.
Comparisons between sorafenib therapy and best supportive care treatment on (A) time to recurrence; (B) time to progression; (C) median overall survival.
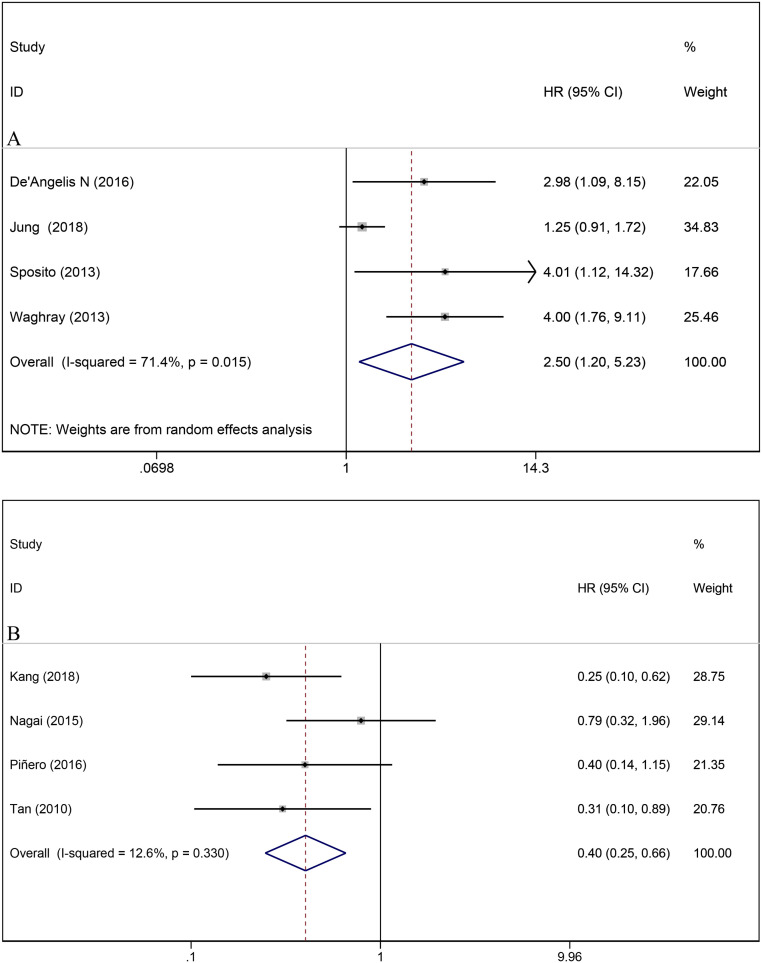
Eight studies provided data on multivariate analysis for OS after post-LT HCC recurrence. The pooled results showed that sorafenib treatment was correlated with a better OS when compared with BSC (BSC vs sorafenib: HR = 2.50, 95% CI, 1.20-5.23, I 2 = 71.4%, P = .015; sorafenib vs BSC: HR = 0.40, 95% CI, 0.25-0.66, I 2 = 12.6%, P < .0001) (Figure 4). The sensitivity analysis confirmed that the results of OS were robust, since no individual study dominantly affected the overall HR.
Figure 4.
Forest plot for the correlation between sorafenib treatment and overall survival in patients with hepatocellular carcinoma recurrence after liver transplantation; (A) best supportive care versus sorafenib; (B) sorafenib versus best supportive care.
Publication Bias
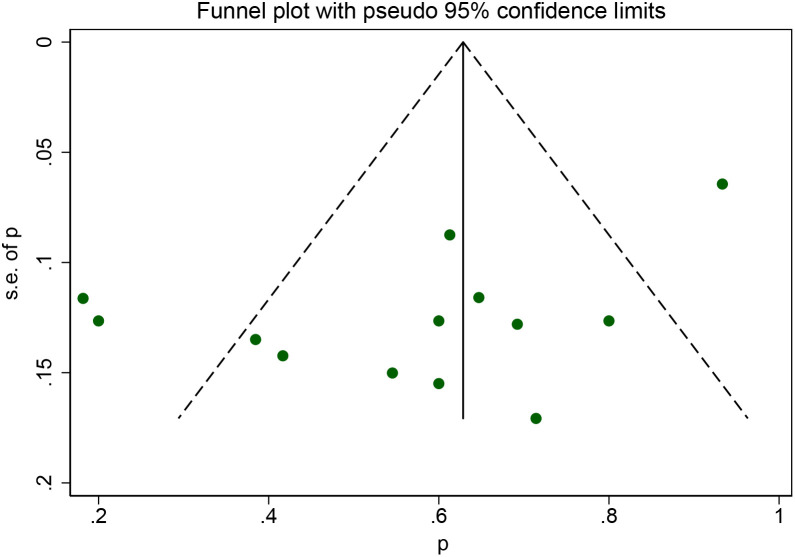
Begg’s funnel plot and Egger’s test were applied to estimate possible publication bias for the 1-year survival rate. The result of Egger’s test showed the existence of publication bias for the 1-year survival rate (P = .037) (Figure 5).
Figure 5.
Publication bias of 1-year survival rate.
DISCUSSION
Before the introduction of sorafenib, no chemotherapy has been proven to be effective in improving survival for patients with HCC recurrence after LT.34 Sorafenib is the first agent that demonstrated improvement in OS for patients with advanced HCC, and is nowadays considered as standard therapy for recurrence patients.35 The objective of the present meta-analysis was to provide additional information for the field of sorafenib treatment for patients with post-LT HCC recurrence. Our study showed a median OS of 12.8 months for patients who received sorafenib therapy after HCC recurrence. This result was much higher than that reported for other systemic treatments,8,36 which only demonstrated a median OS of 16.8 weeks for patients with HCC recurrence. This highlighted the potential of sorafenib in prolonging the survival of patients with post-LT HCC recurrence.
An overall 1-year survival rate of 56.8% was detected in our study, which was lower than that reported for a previous meta-analysis (63%).37 However, our studies included five more publications than the former report, which would be preferable in meta-analysis. It is suggested that the variability in patients’ demographic, clinical, and biological characteristics and sorafenib treatment might affect patients’ survival. Twenty-two variables were assessed, and our study further confirmed that male gender, longer median TTP, and an increase in adverse events were positively correlated with the 1-year survival rate.38-40 In addition, the present study demonstrated that patients with longer median duration of sorafenib and achieving a partial response after sorafenib therapy correlated with an increase in 1-year survival rate. These surrogate and clinical markers may be effective in predicting survival after sorafenib treatment.
Despite the potential efficacy on survival, the safety profile of sorafenib treatment is of great concern. The overall percentages of patients requiring dose reduction and temporary discontinuation due to adverse events during sorafenib treatment were 47.8 and 43.3%, respectively. However, a majority of patients could tolerate the treatment after the adjustment. This finding along with other studies in clinical practice suggested that dose-adjusted sorafenib may have implications for tailored therapy.41,42 The most recorded Grade 3-4 adverse events were hand–foot skin reaction, diarrhea and fatigue, with incidence rates of 11.3, 23.9, and 24.7%, respectively. Yet, none of the above adverse events resulted in death, indicating that sorafenib has a tolerable safety profile in treating post-LT HCC recurrence.
Recently, the combined use of sorafenib with mTOR inhibitors has attracted increasing attention. However, no consensus has been reached in terms of the efficacy of sorafenib plus mTOR inhibitors in post-LT HCC recurrence settings. While several preclinical trials supported the use of combined therapy,43,44 recent phase I and II trials failed to find a superior effect of sorafenib plus mTOR inhibitors on OS compared with sorafenib alone in treating post-LT HCC recurrence.45,46 Instead, higher incidences of adverse events were found with the use of combined treatment.46 Moreover, the meta-analysis of Mancuso et al. did not find a significant association between mTOR inhibitors and patients’ survival (P = .682); on the contrary, all episodes of fatal bleeding were associated with the use of mTOR inhibitors therapy.37 Our study found similar results (P = .377). In addition, three of the included studies provided data on multivariate analysis for the association between mTOR inhibitors treatment and OS.17,19,24 The pooled result showed that the use of mTOR inhibitors did not have a significant correlation with OS (HR = 0.62, 95% CI, 0.37-1.04, P = .071) (data not shown). Taken together, the use of sorafenib in combination with mTOR inhibitors should be discouraged or applied with caution.
In addition, our study addressed a specific question, which was, regardless of the previous treatment for post-LT HCC recurrence, once HCC recurrence has reached the UP stage, which treatment, sorafenib or BSC, results in better patient survival. Results from our analysis showed that patients managed by sorafenib treatment achieved a better OS compared with patients who received BSC. Since significantly longer TTR was found in the sorafenib group, the significant improvement in OS we observed for sorafenib treatment may be due to the late HCC recurrence. Late recurrence (>12 months) has been regarded as an independent prognostic factor related to better survival on a multivariable analysis.9,18,27 Thus, it is possible that TTR can be a selection criterion of sorafenib treatment candidates.
Several limitations need to be taken into consideration. The inclusion of low methodology quality and small sample size studies are the first two reasons weakening the credibility of the present meta-analysis. However, high-quality studies within our field of interest was quite rare; therefore, it was unavoidable for us to include the relatively low-quality studies. Furthermore, the results of this meta-analysis certainly need to be interpreted with caution due to the small number of patients. Though 23 publications were enrolled, they only contained a total of 411 patients, and each group of analysis contained an even smaller sample size. Prospective randomized controlled trials with a larger sample size, more rigorous study design, and higher methodology quality are needed to confirm the efficacy of sorafenib treatment in a post-LT HCC recurrence setting.
CONCLUSION
Results of this meta-analysis showed that sorafenib treatment in patients with HCC recurrence after LT provided a 1-year survival rate of 56.8%, with an overall TTP of 6.0 months. Taken together, sorafenib therapy seems to be associated with an acceptable safety profile and survival benefit in patients with post-LT HCC recurrence. In the setting of untreatable post-LT HCC recurrence, sorafenib treatment significantly and independently improved OS compared with BSC. More data are required to verify the current findings.
Funding Statement
Author Zhao Li received funding form the National Natural Science Foundation of China (No. 81502509).
Footnotes
Ethics Committee Approval: N/A.
Informed Consent: N/A.
Peer-review: Externally peer-reviewed.
Conflict of Interest: The authors have no conflict of interest to declare.
References
- 1.. Ferlay J, Soerjomataram I, Dikshit R. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer . 2015;136(5):E359–E386.. 10.1002/ijc.29210) [DOI] [PubMed] [Google Scholar]
- 2.. Parkin DM. Global cancer statistics in the year 2000. Lancet Oncol. 2001;2(9):533–543.. 10.1016/S1470-2045(01)00486-7) [DOI] [PubMed] [Google Scholar]
- 3.. Yao FY, Ferrell L, Bass NM. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology . 2001;33(6):1394–1403.. 10.1053/jhep.2001.24563) [DOI] [PubMed] [Google Scholar]
- 4.. Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet . 2018;391(10127):1301–1314.. 10.1016/S0140-6736(18)30010-2) [DOI] [PubMed] [Google Scholar]
- 5.. Davis E, Wiesner R, Valdecasas J, Kita Y, Rossi M, Schwartz M. Treatment of recurrent hepatocellular carcinoma after liver transplantation. Liver Transpl. 2011;17(suppl 2):S162–S166.. 10.1002/lt.22361) [DOI] [PubMed] [Google Scholar]
- 6.. Welker MW, Bechstein WO, Zeuzem S, Trojan J. Recurrent hepatocellular carcinoma after liver transplantation - an emerging clinical challenge. Transpl Int. 2013;26(2):109–118.. 10.1111/j.1432-2277.2012.01562.x) [DOI] [PubMed] [Google Scholar]
- 7.. Chok KSh. Management of recurrent hepatocellular carcinoma after liver transplant. World J Hepatol. 2015;7(8):1142–1148.. 10.4254/wjh.v7.i8.1142) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.. de’Angelis N, Landi F, Carra MC, Azoulay D. Managements of recurrent hepatocellular carcinoma after liver transplantation: a systematic review. World J Gastroenterol. 2015;21(39):11185–11198.. 10.3748/wjg.v21.i39.11185) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.. De’Angelis N, Landi F, Nencioni M. Role of sorafenib in patients with recurrent hepatocellular carcinoma after liver transplantation. Prog Transplant . 2016;26(4):348-355. 10.1177/1526924816664083) [DOI] [PubMed] [Google Scholar]
- 10.. Sotiropoulos GC, Nowak KW, Fouzas I. Sorafenib treatment for recurrent hepatocellular carcinoma after liver transplantation. Transplant Proc. 2012;44(9):2754–2756.. 10.1016/j.transproceed.2012.09.022) [DOI] [PubMed] [Google Scholar]
- 11.. Sposito C, Mariani L, Germini A. Comparative efficacy of sorafenib versus best supportive care in recurrent hepatocellular carcinoma after liver transplantation: a case-control study. J Hepatol. 2013;59(1):59–66.. 10.1016/j.jhep.2013.02.026) [DOI] [PubMed] [Google Scholar]
- 12.. Yoon DH, Ryoo BY, Ryu MH. Sorafenib for recurrent hepatocellular carcinoma after liver transplantation. Jpn J Clin Oncol. 2010;40(8):768–773.. 10.1093/jjco/hyq055) [DOI] [PubMed] [Google Scholar]
- 13.. Moher D, Liberati A, Tetzlaff J, Altman DG.PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ . 2009;339:b2535. 10.1136/bmj.b2535) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605.. 10.1007/s10654-010-9491-z) [DOI] [PubMed] [Google Scholar]
- 15.. De Simone P, Crocetti L, Pezzati D. Efficacy and safety of combination therapy with everolimus and sorafenib for recurrence of hepatocellular carcinoma after liver transplantation. Transplant Proc. 2014;46(1):241–244.. 10.1016/j.transproceed.2013.10.035) [DOI] [PubMed] [Google Scholar]
- 16.. Gomez-Martin C, Bustamante J, Castroagudin JF. Efficacy and safety of sorafenib in combination with mammalian target of rapamycin inhibitors for recurrent hepatocellular carcinoma after liver transplantation. Liver Transpl. 2012;18(1):45–52.. 10.1002/lt.22434) [DOI] [PubMed] [Google Scholar]
- 17.. Invernizzi F, Iavarone M, Zavaglia C. Experience with early sorafenib treatment with mTOR inhibitors in hepatocellular carcinoma recurring after liver transplantation. Transplantation . 2020;104(3):568–574.. 10.1097/TP.0000000000002955) [DOI] [PubMed] [Google Scholar]
- 18.. Jung DH, Tak E, Hwang S. Antitumor effect of sorafenib and mammalian target of rapamycin inhibitor in liver transplantation recipients with hepatocellular carcinoma recurrence. Liver Transpl. 2018;24(7):932–945.. (doi 10.1002/lt.25191) [DOI] [PubMed] [Google Scholar]
- 19.. Kang SH, Cho H, Cho EJ. Efficacy of sorafenib for the treatment of post-transplant hepatocellular carcinoma recurrence. J Korean Med Sci. 2018;33(45):e283–e283.. 10.3346/jkms.2018.33.e283) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.. Kim R, El-Gazzaz G, Tan A. Safety and feasibility of using sorafenib in recurrent hepatocellular carcinoma after orthotopic liver transplantation. Oncology . 2010;79(1-2):62–66.. 10.1159/000319548) [DOI] [PubMed] [Google Scholar]
- 21.. López Ortega S, González Grande R, Santaella Leiva I, De la Cruz Lombardo J, Jiménez Pérez M. Efficacy and safety of sorafenib after liver transplantation: experience in our center. Transplant Proc. 2020;52(2):540–542.. 10.1016/j.transproceed.2019.12.016) [DOI] [PubMed] [Google Scholar]
- 22.. Martin RC 2nd, Bruenderman E, Cohn A. Sorafenib use for recurrent hepatocellular cancer after resection or transplantation: observations from a US regional analysis of the GIDEON registry. Am J Surg. 2017;213(4):688–695.. 10.1016/j.amjsurg.2016.10.006) [DOI] [PubMed] [Google Scholar]
- 23.. Na GH, Hong TH, You YK, Kim DG. Clinical analysis of patients with hepatocellular carcinoma recurrence after living-donor liver transplantation. World J Gastroenterol. 2016;22(25):5790–5799.. 10.3748/wjg.v22.i25.5790) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.. Nagai S, Mangus RS, Kubal CA. Prognosis after recurrence of hepatocellular carcinoma in liver transplantation: predictors for successful treatment and survival. Clin Transpl . 2015;29(12):1156–1163.. 10.1111/ctr.12644) [DOI] [PubMed] [Google Scholar]
- 25.. Pfeiffenberger J, Koschny R, Hoffmann K. Sorafenib treatment is save and may affect survival of recurrent hepatocellular carcinoma after liver transplantation. Langenbecks Arch Surg. 2013;398(8):1123–1128.. 10.1007/s00423-013-1114-1) [DOI] [PubMed] [Google Scholar]
- 26.. Pfiffer TE, Seehofer D, Nicolaou A, Neuhaus R, Riess H, Trappe RU. Recurrent hepatocellular carcinoma in liver transplant recipients: parameters affecting time to recurrence, treatment options and survival in the sorafenib era. Tumori . 2011;97(4):436–441.. 10.1700/950.10394) [DOI] [PubMed] [Google Scholar]
- 27.. Pinero F, Marciano S, Anders M. Sorafenib for recurrent hepatocellular carcinoma after liver transplantation: a South American experience (Sorafenib para el tratamiento de la recurrencia de hepatocarcinoma post trasplante hepatico: experiencia Sudamericana). Acta Gastroenterol Latinoam. 2016;46(4):300–309.. [Google Scholar]
- 28.. Staufer K, Fischer L, Seegers B, Vettorazzi E, Nashan B, Sterneck M. High toxicity of sorafenib for recurrent hepatocellular carcinoma after liver transplantation. Transpl Int. 2012;25(11):1158–1164.. 10.1111/j.1432-2277.2012.01540.x) [DOI] [PubMed] [Google Scholar]
- 29.. Tan WF, Qiu ZQ, Yu Y. Sorafenib extends the survival time of patients with multiple recurrences of hepatocellular carcinoma after liver transplantation. Acta Pharmacol Sin. 2010;31(12):1643–1648.. 10.1038/aps.2010.124) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.. Vitale A, Boccagni P, Kertusha X. Sorafenib for the treatment of recurrent hepatocellular carcinoma after liver transplantation? Transplant Proc. 2012;44(7):1989–1991.. 10.1016/j.transproceed.2012.06.046) [DOI] [PubMed] [Google Scholar]
- 31.. Waghray A, Balci B, El-Gazzaz G. Safety and efficacy of sorafenib for the treatment of recurrent hepatocellular carcinoma after liver transplantation. Clin Transpl . 2013;27(4):555–561.. 10.1111/ctr.12150) [DOI] [PubMed] [Google Scholar]
- 32.. Weinmann A, Niederle IM, Koch S. Sorafenib for recurrence of hepatocellular carcinoma after liver transplantation. Dig Liver Dis. 2012;44(5):432–437.. 10.1016/j.dld.2011.12.009) [DOI] [PubMed] [Google Scholar]
- 33.. Zavaglia C, Airoldi A, Mancuso A. Adverse events affect sorafenib efficacy in patients with recurrent hepatocellular carcinoma after liver transplantation: experience at a single center and review of the literature. Eur J Gastroenterol Hepatol. 2013;25(2):180–186.. 10.1097/MEG.0b013e328359e550) [DOI] [PubMed] [Google Scholar]
- 34.. Nowak AK, Chow PKH, Findlay M. Systemic therapy for advanced hepatocellular carcinoma: a review. Eur J Cancer . 2004;40(10):1474–1484.. 10.1016/j.ejca.2004.02.027) [DOI] [PubMed] [Google Scholar]
- 35.. Llovet JM, Ricci S, Mazzaferro V. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390.. 10.1056/NEJMoa0708857) [DOI] [PubMed] [Google Scholar]
- 36.. Jeong-Ok L, Dae-Young K, Joo Han L. Palliative chemotherapy for patients with recurrent hepatocellular carcinoma after liver transplantation. J Gastroenterol Hepatol. 2010;24(5):800–805.. [DOI] [PubMed] [Google Scholar]
- 37.. Mancuso A, Mazzola A, Cabibbo G. Survival of patients treated with sorafenib for hepatocellular carcinoma recurrence after liver transplantation: a systematic review and meta-analysis. Dig Liver Dis. 2015;47(4):324–330.. 10.1016/j.dld.2015.01.001) [DOI] [PubMed] [Google Scholar]
- 38.. Iavarone M, Cabibbo G, Piscaglia F. Field-practice study of sorafenib therapy for hepatocellular carcinoma: a prospective multicenter study in Italy. Hepatology . 2011;54(6):2055–2063.. 10.1002/hep.24644) [DOI] [PubMed] [Google Scholar]
- 39.. Shah DR, Shah RR, Morganroth J. Tyrosine kinase inhibitors: their on-target toxicities as potential indicators of efficacy. Drug Saf. 2013;36(6):413–426.. 10.1007/s40264-013-0050-x) [DOI] [PubMed] [Google Scholar]
- 40.. Mancuso A, Zavaglia C, Bai F, Puoti M, Belli LS. Letter: Sorafenib hepatotoxicity may be enhanced during treatment of advanced hepatocellular carcinoma in HIV-infected patients. Aliment Pharmacol Ther. 2013;38(11-12):1414–1416.. 10.1111/apt.12536) [DOI] [PubMed] [Google Scholar]
- 41.. Abou-Alfa GK. Sorafenib use in hepatocellular carcinoma: more questions than answers. Hepatology . 2014;60(1):15–18.. 10.1002/hep.27044) [DOI] [PubMed] [Google Scholar]
- 42.. Sacco R, Gadaleta-Caldarola G, Galati G, Lombardi G, Mazza G, Cabibbo G. European Association for the study of the liver hepatocellular carcinoma summit 2014: old questions, new (or few) answers? Future Oncol . 2014;10(10):1719–1721.. 10.2217/fon.14.102) [DOI] [PubMed] [Google Scholar]
- 43.. Wang Z, Zhou J, Fan J. Effect of rapamycin alone and in combination with sorafenib in an orthotopic model of human hepatocellular carcinoma. Clin Cancer Res . 2008;14(16):5124-5130. 10.1158/1078-0432.CCR-07-4774) [DOI] [PubMed] [Google Scholar]
- 44.. Piguet AC, Saar B, Hlushchuk R. Everolimus augments the effects of sorafenib in a syngeneic orthotopic model of hepatocellular carcinoma. Mol Cancer Ther. 2011;10(6):1007–1017.. 10.1158/1535-7163.MCT-10-0666) [DOI] [PubMed] [Google Scholar]
- 45.. Koeberle D, Dufour JF, Demeter G. Sorafenib with or without everolimus in patients with advanced hepatocellular carcinoma (HCC): a randomized multicenter, multinational phase II trial (SAKK 77/08 and SASL 29). Ann Oncol . 2016;27(5):856-861. 10.1093/annonc/mdw054) [DOI] [PubMed] [Google Scholar]
- 46.. Finn RS, Poon RTP, Yau T. Phase I study of everolimus in combination with sorafenib in patients with advanced hepatocellular carcinoma (HCC). J Clin Oncol . 2011;29(15_Suppl):4074. 10.1200/jco.2011.29.15_suppl.4074) [DOI] [Google Scholar]