Abstract
Cryptococcus neoformans is an opportunistic fungal pathogen that causes life-threatening infections of the central nervous system. Existing therapies include amphotericin B, fluconazole, and flucytosine, which are limited by toxic side effects and the emergence of drug resistance. We recently demonstrated that the protein phosphatase calcineurin is required for growth at 37°C and virulence of C. neoformans. Because calcineurin is the target of potent inhibitors in widespread clinical use, cyclosporine and FK506 (tacrolimus), it is an attractive drug target for novel antifungal agents. Here we have explored the synergistic potential of combining the calcineurin inhibitor FK506 or its nonimmunosuppressive analog, L-685,818, with other antifungal agents and examined the molecular basis of FK506 action by using genetically engineered fungal strains that lack the FK506 target proteins FKBP12 and calcineurin. We demonstrate that FK506 exhibits marked synergistic activity with the H+ATPase inhibitor bafilomycin A1 via a novel action distinct from calcineurin loss of function. FK506 also exhibits synergistic activity with the pneumocandin MK-0991/caspofungin acetate (formerly L-743,873), which targets the essential β-1,3 glucan synthase, and in this case, FK506 action is mediated via FKBP12-dependent inhibition of calcineurin. Finally, we demonstrate that FK506 and fluconazole have synergistic activity that is independent of both FKBP12 and calcineurin and may involve the known ability of FK506 to inhibit multidrug resistance pumps, which are known to export azoles from fungal cells. In summary, our studies illustrate the potential for synergistic activity of a variety of different drug combinations and the power of molecular genetics to define the mechanisms of drug action, as well as identify a novel action of FK506 that could have profound implications for therapeutic or toxic effects in other organisms, including humans.
Cryptococcus neoformans is a common cause of life-threatening opportunistic infections in immunocompromised hosts, especially in patients infected with the human immunodeficiency virus and recipients of organ transplants (5, 29). Although azoles and amphotericin B are currently acceptable therapies for patients with cryptococcal meningitis, the success of these treatments remains suboptimal. New antifungal agents are needed for the effective management of this pathogenic fungus. Evaluation of the synergistic activity of new antifungal compounds through in vitro susceptibility testing can help to establish guidelines for the potential clinical application of new therapies.
Virulence factors that have been defined for C. neoformans and that could be targeted for therapeutic intervention include production of a polysaccharide capsule, synthesis of melanin, prototrophy, and ability to grow at 37°C (13, 24, 42, 47). We recently demonstrated that the protein phosphatase calcineurin is required for C. neoformans growth at 37°C, and as a consequence, mutant strains lacking calcineurin are avirulent in animal model systems (8, 40). Calcineurin is the target of the immunosuppressive antifungal drugs cyclosporine (CsA) and FK506, and these agents are toxic to C. neoformans (7, 39, 40). Because immunosuppression predisposes to infection by C. neoformans and both CsA and FK506 exacerbate C. neoformans meningitis (39, 41), these potent immunosuppressive agents cannot be used alone to treat fungal meningitis. A variety of nonimmunosuppressive derivatives of both CsA and FK506 that retain antifungal activity have been identified (7, 39), and we have explored here the use of FK506 and its nonimmunosuppressive analog, L-685,818 (the C-18 hydroxy, C-21 ethyl derivative of FK506), in combination with other antifungal agents that might allow lower, less-toxic doses of FK506 or its analogs to be employed in patients.
Previous studies with the model yeast Saccharomyces cerevisiae demonstrated that mutations that impair the function of the vacuolar H+ATPase (V-ATPase) reduce the ability of the cell to withstand cation stress and render calcineurin essential via its ability to regulate the expression and function of cation pumps on the plasma membrane (17, 19, 36). In this case, mutations that impair the function or assembly of the V-ATPase were lethal with mutations in the calcineurin catalytic or regulatory subunit and also conferred sensitivity to the calcineurin inhibitors CsA and FK506. It might be possible to achieve this same lethal effect pharmacologically by applying two drugs in combination: one that inhibits the V-ATPase (bafilomycin A1) and a second to inhibit calcineurin (CsA, FK506, or an analog).
Bafilomycin A1 is a macrolide antibiotic that inhibits the V-ATPase in several organisms (2). Although this class of antibiotic is considered a highly-specific inhibitor of V-ATPase (nanomolar concentration) (2, 18), recent studies have shown that the plasma membrane ATPase (P-ATPase) and the ATP-binding cassette (ABC) transporters are also sensitive to bafilomycin A1 (micromolar concentration) (20, 48). The high specificity of bafilomycin A1 in inhibiting V-ATPase and P-ATPase makes this group of compounds especially interesting, since the H+ATPase pumps are considered promising new antifungal targets (12, 43). In particular, the P-ATPase is encoded by the PMA1 gene and controls both efflux and influx of cations (H+, Ca2+, Na+, and K+) across the plasma membrane. The fungal Pma1 enzyme differs considerably from the homologous mammalian and plant enzymes, especially in transmembrane segments 1, 2, 3, and 4 (32). Moreover, site-directed mutagenesis of these regions frequently results in lethal mutations in S. cerevisiae, demonstrating the importance of these fungus-specific domains to function (31, 34). Taken together, these observations suggest that H+ATPase pumps can be considered new targets for the development of new antifungal agents.
In previous studies, we found that S. cerevisiae vph6 mutant strains, which have defects in V-ATPase assembly and function, are hypersensitive to the calcineurin inhibitors CsA and FK506 (19). The immunosuppressive drugs CsA and FK506 inhibit calcineurin in complex with cyclophilin A and FKBP12, respectively (26). In wild-type yeast strains, CsA or FK506 inhibits calcineurin, resulting in Li+ sensitivity (3, 17, 36). These findings suggest that calcineurin might modulate the activity of enzymes that regulate cation transport. Withee et al. have recently shown that S. cerevisiae calcineurin mutant strains are hypersensitive to Li+, while mutant strains with defects in the P-ATPase Pma1 are resistant to Li+, suggesting that calcineurin and Pma1 may have opposing effects on intracellular Li+ transport (52).
In C. neoformans, CsA, FK506 and its nonimmunosuppressive analog, L-685,818, are toxic in vitro (39). Moreover, because C. neoformans FK506-resistant mutant strains are also resistant to the FK506 analog L-685,818 (C-18 hydroxy, C-21 ethyl FK506 derivative), these two drugs have similar mechanisms of action via inhibition of calcineurin (39). In this study, we provide evidence that the calcineurin inhibitor FK506 and the V-ATPase inhibitor bafilomycin A1 have a synergistic effect on C. neoformans in vitro. Surprisingly, mutants lacking calcineurin are not hypersensitive to bafilomycin A1, and the combination of FK506 plus bafilomycin A1 still exhibits synergistic activity in mutant strains lacking calcineurin. These findings suggest that calcineurin inhibition is not the action of FK506 in this case. On the other hand, mutants lacking FKBP12 are resistant to bafilomycin A1 plus FK506. Taken together, these findings reveal that a novel aspect of FK506 action enhances bafilomycin A1 toxicity in C. neoformans.
Recently, Marchetti et al. have shown that the combination of fluconazole with CsA or FK506 results in a powerful fungicidal effect against Candida albicans in vitro and in vivo (27; O. Marchetti, J. M. Entenza, D. Sanglard, J. Bille, M. P. Glauser, and P. Moreillon, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. J-50, 1998; O. Marchetti, P. Mareillon, M. P. Glauser, J. Bille, and D. Sanglard, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. J-119, 1998). We have found that the combinations of FK506 plus fluconazole and L-685,818 plus fluconazole have synergistic activities against C. neoformans strains in vitro. In this case, mutations in the FK506 target protein FKBP12 and calcineurin had no effect on synergistic action, indicating that FK506 and L-685,818 enhance fluconazole action in C. neoformans by an FKBP12- and calcineurin-independent mechanism. This mechanism may be mediated via the known inhibitory action of FK506 on multidrug resistance pump function, given that fluconazole is known to be extruded by multidrug resistance pumps in azole-resistant fungal strains (14, 50).
Finally, the pneumocandin MK-0991/caspofungin acetate represents a new class of antifungal drugs that target the enzyme 1,3-β-glucan synthase, which is an essential enzyme involved in the synthesis of the fungal cell wall (11). Previous studies have shown that pneumocandins possess in vitro activity against C. albicans fluconazole-sensitive and -resistant isolates (38), Candida glabrata (51), and clinically important molds (9) and that this action is mediated via inhibition of 1,3-β-glucan synthase (10, 23). In this study, we combined the pneumocandin MK-0991/caspofungin acetate with FK506 or L-685,818 and demonstrated synergistic activity against C. neoformans in vitro. In this case, mutant strains lacking FKBP12 are resistant to this synergistic effect, and calcineurin mutants exhibit increased caspofungin sensitivity, demonstrating that FKBP12-dependent inhibition of calcineurin by FK506 enhances pneumocandin action in C. neoformans. In the yeast S. cerevisiae, the FKS1 and FKS2 genes encode 1,3-β-glucan synthase (11, 28), and the FKS2 gene is transcriptionally regulated by calcineurin (28, 54). As a consequence, fks1 mutant strains are markedly sensitive to calcineurin inhibition by CsA or FK506 (15, 28). Finally, mutations in the cps1 gene, which encodes a 1,3-β-glucan synthase in Schizosaccharomyces pombe, similarly confer sensitivity to CsA and to the pneumocandin papulacandin B (25). Our findings suggest that the single essential gene encoding 1,3-β-glucan synthase in C. neoformans, FKS1 (49), may be similarly regulated by calcineurin.
MATERIALS AND METHODS
Compounds.
CsA was purchased from Alexis Corporation. FK506 was obtained from Fujisawa, and L-685,818, bafilomycin, and MK-0991/caspofungin acetate (L-743,873) were obtained from Merck & Co., Fluconazole was obtained from Pfizer, and LiCl was obtained from Sigma (St. Louis, Mo.). Stock solutions were prepared in dimethyl sulfoxide at 10 mg/ml for CsA, FK506, and L-685,818 and 2 mg/ml for bafilomycin. Stock solutions of MK-0991/caspofungin acetate, fluconazole, and LiCl were made in sterile distilled water at 10, 10, and 1 mg/ml, respectively. The final drug concentrations that were tested were as follows: CsA, from 10 to 0.156 μg/ml (7 dilutions); FK506 and L-685,818, from 25 to 0.39 μg/ml (7 dilutions); fluconazole and MK-0991/caspofungin acetate, from 100 to 0.09 μg/ml (11 dilutions); bafilomycin, from 62.2 to 0.06 μg/ml or from 100 to 0.09 μM (11 dilutions); and LiCl, from 42.39 to 0.041 μg/ml or from 1 mM to 0.9 μM (11 dilutions).
Strains.
Cryptococcus neoformans var. grubii serotype A strain H99 (wild type) is a clinical reference strain for the Duke University Mycology Research Unit. The isogenic C. neoformans strains AO4 (Δcna1) (AO4:MATα Δcna1::ADE2 ade2) (40) and MCC1 (Δfrr1) (MCC1:MATα Δfrr1::ADE2 ade2) (6), derived from H99, are calcineurin and FKBP12 mutants, respectively. Cryptococcus neoformans var. neoformans serotype D strain JEC21 (wild type) was provided by J. Edman (University of California, San Francisco) (33). The isogenic C. neoformans calcineurin mutant strain (Δcna1) (MCC2:MATα Δcna1::ADE2 ade2) and the isogenic C. neoformans FKBP12 mutant strain (C21F3 [frr1-3]) were obtained as previously described (6, 8, 40).
Checkerboard broth microdilution method for synergistic study.
Drug interactions were assessed with a checkerboard titration, according to the recommendations of the National Committee for Clinical Laboratory Standards for in vitro susceptibility testing (37). Briefly, the in vitro susceptibility testing was performed in RPMI 1640 medium (Sigma) with l-glutamine, but without sodium bicarbonate, and buffered at pH 7.0 with 0.165 M morpholinepropanesulfonic acid (MOPS). Aliquots of 50 μl of each drug (and in case of the single-drug control, 50 μl of that drug and 50 μl of sterile water) at a concentration of 4 times the target final concentration were dispensed in wells of a microtiter plate (96-well flat-bottom Cell Culture Cluster; Costar, Cambridge, Mass.) to provide 77 drug combinations. Additional rows were used to determine the MIC of each agent alone and for the growth control well (drug free). The yeast inocula (100 μl), prepared according to the proposed standard (37), were added to each well, and the microtiter plates were incubated at 30°C without shaking. Readings were performed following 72 h of incubation. Before the readings, each plate was shaken for 5 min with an Easy-Shaker EAS 2/4 (SLT, Lab-instruments, Salzburg, Austria), and the optical density at 490 nm of each well was read on a microtiter reader (Titerek Multiskan MC; Flow Laboratories, Huntsville, Ala.).
The MIC of both drugs, alone or in combination, was defined as the lowest drug concentration in a well which produced an inhibition or decrease in absorbance of ≤80% compared with that of the growth control well. Drug interactions were classified as synergistic, additive, autonomous, or antagonistic on the basis of the fractional inhibitory concentration (FIC) index. The FIC index is the sum of the FICs for each of the drugs, which in turn is defined as the MIC of each drug when used in combination divided by the MIC of the drug when used alone. The interaction was defined as synergistic if the FIC index was <1.0, additive if the FIC index was 1.0, autonomous if the FIC index was between 1.0 and 2.0, and antagonistic if the FIC index was >2.0. Each drug combination was retested. No significant differences (less than twofold dilutions) were found in two independent experiments.
FKBP12-calcineurin interactions in vitro.
FKBP12 bound to Affi-Gel beads was used for FKBP12-calcineurin binding assays as described previously (4). Incubation mixtures contained 800 μl of cryptococcal cell extract from strain MCC1 (serotype A frr1::ADE2 CNA1) (4 mg of protein) (6) and 40 μl of FKBP12–Affi-Gel beads (50% [vol/vol] suspension). FK506 and the FK506 analog L-685,818 were added where indicated to a final concentration of 20 μM. The binding mixtures were incubated at 4°C on a nutator shaker for 1 to 2 h. The Affi-Gel beads were collected by centrifugation for 10 s and washed four times with lysis buffer (20 mM Tris-Cl [pH 7.4]–10 mM KCl). Bound proteins were eluted from the beads by boiling for 4 min in 30 μl of sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer, fractionated by 12.5% polyacrylamide SDS-PAGE, and transferred to a nitrocellulose membrane. The membrane was blocked overnight in a block-wash buffer (10 mM imidazole [pH 7.3], 100 mM KCl, 5 mM CaCl2, 5% bovine serum albumin, 0.05% Tween 20, 0.02% NaN3), transferred to fresh buffer containing 106 cpm of 125I-calmodulin, and incubated at room temperature for 2 h with gentle agitation. The membrane was washed twice in block-wash buffer, air dried, and exposed to film overnight at −80°C.
RESULTS
FK506 and L-685,818 promote FKBP12 binding to C. neoformans calcineurin.
We previously demonstrated that the immunosuppressive antifungal drug FK506 and the nonimmunosuppressive analog L-685,818 have antifungal activity against C. neoformans and presented genetic and molecular biological evidence that the protein phosphatase calcineurin is the common target of the FKBP12-FK506 and FKBP12–L-685,818 complexes in fungal cells (6, 39, 40). Here we provide additional biochemical evidence that supports this mechanism of drug action and provides further insights into the action of the L-685,818 drug analog.
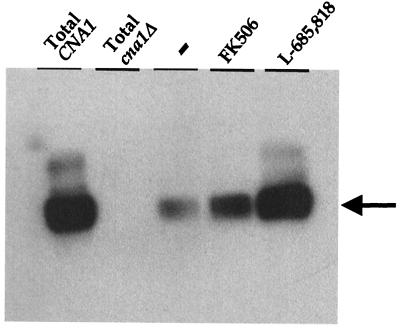
Affinity chromatography was used to demonstrate that FK506 and the FK506 analog L-685,818 mediate interactions between the FKBP12 protein and C. neoformans calcineurin in vitro (Fig. 1). Purified FKBP12 protein was covalently attached to Affi-Gel beads and then incubated with C. neoformans total protein extract in the presence or absence of either FK506 or L-685,818. Total protein extract was used from a strain in which the FKBP12 gene had been disrupted (MCC1 [frr1::ADE2]) (6), and which therefore lacks endogenous FKBP12, to reduce competition for calcineurin binding to the exogenous FKBP12 affinity matrix. Proteins which associated with the FKBP12, FKBP12-FK506, or FKBP12–L-685,818 affinity matrices were eluted, fractionated by SDS-PAGE, and transferred to nitrocellulose membranes. The calcineurin A catalytic subunit was detected via its ability to associate with 125I-calmodulin in an overlay blot. The specificity and sensitivity of this approach were demonstrated in control overlay blots. A prominent calmodulin binding protein of ∼60 kDa was readily detected in total protein extract from wild-type cells expressing calcineurin A and was missing in total protein extract from an isogenic mutant strain in which the CNA1 gene encoding calcineurin A is disrupted (Fig. 1, lanes 1 and 2).
FIG. 1.
FK506 and L-685,818 promote FKBP12 binding to cryptococcal calcineurin. Binding assays with purified FKBP12 immobilized on Affi-Gel beads were performed with equal amounts of protein extract from serotype A frr1::ADE2 CNA1 strain MCC1, in the absence (−) or presence of 20 μM FK506 and L-685,818. The migration position of the Cna1 protein is indicated by an arrow. Total protein extracts from serotype A CNA1 wild-type and cna1 mutant strains served as controls for the identity and electrophoretic mobility of the calcineurin A catalytic subunit Cna1.
As shown in Fig. 1, calcineurin readily associated with both the FKBP12-FK506 and the FKBP12–L-685,818 affinity matrices, whereas only modest binding to FKBP12 alone was observed. Because the cell extracts used for the affinity chromatography contained equal amounts of calcineurin A, the affinities of the FKBP12, FKBP12-FK506, and FKBP12–L-685,818 complexes for cryptococcal calcineurin could be compared. Interestingly, the L-685,818 analog promoted the FKBP12-calcineurin interaction to a greater extent than FK506 itself (Fig. 1 [compare FK506 and L-685,818]). This observation suggests that either or both the C-18 hydroxyl- or the C-21 allyl-to-ethyl group substitutions may increase the affinity of the FKBP12-drug complex for cryptococcal calcineurin in vitro.
FK506 and the V-ATPase inhibitor bafilomycin A1 have marked synergistic antifungal activity against C. neoformans.
Previous studies have revealed that therapeutic levels of the calcineurin inhibitors FK506 and CsA do not confer protection against and in fact exacerbate C. neoformans meningitis in experimental animals (39, 41). Moreover, organ transplant recipients treated with either FK506 or CsA as part of their immunosuppressive therapy continue to present with C. neoformans infections, both extraneurally and within the central nervous system. Likely, two factors account for these observations. First, CsA does not cross the blood-brain barrier, and FK506 does so relatively poorly (∼10%). Second, cell-mediated immunosuppression predisposes to cryptococcal infection, and these agents have profound effects on cell-mediated immunity. We have therefore focused on nonimmunosuppressive derivatives of FK506 and CsA as candidate antifungal drugs (7, 39). Although nonimmunosuppressive analogs have been identified, limited trials with one such agent, L-685,818, conferred only a modest fivefold decrease in C. neoformans cell counts in the cerebrospinal fluid of immunosuppressed rabbits, and this antifungal activity was not enhanced by direct intracerebral delivery of the agent (39). Here we have explored the possible synergistic actions of FK506 and the FK506 analog L-685,818 in combination with other antifungal agents under conditions (growth at 24°C or 30°C) in which calcineurin is not normally essential for vegetative growth.
We have discovered that the calcineurin inhibitor FK506 and the nonimmunosuppressive analog L-685,818 are dramatically synergistic with the antifungal action of the drug bafilomycin A1, which is an inhibitor of the V-ATPase pump. These studies were predicated on the earlier discovery that mutations in both calcineurin and the V-ATPase are lethal in the model yeast S. cerevisiae (17, 19). Here we sought to achieve the same effect with two pharmacological inhibitors in C. neoformans. As shown in Table 1, FK506 and bafilomycin A1 are potently synergistic in vitro against the C. neoformans serotype A clinical isolate H99 (wild type). The FIC index for FK506 plus bafilomycin A1 was 0.008 at 30°C (Table 1). To illustrate how dramatic this synergistic activity is, the MICs of FK506 and bafilomycin A1 given alone are 12.5 and >62 μg/ml, respectively, whereas given in combination, the MICs of FK506 and bafilomycin A1 decrease to ≤0.39 and ≤0.06 μg/ml, respectively. Thus, in combination, the dose of FK506 can be decreased ∼33-fold and the dose of bafilomycin A1 can be decreased ∼1,000-fold to obtain an inhibitory end point. Similar synergistic activity was observed between FK506 and bafilomycin A1 in the serotype D strain JEC21 (wild type) (see Table 3).
TABLE 1.
Synergistic in vitro activities of FK506 combined with bafilomycin A1, fluconazole, LiCl, and MK-0991/caspofungin acetate against serotype A C. neoformans wild-type strain H99 and calcineurin (Δcna1) and FKBP12 (Δfrr1) mutant strains at 30°C
| Strain type | MIC (μg/ml) ofa:
|
FIC index
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Agent alone
|
Agents combined
|
||||||||||||
| FK | BF | FL | Li | MK | FK+BF | FK+FL | FK+Li | FK+MK | FK+BF | FK+FL | FK+Li | FK+MK | |
| H99 | 12.5 | >62.2 | 6.25 | >42 | 12.5 | ≤0.39–≤0.06 | ≤0.39–1.56 | 25–>42 | ≤0.39–6.25 | 0.008 | 0.25 | 2.0 | 0.51 |
| Δcna1 mutant | >25 | >62.2 | 6.25 | >42 | 6.25 | ≤0.39–≤0.06 | ≤0.39–1.56 | 25–>42 | ≤0.39–6.25 | 0.008 | 0.25 | 1.5 | 1.00 |
| Δfrr1 mutant | >25 | >62.2 | 6.25 | >42 | 25 | ≤0.39–62.2 | ≤0.39–1.56 | 25–>42 | ≤0.39–25 | 1.00 | 0.25 | 1.5 | 1.00 |
FK, FK506; BF, bafilomycin A1; FL, fluconazole; Li, LiCl; MK, MK-0991/caspofungin acetate. For calculation purposes, MICs of >25, >42, >62.2, ≤0.39, and ≤0.06 μg/ml were assumed to be 50, 84, 124.4, 0.19, and 0.03 μg/ml, respectively.
TABLE 3.
Synergistic in vitro activities of FK506 combined with bafilomycin A1 against the serotype D C. neoformans wild type strain and calcineurin (Δcna1) and FKBP12 (Δfrr1-3) mutant strains at 30°C
| Strain type | MIC (μg/ml) ofa:
|
FIC index
|
||
|---|---|---|---|---|
| Agent alone
|
Agents combined
|
|||
| FK | BF | FK + BF | FK + BF | |
| Wild type | 12.5 | >0.31 | ≤0.78–≤0.0003 | 0.03 |
| Δcna1 mutant | >25 | >0.31 | ≤0.78–≤0.0003 | 0.007 |
| Δfrr1-3 mutant | >25 | >0.31 | >25–>0.31 | 2.0 |
FK, FK506; BF, bafilomycin A1. Note that drug dilutions of bafilomycin A1 for these experiments were from 0.31 to 0.0003 μg/ml, or from 0.5 to 0.0004 μM (11 dilutions). For calculation purposes, MICs of >25, >0.31, ≤0.78, and ≤0.0003 μg/ml, were assumed to be 50, 0.62, 0.39, and 0.00015 μg/ml, respectively.
We next sought to test our original hypothesis that the mechanism of synergistic action of FK506 and bafilomycin A1 results from a combined pharmacological inhibition of calcineurin and the V-ATPase. For this purpose, we employed an isogenic set of strains consisting of wild-type and mutant strains lacking the known FK506 target proteins FKBP12 and calcineurin because of targeted gene replacements (cna1::ADE2 or frr1::ADE2) or a point mutation in the FKBP12 gene (frr1-3 [W60R]) that destabilizes the protein and confers FK506 resistance (strain C21F3) (6, 40). We found that FKBP12 was required for FK506 synergistic action with bafilomycin A1 (Table 1, FIC index = 1.00 in the frr1::ADE2 mutant strain lacking FKBP12; Table 3, FIC index = 2.0 in the frr1-3 mutant strain lacking FKBP12). Inhibition of FKBP12 by FK506 was not responsible for the effect, since an FKBP12 mutant strain was not hypersensitive to bafilomycin A1 in the absence of FK506.
We then made the surprising observation that a mutant strain lacking calcineurin, which should be equivalent to wild-type cells exposed to FK506, was no more sensitive to bafilomycin A1 than wild-type cells (Table 1). This was the case in both serotype A (Table 1) and serotype D (Table 3) calcineurin mutant strains. This was in marked contrast to the findings in the model yeast S. cerevisiae, in which either mutation or inhibition of calcineurin rendered the cell inviable in combination with loss of V-ATPase action (19). These results suggest that FK506 was synergistic with bafilomycin A1 in C. neoformans, but not via inhibition of calcineurin. This conclusion was further supported by studies with the structurally distinct calcineurin inhibitor CsA, which also inhibits C. neoformans calcineurin (7, 40). CsA and bafilomycin A1 exhibited no synergistic activity against C. neoformans H99 (FIC, >1.00 [data not shown]), providing further evidence that calcineurin inhibition is not the mechanism of FK506 synergistic action in combination with bafilomycin A1 at 30°C.
We further note that the FK506 analog L-685,818 also exhibited synergistic activity with bafilomycin A1, but in this case, synergistic activity could only be demonstrated in the mutant strain lacking calcineurin and not in either the wild-type strain or the FKBP12 mutant strain (Table 2).
TABLE 2.
Synergistic in vitro activities of L-685,818 combined with bafilomycin A1, fluconazole, LiCl, and MK-0991/caspofungin acetate against serotype A C. neoformans wild-type strain H99 and calcineurin (Δcna1) and FKBP12 (Δfrr1) mutant strains at 30°C
| Strain type | MIC (μg/ml) ofa:
|
FIC index
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Agent alone
|
Agents combined
|
||||||||||||
| L6 | BF | FL | Li | MK | L6+BF | L6+FL | L6+Li | L6+MK | L6+BF | L6+FL | L6+Li | L6+MK | |
| H99 | >25 | >62.2 | 6.25 | >42 | 25 | ≤0.39–>62.2 | 6.25–3.12 | >25–>42 | ≤0.39–6.25 | 1.00 | 0.62 | 2.0 | 0.25 |
| Δcna1 mutant | >25 | >62.2 | 6.25 | >42 | 6.25 | ≤0.39–≤0.06 | ≤0.39–1.56 | >25–>42 | ≤0.39–6.25 | 0.008 | 0.25 | 2.0 | 1.00 |
| Δfrr1 mutant | >25 | >62.2 | 6.25 | >42 | 25 | ≤0.39–>62.2 | ≤0.39–3.12 | >25–>42 | ≤0.39–25 | 1.00 | 0.50 | 2.0 | 1.00 |
L6, L-685,818; BF, bafilomycin A1; FL, fluconazole; Li, LiCl; MK, MK-0991/caspofungin acetate. For calculation purposes, MICs of >25, >42, >62.2, ≤0.39, and ≤0.06 μg/ml were assumed to be 50, 84, 124.4, 0.19, and 0.03 μg/ml, respectively.
FK506 has synergistic antifungal activity with the β-1,3, glucan synthase inhibitor pneumocandin MK-0991/caspofungin acetate via FKBP12-dependent inhibition of calcineurin.
Enzymes that mediate fungal cell wall biosynthesis are attractive targets for novel antifungal drugs, because these enzymes are present only in fungal and not host cells. The pneumocandins are a novel class of antifungal agents that target the enzyme β-1,3 glucan synthase (10, 23). Several pneumocandin or echinocandin B analogs are nearing Food and Drug Administration approval; these agents have potent antifungal activities against a variety of different fungi, including C. albicans, but interestingly they have little or no activity against C. neoformans strains. Although the FKS1 gene encoding β-1,3 glucan synthase is essential in C. neoformans (49), it may differ in regulation or structure from those of other fungi, rendering the present pneumocandins less active against this pathogenic fungus. Drugs that enhance the activity of the pneumocandins against C. neoformans might therefore be of clinical utility.
Studies of the model yeasts S. cerevisiae and S. pombe provide a rational basis for testing the possible synergistic action of calcineurin inhibitors combined with pneumocandins. β-1,3 Glucan synthase is encoded by two genes, FKS1 and FKS2, in S. cerevisiae (11, 15, 28). The two gene products have a redundant essential function, and hence fks1 or fks2 single-mutant strains are viable, whereas fks1 fks2 double-mutant strains are inviable. Most interestingly, expression of the FKS2 gene is transcriptionally induced in response to a mating pheromone, high temperature, and calcium ions, and FKS2 expression is dependent on calcineurin (15, 28, 54). As a consequence, fks1 mutant strains are markedly sensitive to the calcineurin inhibitors FK506 and CsA, because calcineurin inhibition in fks1 mutant strains blocks expression of the one remaining essential β-1,3 glucan synthase gene, FKS2. In S. pombe, mutations in a homologous gene encoding β-1,3 glucan synthase, cps1, confer resistance to papulacandin B and marked hypersensitivity to calcineurin inhibition by CsA and result in a temperature-sensitive growth defect (25). Furthermore, Franzot et al. have shown that MK-0991/caspofungin acetate enhances the activities of amphotericin B and fluconazole against C. neoformans in vitro (16). These observations indicate that calcineurin plays an intimate role in regulating expression or function of β-1,3 glucan synthase in model yeasts.
We found that a β-1,3 glucan synthase inhibitor, the pneumocandin MK-0991/caspofungin acetate, exhibited synergistic antifungal activity when combined with the calcineurin inhibitor FK506. The FIC index of FK506 combined with MK-0991/caspofungin acetate was 0.51 in the wild-type serotype A strain H99 (Table 1). Similarly, the FK506 analog L-685,818 also exhibited synergistic activity with MK-0991/caspofungin acetate, with an FIC index of 0.25 (Table 2). Most interestingly, the synergistic activity of both FK506 and L-685,818 with the pneumocandin MK-0991/caspofungin acetate was dependent on both FKBP12 and calcineurin, because no synergistic activity (FIC index = 1.00) was observed in the mutant strains lacking FKBP12 or calcineurin (Tables 1 and 2). Moreover, the toxic actions of MK-0991/caspofungin acetate were increased to similar extents by either inhibition of calcineurin with FK506 (MIC decreased from 12.5 and 25 μg/ml to 6.25 μg/ml) or in the mutant strain lacking calcineurin (Δcna1 [MIC again decreased from 12.5 and 25 μg/ml to 6.25 μg/ml) (Tables 1 and 2). These findings reveal that FK506 is synergistic with the pneumocandin MK-0991/caspofungin acetate via FKBP12-dependent inhibition of calcineurin and suggest that, as in the model yeasts S. cerevisiae and S. pombe, calcineurin function and β-1,3 glucan synthase function may be related in C. neoformans.
FK506 has synergistic antifungal activity with fluconazole via a mechanism that is independent of the FK506 target proteins FKBP12 and calcineurin.
One of the most exciting developments in antifungal drug therapy has been the clinical use of fluconazole and other related azoles. These compounds inhibit the enzyme 14-α-demethylase that catalyzes an intermediate step in the synthesis of the membrane sterol ergosterol. Sanglard and colleagues recently reported that the calcineurin inhibitors FK506 and CsA are synergistic with fluconazole against C. albicans, both in vitro and in animal models of candidiasis, to produce a fungicidal response (27; Marchetti et al., 38th ICAAC, abstr. J-50 and J-119). Some evidence suggests that the mechanism of synergistic activity results from the inhibition of multidrug resistance pumps by FK506, thus increasing intracellular concentrations of fluconazole to a fungicidal level (27). FK506 and CsA are known to inhibit multidrug resistance pump functions in both humans and yeasts (1, 21, 30, 35, 46).
We found that FK506 and fluconazole exhibited synergistic activity, with an FIC index of 0.25 against C. neoformans (wild type) (Table 1). Combined use of these two agents resulted in an ∼30-fold decrease in the MIC of FK506 and a 4-fold decrease in the MIC of fluconazole. The nonimmunosuppressive FK506 analog L-685,818 also exhibited synergistic activity with fluconazole (Table 2). Furthermore, the synergistic activities of FK506 combined with fluconazole were identical in both the wild-type H99 strain and in the mutant strains lacking either calcineurin (Δcna1) or FKBP12 (Δfrr1). Thus, neither FKBP12 nor calcineurin is involved in the synergistic action of FK506 plus fluconazole. These findings are in accord with a model in which FK506 enhances fluconazole action by inhibiting the function of one or more multidrug resistance pumps that normally extrude fluconazole and thereby increases the functional intracellular concentration of fluconazole.
FK506 has synergistic antifungal activity with Li+ cations, but not at doses that can be achieved in vivo.
In previous studies, we found that C. neoformans mutants lacking calcineurin were markedly sensitive to inhibition by Li+ cations, which was similar to the model yeast S. cerevisiae (36, 40). Furthermore, exposure of yeasts to CsA or FK506 conferred marked sensitivity to Li+ cations. Because Li+ has been widely employed to treat and prevent bipolar disorder in humans, we explored whether there might be therapeutic potential to a synergistic combination of Li+ with FK506.
In previous studies, we found that a relatively high concentration of LiCl, 50 mM (2.11 mg/ml), was required for a toxic effect in strains lacking calcineurin activity. Here, we tested whether the therapeutic levels of Li+ that can be achieved in patients (0.5 to 2 mM, or 21.19 to 84.78 μg/ml) might also be synergistic under our in vitro conditions for synergistic antifungal action. However, at the concentrations of LiCl we tested (from 1 mM to 0.9 μM, or from 42.39 to 0.041 μg/ml), no enhancement of the toxic effects of FK506 or L-685,818 was apparent (Tables 1 and 2 [FIC index = 1.50 to 2.0). Given that the therapeutic window for lithium action is quite narrow, these findings indicate that simple combination of Li+ cations and FK506 will not likely be of therapeutic benefit in the concentrations that can be tolerated in patients.
DISCUSSION
C. neoformans is an opportunistic fungal pathogen in AIDS patients, and the numbers of azole-resistant isolates are increasing. Thus, the identification of novel drug targets for this fungus is of paramount importance. We recently found that calcineurin is required for C. neoformans growth at 37°C and under other stress conditions. As a consequence, calcineurin is required for virulence in vivo (8, 40). As in S. cerevisiae, calcineurin also controls Na+ and Li+ homeostasis in C. neoformans. Calcineurin plays an essential role in cell cycle regulation in Aspergillus nidulans (45) and is involved in hyphal growth and vegetative growth in Neurospora crassa (22, 44), and S. pombe mutants lacking calcineurin have defects in cytokinesis, cell polarity, mating, and growth at 22°C (53). In summary, calcineurin plays an important role in normal growth, morphology, mating, and virulence in fungi and is an excellent candidate for antifungal drug development.
CsA and FK506 are toxic to pathogenic fungi such as C. albicans, C. neoformans, and A. fumigatus (39; Marchetti et al., 38th ICAAC, abstr. J-50 and J-119). CsA binds to cyclophilin A, whereas FK506 binds to the immunophilin FKBP12. The target of the CsA-cyclophilin A and FK506-FKBP12 complexes in both mammals and fungi is calcineurin (3, 26). The in vivo immunosuppressive activity of CsA and FK506 exacerbates cryptococcal infections in a rabbit model of C. neoformans meningitis, likely because immunosuppressive activity outweighs any beneficial antifungal activity (39, 40). We have identified nonimmunosuppressive CsA and FK506 analogs that retain antifungal activity against C. albicans and C. neoformans (7, 39), but impressive in vivo data are not yet available.
Here we demonstrate that the combination of calcineurin inhibitors plus known antifungal agents (fluconazole, pneumocandins, or bafilomycin A1) exhibits marked synergistic activity against C. neoformans. Molecular genetic studies have demonstrated novel features of FK506 action that could be exploited for antifungal drug design. As a consequence, a combination of mutations in calcineurin and V-ATPase subunits or assembly factors is lethal in S. cerevisiae. Here we tested whether the same lethal effect could be achieved by combined use of drugs that inhibit these target proteins: FK506 to inhibit calcineurin and bafilomycin A1 to inhibit V-ATPase.
Both calcineurin and V-ATPase regulate cation influx and efflux in fungi. In this study, we demonstrated that our hypothesis is correct: FK506 plus bafilomycin A1 is a very potent combination in vitro against C. neoformans. In our testing to determine if FKBP12 is involved in this mechanism of action, we used two FKBP12 mutants: the C. neoformans Δfrr1 mutant derived from the serotype A H99 strain (Table 1) and the C. neoformans Δfrr1-3 mutant derived from the serotype D JEC21 strain (Table 3). In both cases, FKBP12 mutant strains were completely resistant to the synergistic activity of FK506 plus bafilomycin A1. Therefore, FKBP12 is required for the action of FK506 plus bafilomycin. Next, we tested if calcineurin is involved by testing (i) the activity of CsA plus bafilomycin A1, (ii) the action of bafilomycin A1 in calcineurin mutant strains, and (iii) the synergistic activity of FK506 plus bafilomycin A1 in mutants lacking calcineurin (Tables 1 and 3). First, CsA and bafilomycin A1 do not exhibit synergistic activity against H99 strain (data not shown). Second, calcineurin mutants are not hypersensitive to bafilomycin A1. Third, calcineurin is not required for the activity of FK506 plus bafilomycin A1, since the calcineurin mutant is still potently inhibited by the combination (Tables 1 and 3).
Taken together, these findings indicate that FK506 synergistic action with bafilomycin requires FKBP12 but is distinct from calcineurin inhibition or mutation. Several possible models can be invoked. First, the physiology of cells lacking calcineurin might differ from that of cells in which calcineurin has been rapidly inhibited by FK506. Second, FKBP12 but not calcineurin could be required for function or expression of a novel drug target. Third, FK506 inhibition of both calcineurin and a second target could be required to enhance bafilomycin toxicity. Finally, there could be a novel target of the FKBP12-FK506 complex. In the last model, calcineurin could compete for FKBP12-ligand complexes with another target. This could explain the paradoxical finding that L-685,818 is synergistic with bafilomycin A1 in the calcineurin mutant but not the wild-type strain (Table 2). Because the FKBP12–L-685,818 complex has a higher affinity for calcineurin than FK506 (Fig. 1), in the wild-type cell more FKBP12–L-685,818 could be bound to calcineurin. The calcineurin mutation would free L-685,818 to bind another target. Further studies will be required to test these and other models.
FK506 and the pneumocandin MK-0991/caspufungin acetate exhibit synergistic activity against C. neoformans that is dependent upon FKBP12-dependent inhibition of calcineurin by FK506. The pneumocandins inhibit the fungal cell wall biosynthetic enzyme β-1,3 glucan synthase, which is encoded by a single essential gene in C. neoformans (49). While the pneumocandins are quite active against C. albicans and other fungal pathogens, C. neoformans is relatively resistant to the pneumocandins. Although the Fks1 proteins from C. albicans, S. cerevisiae, A. fumigatus, and C. neoformans have high degrees of similarity, the function and regulation of C. neoformans glucan synthase may be quite distinct, or C. neoformans may differ in permeability to pneumocandins. Calcineurin is known to regulate the FKS2 gene encoding β-1,3 glucan synthase in S. cerevisiae, and mutants with alterations in the cps1 gene in S. pombe encoding the β-1,3 glucan synthase are hypersensitive to CsA. Our findings reveal that the activity of pneumocandins is increased by calcineurin inhibition or mutation, suggesting a functional link between calcineurin and β-1,3 glucan synthase expression or regulation in C. neoformans. Further studies to address whether the FKS1 gene is transcriptionally regulated by calcineurin are in progress. Finally, our findings suggest that combination drug therapy may be a viable option to enhance the activity of the pneumocandin class of drugs against fungi such as C. neoformans.
We have also found that FK506 and fluconazole have synergistic activity against C. neoformans. These studies were predicated on the earlier reports of Sanglard and colleagues that CsA and FK506 are synergistic in combination with fluconazole against C. albicans, both in vitro and in vivo (27; Marchetti et al., 38th ICAAC, abstr. J-50 and J-119). While the mechanism of action is not entirely known, recent studies with C. albicans suggest that FK506 increases the intracellular concentration of fluconazole, likely via its known ability to inhibit multidrug resistance pump functions (27). We have demonstrated that the synergistic action of FK506 and fluconazole in C. neoformans is independent of both FKBP12 and calcineurin and that FKBP12 and calcineurin mutant strains are no more sensitive to fluconazole than isogenic wild-type strains. Thus, FK506 is clearly not acting in C. neoformans by inhibiting either FKBP12 or calcineurin. Our working hypothesis is that FK506 inhibits the activity of one or more multidrug resistance pump homologs that normally function to extrude fluconazole from the cell. This drug combination might have potential to enhance activity against azole-resistant C. neoformans strains or make fluconazole treatment more fungicidal against C. neoformans. Further studies will be necessary to establish the molecular mechanism of FK506 and fluconazole synergistic action against both C. albicans and C. neoformans.
Finally, we demonstrated that FK506 was not synergistic with Li+ cations at therapeutic doses of lithium chloride that can be achieved in vivo. Calcineurin does play a clear role in cation stress tolerance in C. neoformans, and strains in which calcineurin was mutated or inhibited were sensitive to 50 mM LiCl. However, therapeutic doses of lithium achieved in human patients are in the range of 0.5 to 2 mM, which is not sufficient to render calcineurin essential in C. neoformans cells under the in vitro culture conditions in which we have tested. These observations suggest that FK506 plus lithium will not likely be a viable therapeutic option in humans.
In conclusion, our studies illustrate the value of drug combinations with existing drugs and new potential antifungal agents. It remains to be tested whether these synergistic activities confer a beneficial effect in animal models of cryptococcal meningitis. In our opinion, a focus for future antifungal therapeutics will be the study and use of drug combinations for these eucaryotic pathogens that require fungicidal regimens in severely immunosuppressed patients.
ACKNOWLEDGMENTS
We thank Myra Kurtz at Merck & Co. for generously supplying bafilomycin A1, Ken Bartizal at Merck & Co. for supplying L-685,818, and Fujisawa pharmaceuticals for supplying FK506. We thank Tony Means and Elizabeth MacDougall for generously providing 125I-calmodulin.
These studies were supported by R01 grant AI41937 from the NIAID (J.H., M.E.C., and J.R.P.), supplement AI41937-01S1 (M.C.C.), P01 grant AI44975 from the NIAID to the Duke University Mycology Research Unit, and K01 award CA77075 from the NCI (to M.E.C.). Joseph Heitman is a Burroughs Wellcome Scholar in Molecular Pathogenic Mycology and an associate investigator of the Howard Hughes Medical Institute.
Maurizio Del Poeta and M. Cristina Cruz contributed equally to this study.
REFERENCES
- 1.Arceri R J, Stieglitz K, Bierer B E. Immunosuppressants FK506 and rapamycin function as reversal agents of the multidrug resistance phenotype. Blood. 1992;80:1528–1536. [PubMed] [Google Scholar]
- 2.Bowman E J, Siebers A, Altendorf K. Bafilomycins: a class of inhibitors of membrane ATPases from microorganisms, animal cells and plant cells. Proc Natl Acad Sci USA. 1988;85:7972–7976. doi: 10.1073/pnas.85.21.7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Breuder T, Hemenway C S, Movva N R, Cardenas M E, Heitman J. Calcineurin is essential in cyclosporin A- and FK506-sensitive yeast strains. Proc Natl Acad Sci USA. 1994;91:5372–5376. doi: 10.1073/pnas.91.12.5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cardenas M, Hemenway C, Muir R S, Ye R, Fiorentino D, Heitman J. Immunophilins interact with calcineurin in the absence of exogenous immunosuppressive ligands. EMBO J. 1994;13:5944–5957. doi: 10.1002/j.1460-2075.1994.tb06940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cox G M, Perfect J R. Fungal infections. Curr Opin Infect Dis. 1993;6:422–426. [Google Scholar]
- 6.Cruz M C, Cavallo L M, Görlach J M, Cox G, Perfect J R, Cardenas M E, Heitman J. Rapamycin antifungal action is mediated via conserved complexes with FKBP12 and TOR kinase homologs in Cryptococcus neoformans. Mol Cell Biol. 1999;19:4101–4112. doi: 10.1128/mcb.19.6.4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cruz M C, Del Poeta M, Wang P, Wenger R, Zenke G, Quesniaux V F J, Movva N R, Perfect J R, Cardenas M E, Heitman J. Immunosuppressive and nonimmunosuppressive cyclosporine analogs are toxic to the opportunistic fungal pathogen Cryptococcus neoformans via cyclophilin-dependent inhibition of calcineurin. Antimicrob Agents Chemother. 2000;44:143–149. doi: 10.1128/aac.44.1.143-149.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cruz M C, Sia R A, Olson M, Cox G M, Heitman J. Comparison of the roles of calcineurin in physiology and virulence in serotype D and serotype A strains of Cryptococcus neoformans. Infect Immun. 2000;68:982–985. doi: 10.1128/iai.68.2.982-985.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Del Poeta M, Schell W A, Perfect J R. In vitro antifungal activity of pneumocandin L-743,872 against a variety of clinically important molds. Antimicrob Agents Chemother. 1997;41:1835–1836. doi: 10.1128/aac.41.8.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Douglas C M, D'Ippolito J A, Shei G J, Meinz M, Onishi J, Marrinan J A, Li W, Abruzzo G K, Flattery A, Bartizal K, Mitchell A, Kurtz M B. Identification of the FKS1 gene of Candida albicans as the essential target of the 1,3-β-d-glucan synthase inhibitors. Antimicrob Agents Chemother. 1997;41:2471–2479. doi: 10.1128/aac.41.11.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Douglas C M, Foor F, Marrinan J A, Morin N, Nielsen J B, Dahi A M, Mazur P, Baginsky W, Li W, et al. The Saccharomyces cerevisiae FKS1 (ETG1) gene encodes an integral membrane protein which is a subunit of 1,3-beta-D-glucan synthase. Proc Natl Acad Sci USA. 1994;20:12907–12911. doi: 10.1073/pnas.91.26.12907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dröse S, Altendorf K. Bafilomycins and concanamycin as inhibitors of V-ATPases and P-ATPases. J Exp Biol. 1997;200:1–8. doi: 10.1242/jeb.200.1.1. [DOI] [PubMed] [Google Scholar]
- 13.Dykstra M A, Friedman L, Murphy J W. Capsule size of Cryptococcus neoformans: control and relationship to virulence. Infect Immun. 1977;16:129–135. doi: 10.1128/iai.16.1.129-135.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Egner R, Rosenthal F E, Kralli A, Sanglard D, Kuchler K. Genetic separation of FK506 susceptibility and drug transport in the yeast Pdr5 ATP-binding cassette multidrug resistance transporter. Mol Biol Cell. 1998;9:523–543. doi: 10.1091/mbc.9.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eng W K, Faucette L, McLaughlin M M, Cafferkey R, Koltin Y, Morris R A, Young P R, Johnson R K, Livi G P. The yeast FKS1 gene encodes a novel membrane protein, mutations in which confer FK506 and cyclosporin A hypersensitivity and calcineurin-dependent growth. Gene. 1994;151:61–71. doi: 10.1016/0378-1119(94)90633-5. [DOI] [PubMed] [Google Scholar]
- 16.Franzot S P, Casadevall A. Pneumocandin L-743,872 enhances the activities of amphotericin B and fluconazole against Cryptococcus neoformans in vitro. Antimicrob Agents Chemother. 1997;41:331–336. doi: 10.1128/aac.41.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garrett-Engele P, Moilanen B, Cyert M S. Calcineurin, the Ca2+/calmodulin-dependent protein phosphatase, is essential in yeast mutants with cell integrity defects and in mutants that lack a functional vacuolar H+-ATPase. Mol Cell Biol. 1995;15:4103–4114. doi: 10.1128/mcb.15.8.4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanada H, Moriyama Y, Maede M, Futai M. Kinetic studies of chromaffin granule H+ATPase and effects of bafilomycin A1. Biochem Biophys Res Commun. 1990;170:873–878. doi: 10.1016/0006-291x(90)92172-v. [DOI] [PubMed] [Google Scholar]
- 19.Hemenway C S, Dolinski K, Cardenas M E, Hiller M A, Jones E W, Heitman J. vph6 mutants of Saccharomyces cerevisiae require calcineurin for growth and are defective in vacuolar H(+)-ATPase assembly. Genetics. 1995;141:833–844. doi: 10.1093/genetics/141.3.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hunke S, Dröse S, Schneider E. Vanadate and bafilomycin A1 are potent inhibitors of the ATPase activity of the reconstituted bacterial ATP-binding cassette transporter for maltose (MalFGK2) Biochem Biophys Res Commun. 1995;216:589–594. doi: 10.1006/bbrc.1995.2663. [DOI] [PubMed] [Google Scholar]
- 21.Jachez B, Boesch D, Grassberger M A, Loor F. Reversion of the P-glycoprotein-mediated multidrug resistance of cancer cells by FK506 derivatives. Anticancer Drugs. 1993;4:223–229. doi: 10.1097/00001813-199304000-00015. [DOI] [PubMed] [Google Scholar]
- 22.Kothe G O, Free S J. Calcineurin subunit B is required for normal vegetative growth in Neurospora crassa. Fungal Genet Biol. 1998;23:248–258. doi: 10.1006/fgbi.1998.1037. [DOI] [PubMed] [Google Scholar]
- 23.Kurtz M B, Abruzzo G K, Flattery A, Bartizal K, Marrinan J A, Li W, Milligan J, Nollstadt K, Douglas C M. Characterization of echinocandin-resistant mutants of Candida albicans: genetic, biochemical, and virulence studies. Infect Immun. 1996;64:3244–3251. doi: 10.1128/iai.64.8.3244-3251.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwon-Chung K J, Polacheck I, Popkin T J. Melanin-lacking mutants of Cryptococcus neoformans and their virulence for mice. J Bacteriol. 1982;150:1414–1421. doi: 10.1128/jb.150.3.1414-1421.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ishiguro J, Saitou A, Durán A, Ribas J C. cps1+, a Schizosaccharomyces pombe gene homolog of Saccharomyces cerevisiae FKS genes whose mutation confers hypersensitivity to cyclosporin A and papulacandin B. J Bacteriol. 1997;179:7653–7662. doi: 10.1128/jb.179.24.7653-7662.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu J, Farmer J D, Lane W S, Friedman J, Weissman I, et al. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991;66:807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- 27.Maesaki S, Marichal P, Hossain M A, Sanglard D, Vanden Bossche H, Kohno S. Synergic effects of tacrolimus and azole antifungal agent against azole-resistant Candida albicans strains. J Antimicrob Chemother. 1998;42:747–753. doi: 10.1093/jac/42.6.747. [DOI] [PubMed] [Google Scholar]
- 28.Mazur P, Morin N, Baginsky W, El-Sherbeini M, Clemas J A, Nielsen J B, Foor F. Differential expression and function of two homologous subunits of yeast 1,3-β-d-glucan synthase. Mol Cell Biol. 1995;15:5671–5681. doi: 10.1128/mcb.15.10.5671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mitchell T G, Perfect J R. Cryptococcosis in the era of AIDS—100 years after the discovery of Cryptococcus neoformans. Clin Microbiol Rev. 1995;8:515–548. doi: 10.1128/cmr.8.4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mizuno K, Furuhashi Y, Misawa T, Iwata M, Kawai M, Kikkawa F, Kano T, Tomoda Y. Modulation of multidrug resistance by immunosuppressive agents: cyclosporin analogues, FK506 and mizoribine. Anticancer Res. 1992;12:21–25. [PubMed] [Google Scholar]
- 31.Monk B C, Feng W C, Marshall C J, Seto-Young D, Na S, Haber J E, Perlin D S. Modeling a conformationally sensitive region of the membrane sector of the fungal plasma membrane proton pump. J Bioenerg Biomembr. 1994;26:101–115. doi: 10.1007/BF00763222. [DOI] [PubMed] [Google Scholar]
- 32.Monk B C, Perlin D S. Fungal membrane proton pumps as promising new antifungal targets. Crit Rev Microbiol. 1994;20:209–223. doi: 10.3109/10408419409114555. [DOI] [PubMed] [Google Scholar]
- 33.Moore T D E, Edman J C. The α-mating type locus of Cryptococcus neoformans contains a peptide pheromone gene. Mol Cell Biol. 1993;13:1962–1970. doi: 10.1128/mcb.13.3.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Na S, Perlin D S, Seto-Young D, Wang G, Haber J B. Characterization of yeast plasma membrane H(+)-ATPase mutant pma1-A135V and its revertants. J Biol Chem. 1993;268:11792–11797. [PubMed] [Google Scholar]
- 35.Naito M, Oh-hara T, Yamazaki A, Danki T, Tsuruo T. Reversal of multidrug resistance by an immunosuppressive agent, FK506. Cancer Chemother Pharmacol. 1992;29:195–200. doi: 10.1007/BF00686252. [DOI] [PubMed] [Google Scholar]
- 36.Nakamura T, Liu Y, Hirata D, Namba H, Harada S, Hirokawa T, Miyakawa T. Protein phosphatase type 2B (calcineurin)-mediated, FK506-sensitive regulation of intracellular ions in yeast is an important determinant for adaptation to high salt stress conditions. EMBO J. 1993;12:4063–4071. doi: 10.1002/j.1460-2075.1993.tb06090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.National Committee for Clinical Laboratory Standards. Reference method for broth dilution susceptibility testing of yeasts. Approved standard M27-A. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 38.Nelson P W, Lozano-Chiu M, Rex J H. In vitro growth-inhibitory activity of pneumocandins L-733,560 and L-743,872 against putatively amphotericin B- and fluconazole-resistant Candida isolates: influence of assay conditions. J Med Vet Mycol. 1996;35:285–287. [PubMed] [Google Scholar]
- 39.Odom A, Del Poeta M, Perfect J, Heitman J. The immunosuppressant FK506 and its nonimmunosuppressive analog L-685,818 are toxic to Cryptococcus neoformans by inhibition of a common target protein. Antimicrob Agents Chemother. 1997;41:156–161. doi: 10.1128/aac.41.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Odom A, Muir S, Lim E, Toffaletti D L, Perfect J, Heitman J. Calcineurin is required for virulence of Cryptococcus neoformans. EMBO J. 1997;16:2576–2589. doi: 10.1093/emboj/16.10.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perfect J R, Durack D T. Effects of cyclosporine in experimental cryptococcal meningitis. Infect Immun. 1985;50:22–26. doi: 10.1128/iai.50.1.22-26.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perfect J R, Toffaletti D L, Rude T H. The gene encoding phosphoribosylaminoimidazole carboxylase (ADE2) is essential for growth of Cryptococcus neoformans in cerebrospinal fluid. Infect Immun. 1993;61:4446–4451. doi: 10.1128/iai.61.10.4446-4451.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perlin D S, Seto-Young D, Monk B C. The plasma membrane H(+)-ATPase of fungi. A candidate drug target? Ann N Y Acad Sci. 1997;834:609–617. doi: 10.1111/j.1749-6632.1997.tb52330.x. [DOI] [PubMed] [Google Scholar]
- 44.Prokisch H, Yarden O, Dieminger M, Tropschug M, Barthelmess I B. Impairment of calcineurin function in Neurospora crassa reveals its essential role in hyphal growth, morphology and maintenance of the apical Ca2+ gradient. Mol Gen Genet. 1997;256:104–114. doi: 10.1007/s004380050551. [DOI] [PubMed] [Google Scholar]
- 45.Rasmussen C, Garen C, Brining S, Kincaid R L, Means R L, Means A R. The calmodulin-dependent protein phosphatase catalytic subunit (calcineurin A) is an essential gene in Aspergillus nidulans. EMBO J. 1994;13:2545–2552. doi: 10.1002/j.1460-2075.1994.tb06544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raymond M, Ruetz S, Thomas D Y, Gros P. Functional expression of P-glycoprotein in Saccharomyces cerevisiae confers cellular resistance to the immunosuppressive and antifungal agent FK520. Mol Cell Biol. 1994;14:277–286. doi: 10.1128/mcb.14.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rhodes J C, Polacheck I, Kwon-Chung K J. Phenoloxidase activity and virulence in isogenic strains of Cryptococcus neoformans. Infect Immun. 1982;36:1175–1184. doi: 10.1128/iai.36.3.1175-1184.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sharom J F, Yu X, Chu J W K, Doige C A. Characterization of the ATPase activity of P-glycoprotein from multidrug-resistant Chinese hamster ovary cells. Biochem J. 1995;308:381–390. doi: 10.1042/bj3080381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thompson J R, Douglas C M, Li W, Jue C K, Pramanik B, Yuan X, Rude T H, Toffaletti D L, Perfect J R, Kurtz M. A glucan synthase FKS1 homolog in Cryptococcus neoformans is single copy and encodes an essential function. J Bacteriol. 1999;181:444–453. doi: 10.1128/jb.181.2.444-453.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vanden Bossche H, Dromer F, Improvisi I, Lozano-Chiu M, Rex J H, Sanglard D. Antifungal drug resistance in pathogenic fungi. Med Mycol. 1998;36:119–128. [PubMed] [Google Scholar]
- 51.Vazquez J A, Lynch M E, Boikov D, Sobel J D. In vitro activity of a new pneumocandin antifungal, L-743,872, against azole-susceptible and -resistant Candida species. Antimicrob Agents Chemother. 1997;41:1612–1614. doi: 10.1128/aac.41.7.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Withee J L, Sen R, Cyert M S. Ion tolerance of Saccharomyces cerevisiae lacking the Ca2+/CaM-dependent phosphatase (calcineurin) is improved by mutation in URE2 or PMA1. Genetics. 1998;149:865–878. doi: 10.1093/genetics/149.2.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yoshida T, Toda T, Yanagida M. A calcineurin-like gene ppb1+ in fission yeast: mutant defects in cytokinesis, cell polarity, mating and spindle pole body positioning. J Cell Sci. 1994;107:1725–1735. doi: 10.1242/jcs.107.7.1725. [DOI] [PubMed] [Google Scholar]
- 54.Zhao C, Jung U S, Garrett-Engele P, Roe T, Cyert M S, Levin D E. Temperature-induced expression of yeast FKS2 is under the dual control of protein kinase C and calcineurin. Mol Cell Biol. 1998;18:1013–1022. doi: 10.1128/mcb.18.2.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]