Abstract
Objectives
The present study aimed to identify associations between extremes in body weight status (underweight and excess body weight) before a COVID-19 diagnosis and clinical outcomes in patients infected with SARS coronavirus type 2.
Methods
A multicenter cohort study was conducted in eight different states in northeastern Brazil. Demographic, clinical (previous diagnosis of comorbidities), and anthropometric (self-reported weight and height) data about individuals who tested positive for COVID-19 were collected. Outcomes included hospitalization, mechanical ventilation, and death. Multivariable logistic regression models, adjusted based on age, sex and previous comorbidities, were used to assess the effects of extremes in body weight status on clinical outcomes.
Results
A total of 1308 individuals were assessed (33.6% were elderly individuals). The univariable analyses showed that only hospitalization was more often observed among underweight (3.2% versus 1.2%) and overweight (68.1% versus 63.3%) individuals. In turn, cardiovascular diseases were more often observed in all clinical outcomes (hospitalization: 19.7% versus 4.8%; mechanical ventilation: 19.9% versus 13.5%; death: 21.8% versus 14.1%). Based on the multivariable analysis, body weight status was not associated with risk of hospitalization (underweight: odds ratio [OR]: 1.10; 95% confidence interval [CI] 95%, 0.50–2.41 and excess body weight: OR: 0.81; 95 CI, 0.57–1.14), mechanical ventilation (underweight: OR: 0.92; 95% CI, 0.52–1.62 and excess weight: OR: 0.90; 95% CI, 0.67–1.19), and death (underweight: OR: 0.61; 95% CI, 0.31–1.20 and excess body weight: OR 0.88; 95% CI, 0.63–1.23).
Conclusions
Being underweight and excess body weight were not independently associated with clinical outcomes in patients with COVID-19 in the herein analyzed cohort. This finding indicates that the association between these variables may be confounded by both age and comorbidities.
Keywords: Body mass index, Coronavirus, Hospitalization, Mechanical ventilation, Mortality
Introduction
The pandemic caused by the SARS coronavirus type 2 (CoV-2) viral infection currently plagues the world. Brazil, which has significant territorial dimension and geographic differences in addition to peculiar political issues associated with public health management, has experienced a critical situation that has exacerbated viral transmissibility. This factor resulted in hospital overcrowding in several states, and compromised the quality of health care provided to affected individuals [1,2]. Brazil ranked first in the number of new infected cases and deaths worldwide during the first half of 2021, and the mortality rate reached 2.5%. The northeastern region, which is one of the poorest regions in the country and concentrates approximately 26% of the Brazilian population, accounts for 10.7% and 10.4% of the total number of COVID-19 cases and deaths, respectively, noted in Brazil [2].
Overall, the first COVID-19 symptoms emerge after 5.2 d of incubation, and can last 14 d on average. These symptoms present great variability in time and clinical manifestations, and often affect the respiratory tract among other organs and systems [3]. There is evidence that this disease shows a higher prevalence among individuals with chronic noncommunicable diseases (NCDs), such as diabetes, hypertension, respiratory diseases, and cancer [4]. Although there is no consensus in the literature, extremes in body weight status (i.e., being underweight and excessive body weight) can also be associated with morbidity and mortality rates resulting from SARS-COV-2 infection regardless of the concomitant incidence of other associated comorbidities [5,6].
Although the effects of being underweight on the clinical evolution of patients with COVID-19 remains poorly explored, underweight prevalence in hospitalized patients is often high and leads to a negative prognosis, because this condition is associated with a longer hospitalization time, as well as a higher rehospitalization frequency and death rate [7,8]. On the other hand, individuals with obesity appear to be more likely to evolve to the most severe form of the disease, remain hospitalized for longer periods of time, and require respiratory support upon hospitalization [9,10].
Of note, body mass index (BMI) is the anthropometric indicator most widely used to assess nutritional status in epidemiologic and clinical studies. However, physiological and metabolic changes are inherent to the aging process and should also be taken into consideration, because they have direct implications on the nutritional status and body composition of elderly individuals. Thus, using specific cutoff points for this population is necessary [11]. Nevertheless, several studies conducted with patients with COVID-19 have used the same BMI cutoff point for both adult and elderly individuals; thus, these studies made the mistake of evaluating different groups as equals [12], [13], [14].
Although references were used to classify the nutritional status of each age group [15,16], the literature still lacks consensus about the most adequate BMI cutoff point to be adopted at the time to assess the nutritional status of elderly individuals. Thus, classifying the nutritional status of individuals in this age group based on BMI allows for a comparison of results in a given research with those derived from other studies, as well as an acknowledgment that the body changes observed in this population must be interpreted with caution.
History of unintentional weight loss before hospitalization is another nutritional status indicator that appears to be an independent factor for worse clinical prognoses and death in several pathological processes [17,18], and may be associated with nondiagnosed preexisting diseases [19]. Moreover, this indicator is associated with functional decline, frailty, and a higher risk of infections in the elderly population [20], and should be carefully investigated in patients with COVID-19.
The likely association between being previously underweight or excess body weight and worse clinical outcomes resulting from COVID-19 [5,6] and the peculiarities of northeastern Brazil [1] are important to take into consideration, because this region presents high urban poverty levels [21] that are capable of influencing the epidemiologic pattern in wealthier regions. Therefore, the aim of the current study was to investigate associations between being underweight or excess body weight before a COVID-19 diagnosis and clinical outcomes in patients infected with SARS-CoV-2 in northeastern Brazil.
Methods
A multicenter cohort study was conducted by the Nutrition and COVID-19 Study Group in northeastern Brazil (GENSCoV-BR). The study was coordinated by the Nutrition School of Federal University of Alagoas, and included the states of Alagoas, Bahia, Sergipe, Pernambuco, Paraíba, Rio Grande do Norte, Piauí, and Maranhão. The study is part of a project called “Clinical, nutritional, and sociodemographic aspects associated with mortality rates in patients with COVID-19: Multicenter study in northeastern Brazil”.
The research project was approved by the research ethics committees of all collaborating centers, which were responsible to coordinate the research in different federal units (protocol number 4.090.285/2020). Both ethical and bioethical principles were respected at all research stages and in compliance with the Brazilian legislation and the Declaration of Helsinki.
Population and sample
The main study followed a convenient sampling plan of a nonprobabilistic model by taking into consideration an estimated mean prevalence of 20% obesity in adult individuals who live in the capitals of the northeastern Brazilian states [22]. The margin of error was 2%, the confidence interval (CI) was 99%, and the total sample consisted of 2647 individuals.
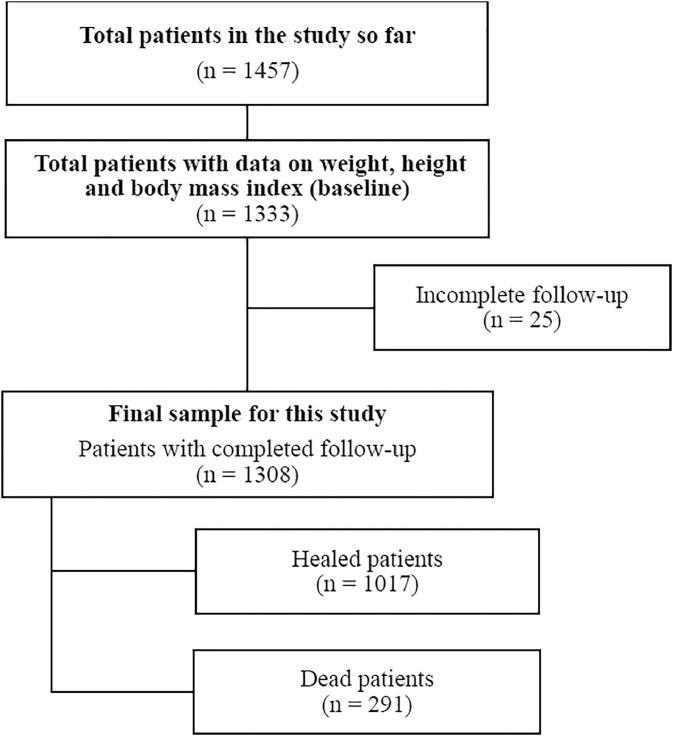
The investigated population consisted of individuals treated for COVID-19 after a laboratory diagnosis based on protocols followed by partner health care services (i.e., public and private hospitals, emergency care units, basic health care units, and flu-like syndrome units). The sample analyzed in the present study consisted of 1308 individuals, because many patients did not want to participate in the study and their decision was respected. In addition, collecting data during the daily routine of health care services was difficult (Fig. 1 ).
Fig. 1.
Flowchart of patient inclusion and final sample size.
Male and female individuals age ≥18 y diagnosed with an infection caused by SARS-CoV-2 as confirmed by a laboratory test (reverse transcription polymerase chain reaction, point-of-care testing, reverse transcription loop-mediated isothermal amplification, or SARS-CoV-2 antigen test) and who presented anthropometric data to establish their body weight status, received hospital or at-home treatment for COVID-19, and completed the follow up until cured or death were included in the study. Patients with a suspected COVID-19 infection whose diagnosis was not confirmed by at least one laboratory test were not included in the study.
Experimental procedures
Included patients were identified and selected by health care professionals from partner hospitals by taking into consideration a laboratory test-based COVID-19 diagnosis in their medical records. Individuals were also identified through a positive COVID-19 diagnosis after attending emergency services, which are considered a health care system gateway entry, regardless of whether they were referred for hospitalization or at-home isolation.
After patients were identified, researchers contacted them or their legal guardians by phone to invite them to voluntarily participate in the study. Patients who agreed to participate in the study were asked to electronically sign the informed consent form and complete the data collection form based on a guided interview. In addition, patient information was collected from their medical records, which are kept at the health care services they were treated at.
Data deriving from the medical records of hospitalized patients or information on the health condition of patients undergoing home treatment were collected in both a retrospective and prospective manner. Data deriving from medical records were collected up to the time of hospital discharge or death. Patients undergoing at-home treatment were followed in a remote manner, either by telephone or virtual messaging application, until the end of the quarantine time (21 d in the current study) or symptom relief, whichever occurred last. Patients who needed hospitalization at some point despite having started treatment at home had their data collected from their medical records.
Demographic, clinical, and anthropometric data
Demographic and clinical data about patients undergoing hospital or at-home treatment were collected. With respect to data collected through remote interviews, variables were categorized as age (elderly age ≥65 years [yes or no]), sex (male/female), black skin color/race (yes/no), and unintentional weight loss regardless of COVID-19 diagnosis (yes/no). Patients who reported having lost >5% of their usual body weight during the previous 6 mo were categorized as having unintentional weight loss without any specific reason, such as advanced chronic disease or treatment, or known cause [23]. Information about the previous medical diagnosis of comorbidities, such as systemic arterial hypertension (SAH), diabetes mellitus (DM), cardiovascular disease (CVD), chronic obstructive pulmonary disease (COPD), and asthma/other respiratory diseases, was also collected.
Incidence of the following clinical events was assessed based on data obtained in health care service records: Need of hospitalization due to COVID-19 (yes/no), need of mechanical ventilation during treatment (yes/no), and death (yes/no). As for anthropometric data, information on current self-reported height and weight was used to calculate patients’ BMI.
In the current study, participants' BMI was classified in two different ways. First, BMI was categorized based on standards recommended by the World Health Organization (WHO) [15] for adults and by Lipschitz [16] for elderly individuals. The categorization was done to unify the nutritional status analysis between adult and elderly individuals by respecting changes in body composition inherent to the aging process. Thus, adult individuals with a BMI ≥25.0 kg/m² and elderly individuals with a BMI >27.0 kg/m² were classified as having excessive body weight. Accordingly, adult individuals with a BMI <18.5 kg/m² and elderly individuals with a BMI <22.0 kg/m² were classified as underweight. Second, since Lipschitz's classification does not establish a cutoff point for overweight and obese, as well as to allow for a comparison of data from the current research with those of other studies, all participants were also classified according to standards established by the WHO (regardless of age), namely being overweight (BMI ≥25.0 kg/m² and ≤29.9 kg/m²) and obese (BMI ≥30.0 kg/m²).
Statistical analysis
The Statistical Package for Social Sciences software, version 21.0 (IBM Inc, Chicago, IL) was used for the statistical analysis, which adopted an alpha value of 5%. Absolute and relative frequencies were calculated for categorical variables. A χ2 or Fisher's exact test was applied to check for a univariable association between categorical variables depending on the expected frequency of counts for each cell. A multivariable binary logistic model, adjusted based on age, sex, color/race, federal unit, as well as diagnosis of SAH, DM, CVD, COPD, and asthma/other respiratory diseases, was used to calculate the adjusted odds ratio (ORs) between the BMI categories presenting any of the investigated outcomes (hospitalization, mechanical ventilation, and/or death).
The BMI categories were always included as independent variable in the adopted models, and normal weight was used as the reference category. Unintentional weight loss was also included as an independent variable. To further explore the associations between BMI category and clinical outcomes and the possible modification effects of age category (age <65 y or >65 y) in this relationship (between BMI categories and clinical outcomes), an interaction analysis were conducted. In this analysis, the full multivariable model received an interaction term “age_group*BMI_category” to assess whether the effects of BMI categories in the clinical outcomes differed between the two age groups. If the interaction term showed a significant P-value, evidence of modification of effects was considered.
Results
In total, 439 of1308 evaluated patients (33.6%) were elderly individuals, of whom 672 (51.4%) were men. Excessive body weight was observed in 872 patients (66.9%), underweight in 35 patients (2.7%), and a history of weight loss was reported by 404 patients with data available (35%). Moreover, 424 patients (32.4%) were also classified as obese according to the WHO's standards, and 983 patients (75.2%) required hospitalization to treat COVID-19, but the others did not meet the clinical criteria for hospitalization and underwent treatment at home. In addition, 291 individuals (22.2%) died. The sociodemographic, clinical, and anthropometric features of patients included in the current study are described in Table 1 .
Table 1.
Characterization of patients who underwent treatment for COVID-19
| N = 1308 | % | |
|---|---|---|
| Age ≥65 y | 439 | 33.6 |
| Male | 672 | 51.4 |
| Black color/race (n = 1210) | ||
| No | 315 | 26.0 |
| Yes | 895 | 74.0 |
| Federal unit | ||
| Alagoas | 348 | 26.6 |
| Bahia | 183 | 14.0 |
| Maranhão | 41 | 3.1 |
| Paraíba | 215 | 16.4 |
| Pernambuco | 253 | 19.3 |
| Piauí | 40 | 3.1 |
| Rio Grande do Norte | 116 | 8.9 |
| Sergipe | 112 | 8.6 |
| Systemic arterial hypertension | 653 | 49.9 |
| Diabetes mellitus | 383 | 29.3 |
| Cardiovascular disease (n = 1292) | 204 | 15.8 |
| Chronic obstructive pulmonary disease (n = 1301) | 64 | 4.9 |
| Asthma/other respiratory diseases | 172 | 13.1 |
| BMI category* (n = 1304) | ||
| Underweight | 35 | 2.7 |
| Normal weight | 397 | 30.4 |
| Excess body weight | 872 | 66.9 |
| BMI category† (n = 1305) | ||
| Underweight | 79 | 6.1 |
| Normal weight | 419 | 32.1 |
| Overweight | 807 | 61.8 |
| Obesity | 424 | 32.4 |
| Unintentional weight loss (n = 1132) | 404 | 35.7 |
| Hospitalization | 983 | 75.2 |
| Mechanical ventilation | 469 | 35.9 |
| Death | 291 | 22.2 |
BMI, body mass index
Classified using World Health Organization category for adults and Lipschitz for the elderly (age ≥65 y): Having excessive body weight was considered BMI ≥25.0 kg/m² for adults and BMI >27.0 kg/m² for the elderly.
Classified according to World Health Organization for all individuals (BMI ≥25.0 kg/m² and ≤29.9 kg/m² for overweight and BMI ≥30 kg/m² for obesity). Unintentional weight loss: Loss of >5% of usual body weight in previous 6 mo without any specific reason or known cause.
Based on the univariable analysis, age ≥65 y; male sex; black color/race; incidence of SAH, DM, CVD, COPD, and asthma/other respiratory diseases; BMI categories; obesity; and unintentional weight loss were associated with the need for hospitalization. Variables, such as age ≥65 y, male sex, DM, CVD, and asthma/other respiratory disease, have shown significant association with the need for mechanical ventilation. Age ≥65 y, male sex, SAH, CVD and asthma/other respiratory diseases were significantly associated with death. A previous COPD diagnosis was more often recorded among patients who required hospitalization, but a previous asthma diagnosis was negatively associated with events, such as hospitalization, mechanical ventilation, and death. In addition, unintentional weight loss was more often observed in the group of patients who required hospitalization, although not associated with mechanical ventilation or death (Table 2 ).
Table 2.
Univariable cross-analysis between predictor variables and observed clinical events (n = 1308)
| Clinical events |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Hospitalization |
Mechanical ventilation |
Death |
|||||||
| No, % | Yes, % | P-value | No, % | Yes, % | P-value | No, % | Yes, % | P-value | |
| Age ≥65 y | 7.1 | 42.7 | < 0.01 | 29.6 | 40.7 | < 0.01 | 29.7 | 47.1 | < 0.01 |
| Male | 35.1 | 57.0 | < 0.01 | 42.0 | 68.2 | < 0.01 | 43.8 | 78.0 | < 0.01 |
| Black color/race | 67.2 | 76.5 | < 0.01 | 74.3 | 73.4 | 0.78 | 73.5 | 75.6 | 0.52 |
| Systemic arterial hypertension | 19.3 | 60.5 | < 0.01 | 48.4 | 52.7 | 0.14 | 48.3 | 55.7 | 0.02 |
| Diabetes mellitus | 7.7 | 36.7 | < 0.01 | 26.6 | 34.1 | < 0.01 | 28.6 | 31.6 | 0.34 |
| Cardiovascular disease (n = 1292) | 4.8 | 19.7 | < 0.01 | 13.5 | 19.9 | < 0.01 | 14.1 | 21.8 | < 0.01 |
| Chronic obstructive pulmonary disease (n = 1301) | 0.9 | 6.3 | < 0.01 | 5.4 | 4.1 | 0.35 | 4.8 | 5.2 | 0.76 |
| Asthma or other respiratory disease | 22.0 | 10.1 | < 0.01 | 15.9 | 8.3 | < 0.01 | 14.7 | 7.9 | < 0.01 |
| BMI category* (n = 1304) | 0.01 | 0.70 | 0.50 | ||||||
| Underweight | 1.2 | 3.2 | 2.9 | 2.4 | 3.0 | 1.7 | |||
| Normal weight | 35.5 | 28.7 | 29.8 | 31.6 | 30.2 | 31.4 | |||
| Excess body weight | 63.3 | 68.1 | 67.3 | 66.0 | 66.9 | 66.9 | |||
| BMI category† (n = 1305) | < 0.01 | 0.35 | 0.39 | ||||||
| Underweight | 1.5 | 7.6 | 5.7 | 6.6 | 6.1 | 5.8 | |||
| Normal weight | 35.8 | 30.8 | 31.0 | 34.1 | 31.2 | 35.4 | |||
| Overweight | 62.7 | 61.5 | 63.3 | 59.3 | 62.7 | 58.8 | |||
| Obesity | 26.5 | 34.5 | < 0.01 | 32.4 | 32.4 | 0.99 | 32.8 | 30.9 | 0.53 |
| Unintentional weight loss (n = 1132) | 30.9 | 37.7 | 0.03 | 34.7 | 37.6 | 0.32 | 36.6 | 32.5 | 0.24 |
BMI, body mass index
Classified using World Health Organization category for adults and Lipschitz for the elderly (age ≥65 y): Having excessive body weight was considered BMI ≥25.0 kg/m² for adults and BMI >27.0 kg/m² for the elderly.
Classified according to World Health Organization for all individuals (BMI ≥25.0 kg/m² and ≤29.9 kg/m² for overweight and BMI ≥30 kg/m² for obesity). Unintentional weight loss: Loss of >5% of usual body weight in previous 6 mo without any specific reason or known cause.
Being underweight was more often observed among patients who required hospitalization when the BMI of adult and elderly individuals was classified based on cutoff points established by the WHO [13] and Lipschitz [14], respectively. On the other hand, excess body weight was more frequent among patients who underwent at-home treatment and successfully healed (Table 2). However, based on the classification by the WHO [13], none of the assessed patients presented an association between BMI categories and hospitalization, regardless of age.
The multivariable analysis was conducted to identify associations of BMI categories, obesity diagnosis, and history of unintentional weight loss with the investigated clinical events adjusted based on sex, age, and other risk factors. Based on this analysis, neither the BMI categories nor the history of unintentional weight loss were associated with a risk of hospitalization, mechanical ventilation, and death (Table 3 ). Based on the univariate sensitivity analyses, age and BMI categories did not show significant interaction with outcomes, such as hospitalization (P = 0.13), mechanical ventilation (P = 0.93), and death (P = 0.56). Based on the multivariable sensitivity analysis adjusted based on other risk factors and federal units, there was significant interaction between age and BMI categories (P = 0.03) because underweight adult individuals were at a greater risk of hospitalization than underweight elderly ones. However, none of the individual OR values were statistically significant (OR: 8.06; 95% CI, 0.96–67.60; P = 0.05 versus OR: 0.59; 95% CI, 0.22–1.57]; P = 0.29, respectively). Besides, the excess body weight and age categories did not show interaction with the evaluated outcomes.
Table 3.
Multivariable analysis between BMI category at the beginning of the follow up and different outcomes
| Clinical events* |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Hospitalization |
Mechanical ventilation |
Death |
|||||||
| OR | 95% CI | P-value | OR | 95% CI | P-value | OR | 95% CI | P-value | |
| BMI category† (n = 1190) | 0.41 | 0.77 | 0.34 | ||||||
| Normal weight | 1 | ― | ― | 1 | ― | ― | 1 | ― | ― |
| Underweight | 1.10 | 0.50–2.41 | 0.81 | 0.92 | 0.52–1.62 | 0.78 | 0.61 | 0.31–1.20 | 0.15 |
| Excess weight | 0.81 | 0.57–1.14 | 0.23 | 0.90 | 0.67–1.19 | 0.47 | 0.88 | 0.63–1.23 | 0.47 |
| Obesity‡ (n = 1194) | 0.83 | 0.59–1.16 | 0.28 | 1.11 | 0.84–1.47 | 0.44 | 0.92 | 0.66–1.28 | 0.63 |
| Unintentional weight loss (n = 1051) | 0.97 | 0.69–1.36 | 0.87 | 1.11 | 0.83–1.47 | 0.47 | 0.78 | 0.55–1.10 | 0.17 |
BMI, body mass index; CI, confidence interval; OR, odds ratio
Analyses adjusted for age, sex, ethnicity, federal unit, and diagnoses of hypertension, diabetes, cardiovascular diseases, chronic obstructive pulmonary disease, and asthma/other respiratory diseases
Classified using World Health Organization category for adults and Lipschitz for the elderly (age ≥65 y): Having excessive body weight was considered BMI ≥25.0 kg/m² for adults and BMI >27.0 kg/m² for the elderly.
Classified according to World Health Organization for all individuals (BMI ≥30.0 kg/m²). Unintentional weight loss: Loss of >5% of usual body weight in previous 6 mo without any specific reason or known cause.
Discussion
The aim of the current research was to investigate whether being underweight and excess body weight were associated with clinical complications of COVID-19 in northeastern Brazil. Based on the univariable analyses, only hospitalization was associated with BMI categories, obesity, and unintentional weight loss (featured by loss >5% of usual body weight in previous 6 mo, without intention or apparent reason). Nevertheless, the multivariable analysis indicated that none of the BMI categories were risk factors for hospitalization, mechanical ventilation, or death. Therefore, the gross effect of body weight status on clinical complications caused by COVID-19 in this sample was confounded by both age and other known risk factors, such as NCDs.
With respect to the univariable association between being underweight and hospitalization, although quite complex, the link between malnutrition (either acute or chronic) and infection has been repeatedly corroborated and long been acknowledged by the WHO [24,25]. Previous evidence highlighted in other pathological processes points out that being underweight impairs patients’ immune function, mainly cell-mediated immunity, a fact that turns underweight individuals in a group at a higher risk of evolving toward adverse results and presenting with the worst prognosis [26]. Few studies have shown the effect of being underweight on negative COVID-19 outcomes. According to Kim et al., underweight patients were at a higher risk of presenting outcomes, such as mechanical ventilation and death, after adjusting based on age, sex, race/ethnicity, incidence of comorbidities, smoking habit, and hospital type [27]. Of note, being underweight is just one of the malnutrition indicators used for adult individuals. Most recently, a meta-analysis comprised of studies that used nutritional risk screening tools among hospitalized patients with COVID-19 pointed toward an increased risk of death in patients at risk of malnutrition [28]. This finding can be explained by patients’ increased resting energy expenditure, reduced oral intake [28,29], and impaired immune system [30].
Based on the current results, excess body weight has shown only association with hospitalization in the univariable analysis. The frequency of excess body weight in patients with COVID-19 was similar to that observed for the rest of the population in the capitals of northeastern Brazilian states, which ranged from 50.3% to 59.5%, according to data derived from the largest population survey focused on identifying risk and protective factors for chronic diseases in Brazil [22]. The highest frequency of individuals with excess body weight was observed among those who remained in isolation at home and who did not require hospitalization. This finding diverges from most publications on this topic. Of note, some studies that identified excess body weight as a risk factor for clinical outcomes of COVID-19 disregarded the preexistence of NCDs, which comprise well-known risk factors, such as DM, SAH, and chronic kidney disease, that can be primary or secondary to being overweight [6,31]. The aforementioned studies included almost exclusively hospitalized or critically ill patients in their samples, and disregarded the prevalence of excess body weight in patients affected by a mild form of COVID-19 who did not require hospitalization [27,32]. Some studies even used the WHO's criteria (BMI ≥25kg/m²) [15] as cutoff points to classify being overweight in elderly individuals, as well as overestimated the prevalence of being overweight/obese in this age group, which accounts for most patients hospitalized due to COVID-19 [6,27,32].
Studies that only focus on assessing hospitalized individuals (rather than individuals treated both at home and at the hospital) and according to which obesity is associated with worse outcomes may present some collider bias type, in which the single condition hospitalized may induce confounding associations between obesity and death because the incidence of preexisting chronic diseases (closely associated with obesity and age) can favor the most severe forms of COVID-19 and lead to hospitalizations [33]. Limiting the sample to individuals who experienced events, such as hospitalization, and disregarding the fact that variable obesity is directly influenced by the other two variables (NCDs and age) leads to colliding bias in many studies about COVID-19, as well as induces results and distorts the real associations between these variables in the assessed sample [34].
Of additional note, results presented in most of these studies contrast with the obesity paradox in critically ill patients. According to this paradox, patients with a higher weight could have better metabolic reserve to tolerate a given inflammatory condition due to high catabolism, as observed for pneumonia caused by SARS-CoV-2 infection (this being the protective factor regarding mortality when adjusted by classical risk factors) [33]. Interestingly, no association pattern has emerged when the association between obesity and clinical outcomes in the herein investigated sample was adjusted based on regular risk factors (i.e., NCDs). This finding indicates a total lack of association between obesity and clinical outcomes in the current sample, neither in the univariable analysis nor the adjusted one.
Different findings from different cutoff points established to classify body weight status based on the BMI of adult and elderly individuals were another relevant aspect observed in the current study. BMI presented a good correlation with morbidity and mortality rates when cutoff points specific to age were taken into consideration [35] due to metabolic- and body composition-related changes inherent to the aging process that require the adoption of specific cutoff points for this population, mainly if one aims at predicting death associated with excess body weight. The present study disregarded the existence of specific and consolidated cutoff points set for the elderly population, and applied the WHO's criteria [15] to all individuals. Consequently, there was an increased prevalence of overweight/obese individuals in the analyzed sample. This factor may have induced observers to overestimate this variable as a risk factor for COVID-19 complications in hospitalized patients.
On the other hand, adipose tissue accumulation in the abdominal region triggers mild inflammatory processes, increases angiotensin-converting enzyme 2 (ACE-2) expression by adipocytes, and hyperactivates the renin-angiotensin system, which leads to endothelial dysfunction. These factors may contribute to the worsening of the disease and the emergence of symptoms in patients with COVID-19, mainly when associated with other preexisting risk factors (e.g., age, NCDs, and immunodeficiency) capable of contributing to the most severe forms of this disease [36], [37], [38].
Unintentional weight loss in the current study was more often observed in patients who needed hospitalization throughout the disease course, although this association was not significant in the adjusted analysis. Previous evidence has shown that patients infected with SARS-CoV-2 often experience acute malnutrition scenario days before hospitalization [39], mainly the most severe cases. Inflammatory storm and an inevitable trend toward weight loss were also reported [40]. Moreover, the incidence of preexisting chronic diseases can contribute to nutritional impairment [40]. Of note, ACE-2 is also found in the skeletal muscle, a fact that leads individuals with COVID-19 to have myalgia and muscle loss [41]. Data capable of explaining the role played by ACE-2 in inducing weight loss during COVID-19 remain scarce; however, its main function is well-known to lie on regulating the renin-angiotensin system, which leads to vasoconstriction, increased sodium absorption, and inflammation, as well as contributes to patients’ hyperinflammatory and catabolic state. In addition, ACE-2 overexpression appears to increase myocellular and adipose insulin sensitivity and favor weight loss [42]. Furthermore, the acute malnutrition condition observed in patients with COVID-19 infection [28,39] leads to adipose tissue mobilization due to lipolysis induced by increased adiponectin and decreased leptin secretion [43]. Patients’ inflammatory state induces the release of cortisol and adrenergic hormones, which can also lead to increased fat oxidation and decreased adipose tissue rates [44].
The method used to collect weight and height data and calculate patients’ BMI (i.e., self-reported information) and the use of this indicator as the only marker of nutritional status were important limitations of the current study. The self-reporting strategy was adopted for anthropometric assessment purposes to avoid physical contact with patients infected with COVID-19, as well as avoid the use of the same assessment instrument by several patients and enable the collection of data about several patients who were unable to walk to the assessment equipment (scale and stadiometer) due to disease worsening. Thus, although the number of assessed patients increased, mitigating the likelihood of contamination by health care professionals and ruling out the risk of spreading SARS-CoV-2, a practice recommended by international guidelines [45,46], was possible. Of note, in addition to the aforementioned reasons for using the selected method, the direct measurement of these variables and other body composition measurements could not be performed during the peak of the COVID-19 pandemic in Brazil due to health care professionals’ workload, the need to restrict physical contact, and the implementation of airborne precautions in hospital environments. Despite these limitations, the measurements are validated and used to assess nutritional status in national and international epidemiologic and clinical studies [47], [48], [49], [50], [51]. In addition, the measurements provided the study with a more homogeneous nutritional status assessment among all research centers based on scientific techniques and procedures, taking into consideration the reality and routine of health care services within the COVID-19 pandemic context.
However, measurement information self-reported by patients with COVID-19 should be used with caution, because these patients present heterogeneous features, such as age. Hence, self-reported body weight may not be efficient in reflecting the current weight of these patients. On the other hand, although an important indicator of body weight status, BMI does not differentiate body compartments (muscle and adipose reserve) or reflect fat distribution in the human body. Thus, BMI is more sensitive in association with other anthropometric measurements capable of evaluating body composition [52,53]. Yet, this assessment tool may be more valid than the use of medical records in health care systems, which do not indicate the adopted method. Thus, different measurement methods may be used in the same study, such as visual observation performed by untrained health care professionals.
Other limitations worth highlighting are the lack of individual socioeconomic information about patients and the adoption of the convenience sampling method in a study conducted in one of the poorest regions of Brazil, experiencing unfavorable socioeconomic and political context. However, of note, obtaining individual socioeconomic data capable of accurately reflecting the economic conditions individuals live in requires adopting extensive data collection protocols that can be considered invasive and uncomfortable. Although these protocols were described in the data collection protocol of the current study, they were not completed by most participants and mainly by the most severe cases. Thus, participants’ socioeconomic conditions were not taken into consideration in the current study to ensure a satisfactory sample size and the quality of the herein performed analyses, as well as to give strength and reliability to the assessed data. Of note, although the sampling plan was featured as of the convenience type, the sample distribution proportionality among the collaborating centers was respected and different health care services were included to recruit patients from different social strata with different intensities of COVID-19, either hospitalized or treated at home.
Conclusions
BMI categories, obesity, and unintentional weight loss, as observed in the herein assessed sample, were overall associated with patients hospitalized with COVID-19. Based on the adjusted analysis, being underweight, excess body weight, and obesity were not independently associated with the clinical outcomes in these patients. This finding indicates that the association among these variables was confounded by both age and traditional risk factors, such as NCDs.
Footnotes
Supplementary material associated with this article can be found, in the online version at doi:10.1016/j.nut.2022.111677.
Appendix. Supplementary materials
References
- 1.Zimerman A, Lopes RD, D’Ávila A, Rohde LE, Zimerman LI. COVID-19 in Brazil: The headlines should be about science. Lancet. 2020;396:1803. doi: 10.1016/S0140-6736(20)32375-8. [DOI] [PubMed] [Google Scholar]
- 2.Ministério da Saúde Brasil. Painel de casos de doença pelo coronavírus 2019 (COVID-19) no Brasil, 2021. Available at: https://covid.saude.gov.br. Accessed April 26, 2021.
- 3.Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou F, Yu T, Du R, Fan G, Liu Y, Xiang J, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim SY, Yoo DM, Min C, Wee JH, Kim JH, Choi HG. Analysis of mortality and morbidity in COVID-19 patients with obesity using clinical epidemiological data from the Korean Center for Disease Control & Prevention. Int J Environ Res Public Health. 2020;17:9336. doi: 10.3390/ijerph17249336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu Z, Hasegawa K, Ma B, Fujiogi M, Camargo CA, Jr, Liang L. Association of obesity and its genetic predisposition with the risk of severe COVID-19: Analysis of population-based cohort data. Metabolism. 2020;112 doi: 10.1016/j.metabol.2020.154345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giorno RD, Quarenghi M, Stefanelli K, Capelli S, Giagulli A, Quarleri L, et al. Nutritional risk screening and body composition in COVID-19 patients hospitalized in an internal medicine ward. Int J Gen Med. 2020;13:1643–1651. doi: 10.2147/IJGM.S286484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fedele D, Francesco A, Riso S, Collo A. Obesity, malnutrition, and trace element deficiency in the coronavirus disease (COVID-19) pandemic: An overview. Nutrition. 2021;81 doi: 10.1016/j.nut.2020.111016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson MR, Geleris J, Anderson DR, Zucker J, Nobel YR, Freedberg D, et al. Body mass index and risk for intubation or death in SARS-CoV-2 Infection: A retrospective cohort study. Ann Intern Med. 2020;173:782–790. doi: 10.7326/M20-3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moriconi D, Masi S, Rebelos E, Virdis A, Manca ML, Marco S, et al. Obesity prolongs the hospital stay in patients affected by COVID-19, and may impact on SARS-COV-2 shedding. Obes Res Clin Pract. 2020;14:205–209. doi: 10.1016/j.orcp.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Souza R, Fraga JSD, Gottschall CBA, Busnello FM, Rabito EI. Avaliação antropométrica em idosos: Estimativas de peso e altura e concordância entre classificações de IMC. Rev Bras Geriatr e Gerontol. 2013;16:81–90. [Google Scholar]
- 12.Simonnet A, Chetboun M, Poissy J, Raverdy V, Noulette J, Duhamel A, et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity. 2020;28:1195–1199. doi: 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hendren NS, de Lemos JA, Ayers C, Das SR, Rao A, Carter S, et al. Association of body mass index and age with morbidity and mortality in patients hospitalized with COVID-19: Results from the American Heart Association COVID-19 Cardiovascular Disease Registry. Circulation. 2021;143:135–144. doi: 10.1161/CIRCULATIONAHA.120.051936. [DOI] [PubMed] [Google Scholar]
- 14.Cariou B, Hadjadj S, Wargny M, Pichelin M, Al-Salameh A, Allix I, et al. Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: The CORONADO study. Diabetologia. 2020;63:1500–1515. doi: 10.1007/s00125-020-05180-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization . World Health Organization; Geneva, Switzerland: 1995. Physical status: The use and interpretation of anthropometry. [PubMed] [Google Scholar]
- 16.Lipschitz DA. Screening for nutritional status in the elderly. Primary Care. 1994;21:55–67. [PubMed] [Google Scholar]
- 17.England BR, Baker JF, Sayles H, Michaud K, Caplan L, Davis LA, et al. Body mass index, weight loss, and cause-specific mortality in rheumatoid arthritis. Arthritis Care Res (Hoboken) 2018;70:11–18. doi: 10.1002/acr.23258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pack QR, Rodriguez-Escudero JP, Thomas RJ, Ades PA, West CP, Somers VK, et al. The prognostic importance of weight loss in coronary artery disease: A systematic review and meta-analysis. Mayo Clin Proc. 2014;89:1368–1377. doi: 10.1016/j.mayocp.2014.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bosch X, Monclús E, Escoda O, Guerra-García M, Moreno P, Guasch N, et al. Unintentional weight loss: Clinical characteristics and outcomes in a prospective cohort of 2677 patients. PloS One. 2017;12 doi: 10.1371/journal.pone.0175125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gaddey HL, Holder K. Unintentional weight loss in older adults. Am Fam Physician. 2014;89:718–722. [PubMed] [Google Scholar]
- 21.Kerr L, Kendall C, Silva AA, Aquino EM, Pescarini JM, Almeida RL, et al. COVID-19 in Northeast Brazil: Achievements and limitations in the responses of the state governments. Ciên Saúde Coletiva. 2020;25:4099–4120. doi: 10.1590/1413-812320202510.2.28642020. [DOI] [PubMed] [Google Scholar]
- 22.Ministério da Saúde Brasil Vigitel Brasil 2019: Vigilância de Fatores de Risco e Proteção para Doenças Crônicas por Inquérito Telefônico; Brasília; 2020. p. 137. [Google Scholar]
- 23.Wong CJ. Involuntary weight loss. Med Clin North Am. 2014;98:625–643. doi: 10.1016/j.mcna.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 24.Schaible UE, Kaufmann SH. Malnutrition and infection: Complex mechanisms and global impacts. PLoS Med. 2007;4:e115. doi: 10.1371/journal.pmed.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berger MM. Nutrition status affects COVID-19 patient outcomes. JPEN J Parenter Enteral Nutr. 2020;44:1166–1167. doi: 10.1002/jpen.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Calder PC. Feeding the immune system. Proc Nutr Soc. 2013;72:299–309. doi: 10.1017/S0029665113001286. [DOI] [PubMed] [Google Scholar]
- 27.Kim TS, Roslin M, Wang JJ, Kane J, Hirsch JS, Kim EJ, et al. Body mass index as a risk factor for clinical outcomes in patients hospitalized with COVID-19 in New York. Obesity (Silver Spring) 2021;29:279–284. doi: 10.1002/oby.23076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abate SM, Checkol YA, Estifanos MB, Abate KH, Kabthymer RH. Prevalence and outcomes of malnutrition among hospitalized COVID-19 patients: A systematic review and meta-analysis. Clin Nutr ESPEN. 2021;43:174–183. doi: 10.1016/j.clnesp.2021.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bagni UV, Ribeiro KD, Bezerra DS, Barros DC, Fittipaldi AL, Araújo RG, et al. Anthropometric assessment in ambulatory nutrition amid the COVID-19 pandemic: Possibilities for the remote and in-person care. Clin Nutr ESPEN. 2021;41:186–192. doi: 10.1016/j.clnesp.2020.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Savino W, Dardenne M. Nutritional imbalances and infections affect the thymus: Consequences on T-cell-mediated immune responses. Proc Nutr Soc. 2010;69:636–643. doi: 10.1017/S0029665110002545. [DOI] [PubMed] [Google Scholar]
- 31.Knight JA. Diseases and disorders associated with excess body weight. Ann Clin Lab Sci. 2011;41:107–121. [PubMed] [Google Scholar]
- 32.Al-Salameh A, Lanoix JP, Bennis Y, Andrejak C, Brochot E, Deschasse G, et al. The association between body mass index class and coronavirus disease 2019 outcomes. Int J Obes (Lond) 2021;45:700–705. doi: 10.1038/s41366-020-00721-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sperrin M, Candlish J, Badrick E, Renehan A, Buchan I. Collider bias is only a partial explanation for the obesity paradox. Epidemiology. 2016;27:525–530. doi: 10.1097/EDE.0000000000000493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Griffith GJ, Morris TT, Tudball MJ, Herbert A, Mancano G, Pike L, et al. Collider bias undermines our understanding of COVID-19 disease risk and severity. Nat Commun. 2020;11:5749. doi: 10.1038/s41467-020-19478-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Global BMI Mortality Collaboration. Di Angelantonio E, Bhupathiraju S, Wormser D, Gao P, Kaptoge S, et al. Body-mass index and all-cause mortality: Individual-participant-data-meta-analysis of 239 prospective studies in four continents. Lancet. 2016;388:776–786. doi: 10.1016/S0140-6736(16)30175-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanchis-Gomar F, Lavie CJ, Mehra MR, Henry BM, Lippi G. Obesity and outcomes in COVID-19: When an epidemic and pandemic collide. Mayo Clin Proc. 2020;95:1445–1453. doi: 10.1016/j.mayocp.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pasquarelli-do-Nascimento G, Braz-de-Melo HA, Faria SS, Santos IO, Kobinger GP, Magalhães KG. Hypercoagulopathy and adipose tissue exacerbated inflammation may explain higher mortality in COVID-19 patients with obesity. Front Endocrinol (Lausanne) 2020;11:530. doi: 10.3389/fendo.2020.00530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ji W, Lee R, Huh K, Kang M, Hwang IC, Radnaabaatar M, et al. Overweight and obesity are risk factors for coronavirus disease 2019: A propensity score-matched case-control study. Endocrinol Metab (Seoul) 2021;36:196–200. doi: 10.3803/EnM.2020.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao X, Li Y, Ge Y, Shi Y, Lv P, Zhang J, et al. Evaluation of nutrition risk and its association with mortality risk in severely and critically ill COVID-19 patients. JPEN J Parenter Enteral Nutr. 2021;45:32–42. doi: 10.1002/jpen.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu G, Zhang S, Mao Z, Wang W, Hu H. Clinical significance of nutritional risk screening for older adult patients with COVID-19. Eur J Clin Nutr. 2020;74:876–883. doi: 10.1038/s41430-020-0659-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morley JE, Kalantar-Zadeh K, Anker SD. COVID-19: A major cause of cachexia and sarcopenia? J Cachexia Sarcopenia Muscle. 2020;11:863–865. doi: 10.1002/jcsm.12589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li L, Spranger L, Soll D, Beer F, Brachs M, Spranger J, et al. Metabolic impact of weight loss induced reduction of adipose ACE-2–potential implication in COVID-19 infections? Metabolism. 2020;113 doi: 10.1016/j.metabol.2020.154401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Romero MM, Fernández-López JA, Esteve M, Alemany M. Different modulation by dietary restriction of adipokine expression in white adipose tissue sites in the rat. Cardiovasc Diabetol. 2009;8:42. doi: 10.1186/1475-2840-8-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ali S, Garcia JM. Sarcopenia, cachexia and aging: Diagnosis, mechanisms and therapeutic options–A mini-review. Gerontology. 2014;60:294–305. doi: 10.1159/000356760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weber TK, Leandro-Mehri VA, Bernasconi I, Oliveira MR. Nutritional therapy in hospital care of in-patients with COVID-19: Evidence, consensus and practice guidelines. Rev Nutr. 2020;33 [Google Scholar]
- 46.Barazzoni R, Bischoff SC, Breda J, Wickramasinghe K, Krznaric Z, Nitzan Z, et al. ESPEN expert statements and practical guidance for nutritional management of individuals with SARS-CoV-2 infection. Clin Nutr. 2020;39:P1631–P1638. doi: 10.1016/j.clnu.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Filippo L, Lorenzo R, D'Amico M, Sofia V, Roveri L, Mele R, et al. COVID-19 is associated with clinically significant weight loss and risk of malnutrition, independent of hospitalisation: A post-hoc analysis of a prospective cohort study. Clin Nutr. 2021;40:2420–2426. doi: 10.1016/j.clnu.2020.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Teixeira IP, Pereira JL, Barbosa JPAS, Mello AV, Onita BM, Fisberg RM, et al. Validity of self-reported body mass and height: relation with sex, age, physical activity, and cardiometabolic risk factors. Rev Bras Epidemiol. 2021;24 doi: 10.1590/1980-549720210043. [DOI] [PubMed] [Google Scholar]
- 49.Moreira AD, Gomes CS, Machado IE, Malta DC, Felisbino-Mendes MS. Cardiovascular health and validation of the self-reported score in Brazil: Analysis of the National Health Survey. Cien Saude Colet. 2020;25:4259–4268. doi: 10.1590/1413-812320202511.31442020. [DOI] [PubMed] [Google Scholar]
- 50.Lima LRM, Freitas RPA, Silva LRD, Medeiros ACQ. Estimation of body mass index from self-reported measures: What is the validity? J Phys Educ. 2018;29:e2907. [Google Scholar]
- 51.Ng SP, Korda R, Clements M, Latz I, Bauman A, Bambrick H, et al. Validity of self-reported height and weight and derived body mass index in middle-aged and elderly individuals in Australia. Aust N Z J Public Health. 2011;35:557–563. doi: 10.1111/j.1753-6405.2011.00742.x. [DOI] [PubMed] [Google Scholar]
- 52.Lipsky LM, Haynie DL, Hill C, Nansel TR, Li K, Liu D, et al. Accuracy of self-reported height, weight, and BMI over time in emerging adults. Am J Prev Med. 2019;56:860–868. doi: 10.1016/j.amepre.2019.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Olfert MD, Barr ML, Charlier CM, Famodu OA, Wenjun Z, Mathews AE, et al. Self-reported vs. measured height, weight and BMI in young adults. Int J Environ Res Public Health. 2018;15:2216. doi: 10.3390/ijerph15102216. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.