Abstract
In many countries, vaccination programs still require dogs to be vaccinated against rabies in addition to Canine distemper virus (CDV), adenovirus (CAV), parvovirus (CPV), parainfluenza virus (CPiV), Leptospira (L) or Canine coronavirus (CCV= Cv). Few vaccines containing all these antigens are commercially available and, unless compatibility between the vaccines was demonstrated, concurrent administration of a DAPPi-L(Cv) vaccine and a vaccine against rabies should not be recommended. This may be of concern for practitioners who wish to vaccinate dogs with all components on the same day. This study aimed at evaluating immunological compatibility between a monovalent rabies vaccine (Rabisin™) and two large combination vaccines against CDV, CAV, CPV, CPiV with 2 leptospira components +Cv (Recombitek® C6/Cv) or with 4 Leptospira components (Recombitek® C8), when injected concomitantly at two separate injection sites.
Fourteen days after administration of the rabies vaccine, with or without concomitant administration of combo vaccines, all dogs had seroconverted against rabies and maintained protective titers over the duration of the study. In addition, 100% of the puppies vaccinated with one or the other combo vaccines seroconverted against CDV, CAV, CPV, CPiV (CCV) and Leptospira, whatever the vaccination group. Lack of immunological interference between Rabisin™ and all components of the Recombitek® C6/Cv or Recombitek® C8 Combo vaccines was demonstrated by non-inferiority analysis, except for CDV in the Recombitek®C8+ Rabisin™ group. Based on these results, a concomitant administration of Rabisin™ with Recombitek® C6/Cv or Recombitek® C8 can be recommended in daily practice, which can be essential for facilitating vaccination compliance.
Keywords: Rabies, Canine distemper virus, Canine adenovirus, Canine parvovirus, Canine parainfluenza virus, Leptospira, Vaccine compatibility
1. Introduction
Beside receiving the basic vaccination course against canine distemper virus (CDV), adenovirus (CAV), parvovirus (CPV), parainfluenza virus (CPiV) and leptospirosis, many dogs in the world still require to be vaccinated against rabies, especially in areas where the disease remains prevalent and/or vaccination is still mandatory. As long as all those vaccines were administered annually, practitioners were prompted to use large combination vaccines, which included the rabies component. But both the epidemiological situation and vaccine recommendation have evolved, making such combinations less adapted.
Indeed, due to successful rabies control, routine vaccination against rabies is no longer recommended in many countries and this component doesn’t need to be systematically included in basic vaccination schemes (Day et al., 2016). Nevertheless, regular vaccination against rabies remains recommended where the disease still causes a threat, especially in Asia, Africa and some areas in South America (Day et al., 2015).
In addition, international vaccination guidelines (Day et al., 2015, [Ford et al., 2017]) recognize that all vaccines do not have same duration of immunity (DOI) and recommend to split vaccines, in particular the rabies ones, as much as possible. As a matter of fact, even if rabies vaccination has to be administered annually by law in some high-risk countries, many rabies vaccines have demonstrated duration of immunity of up to 3 years or even above (Dodds et al., 2020). The same applies to components such as CPV, CDV and CAV which DOI can also, in some extend, be monitored through antibody level follow-up, whereas other components, such as Leptospira (L) and Canine Parainfluenza (CPi), usually require to be readministered every year. As a consequence, veterinarians require manufacturers to provide rabies component as a separate option for allowing more flexibility.
Finally, several studies have shown that some components may interfere with the anti-rabies immune response when administered together in the same syringe, this being likely linked to some physico-chemical interactions (Cliquet et al., 2003).
Such larger combinations are usually leading to decreased duration of immunity of the rabies component. These interferences may also occur, even when vaccines are administered at two different injection sites, unless the opposite has been demonstrated, as it has been shown in previous studies (Bouvet et al., 2018).
However, practitioners are asking for solutions allowing to vaccinate dogs with all components on the same day, in order to make vaccination compliance easier to achieve for dog owners.
The objective of this study was to analyze the lack of immunological interference between a monovalent rabies vaccine (Rabisin™) and two large combination vaccines against canine distemper virus (CDV), adenovirus (CAV), parvovirus (CPV), parainfluenza virus (CPiV) and leptospirosis 2-1 way (Recombitek® C6*) or 4-way (Recombitek® C8*), when injected concomitantly at two separate injection sites. Simultaneous administration (mixing the vaccines and injecting them at the same site) was not tested, since studies have shown that some bacterial components are likely to modify the immune response to other antigens after the vaccines are mixed (Cliquet et al., 2003). Injection of the vaccines at separate sites may prevent such interaction.
2. Materials and methods
2.1. Vaccines
Recombitek®C8: freeze-dried vaccine containing a recombinant canarypox expressing glycoproteins HA and F of the canine distemper virus (CDV) and 3 live attenuated viruses (Canine Adenovirus 2 -CAV2, Canine Parvovirus - CPV and Canine Parainfluenza Virus - CPiV) + liquid vaccine containing 4 inactivated Leptospira serovars (Icterohaemorrhagiae – Li; Canicola – Lc; Grippotyphosa – Lg; Pomona – Lp).
Recombitek®C6/CV: freeze-dried vaccine containing a recombinant canarypox expressing glycoproteins HA and F of the canine distemper virus (CDV) and 4 live attenuated viruses (Canine Adenovirus 2 -CAV2, Canine Parvovirus – CPV, Canine Parainfluenza Virus – CPiV, Canine Coronavirus - CCV) + liquid vaccine containing 2 inactivated Leptospira serovars (Icterohaemorrhagiae – Li; Canicola – Lc).
Rabisin™: aluminum hydroxide adjuvanted inactivated vaccine against rabies.
2.2. Animals
Twenty-five specific pathogen-free (SPF), specific Maternal-Derived Antibody (MDA)-free Beagle puppies over 15 weeks of age were provided by a commercial supplier and allocated randomly into five groups (A to E) of 5 animals each, according to sex and age. All puppies were chipped (Indexel™) and were allowed to acclimatize during 4 days prior to the study.
2.3. Experimental procedure
On days 0 and 21, puppies from Groups A and B received one subcutaneous injection of Recombitek® C8. On the same days, puppies from group C and D received one subcutaneous injection of Recombitek® C6/CV. On day 21, puppies from Group A and C also received an injection of Rabisin™ at a separate injection site. Puppies from Group E only received a single injection of Rabisin™ on day 21.
Blood samples were drawn from all puppies on days 0, 21, 28, 35, 49, 63, and 83 then processed into serum, aliquoted and kept frozen until analysis.
All puppies were checked daily for possible local and general adverse reaction(s) throughout the duration of the study.
2.4. Laboratory testing
Titration of anti-rabies antibodies from Groups A, C and E was carried out in an OIE accredited laboratory for rabies (Laboratoire Départemental 31 EVA, Launaguet / Toulouse, France) by using the fluorescent antibody virus neutralization (FAVN) test according to the technique described by Cliquet et al. (1998).
Titration of anti-CDV and anti-CAV was carried out using viral neutralization assay. Briefly, identical concentrations of virus (200 CCID50 per ml for CDV and 320 CCID50 per ml for CAV) are mixed with 3-fold serial dilutions of sera for a final concentration range of 0.48 log10 to 3.84 log10. Four wells of each sample are evaluated. After 1 (CAV) or 2 (CDV) hours at around 21 °C (CDV) or 37 °C (CAV), the Vero (CDV) or MDCK (CAV) cellular suspension are added in the mixtures in each well and then the plates are placed in incubator at 37 °C for 5 (CDV) or 7 (CAV) days to read the cytopathic effects. The titerss of the sera are calculated using linear regression and angular transformation and expressed as virus neutralizing antibody at 50% (log10 SN50). Sera were considered positive for titerss above 0.48 (log10 SN50).
Titration of anti-CPV and anti-CPiV was carried out with proprietary blocking ELISA. In summary, plates are coated with respectively anti-VP2 (for CPV) or CPiV capture monoclonal antibody. Then, a previously co-incubated mixture of tested sera and a fixed amount of respectively CPV2 or CPi virus are added to the wells. A monoclonal anti-VP2 or anti-CPiV antibody coupled to peroxidase is finally added before being revealed by adding the substrate. The titerss of positive control and test sera are calculated by regression and expressed as titers OD50. OD is inversely proportional to the amount of antibody and titers are expressed as log10OD50 compared to the reference positive sera. Positive threshold was set at 1.00 log10OD50 for CPV and 2.22 log10OD50 for CPiV.
CCV antibodies were titrated according to a serum neutralization-based technique. In brief, serial 1:2 dilutions of the samples to be tested are performed on a plate to which the prediluted reference virus at the required concentration (TCID50/ 50 µl) is added. 100 µl of Crandell-Rees Feline Kidney (CRFK) cells in specific medium are then added to the wells and plates incubated for 5–7 days at 37 °C in a 5 ± 1% CO2 atmosphere. Cytopathic effect is finally determined by examining the plate under a fluorescent microscope. Results are expressed as the highest dilution of serum that protects 50% of the wells against viral infection. Titers above 0.2 (log10 SN50) are considered as positive.
Anti-leptospiral antibodies for serovars Canicola, Icterohaemorrhagiae and Grippotyphosa, were also titrated by a proprietary blocking ELISA as described in a recent publication (Cariou et al., 2020). Antibodies against Leptospira Pomona were titrated by microagglutination technique (MAT) at the Institut Pasteur, National Reference Center for Leptospirosis, Paris (France).
2.5. Statistical analysis
Statistical analyses were carried out using SAS release 9.4 (SAS Institute, Cary, NC) with a one-sided test and level of significance at 0.05, except for analyses with descriptive purposes, which include two-sided 95% confidence intervals.
The primary endpoints for efficacy analysis are the titers measured at different time points (period D0 to D84) for each valence. For each valence, the descriptive statistics along with two-sided 95% confidence intervals (CI) were calculated per day and group on the log-transformed titer (log2 scale for Lp and log10 scale for all other valences). Mean, Standard Deviation (SD), and 95% CI are provided on a log-transformed scale (arithmetic mean and standard deviation) and on the original scale of the antibody titers (geometric mean and geometric standard deviation).
The lack of immunological interference between Rabisin™ and Recombitek® C6 and C8 components was evaluated on the maximum titer obtained for each valence over the measurement period D35 to D49. Non-inferiority was assessed for the group comparisons and the maximum antibody titers. For each group comparison and valence, the maximum loss of serological efficacy used to demonstrate that the association of the test vaccine is non-inferior to the reference vaccine is set to Δ L = 0.602 on the log10-transformed scale for all valences except Lp, and Δ L = 2 on the log2-transformed scale for Lp. The lower limit of the one-sided 1-alpha confidence interval was computed for alpha = 0.05. For each comparison, a Student’s t test was carried out to provide the corresponding p-value. The results of the non-inferiority analyses were also back transformed in order to provide the geometric mean ratio (GMR) and its 1-alpha one-sided confidence interval. The corresponding non-inferiority margins are 25% on the ratio scale.
A secondary endpoint was the immune response for each valence (seroprotection or seroconversion). Protection rates for Rabies (as determined by the WHO cut-off value of 0,5 IU/ml) or response rates (other antigens) along with the two-sided 95% confidence interval were calculated by day (D35, D49, D63, and D84) and group.
3. Results
3.1. Rabies
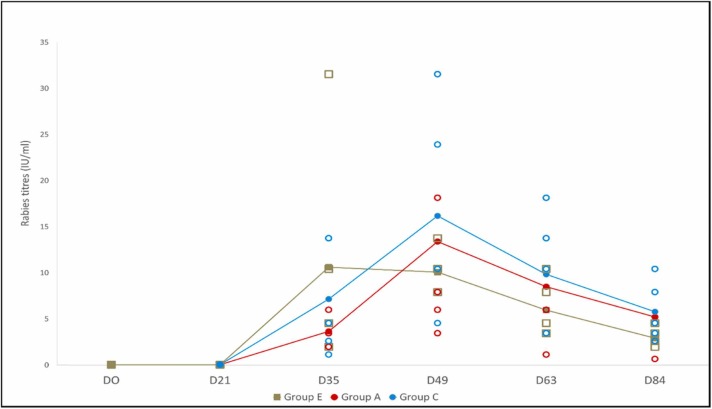
Fourteen days after administration of the rabies vaccine, all dogs had seroconverted, exceeding the WHO protective level against rabies. One single administration of rabies vaccine, alone or concurrently with DAPPi-L4 (Recombitek® C8) or DAPPi/CV-L2 (Recombitek® C6/CV) vaccines, induced high rabies virus- neutralizing titerss in all vaccinated puppies ( Fig. 1). All individual titerss rose above the WHO positivity threshold of 0.5 IU/ml and remained higher than this level throughout the rest of the study. Mean titerss were above 3 IU/ml in all rabies-vaccinated groups whatever the sampling day starting from D35, which is consistent with the ability of puppies older than 12 weeks to respond well to rabies vaccine (Cliquet et al., 2003). There was no significant difference between the three groups, whether vaccinated with Rabisin™ only, or concomitantly with the large Combo vaccines, showing the absence of interference of the DAPPi (CV) and leptospiral components with rabies vaccine-induced seroconversion.
Fig. 1.
Mean and individual rabies antibody titerss after a single vaccination on day 21. Group E puppies received one dose of Rabisin™, whereas, group A and group C puppies received Rabisin™ concomitantly with Recombitek® C8 or Recombitek® C6/CV, respectively.
3.2. Other viral components
All puppies seroconverted against CDV, CAV CPV and CPiV, whatever the group receiving one of the combo vaccines ( Table 1). For CCV, the percentage of responders varied from as low as 20% to up to 40% (Table 1), whatever the group (with or without Rabisin™). One puppy in group D (Recombitek® C6/CV without Rabisin™) had a low response to CDV vaccination and was non-responsive at D84. Similarly, one puppy in group A (Recombitek® C8+Rabisin™) was low responder throughout the duration of the study. This profile can nevertheless be expected for a Canarypox-based CDV vaccine which tends to favor cellular immunity over the humoral one, similarly to some other modified live vaccines (Pardo et al., 1997).
Table 1.
Percentage of seroconversion (with confidence intervals) for CDV, CAV, CPV, CPiV and CCV after two vaccine administrations at D0 and D21. Group A and B were administered Recombitek®C8 alone on D0 and concomitantly with Rabisin™ on D21. Group C and D received Recombitek®C6/CV on day 0 and concomitant administration of Recombitek®C6/CV and Rabisin™ on D21. Group E received Rabisin™ only on D21. It should be noted that one of the puppies in the Rabisin™ vaccinated group was responder against CPiV at D49. Nevertheless, this result should be considered as “false positive”, since the titers was 2.23 log10 OD50, right above the threshold set statistically at 2.22 log10 OD50.
| Valence Threshold | CAV | 0.48 log10 SN50 | CDV | 0.48 log10 SN50 | CPV | 1.00 log 10 OD50 | CPiV | 2.22 log 10 OD50 | CCV | 0.2 log 10 OD50 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Day | Percentage respondersn/N | 95% CI for percentage | Percentage respondersn/N | 95% CI for percentage | Percentage respondersn/N | 95% CI for percentage | Percentage respondersn/N | 95% CI for percentage | Percentage respondersn/N | 95% CI for percentage |
| A | D35 | 100% (5/5) | [56.6–100.0] | 100% (4/4) | (51.0–100.0] | 100% (5/5) | [56.6–100.0] | 100% (5/5) | [56.6–100.0] | ||
| D49 | 100% (5/5) | [56.6–100.0] | 100% (5/5) | [56.6–100.0] | 100% (5/5) | [56.6–100.0] | 100% (5/5) | [56.6–100.0] | |||
| D63 | 100% (5/5) | [56.6–100.0] | 100% (5/5) | [56.6–100.0] | 100% (5/5) | [56.6–100.0] | 100% (5/5) | [56.6–100.0] | |||
| D84 | 100% (5/5) | [56.6–100.0] | 100% (5/5) | [56.6–100.0] | 100% (5/5) | [56.6–100.0] | 100% (5/5) | [56.6–100.0] | |||
| B | D35 | 100% (5/5) | [56.6–100.0] | 100% (5/5) | [56.6–100.0] | 100% (5/5) | [56.6–100.0] | 100% (5/5) | [56.6–100.0] | ||
| D49 | 100% (5/5) | [56.6–100.0] | 100% (5/5) | [56.6–100.0] | 100% (5/5) | [56.6–100.0] | 100% (5/5) | [56.6–100.0] | |||
| D63 | 100% (5/5) | [56.6–100.0] | 100% (5/5) | [56.6–100.0] | 100% (5/5) | [56.6–100.0] | 100% (5/5) | [56.6–100.0] | |||
| D84 | 100% (5/5) | [56.6–100.0] | 100% (5/5) | [56.6–100.0] | 100% (5/5) | [56.6–100.0] | 100% (5/5) | [56.6–100.0] | |||
| C | D35 | 100% (5/5) | [56.6–100.0] | 100% (5/5) | [56.6–100.0] | 100% (5/5) | [56.6–100.0] | 100% (5/5) | [56.6–100.0] | 20% (1/5) | [3.6–62.4] |
| D49 | 100% (5/5) | [56.6–100.0] | 100% (5/5) | [56.6–100.0] | 100% (5/5) | [56.6–100.0] | 100% (5/5) | [56.6–100.0] | 20% (1/5) | [3.6–62.4] | |
| D63 | 100% (5/5) | [56.6–100.0] | 100% (5/5) | [56.6–100.0] | 100% (5/5) | [56.6–100.0] | 100% (5/5) | [56.6–100.0] | 20% (1/5) | [3.6–62.4] | |
| D84 | 100% (5/5) | [56.6–100.0] | 100% (5/5) | [56.6–100.0] | 100% (5/5) | [56.6–100.0] | 100% (5/5) | [56.6–100.0] | 40% (2/5) | [11.8,76.9] | |
| D | D35 | 100% (5/5) | [56.6–100.0] | 100% (5/5) | [56.6–100.0] | 100% (5/5) | [56.6–100.0] | 100% (5/5) | [56.6–100.0] | 40% (2/5) | [11.8,76.9] |
| D49 | 100% (5/5) | [56.6–100.0] | 100% (5/5) | [56.6–100.0] | 100% (5/5) | [56.6–100.0] | 100% (5/5) | [56.6–100.0] | 20% (1/5) | [3.6–62.4] | |
| D63 | 100% (5/5) | [56.6–100.0] | 100% (5/5) | [56.6–100.0] | 100% (5/5) | [56.6–100.0] | 100% (5/5) | [56.6–100.0] | 40% (2/5) | [11.8,76.9] | |
| D84 | 100% (5/5) | [56.6–100.0] | 80% (4/5) | [37.6,96.4] | 100% (5/5) | [56.6–100.0] | 100% (5/5) | [56.6–100.0] | 20% (1/5) | [3.6–62.4] | |
| E | D35 | 0% (0/5) | [0.0–43.4] | 0% (0/5) | [0.0–43.4] | 0% (0/5) | [0.0–43.4] | 0% (0/5) | [0.0–43.4] | 0% (0/5) | [0.0–43.4] |
| D49 | 0% (0/5) | [0.0–43.4] | 0% (0/5) | [0.0–43.4] | 0% (0/5) | [0.0–43.4] | 20% (1/5) | [3.6–62.4] | 0% (0/5) | [0.0–43.4] | |
| D63 | 0% (0/5) | [0.0–43.4] | 0% (0/5) | [0.0–43.4] | 0% (0/5) | [0.0–43.4] | 0% (0/5) | [0.0–43.4] | 0% (0/5) | [0.0–43.4] | |
| D84 | 0% (0/5) | [0.0–43.4] | 0% (0/5) | [0.0–43.4] | 0% (0/5) | [0.0–43.4] | 0% (0/5) | [0.0–43.4] | 0% (0/5) | [0.0–43.4] | |
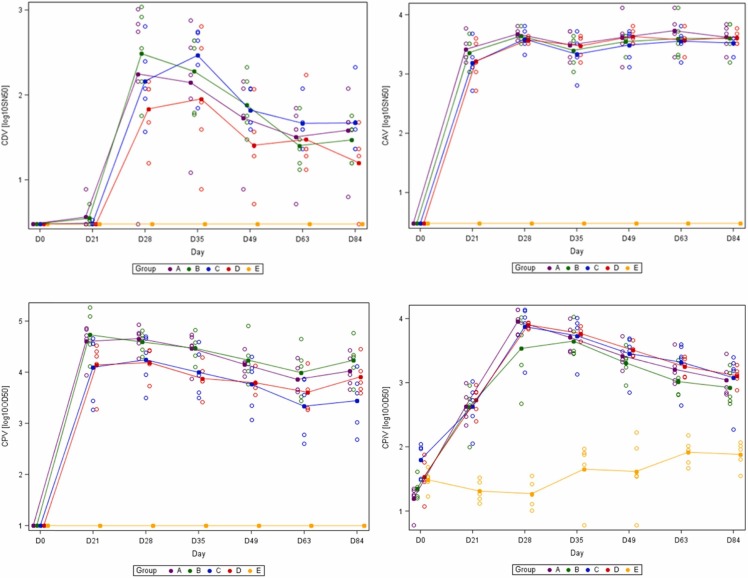
The kinetics of seroneutralizing titerss against CDV and CAV and ELISA titerss against CPV and CPiV are presented in Figs. 2a and 2b. All the puppies were seronegative on day 0 as a result of their SPF unvaccinated status. One injection was sufficient to induce high antibody titerss against CAV in all vaccinated puppies from groups A, B, C and D. Strong antibody titerss rise was observed after single CPV vaccine administration. Seroconversion against CDV usually required two injections. Comparison of mean titerss against CDV, CAV, CPV and CPiV did not show any significant difference between groups A and B, respectively vaccinated with Recombitek® C8 alone or Recombitek® C8 +Rabisin™ as well as between groups C and D respectively vaccinated with Recombitek® C6/CV alone or Recombitek® C6/CV +Rabisin™. It should be noted that one of the non-vaccinated puppies in the control group displayed a CPiV titer of 2.23 log10 OD50, right above the threshold set statistically at 2.22 log 10 OD50 and should be considered as false positive.
Fig. 2.
(a) Mean virus-neutralizing antibody titerss against canine distemper (CDV) and canine adenovirus (CAV). The combined vaccines Recombitek®C8 and Recombitek®C6/CV were administered on days 0 and 28, and the rabies vaccine on day 28. Fig. 2b: Mean virus ELISA antibody titerss against canine parvovirus (CPV) and canine parainfluenza virus (CPiV). The combined vaccines Recombitek®C8 and Recombitek®C6/CV were administered on days 0 and 28, and the rabies vaccine on day 28.
Similarly, there was no significant difference between groups vaccinated with C6/CV alone or C6/CV + Rabisin™ regarding the mean kinetics of ELISA antibodies against CCV (data not shown).
3.3. Leptospiral components
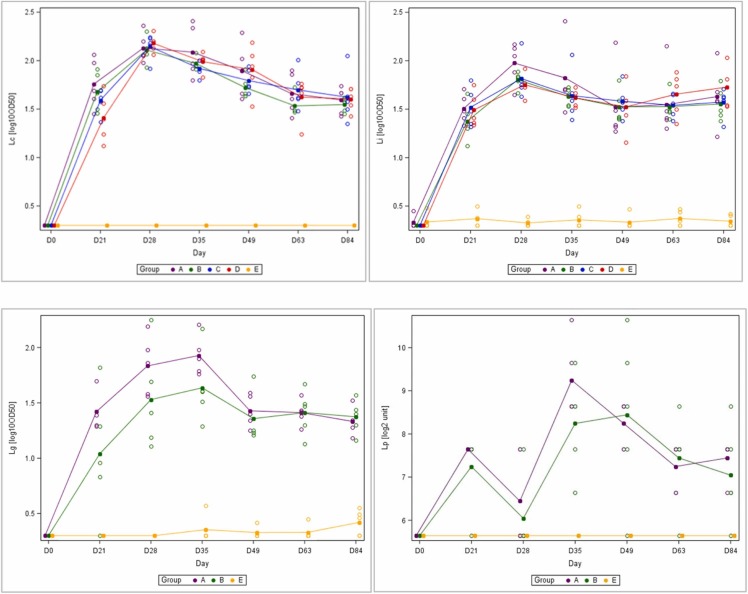
Results for the mean kinetics of antibodies against Leptospira components are presented in Fig. 3, as measured in their respective ELISA test. In all groups, antibodies against Leptospira serovars Canicola and Icterohaemorrhagiae started to raise strongly already from the first injection, peaking at day 28, slightly decreasing till day 63 for serovar Canicola, Grippotyphosa and Pomona. Similar antibody profiles were seen for serovars Grippotyphosa in groups A and B which both were administered the Recombitek®C8 vaccine. Antibody kinetics for serovar Pomona had a slightly different profile, peak being reached after the second injection at D35.
Fig. 3.
mean kinetics of antibodies against Leptospira serogroup Canicola (upper left), serogroup Icterohaemorrhagiae (upper right), serogroup Grippotyphosa (bottom left) and serogroup Pomona (bottom right).
In terms of immune response (percentage of responders), 100% of the puppies responded to vaccination against serovars Canicola and Icterohaemorrhagiae in all groups, except those from group E who only received the rabies vaccine. All puppies responded to vaccination against serovars Grippotyphosa and Pomona in group A and B vaccinated with Recombitek®C8. One puppy became seronegative for serovar Pomona in group B (Recombitek®C8 without Rabisin™) from day 49, despite an early seroconversion, as measured by MAT ( Table 2).
Table 2.
Percentage of seroconversion (with confidence intervals) for Leptospira serogroups Canicola, Icterohaemorrhagiae, Grippotyphosa and Pomona after two administrations of the vaccines at D0 and D21. Group A and B were administered Recombitek®C8 alone on D0 and concomitantly with Rabisin™ on D21. Group C and D received Recombitek®C6/CV on day 0 and concomitant administration of Recombitek®C6/CV and Rabisin™ on D21. Group E received only Rabisin™ on D21.
| Valence Threshold | Lepto. sg. Canicola | 0.3 logl0 OD50 | Lepto. sg. Icterohaem. | 0.48 log10 0D50 | Lepto. sg. Grippotyphosa | 0.43 logl0 ODS0 | Lepto. sg. Pomona | 1/ 100 | |
|---|---|---|---|---|---|---|---|---|---|
| Group | Day | Percentage of respondersn/N | 95% CI for percentage | Percentage of respondersn/N | 95% CI for percentage | Percentage of respondersn/N | 95% CI for percentage | Percentage of respondersn/N | 95% CI for percentage |
| A | D35 | 100% (5/5) | [56.6–100.0] | 100% (4/4) | (51.0–100.0] | 100% (5/5) | [56.6–100.0] | 100% (5/5) | [56.6–100.0] |
| D49 | 100% (5/5) | [56.6–100.0] | 100% (5/5) | [56.6–100.0] | 100% (5/5) | [56.6–100.0] | 100% (5/5) | [56.6–100.0] | |
| D63 | 100% (5/5) | [56.6–100.0] | 100% (5/5) | [56.6–100.0] | 100% (5/5) | [56.6–100.0] | 100% (5/5) | [56.6–100.0] | |
| D84 | 100% (5/5) | [56.6–100.0] | 100% (5/5) | [56.6–100.0] | 100% (5/5) | [56.6–100.0] | 100% (5/5) | [56.6–100.0] | |
| B | D35 | 100% (5/5) | [56.6–100.0] | 100% (5/5) | [56.6–100.0] | 100% (5/5) | [56.6–100.0] | 100% (5/5) | [56.6–100.0] |
| D49 | 100% (5/5) | [56.6–100.0] | 100% (5/5) | [56.6–100.0] | 100% (5/5) | [56.6–100.0] | 80% (4/5) | [37.6,96.4] | |
| D63 | 100% (5/5) | [56.6–100.0] | 100% (5/5) | [56.6–100.0] | 100% (5/5) | [56.6–100.0] | 80% (4/5) | [37.6,96.4] | |
| D84 | 100% (5/5) | [56.6–100.0] | 100% (5/5) | [56.6–100.0] | 100% (5/5) | [56.6–100.0] | 80% (4/5) | [37.6,96.4] | |
| C | D35 | 100% (5/5) | [56.6–100.0] | 100% (5/5) | [56.6–100.0] | ||||
| D49 | 100% (5/5) | [56.6–100.0] | 100% (5/5) | [56.6–100.0] | |||||
| D63 | 100% (5/5) | [56.6–100.0] | 100% (5/5) | [56.6–100.0] | |||||
| D84 | 100% (5/5) | [56.6–100.0] | 100% (5/5) | [56.6–100.0] | |||||
| D | D35 | 100% (5/5) | [56.6–100.0] | 100% (5/5) | [56.6–100.0] | ||||
| D49 | 100% (5/5) | [56.6–100.0] | 100% (5/5) | [56.6–100.0] | |||||
| D63 | 100% (5/5) | [56.6–100.0] | 100% (5/5) | [56.6–100.0] | |||||
| D84 | 100% (5/5) | [56.6–100.0] | 100% (5/5) | [56.6–100.0] | |||||
| E | D35 | 0% (0/5) | [0.0–43.4] | 20% (1/5) | [3.6–62.4] | 20% (1/5) | [3.6–62.4] | 0% (0/5) | [0.0–43.4] |
| D49 | 0% (0/5) | [0.0–43.4] | 0% (0/5) | [0.0–43.4] | 0% (0/5) | [11.8,76.9] | 0% (0/5) | [0.0–43.4] | |
| D63 | 0% (0/5) | [0.0–43.4] | 0% (0/5) | [0.0–43.4] | 20% (1/5) | [3.6–62.4] | 0% (0/5) | [0.0–43.4] | |
| D84 | 0% (0/5) | [0.0–43.4] | 0% (0/5) | [0.0–43.4] | 60% (3/5) | [23.1–88.2] | 0% (0/5) | [0.0–43.4] | |
3.4. Non-inferiority analysis
From a statistical point of view, non-inferiority was demonstrated for all components between corresponding groups (A vs. B and C vs. D), except CDV for which results of the test was non-conclusive ( Table 3). For this latter component, this was related to one puppy in group A (Recombitek®C8 + Rabisin™) being a low responder.
Table 3.
Results of the statistical non inferiority analysis. The maximum loss of serological efficacy used to demonstrate that the association of the test vaccine is non-inferior to the reference vaccine is set to Δ L = 0.602 on the log10-transformed scale for all valences except Lp, for which Δ L = 2 on the log2-transformed scale for Lp.
| Valence | Test vs. reference | Delta | alpha | Arithm. mean difference (log unit) [1-alpha CI) | Geom. mean ratio[1-alpha CI) | Non-inferiority | p-value |
|---|---|---|---|---|---|---|---|
| CAV | A vs. B | -0.602 | 0.05 | 0.086 [− 0.219, +int) | 1.22 [0.60, +int) | Yes | 0.0015 |
| C vs. D | -0.602 | 0.05 | -0.114 [− 0.338, +int) | 0.77 [0.46, +int) | Yes | 0.0019 | |
| CCV | C vs. D | -0.602 | 0.05 | 0.080 [− 0.406, +int) | 1.20 [0.39, +int) | Yes | 0.0155 |
| CDV | A vs. B | -0.602 | 0.05 | -0.132 [− 0.815, +int) | 0.74 [0.15, +int) | No | 0.1184 |
| C vs. D | -0.602 | 0.05 | 0.512 [− 0.198, +int) | 3.25 [0.63, +int) | Yes | 0.0097 | |
| CPV | A vs. B | -0.602 | 0.05 | 0.014 [− 0.329, +int) | 1.03 [0.47, +int) | Yes | 0.0051 |
| C vs. D | -0.602 | 0.05 | 0.120 [− 0.329, +int) | 1.32 [0.47, +int) | Yes | 0.0087 | |
| CPiV | A vs. B | -0.602 | 0.05 | 0.058 [− 0.224, +int) | 1.14 [0.60, +int) | Yes | 0.0012 |
| C vs. D | -0.602 | 0.05 | -0.036 [− 0.371, +int) | 0.92 [0.43, +int) | Yes | 0.0104 | |
| Le | A vs. B | -0.602 | 0.05 | 0.192 [− 0.059, +int) | 1.56 [0.87, +int) | Yes | 0.0009 |
| C vs. D | -0.602 | 0.05 | -0.108 [− 0.238, +int) | O.78 [0.58, +int) | Yes | 0.0001 | |
| Lg | A vs. B | -0.602 | 0.05 | 0.292 [− 0.017, +int) | 1.96 [0.96, +int) | Yes | 0.0003 |
| Li | A vs. B | -0.602 | 0.05 | 0.082 [− 0.312, +int) | 1.21 [0.49, +int) | Yes | 0.0093 |
| C vs. D | -0.602 | 0.05 | 0.008 [− 0.203, +int) | 1.02 [0.63, +int) | Yes | 0.0003 | |
| Lp | A vs. B | -2 | 0.05 | 0.600 [− 0.911, +int) | 1.52 [0.53, +int) | Yes | 0.0063 |
| Rabies | A vs. E | -0.602 | 0.05 | -0.096 [− 0.476, +int) | 0.80 [0.33, +int) | Yes | 0.0192 |
| C vs. E | -0.602 | 0.05 | 0.048 [− 0.295, +int) | 1.12 [0.51, +int) | Yes | 0.0039 |
4. Discussion
Antibody titerss are markers of the immunogenicity of for most vaccine components (Tizard and Ni, 1998) and may also be correlated with protection, as is the case for rabies, CDV and CAV -neutralizing antibodies, as well as CPV ELISA antibodies (Twark and Dodds, 2000, Schultz, 2006, Litster et al., 2012, [Jensen et al., 2015], Decaro and Buonavoglia, 2017). For these antigens, the absence of vaccine interference can be demonstrated by serology. In this study, all vaccinated dogs raised protective titerss against rabies and/or distemper, adenovirus and parvovirus without significant differences between the corresponding groups. In particular, the response to rabies vaccination was not affected by the concomitant administration of the Combo vaccines, even though they are including several leptospiral components. It is also noticeable that response to the parvoviral component was very strong, all puppies seroconverting after a single administration of vaccine. This shows that, in absence of maternal derived antibodies, puppies are able to mount very quickly an effective and protective immunity. For CPiV, despite antibody titerss are not strictly correlated to protection, the comparable antibody kinetics suggests that the different combinations (combos with or without rabies) have equivalent immunogenicity. Of notice, there was an overall poor response to the CCV component which, in conjunction with the poor proof of virulence of street virus, shows that CCV vaccine is probably not very useful. This is in line with the international vaccination guidelines which have classified this valence as “non-recommended”.
For leptospiral components, ELISA test was preferred to MAT when available, as MAT titerss are generally low and short-lived ([Martin et al., 2014], Sykes et al., 2011). Efficacy studies conducted with different leptospiral vaccines, whether combined or not, have shown that most dogs, despite not having any detectable agglutinating antibodies after vaccination, are protected against severe challenge reproducing Leptospira acute disease (Bouvet et al., 2016). Agglutinins are therefore unlikely to be the only protective immune mechanism and not strictly correlated to protection. Other analytical techniques such as serogroup specific ELISA (SumanthKumar et al., 2013) are interesting to better characterize antibody response against Leptospira. Internally developed ELISA tests were therefore used for serogroups Canicola, Icterohaemorrhagiae, Grippotyphosa (Cariou et al., 2020). Due to their higher sensitivity, these tests enable a better follow-up of post-vaccinal antibodies. As an ELISA was still not available for serovar Pomona, MAT remained the test performed for measuring the antibody response. Interestingly, those results were also the most variable over the time, which is consistent with the lower sensitivity of this technique.
The results were consistent for all antigens and demonstrate the absence of interference between the rabies vaccine and the Recombitek®C8 or Recombitek®C6/CV Combo vaccines, when considering the humoral responses. Overall, non-inferiority could be demonstrated for most components, except for Canine distemper, whereby this was related to one low-responder puppy. It is worth noting that those non-inferiority results were already obtained with a limited number of puppies per group, all groups displaying similar antibody profiles and responses to vaccination. In particular, it is of utmost importance that Rabisin™, whether administered alone or concomitantly with large Combo vaccines such as Recombitek®C6CV and Recombitek®C8, was able to induce a rapid and strong immune response in all puppies. In particular, the number of valences did not lead to any interference with the immune response to rabies or each other. This study also confirms the ability of Rabisin™ to induce consistently protective titerss against rabies in puppies, whether it is administered alone or concomitantly with other commonly used vaccines. These findings are also consistent with the results of another study performed in cats where Rabisin™ induced a very strong seroconversion when administered concomitantly at two separate injection sites with a large combination vaccine (Purevax™ RCPChFeLV) (Guiot et al., 2008).
Practitioners can therefore confidently administer these vaccines on the same day, as long as they are administered at two separate injection sites. This will facilitate vaccination compliance, in particular against rabies, over the life of the dog. It will also provide the necessary flexibility for offering vaccine suited to the patient needs. Indeed, practitioners should be encouraged to adapt vaccination schemes to their individual patients’ lifestyles and risks, with core valences including CDV, CAV and CPV for non-rabies countries and CDV, CAV CPV plus rabies in those countries where the disease is still prevalent – whereas non-core vaccines against CPiV, Bordetella and leptospirosis should be administered separately, as it is outlined in vaccination guidelines all over the world. This would enable the practitioner to comply to the general recommendations and local needs, limiting antigenic stimulation where it may not be needed.
Declaration of Competing Interest
J. Bouvet, L. Cupillard, C. Cariou, and F. Oberli are employees of Boehringer Ingelheim Animal health. JC Thibault is a former employee of Boehringer Ingelheim Animal Health and was involved in the set-up of the study, as well as in the evaluation of the results.
Acknowledgments
The authors would like to thank Frederic David for his contribution to the study protocol and the analytical lab team for performing the various tests. Special thanks to Jean-Philippe Tronel for his careful proof-reading and coordination of the co-author team.
Footnotes
*All vaccines are produced by Boehringer Ingelheim Animal Health
References
- Bouvet J., Valfort W., Oberli F., Guigal P.M., Cariou C., Villard S., Cupillard F. Efficacy of a multivalent DAPPi-Lmulti canine vaccine against mortality, clinical signs, infection, bacterial excretion, renal carriage and renal lesions caused by Leptospira experimental challenges. Vaccine Rep. 2016;6:23–28. [Google Scholar]
- Day M.J., Horzinek M.C., Schultz R.D., Squires R.A. WSAVA Guidelines for the vaccination of the dog and the cat. J. Small Anim. Pract. 2016;56(2016) doi: 10.1111/jsap.2_12431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day M.J., Karkare U., Schultz R.D., Squires R., Tsujimoto H. Recommendations on vaccination for Asian small animal practitioners: a report of the WSAVA vaccination guidelines group. J. Small Anim. Pract. 2015;56(2):77–95. doi: 10.1111/jsap.12272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford et al., 2017.Ford R.B., Larson L.B., McClure K.D. AAHA canine vaccination guidelines. J. Am. Anim. Hosp. Assoc. 2017;53(5):243–251. doi: 10.5326/JAAHA-MS-6741. [DOI] [PubMed] [Google Scholar]
- Dodds et al., 2020.Dodds W.Jean, Larson Laurie J., Christine Kris L., et al. Duration of immunity after rabies vaccination in dogs: the rabies challenge fund research study. Can. J. Vet. Res. 2020;84(2):153–158. [PMC free article] [PubMed] [Google Scholar]
- Cliquet F., Verdier Y., Sagné L., Aubert M., Schereffer J.L., Selve M., Wasniewski M., Servat A. Neutralising antibodies titration in 25000 sera of dogs and cats vaccinated against rabies in France, in the framework of the new regulations that offer an alternative to quarantine. Rev. Sci. Tech. Off. Int. Epiz. 2003;22:857–866. doi: 10.20506/rst.22.3.1437. [DOI] [PubMed] [Google Scholar]
- Cliquet F., Aubert M.F., Sagné L. Development of a fluorescent antibody virus neutralizing test (FAVN test) for the quantitation of rabies-neutralising antibody. J. Immunol. Methods. 1998;212:79–87. doi: 10.1016/s0022-1759(97)00212-3. [DOI] [PubMed] [Google Scholar]
- Bouvet et al., 2018.Bouvet J., Cariou C., Poulard A., Oberli F., Cupillard L., Guigal P.M. Compatibility between a rabies vaccine and a combined vaccine against canine distemper, adenovirosis, parvovirosis, parainfluenza virus and leptospirosis. Vet. Immunol. Immunopathol. 2018;205(2018):93–96. doi: 10.1016/j.vetimm.2018.11.001. [DOI] [PubMed] [Google Scholar]
- Cariou et al., 2020.Cariou C., Herbet G., Ripart P., Martin-Cagnon N., Bouvet J., Schneider M., Guiot A.L., Cupillard L. Development of antibody ELISA specific of Leptospira interrogans serovar Grippotyphosa, Canicola, and Icterohaemorrhagiae to monitor vaccine immunogenicity. Vet. Immunol. Immunopathol. 2020;219 doi: 10.1016/j.vetimm.2019.109960. [DOI] [PubMed] [Google Scholar]
- Pardo et al., 1997.Pardo C., Bauman J.E., Mackowiak M. Protection of dogs against canine distemper by vaccination with a canarypox virus recombinant expressing canine distemper virus fusion and hemagglutinin glycoproteins. Am. J. Vet. Res. 1997;58(8):833–836. [PubMed] [Google Scholar]
- Twark L., Dodds W.J. Clinical use of serum parvovirus and distemper virus antibody titers for determining revaccination strategies in healthy dogs. J. Am. Vet. Med. Assoc. 2000;217:1021–1024. doi: 10.2460/javma.2000.217.1021. [DOI] [PubMed] [Google Scholar]
- Schultz R.D. Duration of immunity for canine and feline vaccines: a review. Vet. Microbiol. 2006;117:75–79. doi: 10.1016/j.vetmic.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Tizard I., Ni Y. Use of serologic testing to assess immune status of companion animals. J. Am. Vet. Med. Assoc. 1998;213:54–60. [PubMed] [Google Scholar]
- Litster A., Nichols J., Volpe A. Prevalence of positive antibody test results for canine parvovirus (CPV) and canine distemper virus (CDV) and response to modified live vaccination against CPV and CDV in dogs entering animal shelters. Vet. Microbiol. 2012;157:86–90. doi: 10.1016/j.vetmic.2011.12.030. [DOI] [PubMed] [Google Scholar]
- Jensen et al., 2015.Jensen W.A., Totten J.S., Lappin M.R., Schultz R.D. Use of serologic tests to predict resistance to canine distemper virus–induced disease in vaccinated dogs. J. Vet. Diagn. Investig. 2015;27(5):576–580. doi: 10.1177/1040638715602291. [DOI] [PubMed] [Google Scholar]
- Decaro N., Buonavoglia C. Canine parvovirus post vaccination shedding: interference with diagnostic assays and correlation with host immune status. Vet. J. 2017;221:23–24. doi: 10.1016/j.tvjl.2017.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin et al., 2014.Martin L.E., Wiggans K.T., Wennogle S.A., Curtis K., Chandrashekar R., Lappi M.R. Vaccine-associated Leptospira antibodies in client-owned dogs. J. Vet. Intern. Med. 2014;28:789–792. doi: 10.1111/jvim.12337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes J.E., Hartmann K., Lunn K.F., Moore G.E., Stoddard R.A., Goldstein R.E. ACVIM small animal consensus statement on leptospirosis: diagnosis, epidemiology, treatment, and prevention. Vet. Intern. Med. 2011;25:1–13. doi: 10.1111/j.1939-1676.2010.0654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SumanthKumar R., Pillai R.M., Mukhopadhyay H.K., Antony P.X., Thanislass J., Vivek Srinivas V.M., Vishnupriya S. Seroepidemiology of canine leptospirosis by iELISA and MAT. Vet. World. 2013;6:926–930. [Google Scholar]
- Guiot et al., 2008.Guiot A.L., Cariou C., Krogmann V., Thibault J.C., Poulet H. Compatibility between a rabies vaccine and a combined vaccine against feline rhinotracheitis, FCV, FPLV and FeLV, and Chlamydophila felis. Vet. Rec. 2008;162(21):690–692. doi: 10.1136/vr.162.21.690. [DOI] [PubMed] [Google Scholar]