Abstract
Background:
In Turkey, cytomegalovirus (CMV) seropositivity has been reported to be high, between 85 and 100%. CMV has been responsible for disease exacerbation in inflammatory bowel disease (IBD). We aimed to evaluate the presence of CMV in intestinal tissue by immunohistochemical staining in IBD and non-IBD patient groups, in a country with high CMV seroprevalence.
Methods:
In this prospective cross-sectional study, the presence of intestinal CMV was investigated with tissue immunohistochemistry (IHC) staining, which is accepted as the gold standard method, and with polymerase chain reaction (PCR) in tissue and blood. Patients (≥18 years old, n = 189) who had a colonoscopic biopsy between January and May 2017 were included in the study at our hospital. Clinical, laboratory, endoscopic, and histopathological data of patients were assessed by dividing them into IBD (n = 34) and non-IBD (n = 155) groups.
Results:
In this study, 567 colonic biopsy samples from 189 patients were evaluated. Tissue IHC staining was positive for 3 (1.58%) non-IBD patients. One of them was diagnosed as CMV ileitis. CMV DNA was also detected in 14 plasma (7.40%, <80-469 copies/mL) and 20 tissue samples (10.69%, 7-15 289 copies/mL). Tissue IHC staining is accepted as the gold standard for CMV ileitis, and the sensitivity and specificity of tissue PCR was 33% and 89.67%, while the sensitivity and specificity of plasma PCR was 66.66% and 93.54%, respectively.
Conclusion:
Although CMV seroprevalence is high in Turkey, CMV ileitis was diagnosed in only one non-IBD patient (0.53%). Compared to tissue IHC staining, the sensitivity of tissue and blood CMV PCR was low while their specificity was higher.
Keywords: Cytomegalovirus, immunohistochemistry, polymerase chain reaction, intestinal tissue
INTRODUCTION
Cytomegalovirus (CMV) which is a ubiquitous herpesvirus is frequently acquired during childhood or adolescence. After the viral DNA integrates into the host, lifelong persistence without causing disease can occur in different organs including the gut, as a latent CMV infection. The virus may be reactivated when the immune system is compromised, leading to CMV disease.1 The prevalence of latent CMV infection defined by positive IgG serology ranges from 45% to 100% all over the world.2
In Turkey, CMV seroprevalence has been investigated in various groups and seropositivity has been reported between 85 and 100% according to the “Infectious Diseases and Clinical Microbiology Specialty Society of Turkey (EKMUD)’s CMV Diagnosis, Treatment Consensus Report.3 In Turkey, there are studies reporting that it is 68-82% in the 0-6 age group, 79.5-92% in the 6-14 age group and 90.2-97.8% above the age of 15 years. It was also stated that the seroprevalence increased to 96.8% at the age of 7 and over; therefore, being 7 years old showed a statistically significant correlation with CMV seropositivity.3 Uyar et al. reported 97.3% anti-CMV IgG positivity in 600 pregnant women aged 17-40 years in Northern Turkey and stated that their CMV seroprevalence rate was found similar to other studies in Turkey.4
Most CMV-infected people are asymptomatic. In some immunocompetent cases, CMV infection can rarely present with fever, sore throat, fatigue, and adenopathy. However in immunocompromised patients, CMV disease can occur with severe manifestations, such as encephalitis, retinitis, pneumonia, esophagitis, or ileocolitis.5
In patients with known inflammatory bowel disease (IBD), baseline elevation of inflammatory cytokines such as tumor necrosis factor-α (TNF-α) and interferon-γ promotes reactivation of latent CMV and can subsequently lead to IBD exacerbation.6 Moreover, in this population, CMV enterocolitis can lead to severe complications, including toxic megacolon, fistula formation, perforation, and peritonitis.7
Patients with IBD are often immunosuppressive due to malnutrition, the use of immunosuppressive drugs, and immune dysfunction. CMV’s ability to remain dormant as an “innocent bystander” may sometimes cause confusion in the diagnosis of the disease.8 The differentiation between clinically relevant CMV disease and subclinical CMV reactivation in daily practice is often difficult but essential, since different treatment strategies are required.1 Histopathologic examination of intestinal tissue is the gold-standard diagnostic investigation.1,9
In a high CMV seroprevalence population such as Turkey, we aimed to determine the intestinal presence of CMV with immunohistochemistry (IHC) staining in patients undergoing ileocolonoscopy and biopsy. We also wanted to evaluate these results according to IBD or non-IBD groups, and the diagnostic yield of tissue and blood CMV DNA polymerase chain reaction (PCR) compared to tissue IHC staining.
MATERIALS AND METHODS
Ethical Approval
This research project was approved by the Ethics Board of Dokuz Eylul University on March 16, 2015 (1993GOA, 2015/08-12). Written informed consent of patients was obtained.
Study Design and Patient Selection
To assign the number of patients, calculations were made to determine the sensitivity and selectivity of tissue PCR. Hajian-Tilaki’s sample size estimation formula in diagnostic test studies10 was used to calculate the minimum number of patients by taking tissue PCR’s selectivity as 71.9% and IHC as the gold standard.11 The prevalence of CMV infection in the community was estimated as 90%, and with an error margin of 5%, it was calculated that when sensitivity was taken as 95%, at least 42 people for sensitivity and at least 172 people for selectivity should have been included.
In this prospective cross-sectional study, according to the calculations mentioned earlier, all patients (aged 18 and above) who underwent colonoscopy and biopsy in our hospital’s endoscopy unit, between January and May 2017, were included in the study (n = 293). Patients with acute gastrointestinal (GI) bleeding and unstable clinical conditions and who do not give their consent were excluded from the study (n = 104). We reached 189 patients during the study period.
A questionnaire was filled in by talking face to face with patients before the colonoscopic procedure. The following parameters were recorded: patient’s age, gender, reason for colonoscopy, relevant underlying disease or comorbidities, clinical presentation (bloody diarrhea, abdominal pain, fever, etc.), and treatment history (antiviral drugs, immunosuppressive drugs, chemotherapy, etc.).
Colonoscopy and Biopsy
All colonoscopic procedures were applied under sedation analgesia in the endoscopy unit. The colon mucosa of each patient and if needed the ileal mucosa were evaluated in detail and biopsies were taken from all necessary mucosal changes, such as ulcer, tumor, and inflamed mucosa. The colonic polyps were removed by polypectomy. If the colonic mucosa was normal, colonic biopsies were taken from ascending, transverse, descending, and rectosigmoid part of the colon, sequentially.
Laboratory Protocols
In this study, CMV was investigated by IHC staining in tissue and real-time PCR in blood and tissue samples. Blood samples were taken before the colonoscopic procedure at the endoscopy room.
All biopsy samples were transferred to the laboratory in an hour using appropriate sterile containers and transportation conditions. Biopsy samples were kept in formaldehyde and PCR samples at −80°C, until processing.
Histopathology and IHC
All colonic biopsy samples were evaluated by a GI pathologist (ÖS). All colon biopsies were fixed in buffered formalin, processed routinely, stained with H&E (Sigma-Aldrich Inc. Merck Company, Germany) and evaluated for the presence of colitis, any other pathology, and the presence of CMV inclusion bodies.
Sections were then prepared on lysine-coated slides and stained with primary mouse anti-CMV antibodies (Supplier DAKO, Agilent, USA). The CMV complex was visualized by UltraView Universal DAB Detection Kit (Ventana Medical Systems, Roche Group, USA) and hematoxylin counterstaining. Samples were analyzed with a positive control.
CMV DNA Identification
Viral DNA Extraction
CMV DNA was extracted from both plasma and tissue samples using Qiasymphony DSP Virus/Pathogen Midi kit and Qiasymhony SP extraction device (Qiagen, Germany). Tissue samples were prepared by adding 620 μL tissue lysis buffer (Buffer ATL, Qiagen) and 30 μL enzyme (proteinase K, Qiagen) and incubating at 56°C until the sample was totally digested.
Quantitation of CMV DNA
In vitro nucleic acid amplification test (Artus CMV QS-RGQ Kit, Qiagen) and real-time PCR cycler device (Rotor-Gene Q, Qiagen) were used. The limit of detection was 45 copies/mL for plasma and the linear range was 80 to 1 × 108 copies/mL. Analytical sensitivity of the assay was not determined for tissue samples. The number of copies found in the tissues were stated directly.
Statistical Analyses
Results were analyzed using IBM SPSS-Statistics for Macintosh V22.0 (IBM, USA) software. Ratios were compared with the use of the Chi-square test or Fisher’s exact test. Nonparametric data of <30 were calculated using the Mann–Whitney U test. A two-tailed P-value <.05 was considered to be statistically significant. Nonparametric data of ≥30 were calculated using the independent samples t-test.
RESULTS
Totally 189 patients (105 female, 84 male, mean age: 55.02 ± 14.62 years) were included in the study after excluding 104 patients. These 189 patients were divided into 2 groups as:
IBD group (n = 34; ulcerative colitis [UC], n = 18; Crohn’s disease [CD], n = 16, 20 female, 14 male, mean age: 43.06 years).
Non-IBD group (n = 155, 84 female, 71 male, mean age: 57.65 years).
There was a significant difference between the ages of the 2 groups, P < .001.
Patients had comorbidities as follows: cardiovascular diseases (21.16%), diabetes mellitus (18.51%), malignancy (17.46%), asthma-chronic obstructive pulmonary disease (7.40%), rheumatologic and autoimmune diseases (6.34%), chronic kidney disease (4.23%), tuberculosis (1.05%), and kidney transplantation (0.52%). Twenty-six (13.7%) patients were immunocompromised due to immunosuppressive drugs (n = 25) and recent chemotherapy (n = 1).
Most of the patients (n = 108, 57.1%) had symptoms which was the reason for their colonoscopies. In asymptomatic patients (n = 81, 42.9%) colonoscopies were performed for their own follow-up or screening.
Symptoms of Patients
There was no significant difference between IBD and non-IBD groups in terms of the presence of symptoms (P = .827). Rectal bleeding was significantly more common in the IBD group than in the non-IBD group (n = 29, 32.4% vs 14.83%, P = .016) among the other main symptoms such as abdominal pain (n = 31, 11.7% vs 17.42%, P = .420), constipation (n = 23, 2.94% vs 14.19%, P = .084), diarrhea (n = 23, 20.50% vs 10.32%, P = .142), and weight loss (n = 11, 11.76% vs 4.51%, P = .113). Other rare symptoms were nausea and vomiting (n = 6), anal pain (n = 3), and fever (n = 2). Many of these patients had more than one symptom.
While 16 patients with IBD were asymptomatic, the rest of them (n = 18) had symptoms such as rectal bleeding (n = 8), diarrhea (n = 6), abdominal pain (n = 3), and constipation (n = 1).
There were 4 patients with active diseases (n = 1 UC and n = 3 CD) on systemic corticosteroid (CS) therapy. None of the patients had CS refractory or dependent disease. One of the CD patients had unresponsive disease to infliximab (IFX).
Use of Immunosuppressive Drugs
Twenty-five patients (13.22%, n = 18 in IBD and n = 7 in non-IBD groups, P) were using immunosuppresive drugs, such as monotherapy (n = 15) and combination therapies (dual, n = 7; triple therapies, n = 3). Eighteen patients with IBD (n = 7 UC, n = 11 CD) used CS (n = 6), azathioprine (AZA) (n = 8), and anti-TNF agents (IFX n = 2, adalimumab n = 4) (P < .001) as described in Table 1. The other immunosupressive agents were methotrexate (n = 2), leflunomide (n = 2), and mycophenolate mofetil (n = 1). No patient received antiviral therapy.
Table 1.
Use of the Immunosupressive Drugs
| Immunosupressive Drugs (n) | IBD Group (n = 34), n (%) | Non-IBD Group (n = 155), n (%) | P |
|---|---|---|---|
| CS13 | 7 (20.59) | 7 (4.52) | .005 |
| Azathioprine8 | 8 (23.53) | 0 (0) | <.001 |
| Anti-TNFα7 | 6 (17.65) IFX (n = 2)ADA (n = 4) |
1 (0.65) IFX (n = 1) |
<.001 |
IBD, inflammatory bowel disease; CS, corticosteroid; anti-TNFα, anti-tumor necrosis factor-α; IFX, infliximab; ADA, adalimumab.
Statistically significant differences were marked bold.
Results of Colonoscopy
Colonoscopic findings were completely normal in 59 (31.2%) patients (n = 6 in the IBD group (17.65%) vs n = 53 in the non-IBD group (34.19%), respectively, P = .059).
There were 130 (68.8%) patients with significant colonoscopic findings such as colorectal polyps (n = 74), colonic diverticulae (n = 16), colon cancer (n = 8), angiodysplasia (n = 4), ileal mucosal nodularity (n = 3), lipoma (n = 2), and pseudomembranous colitis (n = 1). Colonoscopic findings were seen in more than 1 patient.
Among clinically significant colonoscopic findings, only mucosal inflammation and mucosal injuries (erosion and/or ulcer) and/or pseudopolyps were found to be more significant in the IBD group compared to the non-IBD group.
Colonoscopic inflammation defined by mucosal edema, hyperemia, and the absence of a vascular pattern was found in 26.15% (n = 34/130) of patients. About 85.71% of patients (n = 24/28) in the IBD group had significantly much more mucosal inflammation compared to the non-IBD group (9.80%, n = 10/102, P < .001). Erosion, ulcer, and/or pseudopolyps were found in 17.69% (n = 23) of patients. About 64.28% of patients with IBD had much more mucosal injury and/or pseudopolyps compared to 4.90% in the non-IBD group (P < .001) (Table 2).
Table 2.
Significant Colonoscopic Findings in IBD and Non-IBD Groups
| Colonoscopic Findings (n = 189), n (%) | IBD (n = 34), n (%) | Non-IBD (n = 155), n (%) | P |
|---|---|---|---|
| Normal 59 (31.22) | 6 (17.65) | 53 (34.19) | .059 |
| Positive findings 130 (68.78) | 28 (87.50) | 102 (65.80) | .059 |
| Inflammation 34 (26.15)Erosions, ulcers, and/or pseudopolyps 23 (17.69) | 24 (85.71) 18 (64.29) |
10 (9.80) 5 (4.90) |
<.001<.001 |
IBD, inflammatory bowel disease.
Statistically significant differences were marked bold.
There was a statistically significant relationship between the symptoms and colonoscopic findings in the study group (P = .037). The presence of diarrhea and rectal bleeding was related to colonoscopic ulcers (P = .041, P = .009, respectively).
Nine of the 16 asymptomatic patients with IBD had active disease according to their colonoscopy results while the other 7 of them were in endoscopic remission according to Mayo endoscopic subscore (≤1).12 All of the symptomatic IBD patients had clinically significant colonoscopic findings.
A statistically significant relationship was found between the use of immunosuppressive drugs and colonic ulcers (P < .001) and inflammation (P < .001) in both the groups.
Tissue H&E and IHC Staining Results
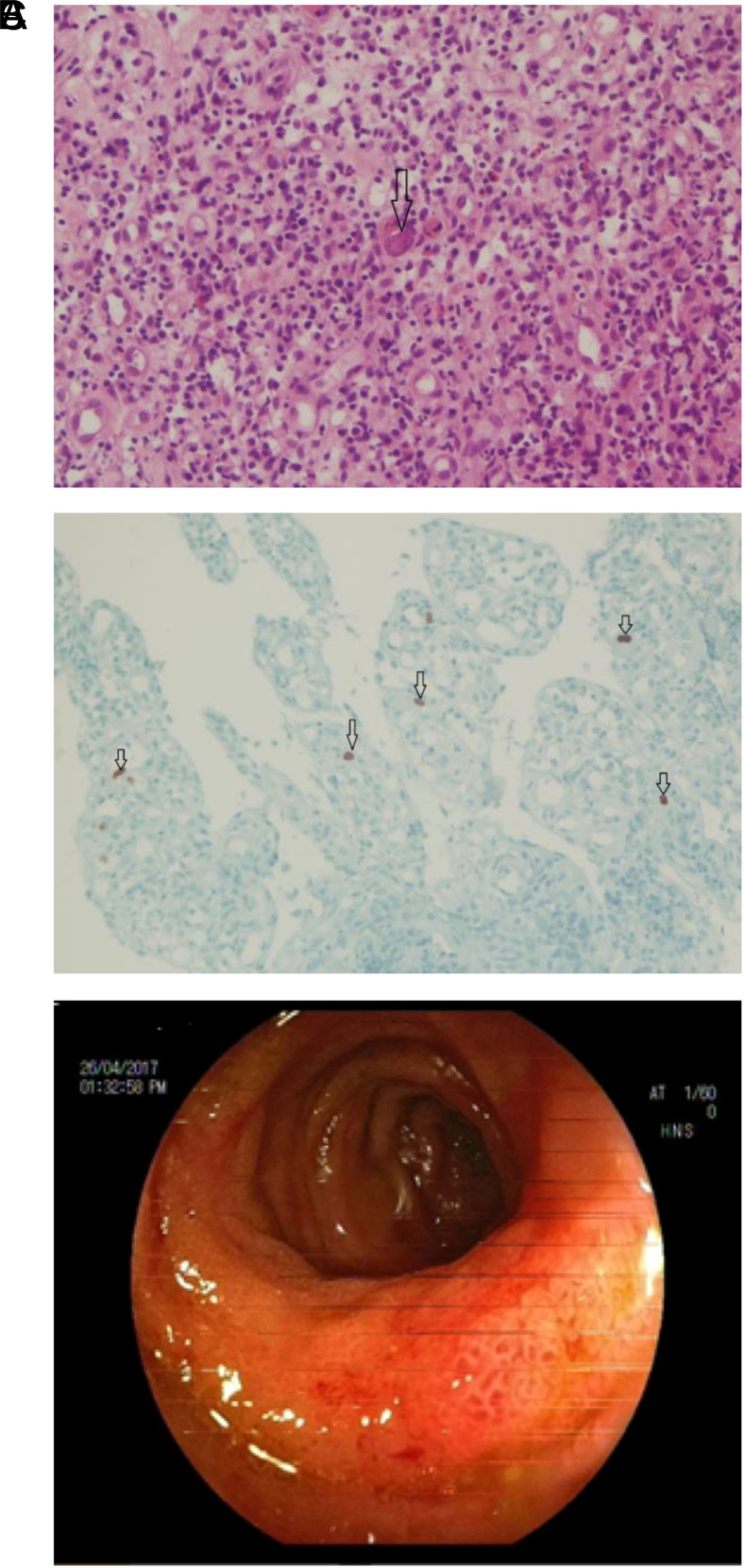
CMV was detected by IHC staining in 3 non-IBD patients (1.58%) (Table 3). Two patients had only one positive cell per biopsy. Blood and tissue CMV DNA could not be detected in these patients. One of 3 who had gastric cancer and chemotherapy had fever and diarrhea. At least 10 positive cells per biopsy in the ileum were detected in this patient using IHC staining and nuclear inclusion bodies (owl’s eye) and neutrophilic infiltration in hematoxylin and eosin (H&E) staining. CMV ileitis was diagnosed based on histopathologic findings after ileocolonoscopic view (Figure 1A–C). İleal tissue CMV DNA was 45 copy/mL whereas plasma CMV DNA level was 469 copy/mL. Neutropenic fever and CMV ileitis was treated with antibiotics and granulocyte colony-stimulating factor (G-CSF) except anti-virals. The patient recovered before the results of histopathology and CMV DNA were obtained.
Table 3.
IHC Staining Positive Cases
| n | Comorbidity/Risk Factors | Symptoms | Histopathology | Immune Positive Cell Number Per Biopsy | CMV DNA PCR (copy/mL) | |
|---|---|---|---|---|---|---|
| Tissue | Blood | |||||
| 1 | - | Rectal bleeding | Tubular adenoma | 1 | - | - |
| 2 | Asthma CS |
Weight loss | Tubular adenoma | 1 | - | 80 |
| 3 | Gastric cancer Chemotherapy |
Fever Diarrhea |
CMV ileitis findings with owl’s eye* | >10 | 45 | 469 |
*The histopathological interpretation of CMV disease with nuclear inclusions and neutrophilic infiltration in H&E staining.
H&E, hematoxylin and eosin; PCR, polymerase chain reaction; CMV, cytomegalovirus; CS, corticosteroid.
Figure 1.
Neutrophilic infiltration and CMV nuclear inclusion in the lamina propria of ileal tissue (arrow) with H&E (A), ×20. Nuclear positive cells (arrows) with CMV IHC stain (B) in ileal tissue, ×20. Mucosa edema and hyperemia in the distal 15 cm segment of the terminal ileum, edema in ileocecal valve, colonoscopic view (C). H&E, hematoxylin and eosin; CMV, cytomegalovirus; IHC, immunohistochemistry.
Tissue PCR Results
A total of 187 tissue samples (n = 33 in the IBD group, n = 154 in the non-IBD group) for CMV DNA PCR were studied. Two samples were not enough to extract CMV DNA. It was found to be positive in 20 (10.69%) tissue samples (range: 7-15 289 copies/mL) (Table 4). Among 33 patients with IBD, 10 (29.41%) had tissue CMV DNA positivity while 10 patients (6.49%) from the non-IBD group (n = 154) were positive. There was a statistically significant relationship between tissue CMV DNA PCR positivity and IBD (P < .001) and especially CD (P < .001) (Table 4).
Table 4.
Tissue CMV DNA PCR Results
| n | CMV DNA (copy/mL) | IHC +/− | IBD type/non-IBD | |
|---|---|---|---|---|
| Tissue | Blood | |||
| 1 | 7 | - | - | non-IBD |
| 2 | 7 | 80 | - | non-IBD |
| 3 | 7 | - | - | IBD-CD |
| 4 | 8 | - | - | non-IBD |
| 5 | 11 | - | - | non-IBD |
| 6 | 11 | - | - | non-IBD |
| 7 | 17 | 82 | - | IBD-CD |
| 8 | 21 | - | - | IBD-UC |
| 9 | 25 | - | - | IBD-UC |
| 10 | 30 | - | - | IBD-CD |
| 11 | 45 | 469 | + | non-IBD |
| 12 | 58 | - | - | IBD-CD |
| 13 | 88 | - | - | IBD-CD |
| 14 | 135 | - | - | IBD-CD |
| 15 | 201 | - | - | non-IBD |
| 16 | 361 | - | - | IBD-UC |
| 17 | 504 | 111 | - | non-IBD |
| 18 | 1044 | - | - | IBD-CD |
| 19 | 1446 | - | - | non-IBD |
| 20 | 15 289 | - | - | non-IBD |
IHC, immunohistochemistry; CMV, cytomegalovirus; IBD, inflammatory bowel disease; UC, ulcerative colitis; CD, Crohn’s disease.
IBD type, CD or UC were given in bold.
Blood PCR Results
A total of 189 plasma samples were studied for CMV DNA by PCR. CMV DNA was detected in 14 (7.40%) of the plasma samples and 4 had the value over the lower limit of quantitation (80 copies/mL). These were 82, 111, 229, and 469 copies/mL. Blood CMV DNA was positive in 4 of 34 IBD patients while 10 of 155 patients from the non-IBD group were found to be positive (6.45%) and it was not statistically significant (P = .284) (Table 4).
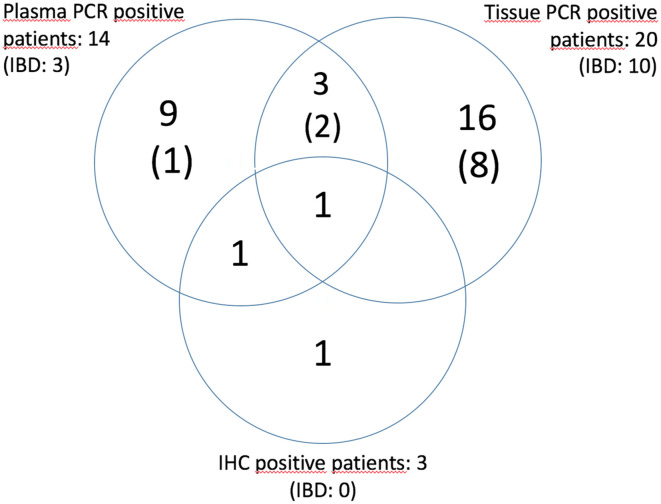
All (IBD) CMV test results can be viewed as a cluster in Figure 2.
Figure 2.
CMV test results (IBD). CMV, cytomegalovirus; IBD, inflammatory bowel disease.
All CMV test results according to the IBD and non-IBD groups can be seen in Table 5. Tissue CMV DNA was significantly positive in the IBD and CD groups.
Table 5.
The Collective Results of CMV Tests
| n | Blood PCR | Tissue PCR | IHC | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| + | − | P | + | − | P | + | − | P | ||
| IBD | + | 4 | 30 | .284* | 10 | 23 | <.001** | 0 | 34 | .999* |
| Non-IBD | - | 10 | 145 | 10 | 144 | 3 | 152 | |||
| UC | + | 3 | 15 | .135* | 3 | 14 | .4* | 0 | 18 | .999* |
| - | 11 | 160 | 17 | 153 | 3 | 168 | ||||
| CD | + | 1 | 15 | .999* | 7 | 9 | <.001** | 0 | 16 | .999* |
| - | 13 | 160 | 13 | 158 | 3 | 170 | ||||
| Total | 189 | 187‡ | 189 | |||||||
*Fisher’s exact chi-square test, **Chi-Square test.
PCR, polymerase chain reaction; IHC, immunohistochemistry; UC, ulcerative colitis; CD, Crohn’s disease; CMV, cytomegalovirus.
Statistically significant relationships were given in bold.
Tissue CMV DNA positivity was significantly related to the diarrhea (P = .013) and the use of immunosuppressive drugs (P = .006), especially for AZA in CD group (P = .035). Tissue CMV DNA was positive in 6 (75%) of 8 patients receiving AZA. The tissue viral load of 6 AZA-treated patients and the other (n = 16) patients were similar (the median CMV DNA was 51.50 copy/mL (quartiles: 10.25-639.00) and 27.50 copy/mL (quartiles: 27.50-99.75), respectively (P = .547).
There was also a statistically significant relationship between tissue CMV-DNA positivity and mucosal injury such as ulcers at colonoscopy for all patients (P = .017) but not for IBD patients (P = .465).
As a result, when IHC staining of CMV was accepted as the diagnostic gold standard, the sensitivity of the tissue CMV DNA PCR was 33% and the specificity was 89.67%, whereas the sensitivity of plasma CMV DNA PCR was 66.66% and specificity was 93.54%.
DISCUSSION
CMV which has worldwide seroprevalence ranging from 56% to 94% has global clinical importance. CMV can be particularly problematic for 2 patient groups: (i) immunocompromised individuals, such as HIV positive, organ transplanted, and receiving chemotherapy and (ii) congenitally infected newborns. Rarely in immunocompetent hosts, it can cause an asymptomatic or mild form of infection. Until now, the presence of CMV has been examined in these risk groups. In this study, we evaluated intestinal CMV disease in 189 patients who are mostly immunocompetent and had routine colonoscopy and biopsy.
Our study population was 57.1% symptomatic. The positivity of IHC staining was 1.5% while CMV DNA was found to be positive 10.69% in intestinal tissue and 7.4% in plasma by PCR. Since intestinal IHC staining is accepted as the gold standard diagnostic method, we evaluated tissue and plasma CMV PCR positivity based on this method.
There was no CMV colitis in the study group.
Among the 26 immunocompromised patients, only 1 patient who had gastric cancer, chemotherapy, neutropenic fever, and diarrhea was diagnosed with CMV ileitis. This patient had a typical finding of enlarged cells with thickened nuclear membrane and large nuclear inclusion bodies (owl’s eyes) by H&E, which is representative of CMV replication, and >10 immune positive cell/biopsy by IHC staining. H&E staining has a high specificity (92–100%), but its sensitivity is in the range of 10–87%.2
CMV ileitis is much rarely seen unlike CMV colitis in immunocompromised patients.13-15 But CMV ileitis can be also seen in immunocompetent ones.16
CMV ileitis was treated with empirical antibiotics and G-CSF due to risk factors (gastric cancer, chemotherapy, and neutropenic fever) before tissue pathology and PCR tests results were obtained. The patient recovered from neutropenia and his gained immunity overcame CMV disease without antiviral treatment. If the patient’s results were obtained concurrently with the clinical findings, antiviral treatment could have been considered.
Apart from the patient with CMV ileitis, 2 other patients also had positive IHC staining (only 1 immune positive cell/biopsy), whereas 1 had a plasma CMV DNA level of 80 copy/mL. Although these patients had positive IHC staining, they were not diagnosed as CMV ileitis and/or colitis. Because these patients were not immunocompromised and did not have symptoms related to the CMV disease, the diagnostic sensitivity and specificity of tissue IHC staining can reach up to 78-93% and 92-100%, respectively.2
The threshold number of positive cell/biopsy by tissue IHC staining is not clearly known. Liao et al.17 classified IHC staining as “rare positive” if <2 cells/biopsy, and “true positive” if ≥2 or more cells/biopsy were positive. They found low CMV PCR positivity in “rare positive” cell cases and significantly higher CMV PCR positivity in “true positive” ones. In addition, when there are more positive cells in IHC staining for CMV, histopathology is found to be more affected.
IBD patients are at high risk for active CMV disease. It is stated that this predisposition may be caused by immunosuppressive therapy, poor nutrition, deterioration of the functions of natural killer cells, and tropism of CMV to inflammation sites.18 UC patients were found to have significantly more CMV disease than patients with Crohn’s disease.
The CMV burden on microscopic examination has been found to be important in patients with IBD. The presence of >5 IHC positive cells per 2 mm tissue is higher in patients with steroid-refractory IBD disease and the presence of >10 IHC positive cells per biopsy has an increased risk of colectomy.1 It has been recommended that anti-viral treatment should be given in the presence of >4 positive cells/biopsy or plasma CMV DNA ≥1000 IU/mL in symptomatic IBD patients, who have ≥1 of these findings: CS refractory disease, splenomegaly, absence of leukocytosis.9
The number of CMV DNA copies/mL of the CMV ileitis cases (469 copies/mL in plasma, and 45 copies/mL in tissue) was not as high as mentioned in the literature. Current studies in this field have been reporting established protocols according to the risk groups of patients. Most of the studies focus on determining a threshold for diagnosis of CMV colitis by tissue PCR, but is there a threshold that we can detect to decide on antiviral therapy? Roblin et al.19 found that tissue CMV DNA load >250 copies/mL for UC patients was predictive of resistance to three successive regimens and so they recommended early initiation of antiviral treatment. In the study of Ciccocioppo et al.,20 CMV DNA peak values found in the biopsies taken from the diseased mucosa of treatment-resistant IBD patients were found to be statistically significant above 1000 copies/mL and it was accepted as the criterion for treatment. Mavropoulou et al.21 confirmed that the cut-off value of tissue CMV DNA is >250 copies/mL for the diagnosis of CMV colitis in patients after allogeneic stem cell transplantation. However, according to the literature, by strengthening natural immunity we can overcome virus reactivation.22 Examples include the use of G-CSF in neutropenic patients and the reduction of immunosuppressive drug doses in organ transplant patients. In our study, the CMV ileitis patient recovered without any antiviral treatment by treating neutropenia and concomitant infections. It has been shown that the preemptive treatment of patients with stable clinical status followed by allogeneic bone marrow and stem cell transplantation can be postponed until the blood CMV DNA is as high as 10 000 copies/mL.23
In our study, there were three patients with tissue CMV DNA results above 1000 copies/mL and 1 of them was above 10 000 copies/mL (Table 4), but none of them had treatment-resistant IBD or bone marrow transplantation and did not need antiviral treatment in clinical follow-up. These findings emphasize the importance of host characteristics and concomitant diseases for treatment decision rather than CMV DNA PCR thresholds.
Plasma CMV DNA levels above 1000 copies/mL have been found to be highly indicative of the development of a clinically symptomatic systemic disease in renal transplant patients.24 Also, according to the guideline of Ljungman et al.25 for organ transplant patients, detection of CMV by PCR alone is insufficient for the diagnosis of CMV GI tract disease. For diagnosis, clinical symptoms, macroscopic mucosal lesions on endoscopy, and the presence of CMV (by culture, histopathology, IHC, or in situ hybridization) in the GI tract biopsy sample should be coexisting. We had only one renal transplant case in our study and the patient’s plasma CMV DNA was negative. But two of our symptomatic cases had high fever with less than 1000 copies/mL in plasma (111 and 469 copies/mL) and intestinal tissue (45 and 504 copies/mL) (Table 4). One of them was the CMV ileitis case and the other one was being investigated as fever of unknown origin who failed to have another diagnosis. Both improved without antiviral treatment. Our study supports the suggestion that Ljungman et al.’s proposition can be applied to patients other than organ transplantation.
The British Society of Gastroenterology (BSG) guidelines recommend using H&E staining and also IHC staining and/or tissue PCR for the diagnosis of the CMV disease in IBD patients.1 The most recent European Crohn’s and Colitis Organization (ECCO) guidelines concur with the BSG guidelines on the use of H&E and IHC staining. According to ECCO guidelines, multiple intranuclear inclusions are usually clinically significant.1 According to the consensus report of EKMUD on CMV disease of IBD patients, it is not necessary to perform blood tests to detect CMV infection in IBD patients.3
The use of immunosuppressive drugs was significantly higher in the IBD group. In our study, a significant relationship was found between AZA use and tissue CMV DNA positivity. In a study by Kishore et al.,26 a positive correlation was also found between AZA use and CMV infection. The reason is that the 6-thioguanine nucleotides, which are the metabolites of the drug, are purine antagonists and inhibit DNA and RNA synthesis in natural killer cells and proliferating CD4–CD8 lymphocytes, which play a major role in protection from CMV infection.27
Immunosuppressive drugs were correlated with positive colonoscopic findings, such as colonic ulcers and inflammation in both IBD and non-IBD groups.
Among significant colonoscopic findings, only mucosal inflammation and mucosal injuries (erosion, ulcer) and/or pseudopolyps were found to be more significant in the IBD group. In our study, the presence of CMV DNA was significantly associated with colonic ulcer. In the study of Suzuki et al.,28 colonoscopic findings specific to CMV colitis were defined as large mucosal defects and ulcers. Therefore, if any ulcer is detected in colonoscopy, biopsy should be performed and CMV colitis should be considered. However, according to Omiya et al.29 antiviral treatment is not required for patients with active UC who have positive CMV DNA in the tissue PCR test and do not have a large ulcer but only latent CMV infection.
The limitation of our study was the small number of positivity with IHC and H&E stainings. Therefore, a relationship between IHC staining and tissue PCR could not be established, and sensitivity and specificity of tissue PCR were found to be low. However, the available data suggest that tissue IHC staining and PCR can detect latent virus. Our suggestion is that demonstrating the presence of CMV in tissue is not sufficient to make a diagnosis and/or plan treatment. The tissue CMV burden and associated macroscopic and histopathological findings are also important. IHC staining should not be routinely studied in patients without ulcers and/or inflammation in histopathology. Patients should be evaluated on a case-by-case basis.
MAIN POINTS
What’s already known about this topic?
CMV colitis is common in immunocompromised patients and is a complicating issue in IBD. CMV’s ability to remain latent causes an uncertainty in the diagnosis.
Immunohistochemistry is the gold standard and PCR is the increasingly accepted method of diagnosis but both can detect latent virus. Clinicians need a consensus on deciding the treatment plan when the virus is spotted in the tissue.
What does this article add?
We determined only one immunocompromised patient with CMV ileitis among mostly immunocompetent patients in a high CMV seroprevalence population.
To demonstrate only CMV in the intestinal tissue is not sufficient to diagnose CMV disease, such as CMV colitis/ileitis. The tissue CMV burden and associated findings such as symptoms, immunodeficiency, and colonoscopic and histopathologic findings are important.
We suggest that patients with suspected CMV disease should be evaluated on a case-by-case basis.
Further studies are needed to determine tissue CMV load in deciding anti-viral treatment.
Funding Statement
The authors declared that this study has received no financial support.
Footnotes
Ethics Committee Approval: This research project was approved by the Ethics Board of Dokuz Eylul University on March 16, 2015 (1993GOA, 2015/08-12).
Informed Consent: Written informed consent of patients was obtained before every procedure.
Peer-review: Externally peer-reviewed.
Author Contributions: Conception - H.H.K., V.A.O.; Design - H.H.K., V.A.O.; Supervision and Funding Acquisition - V.A.O; Materials - H.H.K., H.A., O.S., A.S.; Data Collection and/or Processing – H.H.K., H.A., O.S., A.S.; Analysis and/or Interpretation – H.H.K., V.A.O., O.S., A.S.; Supervision – V.A.O., H.A.; Literature Review – H.H.K., V.A.O., H.A.; Writing-Original Draft – H.H.K.; Critical Review and Editing - V.A.O., H.A., A.S., O.S.
Acknowledgments: This study was supported by Dokuz Eylul University Department of Scientific Research Projects (2016.KB.SAG.017) (but the Department was not involved in the project).
Conflict of Interest: The authors have no conflict of interest to declare.
References
- 1. . Gilmore RB, Taylor KM, Morrissey CO, Gardiner BJ. Cytomegalovirus in inflammatory bowel disease: a clinical approach. Intern Med J. 2020. 10.1111/imj.15085) [DOI] [PubMed] [Google Scholar]
- 2. . Mourad FH, Hashash JG, Kariyawasam VC, Leong RW. Ulcerative colitis and cytomegalovirus infection: from A to Z. J Crohns Colitis. 2020;14(8):1162–1171.. 10.1093/ecco-jcc/jjaa036) [DOI] [PubMed] [Google Scholar]
- 3. .EKMUD (Infectious Diseases and Clinical Microbiology Speciality Society of Turkey). CMV diagnosis, treatment consensus report. https://www.ekmud.org.tr/files/uploads/files/CMV-Uzlasi-Raporu.pdf. Accessed April 2020. [Google Scholar]
- 4. . Uyar Y, Balci A, Akcali A, Cabar C. Prevalence of rubella and cytomegalovirus antibodies among pregnant women in northern Turkey. New Microbiol. 2008;31(4):451–455.. [PubMed] [Google Scholar]
- 5. . Shieh AC, Guler E, Tirumani SH, Dumot J, Ramaiya NH. Clinical, imaging, endoscopic findings, and management of patients with CMV colitis: a single-institute experience. Emerg Radiol. 2020;27(3):277–284.. 10.1007/s10140-020-01750-z) [DOI] [PubMed] [Google Scholar]
- 6. . Rafailidis PI, Mourtzoukou EG, Varbobitis IC, Falagas ME. Severe cytomegalovirus infection in apparently immunocompetent patients: a systematic review. Virol J. 2008;5:47. 10.1186/1743-422X-5-47) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. . Stilwell KT, Estes J, Kurtz MT. CMV Ileitis: to treat or not to treat? Implications of initiating biologic therapy for concurrent Crohn’s disease. Case Rep Gastrointest Med. 2019;2019:4513795. 10.1155/2019/4513795) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. . Umar S, Clarke K, Bilimoria F. Diagnostic yield from colon biopsies in patients with inflammatory bowel disease and suspected cytomegalovirus infection: is it worth it? Ann Gastroenterol. 2017;30(4):429–432.. 10.20524/aog.2017.0153) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. . Kredel LI, Mundt P, van Riesen L. et al. Accuracy of diagnostic tests and a new algorithm for diagnosing cytomegalovirus colitis in inflammatory bowel diseases: a diagnostic study. Int J Colorectal Dis. 2019;34(2):229–237.. 10.1007/s00384-018-3170-z) [DOI] [PubMed] [Google Scholar]
- 10. . Hajian-Tilaki K. Sample size estimation in diagnostic test studies of biomedical informatics. J Biomed Inform. 2014;48:193–204.. 10.1016/j.jbi.2014.02.013) [DOI] [PubMed] [Google Scholar]
- 11. . Burston J, van Hal S, Dubedat S, Lee A. Inclusions or bystanders? CMV PCR sensitivity and specificity in tissue samples. J Clin Virol. 2017;90:38–39.. 10.1016/j.jcv.2017.03.008) [DOI] [PubMed] [Google Scholar]
- 12. . Shah J, Dutta U, Das A. Relationship between Mayo endoscopic score and histological scores in ulcerative colitis: a prospective study. JGH Open. 2020;4(3):382–386.. 10.1002/jgh3.12260) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. . Kato K, Cooper M. Small bowel perforation secondary to CMV-positive terminal ileitis postrenal transplant. BMJ Case Rep. 2019;12(11):e231662. 10.1136/bcr-2019-231662) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. . Navaneethan U, Venkatesh PG, Wang J. Cytomegalovirus ileitis in a patient after liver transplantation-differentiating from de novo IBD. J Crohns Colitis. 2011;5(4):354–359.. 10.1016/j.crohns.2011.01.010) [DOI] [PubMed] [Google Scholar]
- 15. . Michalopoulos N, Triantafillopoulou K, Beretouli E. et al. Small bowel perforation due to CMV enteritis infection in an HIV-positive patient. BMC Res Notes. 2013;6:45. 10.1186/1756-0500-6-45) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. . Tejedor Cerdeña MA, Velasco Guardado A, Fernández Prodomingo A. et al. Cytomegalovirus ileitis in an immunocompetent patient. Rev Esp Enferm Dig. 2011;103(3):154–156.. 10.4321/s1130-01082011000300009) [DOI] [PubMed] [Google Scholar]
- 17. . Liao X, Reed SL, Lin GY. Immunostaining detection of cytomegalovirus in gastrointestinal biopsies: clinicopathological correlation at a large academic health system. Gastroenterol Res. 2016;9(6):92–98.. 10.14740/gr725e) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. . Kandiel A, Lashner B. Cytomegalovirus colitis complicating inflammatory bowel disease. Am J Gastroenterol. 2006;101(12):2857–2865.. 10.1111/j.1572-0241.2006.00869.x) [DOI] [PubMed] [Google Scholar]
- 19. . Roblin X, Pillet S, Oussalah A. Cytomegalovirus load in inflamed intestinal tissue is predictive of resistance to immunosuppressive therapy in ulcerative colitis. Am J Gastroenterol. 2011;106(11):2001–2008.. 10.1038/ajg.2011.202) [DOI] [PubMed] [Google Scholar]
- 20. . Ciccocioppo R, Racca F, Paolucci S. Human cytomegalovirus and Epstein-Barr virus infection in inflammatory bowel disease: need for mucosal viral load measurement. World J Gastroenterol. 2015;21(6):1915–1926.. 10.3748/wjg.v21.i6.1915) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. . Mavropoulou E, Ternes K, Mechie NC. Cytomegalovirus colitis in inflammatory bowel disease and after haematopoietic stem cell transplantation: diagnostic accuracy, predictors, risk factors and disease outcome. BMJ Open Gastroenterol. 2019;6(1):e000258. 10.1136/bmjgast-2018-000258) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. . Hanley PJ, Bollard CM. Controlling cytomegalovirus: helping the immune system take the lead. Viruses. 2014;6(6):2242–2258.. 10.3390/v6062242) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. . Verkruyse LA, Storch GA, Devine SM, Dipersio JF, Vij R. Once daily ganciclovir as initial pre-emptive therapy delayed until threshold CMV load > or =10 000 copies/ml: a safe and effective strategy for allogeneic stem cell transplant patients. Bone Marrow Transplant. 2006;37(1):51–56.. 10.1038/sj.bmt.1705213) [DOI] [PubMed] [Google Scholar]
- 24. . Kühn JE, Wendland T, Schäfer P. Monitoring of renal allograft recipients by quantisation of human cytomegalovirus genomes in peripheral blood leukocytes. J Med Virol. 1994;44(4):398–405.. 10.1002/jmv.1890440416) [DOI] [PubMed] [Google Scholar]
- 25. . Ljungman P, Griffiths P, Paya C. Definitions of cytomegalovirus infection and disease in transplant recipients. Clin Infect Dis. 2002;34(8):1094–1097.. 10.1086/339329) [DOI] [PubMed] [Google Scholar]
- 26. . Kishore J, Ghoshal U, Ghoshal UC. Infection with cytomegalovirus in patients with inflammatory bowel disease: prevalence, clinical significance and outcome. J Med Microbiol. 2004;53(11):1155–1160.. 10.1099/jmm.0.45629-0) [DOI] [PubMed] [Google Scholar]
- 27. . Hookey LC, Depew W, Boag A, Vanner S. 6-Mercaptopurine and inflammatory bowel disease: hidden ground for the cytomegalovirus. Can J Gastroenterol. 2003;17(5):319–322.. 10.1155/2003/824547) [DOI] [PubMed] [Google Scholar]
- 28. . Suzuki H, Kato J, Kuriyama M. Specific endoscopic features of ulcerative colitis complicated by cytomegalovirus infection. World J Gastroenterol. 2010;16(10):1245–1251.. 10.3748/wjg.v16.i10.1245) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. . Omiya M, Matsushita M, Tanaka T, Kawamata S, Okazaki K. The absence of large ulcer predicts latent cytomegalovirus infection in ulcerative colitis with positive mucosal viral assay. Inter Med. 2010;49(21):2277–2282.. 10.2169/internalmedicine.49.3657) [DOI] [PubMed] [Google Scholar]