Abstract
Among the currently used COVID-19 vaccines, the mRNA-based vaccines drew the interest of the scientists because of its potent and versatile nature in mitigating the disease efficiently through increased translation as well as the robust modulation of the innate and adaptive immune responses within the host. The naked or lipid encapsulated mRNAs are usually optimized in order to formulate the vaccine. One of the interesting advantage of using mRNA vaccines is that such platform can even be used to mitigate other infectious diseases like influenza, zika, and rabies. However, the leading COVID-19 mRNA vaccines, i.e., mRNA-1273 and BNT162b2, have already been noticed to possess around 95% efficacy in provoking both the humoral and cell mediated immunity against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, causing the ongoing COVID-19 pandemic.
Keywords: COVID-19 mRNA vaccines, mRNA 1273 vaccine, mRNA BNT162b2 vaccine, Immunity, Vaccine efficiency, Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)
Introduction
Vaccination has long been known as one of the most operative mass public health interventions to control the transmission of infectious diseases like smallpox, polio, measles, mumps, rubella, and tuberculosis [1]. The traditional live attenuated and inactivated vaccines have been noticed to provide long-lasting immunity against these diseases, i.e., duplication of the immune induction by the natural infections [2]. However, the gradual elimination of the immunoglobulins, avoidance of the vaccine-induced immunity by the infectious agent(s), and mutations within the viral antigenic sites appear as major challenges for vaccine development [2, 3]. Fortunately, vaccine development is now incorporating to meet these challenges. Nevertheless, vaccines are currently under development using various platforms (based on the complete knowledge of the viral genomics, host immunity, bioinformatics for screening the target sites) intended to be applied against a number of infectious viral diseases including Herpes Simplex virus infection, Hanta virus infection, severe acute respiratory distress syndrome (SARS), coronavirus (CoV) infection, the Middle East respiratory distress syndrome coronavirus (MERS-CoV) infection, Ebola virus disease (EVD), Zika virus (ZIKV) infection, and against the SARS-CoV-2 and its variants causing the current COVID-19 pandemic which resulted in 6,099,380 deaths out of 472,816,657 infected cases [1–4].
Among the other vaccine development platforms, the mRNA-based technology has got attention upon the increase of the stability of mRNA through high-tech molecular biological advancement with appropriate delivery system maintaining the potency (i.e., inducing the long-lasting immunogenicity, i.e., induction of both humoral and cytotoxic T-cell responses within host) and requires safety of the mRNA vaccine [1]. Indeed, the among the other nucleic acid-based vaccines including plasmid DNA and viral vectors based vaccines, mRNA vaccines (with low-cost manufacture and safe administration capability) have been proven not to generate infectious particles neither to integrate to the genomic DNA of the host cell; rather, the complex antigens are expressed without packaging constraints in situ at the elevated translational level, and the nuclear membrane remains protected [1, 5]. Like the mRNA vaccines, plasmid DNA and viral vector-based vaccines are also recognized by the pattern recognition receptors (PRRs) of the innate immunity, which afterward facilitate the dendritic cells (DCs) to induce the humoral immunity by T cell-B cell interaction to instigate the plasma cells to produces antibodies [1, 6]. Present review discussed the advantages of using the COVID-19 mRNA vaccines along with their mode of actions in the perspective of the sustainability of the host immunity.
SARS-CoV Infection, mRNA Vaccination and the Significance of the mRNA-1273 Vaccines
According to SARS-CoV-2 structural analysis by the group of Huang (2020), the spike (S) glycoprotein with a transmembrane anchor and an intact S1-S2 cleavage site has been noticed to be coated with the polysaccharide molecules, facilitating the viruses to avoid the host protective immunity during entry [1, 7–9]. However, the S protein, a class I fusion glycoprotein, binds to the the host angiotensin converting enzyme 2 (ACE-2) and gets into the host through membrane fusion with the help of the trans-membrane serine protease 2 (TMPRSS2), followed by the release of viral RNA [7]. It is known that the S protein (consisting of the neutralization-sensitive epitopes) is the primary target for the neutralizing antibodies elicited by the administration of COVID-19 vaccines [1, 3, 5–9]. Corbett et al. (2020) identified two proline substitutions (2P) within the prefusion-stabilized S protein, and thus, the S-2P appeared to be more immunogenic (i.e., good target for the neutralizing antibodies) than the wild-type S protein [1, 8]. This finding is significant to develop the vaccine as the S-2P became the target for the antibodies [8].
As stated above, Pfizer/BioNTech successfully developed BNT162b2 mRNA vaccine, and Moderna developed the mRNA-1273 mRNA vaccine to mitigate COVID-19. The work by Greaney et al. (2021) showed that the neutralizing antibodies elicited by the mRNA 1273-vaccine were more targeted to the receptor binding domain (RBD) of the SARS-CoV-2 S protein compared to that of the natural infection, unraveling the fact that the vaccine elicited antibodies could work against a broader range of antigenic epitopes from the emerging SARS-CoV-2 variants [8, 9]. The research-based development of a vaccine starts with the detection of the long-lasting immunity against the viral infection and determines whether the vaccine dosage is safe and immunogenic (through the cell culture/animal study) followed by the clinical trial authorization, i.e., phase I clinical trial involving 20–100 healthy volunteers [10]. Phase II includes the assessment of vaccine safety and immunogenicity, optimization of the vaccine delivery, and ensures the dose response and the vaccine administration schedule, involving separate groups of hundreds of volunteers [10]. Testing for vaccine efficacy is conducted involving thousands of target population with the aim of getting the biological license (i.e., phase III), and finally, the post marketing surveillance is done during phase IV [10]. As stated above, vaccines are expected to trigger the cellular immunity (i.e., elicitation of the antigen specific T cells instigating T cell cytotoxicity against the intracellular pathogen and also inducing the production of IgG, i.e., the generation of humoral immunity) [11]. After commencement of COVID-19 pandemic in the last of December, 2019, until date, several commercial COVID-19 vaccines have been marketed manufactured in different platforms [6, 10]. Of them, ChAdOx1 nCoV19/AZD1222 (Oxford/AstraZeneca) vaccine, BNT162b2 vaccine (Pfizer-BioNTech) and mRNA-1273 vaccine (Moderna), Gam-COVID-Vac-Lyo/Sputnik (Gamaleya) vaccine, BB152/ Covaxin (Bharat Biotech), and CoronaVac (SinoVac) vaccines are in great commercial use [6–12]. The running second wave of the COVID-19 and the SARS-CoV-2 variants have put the mass public health in a vulnerable situation [11–17]. The efficacy of the currently used vaccines is being analyzed through numerous studies [3, 6, 11, 12]. However, almost all the vaccines mentioned above have been found to impart the required efficiency to immunize the individuals [11, 12].
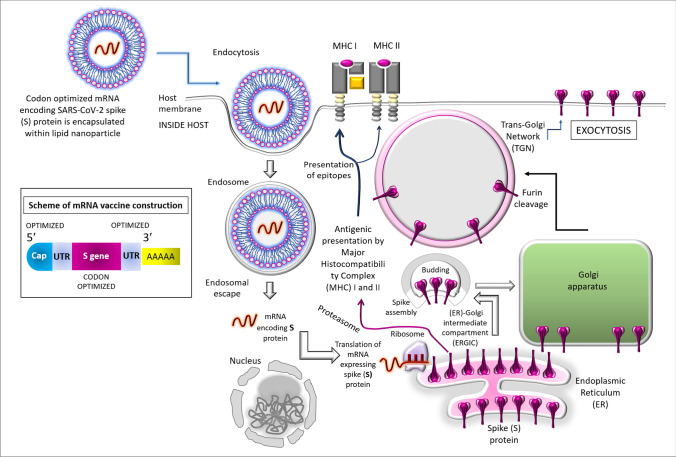
Figure 1 shows how the COVID-19 mRNA vaccine works. The lipid nanoparticles (LNPs) carry the specific SARS-CoV-2 RNA sequence; the mRNA is released into the cytosol where it gets associated with ribosome and translated into the spike (S) proteins which are presented on the surface of the antigen presenting cells (APCs) [1, 6]. T-cells are able to recognize these, and then the B-cells (triggered by the T-helper or Th cells) generate neutralizing antibodies against S proteins [1]. After infection antibodies attached to coronavirus spikes and destroy the virus before it gets into the cell. APC also activates the cytotoxic T cells (Tc) to kill the virally infected cells [1, 6]. The recommended interval between the first and second doses is approximately 3 weeks [1]. Although the active immunity decrease after several months, the memory B- and T-cells are expected to retain the information for a substantial numbers of years (which is actually a concern of further observation) to fight against the SARS-CoV-2 re-infection.
Fig. 1.
Mode of action of vaccines based on RNA platform. In the inset, a scheme mRNA vaccine has been shown where the optimization of relevant codons (especially of the genes encoding spike proteins) is notable. UTR stands for the untranslated regions. The modification of the uridine is done, i.e., converted to N1-methyl-pseudouridine. The main diagram shows how mRNA vaccine facilitates the expression of the spike (S) protein. The mRNA which encodes the full-length S protein is encapsulated into a lipid nanoparticle (LNP) [3, 6, 12]. The encapsulated and in vitro synthesized mRNA enters the host through endocytosis, escapes the endosomal degradation, and translated to produce the S proteins. The nascent S proteins are transported into the lumen of the endoplasmic reticulum (ER) as in case of natural infection [12]. Later the exocytosis takes place, leading to expression of the spike proteins across the plasma membrane. This is to be noted that the intracellularly synthesized protein may undergo degradation within proteasome followed by the entry of the epitopes within the grooves of major histocompatibility complex (MHC) I and II thereby facilitating the antigenic peptide presentation by MHC I and MHC II which in turn, triggers the induction of cellular and humoral immunity, respectively [3, 6, 12]
What Advantages Can Be Achieved from the COVID-19 mRNA Vaccines?
The conventional methods of vaccine development especially using the platforms of heat killed viruses or inactivated viruses are bit longer and expensive process [1]. Pfizer (BioNTech) and Moderna first developed the mRNA vaccine candidates BNT162b2 and mRNA-1273, respectively, and the first clinical trial began on March 2020 which showed up to 95% efficiency [1, 6]. Vaccines developed on the nucleic acid platform (1) induces strong CD8+ T cell response with the concomitant presentation of MHC class-I molecules, resulting in the production of neutralizing antibodies, and (2) the innate immune sensing mechanism is also engaged in the vaccine action by provoking both the innate and adaptive arms of the immune system [1, 6]. The preparation scheme of the COVID-19 mRNA vaccines is rapid and cost-effective which goes a long way to combat the COVID-19 severity in global mass population [1, 15]. During the recent years, the majority of mRNA vaccines have been focused on cancer research as well as an array of infectious agents especially the influenza virus, Ebola virus (EV), Zika virus (notably, the 2013-2016 outbreaks of EV and ZIKV), Streptococcus spp., and Toxoplasma gondii [1, 16, 17]. The capacity to modulate the innate immune sensing mechanism by mRNA vaccines also aids the passive immunization [1, 18].
A number of detailed reports on the mRNA vaccines have been published so far [1, 5, 6]. In principle, mRNA, transcribed (using T7, T3 or Sp6 phage RNA polymerase) from the DNA template consisting of the open reading frame (ORF) encoding the gene of interest, which (consisting of flanking untranslated regions or UTRs, a 5΄ cap (altered nucleotide on the 5΄ end that is known as 7-methylguanylate cap or m7G and 3’poly A tail) is afterward translated for the formation of the protein of interest [1]. Both the non-replicating mRNA and the virally derived self-amplifying and optimized RNAs (which encode both the antigen and the viral replication machinery to facilitate the intracellular RNA amplification for the production of the desired protein) are currently being studied for the vaccine development. Optimization of mRNA has several advantages including the following: (1) mRNA uptake and protection from degradation; (2) facilitating the delivery of mRNA to the target cellular compartments [1].
Advantages Derived from the Structural Stability of the Optimized mRNA
The COVID-19 mRNA vaccines have been proven to possess the safety profile in animals and in human clinical trials [1, 6]. The mRNA-1273 (Moderna Tx, Inc) vaccine consists of nucleoside-modified messenger RNA (modRNA) which encodes the SARS-CoV-2 spike glycoprotein (S), the encapsulating lipid nanoparticles (LNPs, which can also act as the adjuvants to increase the vaccine immunogenecity) complexed with polyethylene glycol (PEG), phosphocholine, and cholesterol, salts (potassium chloride, sodium chloride, buffer (monobasic potassium phosphate plus dibasic sodium phosphate dehydrate), and sucrose (cryoprotectant so that the lipid portion doesn’t become sticky at the storage temperature of −70 °C [6, 18, 19]. Indeed, a DNA fragment consisting of the immunogen open reading frame (ORF) is used as template which is transcribed by the T7 RNA polymerase to generate the mRNA encoding SARS-CoV-2 S (2P) protein [6, 19]. After the enzymatic addition of the Cap structure, mRNA is purified, and encapsulated into the LNPs by means of modified ethanol-drop nanoprecipitation process [6]. PEGs are then mixed with the mRNA (pH at 5.0, acetate buffer), neutralized, and sucrose is added [6]. The final solution is filter sterilized, and the vials filled with the formulated LNP are stored at −70 °C stored at [6, 19].
Indeed, upon administration of the mRNA vaccines, the mRNAs complexed with LNP carriers highly influence their delivery/distribution to the organs within the host [1]. The ex vivo loading of DCs allows the precise control of cellular targets and the mRNA transfection efficiency, facilitating the cell-mediated immune response [1]. mRNA vaccines strongly induce the CD8+ T cell responses with effective presentation of MHC class-I molecules and are fully capable of generating potent neutralizing antibodies response [1, 6]. LNPs may influence the efficiency of expression through the antigen presenting cells (APCs) consisting of the pathogen-associated molecular pattern (PAMPs) [1, 6]. Afterwards, the vaccine triggers the follicular helper T cells for the B cell responses to produce the neutralizing antibodies through the plasma cells [6].
Advantages Based on Immunogenicity Without Side Effects
Self-adjuvanted (LNP) mRNA-based vaccines have been reported to induce balanced immune responses comprising both humoral and cellular effector as well as memory responses [1, 20, 21]. Such self-adjuvanted feature of RNA vaccines may also act the RNA sensing machinery, which in turn mediates protection against the viral infections [1]. The innate immune sensing of mRNA is mediated by (1) the toll-like receptors (TLRs)-3, TLR-7/8, the retinoic acid-inducible gene-I (RIG-I) like receptors, and the melanoma differentiation protein 5 (MDA-5) receptors, which is linked to produce the vaccine response because of its capability to induce the adaptive immunity as well, and is recognized by various cell surfaces, endosomal, and cytosolic innate immune receptors [1]. Indeed, upon administration, the mRNA vaccine is grasped by both non-leukocytic cells and the leukocytic cells mostly the APCs, and the mRNA is then transported to the draining lymph nodes (dLNs) through the migratory DCs with the concomitant expression of the spike (S) protein which is capably presented by APCs, triggering T cell proliferation followed by the stimulation of B cells [21, 22].
APCs, which are recruited to the site of vaccine administration via the innate immune signaling process mediated cytokines, recognize the vaccine antigen which has been processed internally in the cytosol, and the resulting antigenic peptides are presented to other T-cells through the MHC-II receptors [1, 6]. APCs are involved in upregulating the costimulatory molecules bearing CD80 and CD86, expressed in dendritic cells, monocytes and macrophages [1, 6]. In addition, the functions of DCs are also modulated through mRNAs encoding the pro-inflammatory cytokines such as IL-2/or trafficking-associated molecules [1]. Importantly, the induction of both the cell-mediated immunity and the humoral immunity upon the first dose administration (priming dose) and the second dose (booster dose) administration usually does not show any sign of fatigue as detected by the controlled levels of cytokines at the site of injection within dLNs which subside the adverse side effects of vaccination [22].
Efficacy of mRNA Vaccines Against the SARS-CoV-2 Variants
The genomic variations/ mutations are being noticed within the SARS-CoV-2 strains; and already several variants of concern (VOCs) have been identified including (1) the UK variant comprising B.1.1.7 lineage, (2) the South African variant 501Y.V2 of the B.1.351, the B.1.1.248/B1.1.28/P1 (501Y.V3) variant of Brazil, (3) the Indian variant VUI-202012/01 of B.1.1.7 lineage, and (4) the variant in California namely B.1.427/B.1.429 lineage [23]. This is worth to mention that the mutations may change the interaction of the RBD within the S protein that connects the hACE2 receptor facilitating the seepage of the vaccine action [24]. Indeed, the continuous mutations within the S protein is of great concern regarding the vaccine efficiency since the variable epitopes may appear which can make the existing vaccines inappropriate against the viral infection due to the amino acid changes within the ACE2-RBD site [11, 24]. As mentioned earlier, through to the phase III trials, among the mRNA-based platform, BNT162b2 (Biontech/Pfizer) and mRNA-1273 (Moderna) with 95% and 94.1% efficiency, respectively; among Adenovector vaccine platform, Sputnik V (Gamaleya), ChAdOx1-S/AZD1222 (Oxford/AstraZeneca), and Ad26.COV2.S (Janssen) with 91.6%, 81.3%, and 66.9% efficiency, respectively; among the inactivated whole-virus vaccine platform, CoronaVac/PiCoVacc (Sinovac) and BBIBP-CorV (Sinopharm) vaccines with 86% and 78%, respectively, are currently in use worldwide [3, 6, 11, 12, 25, 26]. The Biontech/Pfizer mRNA vaccine and the Moderna mRNA vaccines were found to neutralize the UK mutants while the effectiveness of the Oxford/Astra Zeneca vaccine was much lower than those RNA vaccines [12, 27].
The mRNA vaccines were noticed (1) to reduce the risk for infection irrespective of COVID-19-associated symptoms, (2) to decrease the risk for the transmissible infections taking place between the individuals with both asymptomatic/symptomatic infection, and thus, the vaccines have been found to be very effective for the health care personnel, the first responders, and other front liners who come into close contact with the COVID-19 patients [28]. Indeed, the interventions on the effectiveness findings for both Pfizer-BioNTech’s and Moderna’s mRNA vaccines through a number of observer-blinded, placebo-controlled clinical trials are also showing substantial preventive output in the real-world conditions among working-age adults [28, 29]. However, a disadvantage of using mRNA vaccines may appear through their dependency of ultracold storage requirement, for example, BioNTech/Pfizer COVID-19 vaccine requiring storage at −80 °C and Moderna COVID-19 vaccine requiring storage at −20 °C [30–32]. Another important factor is the instability of mRNA which may be fixed by optimizing the storage temperature and pH, and the incorporation of appropriate excipients, i.e., by encapsulating mRNA in lipid nanoparticles (LNP) during manufacturing in an RNase-free environment [30–33]. Encapsulation of mRNA by LNP imparts protection against degradation by RNases, endonucleases, and 5′ exonucleases [30, 34]. Transportation of mRNA vaccines maintaining their shelf life (which is actually short, i.e., 6 months) is also difficult since special packaging with dry ice is needed which is expensive; and may slow down the distribution of the mRNA vaccines especially in the resource poor settings, rendering the possibility of vaccine wastage [30].
Other Vaccines with Nearly Similar Advantages with That of mRNA Vaccines
This is to be noted that the Janssen Ad26.COV2.S recombinant vaccine consists of stabilizing on underlie on the production of neutralizing antibodies and the induction of the cell-mediated immunity with nearly 70% effectiveness, and interestingly, this vaccine showed higher efficacy against the severely critical COVID-19 cases [12, 15, 34]. The subunit vaccine NVX-CoV2373 (Novavax) is another good candidate vaccine regarding its effectiveness imparted from the high affinity to hACE2 receptor, and sprecially due to its convenient storage temperature 4 °C [12, 35]. The composition and manufacturing scheme have been well explained in the previous reports. Briefly, the antigenic part of such vaccine is mainly a recombinant full-length S protein with stabilizing mutations generated in Sf9 insect cells. Extraction of S protein is done by means of detergent solubilization and chromatographic methods. In mice model, this vaccine with the Matrix-M adjuvant has been noticed to induce high titer of neutralizing antibodies together with the elicitation of cell mediated immunity, i.e., CD4+ T cells, CD8+ T cells, CD4+ follicular helper T cells, and antigen-specific germinal center (GC) B cells in the spleen [12, 35]. In phase III clinical trials, 89.1% effectiveness was noticed in the UK while it was found in a reduced measure in South Africa (60.1%) [36].
Vaccine Effectiveness Against the Variants and the Requirements of Booster Dose
Currently, the COVID-19 vaccine booster doses are strongly recommended and are being administered all around the world since the variants of concern (VOCs) may tolerate or escape the counteracting action of the host immune system induced by the first and second doses of the currently used vaccines [15]. Moderna or Pfizer vaccines have been noticed with reduced activity in case of the Alpha variant (B.1.1.7 lineage), and such effectiveness was even lower against the Beta variant of B.1.351 lineage and the Delta variant of B.1.617.2 lineage [15, 27, 37, 38]. The effectiveness of vaccines was deduced to be around 70% against the omicron variant (of B.1.1.529 lineage) [38]. Therefore, such a reduction in vaccine effectiveness was suggested to be complimented by applying the booster doses which are expected to increase the durability of the neutralized antibodies [39].
The induction of host immunity upon vaccination has been discussed in several reports previously [5, 6, 12, 14, 15]. The humoral memory response, i.e., the IgM and IgG titers against RBD of the S protein after the natural SARS-CoV-2 infection may eventually decrease over time [40]. On the contrary, the RBD-specific memory B cells (eliciting the neutralizing antibodies) may last for long time after vaccination along with boosters which impart the induction of B-cells and T-cell actions not only against the original Wuhan strains but also against the SARS-CoV-2 variants [24, 40–43]. The booster vaccination indeed showed the neutralization of the Omicron variants in all affected patients revealing that Omicron variants, which tend to escape the vaccine induced immunity, can be fully counterbalanced by the booster dose [43]. Finally, so far, 173 candidate vaccines are on preclinical development, 63 on clinical trials, and 7 mRNA candidate vaccines have completed the preclinical development/or now in clinical trials of which two licensed mRNA vaccines, i.e., mRNA-1273 (Moderna) and BNT162b (Pfizer/BioNTech) have completed phase 3 trials [44]. The development strategies, dose optimization, booster formulations, and the side effects have been well analyzed.
Conclusion
COVID-19 vaccines from different platforms are certainly suitable to combat the running pandemic since they are in use in the most parts of the world. Among them, the mRNA vaccines have been noticed with relatively high efficiency (especially with the mRNA-1273 vaccine), and these vaccines require relatively shorter time to manufacture. The intrinsic adjuvant, i.e., the LNPs goes a long way to enhance the immunogenicity of the vaccine. Compared to the heat killed vaccine or the live attenuated vaccines, the mRNA vaccines pose less side effects. The mode of action is based on the in vitro transcription of the target gene followed by the concomitant translation which may facilitate the accurate delivery of the vaccine. Most importantly, the mRNA vaccines may work against the most of the emerging SARS-CoV-2 variants. However, surveillance of the vaccine usage and market complaints continuous examination of the genomic sequences of the SARS-CoV-2 according to geographical locations, and more development in the application of nanoparticles may be required to mitigate the existent gaps (even that is very low) in efficiency of the mRNA-1273 COVID-19 vaccine.
Author Contribution
Conceptualization: RN; literature survey: RN; formal analysis: RN; investigation: RN; resources: RN; writing—original draft preparation: RN; editing: RN; visualization: RN; supervision: RN.
Data Availability
Not applicable.
Code Availability
Not applicable.
Declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Author has read and approved the final manuscript for publication.
Conflict of Interest
The author declare no competing interests.
Footnotes
This article is part of the Topical Collection on COVID-19
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kaushik A. mRNA technology a promising strategy for rapid response vaccine applications against the emerging infectious diseases. Act Sic Microbial. 2021;4(3):199–212. doi: 10.31080/ASMI.2021.04.0797. [DOI] [Google Scholar]
- 2.Rodrigues CMC, Pinto MV, Sadarangani M, Plotkin SA. Whither vaccines? J Infect. 2017;74(Suppl 1):S2–S9. doi: 10.1016/S0163-4453(17)30184-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Noor R. A review on the effectivity of the current COVID-19 drugs and vaccines: are they really working against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants? Curr Clin Micro Rpt. 2021. 10.1007/s40588-021-00172-w [DOI] [PMC free article] [PubMed]
- 4.WHO (World Health Organization) Coronavirus diseases (COVID-19) Dashboard. Updated on 5:51pm CET, 23 March 2022. https://covid19.who.int/ Accessed on 24 Mar 2022.
- 5.Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines - a new era in vaccinology. Nat Rev Drug Discov. 2018;17(4):261–279. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noor R. Developmental status of the potential vaccines for the mitigation of the COVID-19 pandemic and a focus on the effectiveness of the Pfizer-BioNTech and Moderna mRNA vaccines. Curr Clin Microbiol Rep. 2021:1-8. 10.1007/s40588-021-00162-y [DOI] [PMC free article] [PubMed]
- 7.Huang Y, Yang C, Xu Xin-feng, Xu Wei, Liu Shu-wen. Structural and functional properties of SARS-CoV-2 spike protein: potential antivirus drug development for COVID-19. Acta Pharmacol Sin. 2020;41:1141–1149. doi: 10.1038/s41401-020-0485-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corbett KS Edwards D Leist SR Abiona OM Boyoglu-Barnum S Gillespie RA et al. SARS-CoV-2 mRNA vaccine development enabled by prototype pathogen preparedness. bioRxiv [Preprint]. 2020;2020.06.11.145920. 10.1101/2020.06.11.145920 [DOI] [PMC free article] [PubMed]
- 9.Greaney AJ, Loes AN, Gentles LE, Crawford KHD, Starr TN, Malone KD, et al. Antibodies elicited by mRNA-1273 vaccination bind more broadly to the receptor binding domain than do those from SARS-CoV-2 infection. Sci Transl Med. 2021;13(600):eabi9915. doi: 10.1126/scitranslmed.abi9915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khuroo MS, Khuroo M, Khuroo MS, Sofi AA, Khuroo NS. COVID-19 vaccines: a race against time in the middle of death and devastation! J Clin Exp Hepatol. 2020;10(6):610–621. doi: 10.1016/j.jceh.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ura T, Yamashita A, Mizuki N, Okuda K, Shimada M. New vaccine production platforms used in developing SARS-CoV-2 vaccine candidates. Vaccine. 2021;39(2):197–201. doi: 10.1016/j.vaccine.2020.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heinz FX, Stiasny K. Profiles of current COVID-19 vaccines. Wien Klin Wochenschr. 2021;133(7–8):271–283. doi: 10.1007/s00508-021-01835-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Korber B. Tracking changes in SARS-CoV-2 spike: evidence that D614G increases infectivity of the COVID-19 virus. Cell. 2020;182(4):812–827. doi: 10.1016/j.cell.2020.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diaz RS, Vergara TRC. The COVID-19 second wave: a perspective to be explored. Braz J Infect Dis. 2021;25(1):101537. doi: 10.1016/j.bjid.2020.101537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gómez CE, Perdiguero B, Esteban M. Emerging SARS-CoV-2 variants and impact in global vaccination programs against SARS-CoV-2/COVID-19. Vaccines Basel. 2021;9(3):243. doi: 10.3390/vaccines9030243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noor R. A comparative review of pathogenesis and host innate immunity evasion strategies among the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), severe acute respiratory syndrome coronavirus (SARS-CoV) and the Middle East respiratory syndrome coronavirus (MERS-CoV) Arch Microbiol. 2021;7:1–9. doi: 10.1007/s00203-021-02265-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu K Werner AP Moliva JI Koch M Choi A Stewart-Jones GBE et al. mRNA-1273 vaccine induces neutralizing antibodies against spike mutants from global SARS-CoV-2 variants. bioRxiv. 2021;2021.01.25.427948. 10.1101/2021.01.25.427948
- 18.Maruggi G, Zhang C, Li J, Ulmer JB, Yu D. mRNA as a transformative technology for vaccine development to control infectious diseases. Mol Ther. 2019;27(4):757–772. doi: 10.1016/j.ymthe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen N, Xia P, Li S, Zhang T, Wang TT, Zhu J. RNA sensors of the innate immune system and their detection of pathogens. IUBMB Life. 2017;69(5):297–304. doi: 10.1002/iub.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corbett KS, Edwards D, Leist SR, Abiona OM, Boyoglu-Barnum S, Gillespie RA, et al. SARS-CoV-2 mRNA vaccine development enabled by prototype pathogen preparedness. Nature. 2020;586:567–71. doi: 10.1038/s41586-020-2622-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liang Z, Zhu H, Wang X, Jing B, Li Z, Xia X, et al. Adjuvants for coronavirus vaccines. Front Immunol. 2020;11:589833. doi: 10.3389/fimmu.2020.589833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kowalczyk A, Doener F, Zanzinger K, Noth J, Baumhof P, Fotin-Mleczek M, Heidenreich R. Self-adjuvanted mRNA vaccines induce local innate immune responses that lead to a potent and boostable adaptive immunity. Vaccine. 2016;34(33):3882–93. doi: 10.1016/j.vaccine.2016.05.046. [DOI] [PubMed] [Google Scholar]
- 23.Yadav PD Gupta N Nyayanit DA Sahay RR Shete AM Majumdar T Patil S Kaur H Nikam C Pethani J Patil DY Aggarwal N Vijay N Narayan J. Imported SARS-CoV-2 V501Y.V2 variant (B.1.351) detected in travelers from South Africa and Tanzania to India. Travel Med Infect Dis. 2021:102023. 10.1016/j.tmaid [DOI] [PMC free article] [PubMed]
- 24.Noor R. A review on the induction of host immunity by the current COVID-19 vaccines and a brief non-pharmaceutical intervention to mitigate the pandemic. Bull Natl Res Cent. 2022;46:31. doi: 10.1186/s42269-022-00719-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kyriakidis NC, López-Cortés A, González EV, Grimaldos AB, Prado EO. SARS-CoV-2 vaccines strategies: a comprehensive review of phase 3 candidates. NPJ Vaccines. 2021;6(1):28. doi: 10.1038/s41541-021-00292-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pollet J, Chen WH, Strych U. Recombinant protein vaccines, a proven approach against coronavirus pandemics. Adv Drug Deliv Rev. 2021;170:71–82. doi: 10.1016/j.addr.2021.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muik A Wallisch AK Sänger B Swanson KA Mühl J Chen W et al. Neutralization of SARS-CoV-2 lineage B.1.1.7 pseudovirus by BNT162b2 vaccine-elicited human sera. Science. 2021. 10.1126/science.abg6105 [DOI] [PMC free article] [PubMed]
- 28.Thompson MG, Burgess JL, Naleway AL, Tyner HL, Yoon SK, Meece J, Olsho LEW, et al. Interim estimates of vaccine effectiveness of BNT162b2 and mRNA-1273 COVID-19 vaccines in preventing SARS-CoV-2 infection among health care personnel, first responders, and other essential and frontline workers - eight U.S. locations, December 2020-March 2021. Mmwr Morb Mortal Wkly Rep. 2021;70(13):495–500. doi: 10.15585/mmwr.mm7013e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amit S, Regev-Yochay G, Afek A, Kreiss Y, Leshem E. Early rate reductions of SARS-CoV-2 infection and COVID-19 in BNT162b2 vaccine recipients. Lancet. 2021;397(10277):875–877. doi: 10.1016/S0140-6736(21)00448-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uddin MN, Roni MA. Challenges of storage and stability of mRNA-based COVID-19 vaccines. Vaccines Basel. 2021;9(9):1033. doi: 10.3390/vaccines9091033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crommelin DJA, Anchordoquy TJ, Volkin DB, Jiskoot W, Mastrobattista E. Addressing the cold reality of mRNA vaccine stability. J Pharm Sci. 2021;110(3):997–1001. doi: 10.1016/j.xphs.2020.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schoenmaker L, Witzigmann D, Kulkarni JA, Verbeke R, Kersten G, Jiskoot W, Crommelin DJA. mRNA-lipid nanoparticle COVID-19 vaccines: structure and stability. Int J Pharm. 2021;601:120586. doi: 10.1016/j.ijpharm.2021.120586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pascolo S. Synthetic messenger RNA-based vaccines: from scorn to hype. Viruses. 2021;13(2):270. doi: 10.3390/v13020270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sadoff J, Gray G, Vandebosch A, Cárdenas V, Shukarev G, Grinsztejn B, et al. Safety and efficacy of single-dose Ad26 COV2 S vaccine against COVID-19. N Engl J Med. 2021;384(23):2187–2201. doi: 10.1056/NEJMoa2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tian JH, Patel N, Hut R, Zhou H, Weston S, Hammond H, et al. SARS-CoV-2 spike glycoprotein vaccine candidate NVX-CoV2373 immunogenicity in baboons and protection in mice. Nat Common. 2021;12(1):372. doi: 10.1038/s41467-020-20653-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wadman M Cohen J. Novavax vaccine delivers 89 % efficacy against COVID-19 in U.K.—but is less potent in South Africa. Science. 2021. 10.1126/science.abg8101
- 37.Mascellino MT, Di Timoteo F, De Angelis M, Oliva A. Overview of the main anti-SARS-CoV-2 vaccines: mechanism of action, efficacy and safety. Infect Drug Resist. 2021;14:3459–3476. doi: 10.2147/IDR.S315727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Collie S Champion J Moultrie H Bekker LG Gray G. Effectiveness of BNT162b2 vaccine against Omicron variant in South Africa. N Engl J Med. 2021.10.1056/NEJMc2119270 [DOI] [PMC free article] [PubMed]
- 39.Barda N, Dagan N, Cohen C, Hernán MA, Lipsitch M, Kohane IS, Reis BY, Balicer RD. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study. Lancet. 2021;398(10316):2093–2100. doi: 10.1016/S0140-6736(21)02249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gaebler C, Wang Z, Lorenzi JCC, et al. Evolution of antibody immunity to SARS-CoV-2. Nature. 2021;591:639–644. doi: 10.1038/s41586-021-03207-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rubin R. COVID-19 vaccine makers plan for annual boosters, but it’s not clear they’ll be needed. Jama. 2021;326(22):2247–2249. doi: 10.1001/jama.2021.21291. [DOI] [PubMed] [Google Scholar]
- 42.The Lancet Infectious Diseases Emerging SARS-CoV-2 variants: shooting the messenger. Lancet Infect Dis. 2022;22(1):1. doi: 10.1016/S1473-3099(21)00770-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ai J, Zhang H, Zhang Y, Lin K, Zhang Y, Wu J, et al. Omicron variant showed lower neutralizing sensitivity than other SARS-CoV-2 variants to immune sera elicited by vaccines after boost. Emerg Microbes Infect. 2022;11(1):337–343. doi: 10.1080/22221751.2021.2022440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park JW, Lagniton PNP, Liu Y, Xu RH. mRNA vaccines for COVID-19: what, why and how. Int J Biol Sci. 2021;17(6):1446–1460. doi: 10.7150/ijbs.59233. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.