Abstract
Racial health inequities exemplified during the COVID-19 crisis have awakened a sense of urgency among public health and policy experts to examine contributing factors. One potential factor includes the socioeconomic disadvantage of racially segregated neighborhoods. This study quantified associations of neighborhood socioeconomic disadvantage in Chicago, Illinois, as measured by the Area Deprivation Index (ADI), with racial disparities in COVID-19 positivity. A retrospective cohort included 16,684 patients tested for COVID-19 at an academic medical center and five community-based testing sites during Chicago’s “first wave” (March 12, 2020–June 25, 2020). Patients living in Black majority neighborhoods had two times higher odds of COVID-19 positivity relative to those in White majority neighborhoods. The ADI accounted for 20 percent of the racial disparity; however, COVID-19 positivity remained substantially higher at every decile of the ADI in Black relative to White neighborhoods. The remaining disparities (80 percent) suggest a large, cumulative effect of other structural disadvantages in urban communities of color.
The COVID-19 pandemic has revealed a sobering portrait of racial health disparities in the United States. According to the Centers for Disease Control and Prevention, the percentage of positive cases for Black Americans during the first six months of the pandemic was 14 percent—which is two times that of White Americans1—even though there was lower geographic access to testing centers in Black communities.2 Numerous public health and policy experts, as well as professional organizations,3–5 have called for greater attention to the systemic inequalities resulting from structural racism that have contributed to disparities in COVID-19. In particular, the effect of residential segregation by race, combined with compounding economic deprivation over many generations, has been especially damaging to the health of poor and racial minority communities.6,7
Neighborhood disadvantage—the geographic absence or paucity of social and economic resources—directly affects health. Substantial research has documented the health impacts of urban food deserts,8 neighborhood blight (such as broken sidewalks and vacant lots),9 violent crime,10,11 and housing instability,12 as well as limited access to community health resources13,14 such as indoor gyms and pharmacies. Debate remains, however, over the extent to which neighborhood disadvantage accounts for the health disparities experienced by racial minority groups. For example, the Moving to Opportunity study randomized provision of low-poverty housing vouchers to experimentally test the impact of neighborhood conditions on health.15 Its mixed and often modest findings led to decades long debate,16 including discussion of its limitations in disentangling neighborhood disadvantage from other structural disadvantages.
Few studies17 to date have specifically examined associations of neighborhood socioeconomic disadvantage with racial disparities in COVID-19 positivity and outcomes. Gregorio Millett and colleagues found that although only approximately 20 percent of US counties were disproportionately Black, these counties accounted for more than half of the diagnoses and deaths attributed to COVID-19 during the first wave of the pandemic.18 Another study examined the Area Deprivation Index (ADI) at the county level and found no association between the ADI and COVID-19 risk in urban regions, but county-level data may lack sufficient sensitivity to detect meaningful differences in urban contexts.19 For example, the entire city of Chicago, Illinois, belongs to a single county, and analysis at the county level would fail to reflect the fifteen-year life expectancy gap between its most and least disadvantaged neighborhoods.20
One Louisiana study used the ADI at the census tract level and found that people in the highest quintile of neighborhood disadvantage had almost a 40 percent higher risk for COVID-19 compared with those in the lowest quintile of disadvantage,21 but investigators were unable to examine race in analyses. No studies to our knowledge have examined neighborhood disadvantage within a clinical cohort, implementing assessment with a cohesive socioeconomic indicator matched to each patient’s unique residential address at the census block group level.
Similar to prior studies,19,21 we used the ADI, developed by Amy Kind and colleagues,22 to quantify neighborhood disadvantage and specifically test its association with racial disparities in COVID-19 positivity. The ADI is an ideal measure in analyses of neighborhood effects because it is validated to the census block group level,22–24 the closest geographic approximation of a “neighborhood” unit. It also captures socioeconomic disadvantage excluding race in its derivation, which enables explicit testing for associations and interactions between the ADI and neighborhood racial composition. We hypothesized that in explicit analyses of the ADI and neighborhood racial composition, the ADI would account for a significant proportion of the racial disparity in COVID-19 positivity. As a secondary objective, we also tested for associations of the ADI and neighborhood racial composition with COVID-19 intensive care unit (ICU) admission and death.
Study Data And Methods
DATA
We conducted a retrospective cohort study of patients who were tested for COVID-19 at an urban academic medical center or one of its five community-based testing sites in Chicago, Illinois, between March 12, 2020, and June 25, 2020. This is the only academic medical center serving the South and Southeast Sides of Chicago.25 The majority of residents in this region (68.4 percent) identify as non-Hispanic Black, with fewer identifying as non-Hispanic White (13.1 percent) or Hispanic/Latino (11.7 percent).25,26 Universal testing was conducted for all patients who were seen in the medical center’s emergency department or who were directly admitted to the hospital, regardless of symptoms. The medical center also launched a community based testing initiative with the explicit purpose of reaching disadvantaged communities and mitigating racial disparities. Many testing sites were either drive-through or walk-in, located in trusted community spaces, and testing was conducted in collaboration with community-based organizations. Testing sites operated for extended hours during the first six months of the pandemic. The study period was selected to approximate the first wave of the COVID-19 pandemic in Chicago, when racial disparities were most profound.27 Each patient’s residential address was geocoded to the census block group level and paired with the ADI, as well as contemporaneous race and ethnicity data from the Census Bureau’s 2018 American Community Survey five-year estimates. This study was approved with a waiver of informed consent by the University of Chicago Institutional Review Board.
MAIN MEASURES
The primary dependent variable of interest was COVID-19 positivity, which was extracted from the academic medical center’s electronic health record and defined as having a polymerase chain reaction (PCR)–positive clinical sample (Roche cobas 6800 assay). As secondary dependent variables of interest, we also analyzed COVID-19 ICU admission and death, which were defined as having a PCR-positive clinical sample and either admission to the ICU or death at any time during the study period.
The primary independent variables of interest were the ADI and neighborhood racial composition. We used the state-level ADI, which provides a decile ranking for each census block group from 1 to 10.23,28 These state-level rankings are derived by scoring and ordering all census block groups in the state from low to high and then splitting census block groups into ten equal sections. This process creates ten distinct deciles of state ADI, which we implemented as an ordinal variable in all analyses. The ADI, whose construction is described in detail elsewhere,23,28 is composed of the following socioeconomic indicators: educational attainment (percentage of population with less than nine years and percentage of population with twelve or more years of education), income and poverty (median family income, income disparity, percentage of families below the federal poverty level, and percentage of the population below 150 percent of the federal poverty level), household composition (single-parent household rate), employment and labor (occupational composition and unemployment rate), housing characteristics (home ownership rate, median home value, median gross rent, median monthly mortgage, and household crowding), and basic material resources (access to a telephone, plumbing, or motor vehicles). Although the ADI was modeled as an ordinal variable, we have designated the following categories for descriptive purposes: 1–2 (very low disadvantage), 3–4 (low), 5–6 (moderate), 7–8 (high), and 9–10 (very high).
Racial and ethnic composition categories for each census block group were initially constructed on the basis of predominant patterns of residential segregation in Chicago, including White majority, Black majority, Hispanic/Latino majority, and other. A majority was defined as greater than 50 percent of residents identifying with each respective racial or ethnic category. However, because a large proportion (86 percent) of the total patient sample (N = 21,285) lived in either a White or Black majority neighborhood, we limited our analyses to these two racial composition categories (N = 18,246). An additional 1,477 patients (8.1 percent) had missing covariates because of missing marital status (n = 754) and insurance status (n = 808), and 85 patients (0.5 percent) had missing ADI information, yielding a final sample of 16,684 patients.
STATISTICAL ANALYSIS
Descriptive statistics were calculated for all patients and census block groups in the sample. Mixed-effects logistic regression models with a random intercept for census block group were used to examine COVID-19 positivity (primary dependent variable), as well as COVID-19 ICU admission and death (secondary dependent variables), as independent functions of census block group racial composition (independent variable), including ADI (model 2) and race-ADI interaction effects (model 3). Model 2 provides an estimate of the residual direct effect of race,29 whereas model 3 provides an estimate of any potential differences by race in the slope of change across ADI deciles.
All analyses clustered patients within their census block group of residence and adjusted for patients’ sociodemographic characteristics. Selected covariates were captured in the electronic health record and were well documented in prior literature to be associated with health behaviors and outcomes, namely age (continuous), sex, marital status, English language proficiency, and insurance type.30–32 Patient-level race was removed because of collinearity with neighborhood racial composition. However, sensitivity analyses were conducted using patient-level race instead of neighborhood-level race, both with and without inclusion of patients who identified as Hispanic/Latino. Data were analyzed using Stata/SE, release 15.0.
LIMITATIONS
There were several limitations to this study. First, as this was a study of patients tested for COVID-19 at an academic medical center and its five community testing sites, selection bias may have occurred if there were differences in testing behavior for mild or asymptomatic cases. Our study likely underestimated racial disparities in COVID-19 positivity, as prior literature has documented that poor and minority people are less likely to present for care or may present at more advanced stages of disease.33 However, these data likely represent some of the best estimates available for urban, disadvantaged communities of color, as community testing sites were designed to address racial disparities (such as through geographic distribution, extended hours, and testing regardless of symptoms).
Second, although the sample included all patients tested during the study period and reflects nearly 2,000 census block groups in Chicago, the findings are most generalizable to similar urban regions with a high proportion of racially segregated neighborhoods. Third, we were unable to examine multilevel associations between patient-level race and neighborhood-level race because of multicollinearity and heteroscedasticity. In effect, Black patients in our sample predominantly lived in Black majority neighborhoods, whereas White patients in our sample lived in more heterogeneous neighborhood contexts. In addition, the analyses were only performed on the subset of the patient sample living in either Black majority or White majority neighborhoods. However, we conducted sensitivity analyses using patient-level race, regardless of neighborhood racial and ethnic composition, which did not substantively change the results.
Fourth, this analysis was performed using retrospective data collected during the first wave of COVID-19, so the associations might not be generalizable to later waves of the pandemic. Fifth, we treated missing values as missing at random, as prior literature has documented acceptability if less than 10 percent of the sample has missing data.34 However, it is possible that some items were not entirely missing at random.
Finally, we did not control for comorbid conditions. Although some comorbid conditions (such as diabetes and cardiovascular disease) have been associated with higher COVID-19 morbidity and mortality, they have not been associated with an increased risk for infection, which was our primary outcome measure. Importantly, the goal of this study was to examine structural racism and, specifically, one of its most enduring institutions: racialized residential segregation and associated neighborhood disadvantage. As described in prior literature,29 studies of racial disparity should be conscientious about controlling for mediators of racism, as this may inadvertently absorb some of the “legitimate” effect of racism that is being quantified. For this particular study, we conceptualized the disproportionate burden of comorbid conditions in communities of color as a disadvantage imparted by structural racism, which mediates the relationship between neighborhood disadvantage and COVID-19 outcomes.
Study Results
Data on 16,684 patients from 1,992 block groups were analyzed (exhibit 1). Patients were predominantly non-Hispanic Black (67.2 percent), female (60.5 percent), and age thirty-five and older (67.7 percent). A majority of patients (60.2 percent) were insured by Medicaid, Medicare, or both. More than two-thirds (71.4 percent) lived in a neighborhood with a Black majority racial composition, and more than one-quarter (29.9 percent) lived in a neighborhood with an ADI of 9 or 10 (very high disadvantage). However, we observed pronounced differences in the pattern of neighborhood racial composition by ADI decile (p < 0.001) (exhibit 2). For example, only 1.2 percent of patients living in a neighborhood with an ADI of 9 or 10 (very high disadvantage) also lived in a White majority neighborhood. Conversely, 16.7 percent of patients living in a neighborhood with an ADI of 1 or 2 (very low disadvantage) also lived in a Black majority neighborhood. Similar patterns were observed for patient-level race at each ADI decile (online appendix exhibit A1).35 The ADI alone was associated with 10 percent higher adjusted odds (95% confidence interval: 1.08, 1.13; p < 0.001) of COVID-19 positivity for each decile increase in the ADI (appendix exhibit A2).35
EXHIBIT 1.
Characteristics of patients in the study sample of COVID-19 positivity and neighborhood disadvantage in Chicago, Illinois, 2020
| Patient characteristics | Number | Percent |
|---|---|---|
| Age (years) | ||
| Younger than 18 | 1,407 | 8.4 |
| 18–34 | 3,993 | 23.9 |
| 35–49 | 3,254 | 19.5 |
| 50–64 | 3,923 | 23.5 |
| 65–79 | 2,979 | 17.9 |
| 80 or older | 1,128 | 6.8 |
|
| ||
| Female sex | 10,090 | 60.5 |
|
| ||
| Race and ethnicity | ||
| White non-Hispanic | 2,824 | 16.9 |
| Black non-Hispanic | 11,208 | 67.2 |
| Hispanic or Latino | 490 | 2.9 |
| Other | 411 | 2.5 |
| Missing or declined | 1,751 | 10.5 |
|
| ||
| English language proficient | 16,516 | 99.0 |
|
| ||
| Marital status | ||
| Married | 4,094 | 24.5 |
| Not married | 11,474 | 68.8 |
| Missing or declined | 1,116 | 6.7 |
|
| ||
| Insurance type | ||
| Private | 5,771 | 34.6 |
| Medicaid or dual eligible | 5,749 | 34.5 |
| Medicare | 4,282 | 25.7 |
| Other | 3 | 0.0 |
| No insurance | 879 | 5.3 |
|
| ||
| Black majority neighborhood | 11,906 | 71.4 |
|
| ||
| Area Deprivation Index decilea | ||
| 1–2 | 2,761 | 16.6 |
| 3–4 | 2,655 | 15.9 |
| 5–6 | 2,893 | 17.3 |
| 7–8 | 3,387 | 20.3 |
| 9–10 | 4,988 | 29.9 |
|
| ||
| COVID-19 outcomes | ||
| Positive test | 2,174 | 13.0 |
| ICU admission | 308 | 1.8 |
| Death | 92 | 0.6 |
SOURCE Authors’ analysis of patient health records from an urban academic medical center in Chicago. NOTES N = 16,684. ICU is intensive care unit.
Area Deprivation Index (ADI) reflects ranking of neighborhoods by socioeconomic disadvantage at the state level; an ADI of 1 indicates the lowest level of disadvantage, whereas an ADI of 10 indicates the highest level of disadvantage.
EXHIBIT 2. Patients’ neighborhood racial composition in Chicago, Illinois, by Area Deprivation Index (ADI) deciles, 2020.
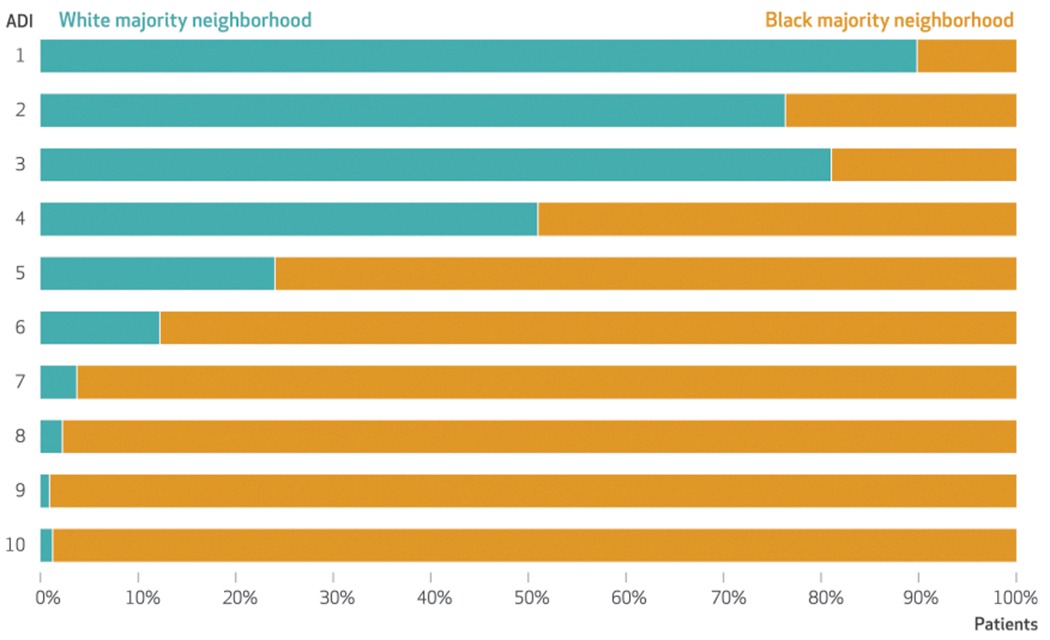
SOURCE Authors’ analysis of patient health records from an urban academic medical center in Chicago. NOTE An ADI of 1 indicates the lowest disadvantage; 10 indicates the highest disadvantage.
Patients living in a Black majority neighborhood had 2.05 times higher adjusted odds (95% CI: 1.77, 2.36; model 1) of COVID-19 positivity relative to those in a White majority neighborhood (exhibit 3). In models including the ADI (model 2), area deprivation accounted for 20 percent of the disparity ([1 – {1.64/2.05}] × 100), with those living in a Black majority neighborhood having 64 percent higher adjusted odds (95 percent CI: 1.36, 1.97) of COVID-19 positivity. Stratified analyses comparing patients in Black and White majority neighborhoods revealed a linear increase in COVID-19 positivity with higher deciles of area deprivation (exhibit 4), but no significant race-ADI interaction effects (model 3) (adjusted odds ratio: 0.94; 95 percent CI: 0.88, 1.01). The average percentage of COVID-19 positivity was substantially higher among those in Black majority neighborhoods compared with White majority neighborhoods at each ADI decile (p < 0.001). For example, people living in White majority neighborhoods with high or very high disadvantage (ADI 7–10) had a positivity of 11.7 percent, whereas those in corresponding Black majority neighborhoods had a positivity of 15.3 percent (exhibit 4).
EXHIBIT 3.
Associations of patients’ neighborhood racial composition and Area Deprivation Index (ADI) with COVID-19 positivity and outcomes in Chicago, Illinois, 2020
| Adjusted odds ratioa |
|||
|---|---|---|---|
| Neighborhood characteristicsb | Model 1: racial composition alone | Model 2: racial composition and ADI | Model 3: race-ADI interaction |
| COVID-19 positivity | |||
| Racial composition | |||
| White majority | Ref | Ref | Ref |
| Black majority | 2.05**** | 1.64**** | 2.10**** |
| ADI (per decile) | —c | 1.05**** | 1.11*** |
| Black majority × ADI | —c | —c | 0.94* |
|
| |||
| COVID-19 ICU admission | |||
| Racial composition | |||
| White majority | Ref | Ref | Ref |
| Black majority | 2.93**** | 2.71**** | 1.79 |
| ADI (per decile) | —c | 1.02 | 0.90 |
| Black majority × ADI | —c | —c | 1.14 |
|
| |||
| COVID-19 death | |||
| Racial composition | |||
| White majority | Ref | Ref | Ref |
| Black majority | 2.68*** | 1.98* | 0.38 |
| ADI (per decile) | —c | 1.07 | 0.60* |
| Black majority × ADI | —c | —c | 1.84** |
SOURCE Authors’ analysis of patient health records from an urban academic medical center in Chicago. NOTES N = 16,684. The reference value is 1.00. ADI is described in the notes to exhibit 1. ICU is intensive care unit.
Multilevel mixed-effects regression models implemented clustering by census block group and adjusted for patient sociodemographic characteristics, including age, sex, marital status, English language proficiency, and insurance type.
Final sample included only patients residing in either White or Black majority neighborhoods.
The model did not include this variable.
p < 0.1
p < 0.05
p < 0.01
p < 0.001
EXHIBIT 4. COVID-19 positivity by Area Deprivation Index (ADI) and neighborhood racial composition in Chicago, Illinois, 2020.
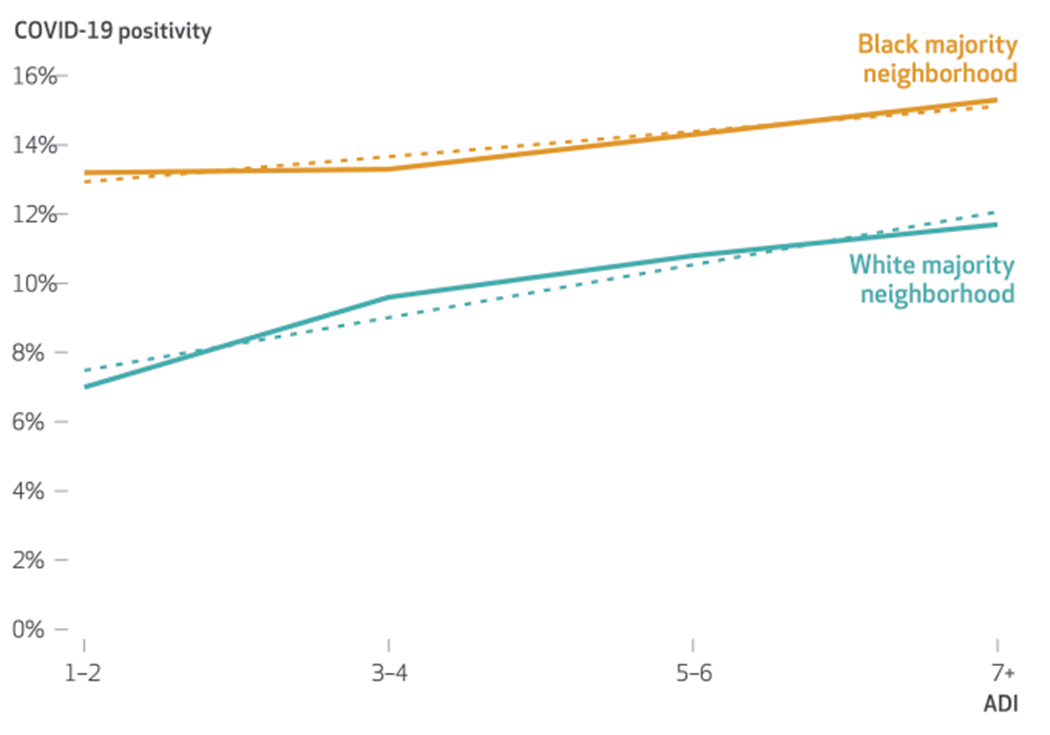
SOURCE Authors’ analysis of patient health records from an urban academic medical center in Chicago. NOTE ADI deciles of 7 or greater were combined in this figure to enhance the precision of descriptive estimates by race, because only a small proportion of patients (2 percent) at these ADI deciles were living in a White majority neighborhood.
We observed similar findings for COVID-19 ICU admission. Patients living in a Black majority neighborhood (model 1) had 2.93 times higher adjusted odds (95% CI: 1.96, 4.36) of COVID-19 ICU admission relative to those in a White majority neighborhood. ADI accounted for 8 percent of the disparity (model 2) (AOR: 2.71; 95% CI: 1.69, 4.35). No significant race-ADI interaction effects (model 3) were detected (AOR: 1.14; 95% CI: 0.90, 1.46) (exhibit 3).
Overall, we observed significant differences in COVID-19 death by neighborhood racial composition. Patients living in a Black majority neighborhood (model 1) had 2.68 times higher adjusted odds (95% CI: 1.38, 5.18) of COVID-19 death relative to those in a White majority neighborhood. Although racial disparities were no longer significant in models accounting for the ADI (model 2), we observed a significant race-ADI interaction effect (model 3) (AOR: 1.84; 95% CI: 1.7, 3.18), indicating a steeper increase in COVID-19 death per level increase in the ADI among patients in a Black majority neighborhood (exhibit 3).
In sensitivity analyses examining patient-level race instead of neighborhood-level race, we observed larger effect sizes for all COVID-19 outcomes (appendix exhibit A3).35 For example, patients identifying as Black had 3.25 times higher adjusted odds (95% CI: 2.73, 3.86) of COVID-19 positivity compared with patients identifying as White. Notably, the ADI accounted for a substantively similar proportion of the disparity (appendix exhibit A3).35 In sensitivity analyses including patient-level ethnicity (appendix exhibit A4),30 patients identifying as Hispanic/Latino had 2.85 times higher adjusted odds (95% CI: 2.23, 3.64) of COVID-19 positivity compared with patients identifying as White; the ADI accounted for 10 percent (1 – [2.58/2.85] × 100) of the disparity.
Discussion
In this study of patients tested for COVID-19 at an academic medical center in Chicago, we found that the Area Deprivation Index accounted for 20 percent of the racial disparity in COVID-19 positivity. In analyses comparing Black and White majority neighborhoods, we observed no significant race and ADI interaction effects, but we did observe dramatic differences in percentage positivity across all deciles of the state ADI. On average, patients living in Black majority neighborhoods with the lowest disadvantage still had a higher percentage positivity than those living in White majority neighborhoods with the highest disadvantage.
The COVID-19 health crisis offers an important case study of neighborhood effects because, as in the Moving to Opportunity experiment,16 effects associated with neighborhood disadvantage were more moderate than expected. Findings remind us that although neighborhood disadvantage undoubtedly plays a substantive role in racial health disparities, economic solutions alone will not resolve the many structural barriers to health equity. We observed a 5.2 percent difference in percentage positivity, even at the same very low deciles of disadvantage (ADI of 1–2) for Black versus White majority neighborhoods. Other structural disadvantages likely make up the remainder of this gap and contribute a sizable, cumulative effect on COVID-19 transmission.
For example, recent literature has described overpolicing and jail cycling as key drivers of COVID-19 infection in Black neighborhoods.36 Eric Reinhart and colleagues found that jail cycling accounted for 55 percent of the variance in case rates across ZIP codes in Chicago.36,37 Other studies have documented a higher rate of essential workers in Black neighborhoods. Jose Figueroa and colleagues noted that employment in food service was significantly associated with higher case rates of COVID-19 in Massachusetts,38 suggesting that even in a low-risk neighborhood context, Black residents may still face a high-risk work environment, given their over-representation in the food service industry. Ultimately, as the COVID-19 pandemic has given rise to growing concern about structural racism and its health effects, it will be imperative to uncover, measure, and quantify the numerous and multilevel mechanisms of disadvantage to inform nuanced solutions.
Of note, we observed a race-ADI interaction effect for COVID-19 death, corresponding to a steeper increase in death for residents of Black neighborhoods at each level of disadvantage. This is in contrast to COVID-19 positivity and ICU admission, for which stratified analyses revealed no interaction effects. It is possible that while COVID-19 positivity is influenced by transmission dynamics, which change in a consistent manner for each race and ethnicity across ADI deciles, death is additionally a function of comorbidity and disease burden, geographic access to resources, health care bias, and other multifactorial risks that widen racial disparities across ADI deciles. We recommend caution, however, in interpreting this finding. Although analyses were statistically powered, only ninety-two deaths were captured in the academic medical center’s electronic health record during the study period. These recorded deaths might not fully reflect events that occurred beyond the study period or might not include unreported events that occurred in a different location (for example, at home or a different hospital) after testing.
Conclusion
The COVID-19 pandemic has raised broad and unprecedented awareness of structural racism and its role in modern health inequity. Although neighborhood socioeconomic disadvantage plays a salient role, we found that its associations with racial disparities in COVID-19 positivity and ICU admission were more moderate than expected. Importantly, COVID-19 positivity was substantially higher in Black majority neighborhoods even at the lowest deciles of area deprivation, suggesting the presence of other structural disadvantages driving case rates. Our study illustrates that single-solution strategies will inevitably fall short. Future research should examine the role of multiple sources of structural racism, with study designs that enable comparative inference. As health care in the US has increasingly committed to health equity as a core ethos, it will be imperative to systematically investigate, quantify, and deconstruct the many structural disadvantages that contribute to racial health inequity in the US. ■
Supplementary Material
Acknowledgments
Elizabeth Tung and Anna Volerman were supported by National Institutes of Health and National Heart, Lung, and Blood Institute Career Development Grants (Tung received Grant No. 1K23HL145090; Volerman received Grant No. 1K23HL143218) in patient-oriented research. Tung, Volerman, and Monica Peek were also supported by the Chicago Center for Diabetes Translation Research (Grant No. P30DK092949), funded by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health. The authors thank Theodore Karrison, from the University of Chicago Biostatistics Laboratory in the Department of Public Health Sciences, for his statistical support and guidance; John Fahrenbach, from the Center for Healthcare Delivery Science and Innovation, for his geostatistical support; and Julie Johnson and Cristina Corrigan, from the Center for Research Informatics, for their COVID-19 data warehouse creation, maintenance, and support. The findings and conclusions in this article are those of the authors and do not reflect the view of the National Institutes of Health, the Department of Health and Human Services, or the United States government.
Contributor Information
Elizabeth L. Tung, Section of General Internal Medicine, University of Chicago, in Chicago, Illinois.
Monica E. Peek, Section of General Internal Medicine, University of Chicago.
Marco A. Rivas, Pritzker School of Medicine, University of Chicago.
Joyce P. Yang, Department of Psychology, University of San Francisco, in San Francisco, California.
Anna Volerman, Sections of General Internal Medicine and Academic Pediatrics, University of Chicago.
NOTES
- 1.Centers for Disease Control and Prevention. Disparities in COVID-19 illness [Internet]. Atlanta (GA): CDC; [last updated 2020 Dec 10; cited 2021 Sep 17]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/community/health-equity/racial-ethnic-disparities/increased-risk-illness.html [Google Scholar]
- 2.Poteat T, Millett GA, Nelson LE, Beyrer C. Understanding COVID-19 risks and vulnerabilities among black communities in America: the lethal force of syndemics. Ann Epidemiol. 2020;47:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Churchwell K, Elkind MSV, Benjamin RM, Carson AP, Chang EK, Lawrence W, et al. Call to action: structural racism as a fundamental driver of health disparities: a presidential advisory from the American Heart Association. Circulation. 2020;142(24):e454–68. [DOI] [PubMed] [Google Scholar]
- 4.Egede LE, Walker RJ. Structural racism, social risk factors, and Covid-19—a dangerous convergence for Black Americans. N Engl J Med. 2020;383(12):e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peek ME, Simons RA, Parker WF, Ansell DA, Rogers SO, Edmonds BT. COVID-19 among African Americans: an action plan for mitigating disparities. Am J Public Health. 2021;111(2):286–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams DR, Collins C. Racial residential segregation: a fundamental cause of racial disparities in health. Public Health Rep. 2001;116(5):404–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang T-C, Park K, Matthews SA. Racial/ethnic segregation and health disparities: future directions and opportunities. Sociol Compass. 2020;14(6):e12794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ver Ploeg M, Breneman V, Farrigan T, Hamrick K, Hopkins D, Kaufman P, et al. Access to affordable and nutritious food-measuring and understanding food deserts and their consequences: report to Congress [Internet]. Washington (DC): Department of Agriculture, Economic Research Service; 2009. Jun [cited 2021 Sep 17]. Available from: https://www.ers.usda.gov/publications/pub-details/?pubid=42729 [Google Scholar]
- 9.South EC, Kondo MC, Cheney RA, Branas CC. Neighborhood blight, stress, and health: a walking trial of urban greening and ambulatory heart rate. Am J Public Health. 2015;105(5):909–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tung EL, Johnson TA, O’Neal Y, Steenes AM, Caraballo G, Peek ME. Experiences of community violence among adults with chronic conditions: qualitative findings from Chicago. J Gen Intern Med. 2018;33(11):1913–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tung EL, Wroblewski KE, Boyd K, Makelarski JA, Peek ME, Lindau ST. Police-recorded crime and disparities in obesity and blood pressure status in Chicago. J Am Heart Assoc. 2018;7(7):e008030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sandel M, Sheward R, Ettinger de Cuba S, Coleman SM, Frank DA, Chilton M, et al. Unstable housing and caregiver and child health in renter families. Pediatrics. 2018;141(2):e20172199. [DOI] [PubMed] [Google Scholar]
- 13.Tung EL, Peek ME, Makelarski JA, Escamilla V, Lindau ST. Adult BMI and access to built environment resources in a high-poverty, urban geography. Am J Prev Med. 2016;51(5):e119–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qato DM, Wilder J, Zenk S, Davis A, Makelarski J, Lindau ST. Pharmacy accessibility and cost-related underuse of prescription medications in low-income Black and Hispanic urban communities. J Am Pharm Assoc (2003). 2017;57(2):162–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ludwig J, Sanbonmatsu L, Gennetian L, Adam E, Duncan GJ, Katz LF, et al. Neighborhoods, obesity, and diabetes—a randomized social experiment. N Engl J Med. 2011;365(16):1509–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sampson RJ. Moving to inequality: neighborhood effects and experiments meet structure. AJS. 2008;114(11):189–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wrigley-Field E, Garcia S, Leider JP, Van Riper D. COVID-19 mortality at the neighborhood level: racial and ethnic inequalities deepened in Minnesota in 2020. Health Aff (Millwood). 2021;40(10):1644–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Millett GA, Jones AT, Benkeser D, Baral S, Mercer L, Beyrer C, et al. Assessing differential impacts of COVID-19 on black communities. Ann Epidemiol. 2020;47:37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kitchen C, Hatef E, Chang H-Y, Weiner J, Kharrazi H. Assessing the impact of Area Deprivation Index on COVID-19 prevalence: a contrast between rural and urban U.S. jurisdictions. medRxiv [preprint on the Internet]. 2020. Oct 11 [cited 2021 Sep 17]. Available from: https://www.medrxiv.org/content/10.1101/2020.10.07.20208462v1.full [DOI] [PMC free article] [PubMed]
- 20.Hunt BR, Tran G, Whitman S. Life expectancy varies in local communities in Chicago: racial and spatial disparities and correlates. J Racial Ethn Health Disparities. 2015;2(4):425–33. [DOI] [PubMed] [Google Scholar]
- 21.K C M, Oral E, Straif-Bourgeois S, Rung AL, Peters ES. The effect of area deprivation on COVID-19 risk in Louisiana. PLoS One. 2020;15(12):e0243028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kind AJH, Buckingham WR. Making neighborhood-disadvantage metrics accessible—the neighborhood atlas. N Engl J Med. 2018;378(26):2456–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kind AJ, Jencks S, Brock J, Yu M, Bartels C, Ehlenbach W, et al. Neighborhood socioeconomic disadvantage and 30-day rehospitalization: a retrospective cohort study. Ann Intern Med. 2014;161(11):765–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chamberlain AM, Finney Rutten LJ, Wilson PM, Fan C, Boyd CM, Jacobson DJ, et al. Neighborhood socioeconomic disadvantage is associated with multimorbidity in a geographically-defined community. BMC Public Health. 2020;20(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chicago Metropolitan Agency for Planning. Community data snapshots [Internet]. Chicago (IL): CMAP; [cited 2021 Sep 17]. Available from: https://www.cmap.illinois.gov/data/community-snapshots [Google Scholar]
- 26.Census Bureau. American Community Survey 5-year estimates, 2018: total population [Internet]. Washington (DC): Census Bureau; [cited 2021 Sep 17]. Available from: https://data.census.gov/cedsci/table?q=&t=Populations%20and%20People&g=0500000US17031%241400000&y=2018&d=ACS%205-Year%20Estimates%20Detailed%20Tables&tid=ACSDT5Y2018.B01003 [Google Scholar]
- 27.Romano SD, Blackstock AJ, Taylor EV, El Burai Felix S, Adjei S, Singleton CM, et al. Trends in racial and ethnic disparities in COVID-19 hospitalizations, by region—United States, March–December 2020. MMWR Morb Mortal Wkly Rep. 2021;70(15):560–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh GK. Area deprivation and widening inequalities in US mortality, 1969–1998. Am J Public Health. 2003;93(7):1137–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cook BL, McGuire TG, Zaslavsky AM. Measuring racial/ethnic disparities in health care: methods and practical issues. Health Serv Res. 2012;47(3 Pt 2):1232–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sorlie PD, Backlund E, Keller JB. US mortality by economic, demographic, and social characteristics: the National Longitudinal Mortality Study. Am J Public Health. 1995;85(7):949–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi L, Lebrun LA, Tsai J. The influence of English proficiency on access to care. Ethn Health. 2009;14(6):625–42. [DOI] [PubMed] [Google Scholar]
- 32.Barnett ML, Hsu J, McWilliams JM. Patient characteristics and differences in hospital readmission rates. JAMA Intern Med. 2015;175(11):1803–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Institute of Medicine. Unequal treatment: understanding racial and ethnic disparities in health care. Washington (DC): National Academies Press; 2003. [PubMed] [Google Scholar]
- 34.Bennett DA. How can I deal with missing data in my study? Aust N Z J Public Health. 2001;25(5):464–9. [PubMed] [Google Scholar]
- 35.To access the appendix, click on the Details tab of the article online.
- 36.Barsky BA, Reinhart E, Farmer P, Keshavjee S. Vaccination plus decarceration—stopping Covid-19 in jails and prisons. N Engl J Med. 2021;384(17):1583–5. [DOI] [PubMed] [Google Scholar]
- 37.Reinhart E, Chen DL. Incarceration and its disseminations: COVID-19 pandemic lessons from Chicago’s Cook County Jail. Health Aff (Millwood). 2020;39(8):1412–8. [DOI] [PubMed] [Google Scholar]
- 38.Figueroa JF, Wadhera RK, Lee D, Yeh RW, Sommers BD. Community-level factors associated with racial and ethnic disparities in COVID-19 rates in Massachusetts. Health Aff (Millwood). 2020;39(11):1984–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


