Abstract
Purpose of Review
RNA therapeutics are a new and rapidly expanding class of drugs to prevent or treat a wide spectrum of diseases. We discuss the defining characteristics of the diverse family of molecules under the RNA therapeutics umbrella.
Recent Findings
RNA therapeutics are designed to regulate gene expression in a transient manner. For example, depending upon the strategy employed, RNA therapies offer the versatility to replace, supplement, correct, suppress, or eliminate the expression of a targeted gene. RNA therapies include antisense nucleotides, microRNAs and small interfering RNAs, RNA aptamers, and messenger RNAs. Further, we discuss the mechanism(s) by which different RNA therapies either reduce or increase the expression of their targets.
Summary
We review the RNA therapeutics approved (and those in trials) to treat cardiovascular indications. RNA-based therapeutics are a new, rapidly growing class of drugs that will offer new alternatives for an increasing array of cardiovascular conditions.
Keywords: RNA therapeutics, Cardiovascular disease, mRNA therapeutics, siRNA therapeutics, Antisense oligonucleotide therapeutics
Introduction
Nucleic acid–based therapies consist of exogenous sequences, either DNA or RNA, that are designed to generate a therapeutic effect in vivo. Although RNA therapeutics have only recently gained notoriety, they have been under development for several decades [1–5]. The initial proof of concept experiments for RNA therapies involved using messenger RNA (mRNA) to artificially express a protein in vivo were performed about three decades ago [6, 7•]. This first work used intramuscular injection to deliver one of three in vitro transcribed mRNAs to mice [6]. Protein expression from the injected mRNAs was verified and proved just as effective as (as judged by the protein levels expressed from the injected nucleic acid) using DNA-encoded vectors [6]. The next key study used a lab-made vasopressin mRNA to transiently correct a rat model of diabetes insipidus [7•]. Since these initial experiments with mRNA, a diverse family of molecules is now covered by the umbrella of RNA therapeutics. RNA therapeutics can contain a diverse mixture of nucleotides and can be single- or double-stranded [8, 9, 10•]. Currently, antisense oligonucleotides (ASOs), small interfering RNAs (siRNAs), microRNAs (miRNAs), RNA aptamers, and mRNAs are all grouped together as RNA therapies [1–9, 10•, 11].
As evidenced by the two COVID-19 vaccines from Moderna and Pfizer/BioNTech, RNA therapies can be designed, developed, evaluated, manufactured, and distributed rapidly [12••, 13••]. A detailed overview covering the theory and functional aspects of different RNA therapies is beyond the scope of this review but is available here [8]. Instead, this review describes the major families of RNA therapies, summarizes the RNA therapeutics currently in use (or in development) for cardiovascular indications (Table 1), and describes the mechanism of action for selected RNA drugs (Figure 1). We also collect and group the different drug and company names with their respective clinical trial identifiers to track each RNA therapeutic over time (Table 1).
Table 1.
RNA therapeutics in clinical trials for cardiovascular disease
| Drug | Type | Clinical trial phase | Condition (phase) | Company | Mechanism of action | NCT# | Aliases |
|---|---|---|---|---|---|---|---|
| Mipomersen | ASO | Marketed; discontinued | Hyperlipoproteinemia type Iia (marketed)/atherosclerosis (discontinued) | Genzyme (Sanofi) | ApoB-100 protein synthesis inhibition | NCT01475825 | ISIS-301012; Kynamro; mipomersen sodium |
| Volanesorsen | ASO |
Marketed; Phase III |
Hyperlipoproteinemia type I (marketed); hypertriglyceridemia; lipodystrophy (III) | Ionis Pharmaceuticals (Akcea Therapeutics) | ApoC-III protein reduction to regulate plasma triglycerides | NCT02300233 | IONIS-304801; IONIS-APOCIII; IONIS-APOCIIIRx; ISIS-304801; ISIS-APOCIII; ISIS-APOCIII-Rx; volanesorsen; volanesorsen sodium; Waylivra |
| Inotersen | ASO |
Marketed; Phase II |
Amyloid polyneuropathy (marketed); amyloidosis, cardiomyopathies (II) | Ionis Pharmaceuticals (Akcea Therapeutics) | Inhibitor of the hepatic production of transthyretin (TTR) protein | NCT03702829 | GSK 2998728; Inotersen; Inotersen sodium; IONIS-TTRRx; ISIS 420915 salt; ISIS-420915; ISIS-GSK1Rx; ISIS-TTRRx; TEGSEDI |
| Eplontersen | ASO | Phase III | Amyloidosis; transthyretin-related hereditary amyloidosis; transthyretin-mediated amyloid cardiomyopathy | Ionis Pharmaceuticals (Akcea Therapeutics) | Reduce the production of transthyretin (TTR) protein | NCT04136171 | AKCEA-TTR-LRx; ION-682884; ION-TTR-LRx; IONIS-TTR-LRx |
| Pelacarsen | ASO | Phase III | Hyperlipoproteinemia | Ionis-Novartis | Reduce apolipoprotein(a) to reduce Lp(a) | NCT04023552 | AKCEA-APO(a)-LRx; IONIS-681257; IONIS-APO(a)-LRx; IONIS-APO-LRx; ISIS 681257; ISIS-APO(a)-LRx; ISIS-APOALRx; Pelacarsen sodium; TQJ 230 |
| Olezarsen (IONIS-APOCIII-LRx) | ASO | Phase III | Hyperlipoproteinemia type I (III); hypertriglyceridemia (III) | Ionis Pharmaceuticals (Akcea Therapeutics) | Inhibit the production of apolipoprotein C-III (ApoC-III) to regulate serum triglycerides | NCT04568434 NCT05079919 | AKCEA-APOCIII-LRx; IONIS-APOCIII-LRx; IONISAPOCIII-LRx, sodium salt; ISIS 678354; ISIS-APOCIII-LRx; Olezarsen sodium |
| IONIS TMPRSS6 LRx | ASO | Phase II; no development reported | Beta-thalassemia (II); thalassemia (NDR) | Ionis Pharmaceuticals | Reduce the production of transmembrane protease serine 6 (TMPRSS6) to increase red blood cell production | IONIS TMPRSS6-Lrx; IONIS-TMPRSS6-LRx; ISIS 702843 | |
| Fesomersen | ASO | Phase II | Thrombosis | Ionis-Bayer | Reduce the production of clotting factor XI | NCT04534114 | BAY-2976217; Factor XI LICA; ION-957943; IONIS-FXI-LRX |
| IONIS AGT LRx | ASO | Phase II | Chronic heart failure; hypertension | Ionis Pharmaceuticals | Reduce production of liver-derived angiotensinogen to decrease blood pressure | IONIS-AGT-LRx; ISIS 757456; ISIS-AGT-LRx | |
| Donidalorsen | ASO | Phase II | COVID 2019 infections; hereditary angioedema | Ionis Pharmaceuticals | Reduce the level of prekallikrein (PKK) | Donidalorsen sodium; IONIS-PKK-LRx; ISIS 721744 | |
| Vupanorsen | ASO | Phase II; Phase I/II | Dyslipidemias; hyperlipoproteinemia type I; hypertriglyceridemia; lipodystrophy; non-alcoholic fatty liver disease; type 2 diabetes mellitus (II); hyperlipoproteinemia type Iia (I/II) | Ionis-Pfizer | Reduce angiopoietin-like 3 protein and reduce triglyceride and LDL-C | AKCEA ANGPTL3 LRx; IONIS ANGPTL3 LRx; ISIS 703802 salt; ISIS-703802; PF 07285557; Vupanorsen sodium | |
| AZD 8233 | ASO |
Phase II Phase I |
Dyslipidemias, hyperlipidemia (II); hypercholesterolemia (I) | Ionis-AstraZeneca | Reduce plasma levels of proprotein convertase subtilisin/kexin type 9 (PCSK9) and LDL-C | AZD-8233; ION-449; IONIS-AZ4-2.5-LRx | |
| CDR 132L | ASO | Phase I | Heart failure | Cardior Pharmaceuticals | Inhibit miR-132 to reduce adverse cardiac remodeling | NCT04045405 | CDR-132L |
| MRG-110 | ASO | Phase I (no development reported) | Heart failure; ischemia; wounds (NDR) | miRagen-viridia | Reduce miR-92a and improve vascularization | NCT03603431 | Anti Mir92a; MRG-110; S95010 |
| SPC 4955 | ASO | Discontinued | Hypercholesterolemia | Roche (Santaris Pharma) | Inhibit ApoB (apolipoprotein B) and reduce LDL-C | NCT01365663 | SPC-4955 |
| SPC 5001 | ASO | Discontinued | Hypercholesterolemia | Roche (Santaris Pharma) | Target proprotein convertase subtilisin/kexin type 9 (PCSK9) expression; reduce LDL-C | NCT01350960 | SPC-5001 |
| IONIS APOARx | ASO | Discontinued | Hyperlipoproteinemia | Ionis Pharmaceuticals | Reduce level of Lp(a) | NCT02160899 | IONIS-APO(a)Rx; IONIS-APOA Rx; ISIS-494372; ISIS-APO(a)RX; ISIS-APOA Rx |
| ISIS CRPRx | ASO | Discontinued | Atrial fibrillation; rheumatoid arthritis | Ionis Pharmaceuticals | Target C-reactive protein (CRP) | NCT01710852 | ISIS-329993; ISIS-CRP; ISIS-CRPRx |
| GTX Drug eluting Coronary Stent System | ASO | Discontinued | Coronary artery disease | Sarepta-Cook Group Incorporated | Target c-myc to reduce arteries reclosing | NCT00777842 | AVI-5126; GTX 5126; Restin-CP |
| Inclisiran | siRNA |
Registered; Phase III; FDA approved |
Hypercholesterolemia (registered)/atherosclerotic cardiovascular disease (III) | Alnylam-Novartis | Inhibit hepatic synthesis of (PCSK9) to lower LDL-C | NCT04929249 NCT03814187 | ALN-60212; ALN-PCSsc; KJX-839; Leqvio; PCSK9si |
| Patisiran | siRNA | Marketed; Phase III | Amyloid polyneuropathy; amyloidosis (marketed); ATTR with cardiomyopathy (III) | Alnylam Pharmaceuticals | Inhibit transthyretin (TTR) synthesis | ALN-18328; ALN-TTR02; GENZ-438027; ONPATTRO; Patisiran sodium; patisiran-LNP; SAR-438037 | |
| Vutrisiran | siRNA | Pre-registration; Phase III | Transthyretin-related hereditary amyloidosis; ATTR with cardiomyopathy | Alnylam Pharmaceuticals | Inhibit transthyretin (TTR) synthesis | ALN-65492; ALN-TTRsc02 | |
| Teprasiran | siRNA | Phase III | Acute kidney injury (patients undergoing cardiac surgery); delayed graft function | Quark Pharmaceuticals | Inhibit p53-mediated cell death | NCT03510897 | AKIi-5; DGFi; I-5NP; QPI-1002; QPI002; Teprasiran sodium |
| Fitusiran | siRNA | Phase III | Hemophilia A; hemophilia B |
Alnylam/ Sanofi |
Target anti-thrombin III to increase thrombin generation | ALN-AT3; ALN-AT3SC; SAR-439774 | |
| ARO APOC3 | siRNA |
Phase III; Phase II |
Dyslipidemias; hypertriglyceridemia (II); hyperlipoproteinemia type I (III) | Arrowhead Pharmaceuticals | Reduce production of apolipoprotein C-III (apoC-III) to lower very-low-density lipoprotein | ARO-APOC3 | |
| Zilebesiran | siRNA | Phase II; no development reported | Hypertension (II); preeclampsia (NDR) | Alnylam Pharmaceuticals | Liver-specific silencing of AGT (angiotensinogen) | NCT04936035 | AGT siRNA;ALN-AGT;ALN-AGT-01 |
| ARO ANG3 | siRNA | Phase II | Dyslipidemias | Arrowhead Pharmaceuticals | Target angiopoietin like protein 3 (ANGPTL3) to lower LDL | NCT04832971 | ARO-ANG3 |
| Olpasiran | siRNA | Phase II | Cardiovascular disorders | Arrowhead-Amgen | Reduce the production of Lp(a) by targeting lipoprotein A (LPA) gene | AMG-890; ARC LPA; ARO LPA; RNAi ARC-LPA | |
| Bamosiran | siRNA | Phase II | Glaucoma; ocular hypertension | Sylentis | Inhibit beta 2 adrenergic receptors to reduce glaucoma | NCT02250612 | SYL-040012 |
| ApoB SNALP | siRNA | Phase I (no development reported) | Hypercholesterolemia | Arbutus Biopharma | Reduce ApoB production to control cholesterol | NCT00927459 | PRO-040201; TKM-ApoB |
| LY 3561774 | siRNA | Phase I | Cardiovascular disorders; dyslipidemias; metabolic disorders |
Dicerna/ Elli lilly |
Target angiopoietin-like protein 3 (ANGPTL3) | NCT04644809 | DCR-CM1; LY-3561774 |
| LY 3819469 | siRNA | Phase I | Cardiovascular disorders; metabolic disorders |
Dicerna/ Elli lilly |
Target the lipoprotein A (LPA) gene | NCT04914546 | DCR-CM2; LY-3819469 |
| SLN 124 | siRNA |
Preclinical; Phase I |
Hemochromatosis; iron overload; polycythemia vera (Pre); beta-thalassemia; myelodysplastic syndromes (I) | Silence therapeutics | Silence TMPRSS6 to control iron level and healthy red blood cell production | NCT04718844 | SLN-124 |
| SLN 360 | siRNA |
Preclinical; Phase I |
Cardiovascular disorders (Pre); dyslipidemias; hyperlipidemia (I) | Silence therapeutics | Target lipoprotein Lp(a) | NCT04606602 | SLN-360 |
| AGN 211745 | siRNA | Discontinued | Age-related macular degeneration, choroid neovascularization | Abbvie (Allergan) |
Target vascular endothelial growth factor receptor-1 expression Reduce pathologic angiogenesis |
NCT00395057 | AGN-211745; AGN-745; Sirna 027 |
| Revusiran | siRNA | Discontinued | Familial amyloid neuropathy; transthyretin-related hereditary amyloidosis | Alnylam Pharmaceuticals | Targets hepatic transthyretin (TTR) production | NCT02319005 | ALN-TTRsc; SAR438714; siTTRsc |
| ALN PCS | siRNA | Discontinued | Hypercholesterolemia | Alnylam Pharmaceuticals | Inhibit PCSK9 synthesis to reduce LDL-C | NCT01437059 | ALN-PCS; ALN-PCS01; ALN-PCS02 |
| Revascularization operation | miRNA | Investigational | Peripheral arterial disease, vascular diseases, peripheral, arterial occlusive diseases, atherosclerosis | Baylor University-National Institute on Aging (NIA) | Study of the role of microRNA-210 in regulating oxidative stress | NCT04089943 | None |
| Pegaptanib | RNA aptamer | Marketed; discontinued; no development reported | Age-related macular degeneration (marketed); choroidal neovascularization; retinal vascular occlusion (discontinued); diabetic macular edema; diabetic retinopathy (NDR) | Eyetech/Pfizer | Directed against vascular endothelial growth factor (VEGF)-165 to prevent ocular neovascularization | Anti-VEGF aptamer; BLO 021; EYE-001; Macugen; NX-1838; Pegaptanib octasodium; pegaptanib sodium | |
| BT 200 | RNA aptamer |
Phase II; Phase I; Preclinical |
Hemophilia A, von Willebrand disease (II); atherosclerosis, stroke (I); arterial thrombosis (Pre) | Band Therapeutics (Guardian Therapeutics) | Inhibit von Willebrand factor binding to platelet glycoprotein GbIb to prevent thrombosis | BT-200 | |
| AON D21 |
RNA/ DNA aptamer |
Phase I | Paroxysmal nocturnal hemoglobinuria | Aptarion Biotech | Complement C5a receptor antagonists | NCT05018403 | AON-D21 |
| Anivamersen-pegnivacogin | RNA aptamer | Discontinued | Acute coronary syndromes; venous thrombosis | Regado Biosciences, Inc. | Target coagulation factor IXa to prevent coagulation | NCT01848106 | Anivamersen; Pegnivacogin; Pegnivacogin/anivamersen; RB 006; RB 006/RB 007; RB 007; REG-1; REG-2; Revolixys |
| Egaptivon pegol |
RNA/ DNA aptamer |
Discontinued | Hemolytic uremic syndrome; thrombosis; thrombotic thrombocytopenic purpura; von Willebrand disease | Archemix Corporation | Inhibits von Willebrand factor binding to platelet receptor glycoprotein Ib to reduce thrombosis | NCT00632242 | ARC1779 |
| AZD 8601 | mRNA | Phase II | Heart failure | Moderna-AstraZeneca |
VEGF-A overexpression |
NCT03370887 | AZD-8601 |
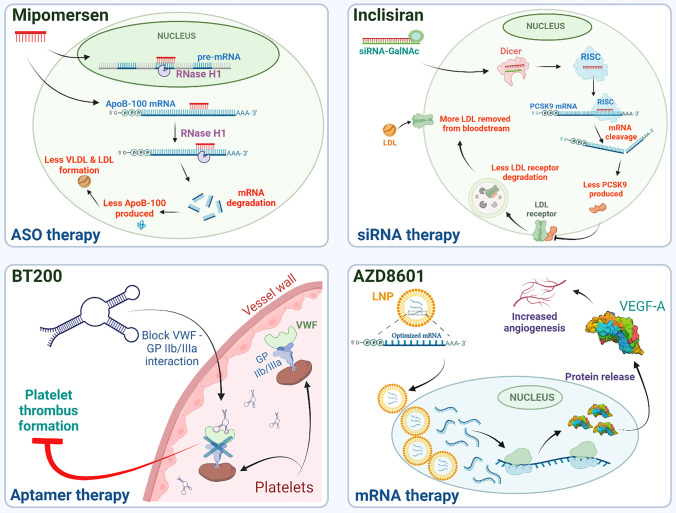
Fig. 1.
Mechanisms of action for selected RNA-based drugs to treat cardiovascular diseases. Top left: Mipomersen is an example of an ASO drug (red) which hybridizes to ApoB-100 mRNA and recruits RNase H1 to cleave the targeted mRNA, preventing apolipoprotein B production, which then reduces the synthesis of VLDL and LDL. Top right: Inclisiran as an siRNA therapy that targets PCSK9 mRNA. The targeting strand is incorporated into RISC complexes which then recognize the targeted mRNA and initiates its cleavage and degradation. This decreases the amount of PCSK9 protein produced, blocking the PCSK9-driven internalization and degradation of LDL receptors. The increased numbers of cell surface LDL receptors then remove more LDL from circulation thereby reducing bloodborne LDL levels. Bottom left: BT200 is an RNA aptamer designed to inhibit aberrant thrombus formation. This aptamer blocks the interaction between VWF (von Willebrand factor) and GP IIb/IIIa receptors on platelet membranes, triggering the blockage of platelet thrombus formation. Bottom right: AZD8601 is an mRNA therapy designed to increase angiogenesis. Optimized VEGF-A mRNAs are packaged in lipid nanoparticles (LNPs) which are endocytosed by the targeted host cells. The mRNA is then translated into VEGF-A protein which increases angiogenesis. Figure generated using BioRender.com
Antisense Oligonucleotide (ASO) Therapeutics
In this section, we briefly discuss ASOs (refer to [14, 15] for more thorough reviews) and highlight the technological aspects that expedited their clinical translation and enabled their development into cardiovascular disease–targeting therapeutics. ASOs are short (18–30 nucleotides in length) synthetic, single-stranded nucleic acids whose sequences are complementary to a cellular RNA target [15]. Importantly, although considered RNA therapeutics, ASOs can be either homo- or mixed polymers consisting of RNA, DNA, and/or LNA (locked nucleic acid) bases [15]. ASOs use base-pairing interactions to (1) disturb or correct the splicing and/or processing of pre-mRNAs or to (2) suppress the translation or (3) induce the degradation of targeted mRNAs [1, 16, 17]. Each of these approaches ultimately modulates the levels of a targeted protein [15]. Many ASOs trigger endogenous RNA degradation pathways by recruiting RNase H1 which is recruited to and degrades the RNA strand of DNA:RNA duplexes [18]. The small size and well-understood principles underpinning ASO sequence design help prevent potential toxicities associated with off-target binding and can be exploited to enhance binding specificity between ASOs and targets [19]. In vivo, ASOs with unmodified phosphodiester bond backbones are rapidly destroyed by serum nucleases and cleared from circulation by the kidneys [20]. Therefore, numerous chemical nucleotide modifications have been introduced to improve the pharmacokinetics and pharmacodynamics of ASOs [9]. For example, phosphodiester linkages of ASOs can be replaced by phosphorothioate linkages to strengthen nuclease resistance and diminish hydrophilicity while maintaining robust RNase H1 activity [9, 21]. Unfortunately, these changes need to be made studiously as certain modified nucleotides were shown to induce a strong immune response and/or lower ASO to target binding affinities when compared to standard unmodified nucleotides [9]. Since it increased ASO stability without affecting ASO targeting, initial base modifications targeted the 2′ position of the ribose sugar. Common modifications include replacing the 2′-hydroxyl moiety with either a 2′-O-methyl, 2′-O-methoxyethyl, 2′-O-aminopropyl, or 2′-fluoro groups to prevent hydrolysis of the ASO [9]. Several other important base modifications or substitutions to alter nucleoside pairing interactions and the molecular conformation of ASO, including the incorporation of LNA bases, restrained ethyl nucleoside analogues, artificial amido-bridged nucleic acids, or other ASO backbone changes [9]. ASOs have also been coupled to ligands (GalNAc for example) to target their delivery to a particular tissue [22]. Finally, helping to reduce production-related costs, due to their heavily modified structures, many ASOs do not require specialized delivery vehicles [9].
Several targets such as proprotein convertase subtilisin/kexin type 9 (PCSK9), lipoprotein(a) (Lp(a)), and ANGPTL3 have been genetically linked to cardiovascular and metabolic diseases [23–25]. In 2019, Pfizer partnered with Akcea Therapeutics (an affiliate of Ionis Pharmaceuticals) to investigate and license AKCEA-ANGPTL3-LRx, an ASO targeting ANGPTL3 [9]. At the same time, Novartis also collaborated with Akcea and Ionis Pharmaceuticals to develop and license AKCEA-APO(a)-LRx, using Ionis’ ligand-conjugated antisense technology platform [9]. Both of these ASOs have entered the Phase II clinical trials (Table 1) and have been showing potential to treat heterozygous familial hypercholesterolemia and atherosclerotic cardiovascular diseases.
As mentioned above, the properties of ASOs and oligonucleotide therapeutics in general allow ASOs to reach every tissue, including the heart, effectively. As with many drugs, ASOs can be targeted to the liver with little or no assistance [9]. Although many ASOs have been approved by the FDA, since 2013 only Mimopersen (Kynamro, Figure 1, top left) has been approved by the FDA as a treatment for a cardiovascular indication (NCT00770146) [26]. Mipomersen is approved as a treatment for homozygous familial hypercholesterolemia (HoFH), a rare genetic disorder where both low-density lipoprotein (LDL) receptor alleles are mutated [26]. Untreated, HoFH leads to reduced clearance of circulating LDL cholesterol in plasma [26]. The Kynamro compound is a “second generation” 2′-O-methoxyethyl chimeric ASO [26]. The ASO is built with phosphorothioate linkages rather than the phosphodiester linkages found in natural RNAs [27]. In addition, the ASO contains DNA nucleotides in the center of the molecule with 2′-O-methoxyethyl-modified RNA nucleotides at the ends [27]. In the liver, this drug initiates the degradation of the mRNA encoding apolipoprotein (Apo)B-100 (Figure 1, top left), a key structural element of LDL and its metabolic precursor, very-low-density lipoprotein [27]. Reduction of ApoB protein then helps reduce LDL cholesterol and lipoprotein(a) (Lp(a)) levels in the blood [27, 28]. A double-blind, randomized, placebo-controlled, Phase III clinical trial (NCT00607373) was completed in 2010 and showed that mipomersen effectively inhibited ApoB protein production by ~25% and reduced LDL cholesterol level in HoFH patients who were already being treated with lipid-lowering drugs [26, 29]. However, several subsequent studies showed the adverse events of Mipomersen in treated patients, including serious injection site reactions and flu-like symptoms [29, 30]. Moreover, a severe risk of liver damage has also been reported. According to liver function tests, around one in three patients receiving Mipomersen exhibited measurable signs of liver toxicity [31–33, 34•]. Therefore, in April 2021, this drug was discontinued on the open market and can only be prescribed in the context of an FDA-approved Risk Evaluation and Mitigation Strategies program.
Another well-known ASO candidate called Volanesorsen (Table 1), which targets the mRNA encoding hepatic apolipoprotein C-III (APOC3), has been shown to reduce plasma triglyceride levels, and has been submitted to the FDA for authorization to market [35, 36]. Ionis Pharmaceuticals and Akcea Therapeutics developed this drug and registered it under the brand name Waylivra. In patients with familial chylomicronemia syndrome (FCS), weekly doses of Volanesorsen markedly reduce triglycerides (1700 mg/dL vs 90 mg/dL compared to placebo treatment) [36]. In 2019, the Phase III APPROACH study also showed mean triglyceride levels decreased 77% in Volanesorsen-treated patients versus an 18% increase in patients in the placebo group [37]. They also revealed that Volanesorsen lowered triglyceride levels below the risk threshold for triglyceride-induced acute pancreatitis [37]. However, since most ASOs can be distributed broadly and accumulate in the liver and kidneys, with half-lives of 2–4 weeks, Volanesorsen showed some evidence of adverse effects associated with thrombocytopenia and risk of bleeding [38, 39]. Despite these side effects, the significant reduction of plasma lipid levels led the European Commission to approve Volanesorsen as the only approved therapy for FCS in 2019 [40].
Numerous second- and third-generation ASOs are currently being developed to treat not only cardiovascular diseases (Table 1), but other life-threatening and rare genetic diseases including spinal muscular atrophy (Spinraza), Duchenne’s muscular dystrophy (Vyondys 53), and hereditary transthyretin amyloidosis (Inotersen) [41–44]. Although the near-term safety of ASOs has been examined in preclinical and clinical trials, the potential consequences of long-term ASO administration still remain unclear [19]. Moreover, some possible adverse effects may happen due to ASO chemistry or downstream effects of target involvement. For these reasons, extended follow-up of patients treated with ASO drugs is required to determine the long-term efficacy and side effects of these ASO therapies. Despite these unknowns, ASOs provide a new approach that has the versatility to improve the quality of life for patients with some previously untreatable diseases.
RNAi: RNA Interference for Gene Silencing
The discovery of RNA interference (RNAi) entirely reshaped how gene expression and regulation was perceived [3, 45]. RNAi is a natural process by which mRNAs are regulated post-transcriptionally [45]. In addition to regulating the expression of endogenous genes, the RNAi pathway also protects an organism from foreign nucleic acids [45]. Targeted, sequence-specific gene silencing offers nearly limitless applications such as defining the function(s) of newly discovered genes, identifying novel and therapeutically relevant genes, and targeting genes previously labeled as “undruggable.” In mammals, RNAi is triggered by short double-stranded RNAs (dsRNAs) from endogenous or exogenous (synthetic RNAs, pathogens) origins. There are two main types of RNAi: small interfering RNAs (siRNAs) and microRNAs (miRNAs) [46]. They both target mRNAs using base-pair recognition and initiate mRNA degradation, which then decreases the levels of the corresponding protein [46]. However, key differences separate the two RNAi mechanisms. For example, siRNAs are perfectly complementary to the targeted mRNA and cause its cleavage and degradation [47], whereas miRNA sequences contain multiple mismatches to their targeted mRNA and initiate mRNA degradation by recruiting decapping enzymes and deadenylases [48]. As they are beyond the scope of this manuscript, other differences and similarities are thoroughly detailed in this review [47].
siRNAs
A standard siRNA drug is a 21- to 25-nucleotide dsRNA. In vivo, these exogenous dsRNAs are trimmed into siRNA precursors by an enzyme called Dicer which leaves a 3′ overhang [49, 50]. The processed siRNA precursor is then loaded into the RNA-induced silencing complex (RISC) which preferentially retains the targeting RNA strand to make the active siRNA [51]. siRNA-mediated gene silencing occurs when a perfectly complementary siRNA sequence triggers the endonuclease “slicing” activity of AGO2 which cleaves the targeted mRNA leading to its degradation thereby reducing protein levels [52]. Therapeutic siRNAs initially faced multiple challenges like immunogenicity, specificity, and instability; however, many studies were performed to optimize the structure and delivery of siRNA drugs. Today, numerous siRNA drugs have obtained FDA approval, while others are currently being tested in clinical trials [8]. Most cardiovascular system–focused siRNA therapeutics or candidate drugs are designed to treat conditions via liver-specific delivery. Inclisiran, sold as Leqvio, (Figure 1, top right) is approved in the EU and was approved by the FDA in the USA as a treatment for primary hypercholesterolemia or mixed dyslipidemia at the end of 2021 53•, 54••]. Inclisiran is an artificial siRNA conjugated with GalNAc on the sense strand to allow for liver-specific delivery [55]. Upon absorption by hepatocytes, Inclisiran targets the mRNA encoding PCSK9 thereby reducing the expression of PCSK9 protein [56]. In so doing, Inclisiran increases cell surface levels of LDL receptor by reducing its turnover which ultimately reduces bloodborne LDL-C levels by increasing the uptake of LDL-C by the liver [53•].
miRNAs
MicroRNAs are short, naturally occurring non-coding RNAs that have vital roles in cellular function via post-transcriptional gene regulation [57, 58]. As mentioned above, miRNAs contain sequence mismatches which can be disadvantageous since they can lead to unwanted off-target effects. However, mismatches can also be beneficial as they can allow for the simultaneous targeting of multiple distinct mRNAs. miRNAs typically bind to the 3′UTR (untranslated region) of mRNAs, and repress their translation or recruit deadenylases and/or decapping enzymes to facilitate the degradation of targeted mRNA(s) [59]. Notably, offering another possible treatment avenue, a small minority of miRNAs have also been reported to upregulate gene expression as reviewed in [60]. Currently, there are no marketed miRNA therapeutics. However, patents and clinical trials for miRNA inhibitors (anti-miRs) and miRNA mimics are on the rise.
miRNA Blockers (Anti-miRs)
Since miRNAs can simultaneously target multiple disease-linked mRNAs and misregulation of miRNAs has been linked to many diseases, repressing well-described miRNAs quickly became an attractive therapeutic approach. Anti-miRs are designed to specifically recognize and inhibit naturally occurring miRNAs. This can be accomplished by targeting miRNAs for degradation or by sequestering the miRNA so it could no longer bind its targets. Both mechanisms can prevent miRNAs from acting on their mRNA targets. Multiple approaches to target miRNAs exist, including antagomiRs (cholesterol-conjugated anti-miRs), locked nucleic acids, and ASOs [61–63]. All of these molecules are designed to bind and sequester miRNAs, thus preventing miRNA-mRNA interactions [64].
miRNA Mimics
miRNA mimics are synthetic RNAs that are patterned after endogenous miRNAs. Unlike anti-miRs which aim at inhibiting miRNAs that are overexpressed in disease, miRNA mimics are designed to replace or supplement the levels of beneficial miRNAs. The therapeutic miRNA mimics are processed similarly to endogenous microRNAs and will reduce the level of specific genes [65]. Current miRNAs mimics in clinical trials are mainly for hepatitis C and different cancers [66].
RNA Aptamers
Unlike other RNA therapeutics, RNA aptamers use their 3D conformation rather than sequence-specific base-pairing to recognize their targets [67]. Similar to an antibody, aptamers (DNA, RNA, or protein-based) bind a desired ligand with very high affinity and selectivity [67]. Although similar in function to protein-based antibodies, RNA aptamer manufacturing is more straightforward, performed entirely in vitro, and more cost-effective compared to protein-based antibodies [68]. RNA aptamers are single-stranded molecules that are isolated using systematic evolution of ligands by exponential enrichment (SELEX) [4, 5]. In SELEX, a pool of RNA is generated, and those binding to desired targets with high specificity are isolated and enriched [4, 5, 68]. RNA aptamers exhibit flexible targeting and have been shown to bind specific molecules, cells, and tissues [68]. In contrast with other RNA therapeutics, RNA aptamers are not restricted to an intracellular target, and they can be designed to bind virtually any molecule in any cell compartment [4, 5]. The binding properties of RNA aptamers also allow them to be conjugated to other therapeutics or delivery vehicles for a tissue-specific delivery [69].
Currently, one RNA aptamer (Pegaptanib) has been approved by the FDA for age-related macular degeneration in 2004 [70]. Pegaptanib is a 28-nucleotide RNA aptamer and functions via binding vascular endothelial growth factor (VEGF) protein and blocking its pro-inflammatory activities in AMD patients, thus preventing serious vision complications [70, 71]. Other candidate RNA aptamer drugs are currently being evaluated in clinical trials. For example, BT200 (Table 1, Figure 1, bottom left) is a pegylated RNA aptamer candidate currently in Phase I or II trials for the treatment of hemophilia A, atherosclerosis, and stroke. BT200 shows promising results and acts as an antithrombotic agent by binding the A1 domain of the von Willebrand factor (VWF), a factor critical for thrombus generation [72•].
Messenger RNA (mRNA) Therapeutics
The proof of concept experiment for mRNA-encoded therapeutics was performed over three decades ago when Wolff et al. showed that administering in vitro transcribed (IVT) mRNA into mouse skeletal muscle resulted in the expression of the protein of interest in vivo [6]. During the 1990s, preclinical trials of IVT mRNA examined a variety of applications including protein replacement and vaccine-based designs to treat or prevent cancer and infectious diseases [6, 11, 73]. Numerous studies quickly discovered several major drawbacks of mRNA therapies, including short RNA half-life and non-specific immunogenicity. The intervening decades have seen the resolution of many of these issues and therapeutic mRNAs are becoming a favored approach. Several universities and pharmaceutical companies including Moderna, BioNTech, Novartis, CureVac, Sanofi Pasteur, Glaxo Smith Kline, AstraZeneca, and Alexion are developing mRNA-based therapeutics [8].
Conceptually, the numerous advantages of IVT mRNA–based therapeutic approaches make them just as or more versatile than other nucleic acid–based therapies. IVT mRNAs are fully functional in the cytoplasm and are rapidly translated to produce the desired proteins [8, 9, 11]. In addition, IVT mRNA–based therapeutics have a better safety profile. Simply, unlike plasmid DNA and certain viral vectors, mRNA therapeutics are incapable of integrating into the genome, and thus eliminate the risk of insertional mutagenesis [11]. Furthermore, IVT mRNA production is relatively manageable and inexpensive; therefore, it has sparked a broad interest in developing this new class of drugs for treatments in oncology, cardiology, endocrinology, hematology, pulmonary medicine, and as vaccines for infectious diseases [8, 9, 11].
Currently, IVT mRNA can be delivered via two approaches. The IVT mRNA can be transferred into the patient’s cells ex vivo, then these modified cells can be delivered back to the patient. The direct delivery of the IVT mRNA to the host using different delivery vehicles is the other alternative [8]. Substantial energy has been invested with the goal of improving the translatability and the in vivo lifespan of IVT mRNA drugs. This includes improvements to optimize structural components of the IVT mRNA including the 5′ cap, 5′- and 3′-untranslated regions, the coding sequences, and the polyadenylated tail of the mRNA. The immune-stimulatory profile of IVT mRNA can be altered and customized based on therapeutics purposes. As an example, for an mRNA-based vaccination strategy, the immune-stimulatory effect associated with IVT mRNA could be considered a benefit as it could help drive antigen-specific cellular and humoral immune responses. However, innate immune activation is a major hurdle for protein replacement therapies; therefore, several approaches aim to create “de-immunized” mRNA have been, and continue to be, developed to overcome this problem [74].
Despite the potential of mRNA therapies to treat cardiovascular diseases, currently only one, named AZD8601 (Figure 1, bottom right), which encodes vascular endothelial growth factor-A (VEGF-A), is being jointly developed by AstraZeneca and Moderna [75]. When given to patients after a heart attack, or those with heart failure, diabetic wound healing problems, or other ischemic vascular diseases, AZD8601 could prove to be a regenerative treatment option [76•]. AZD8601 is VEGF-A165 mRNA in buffered saline [76•]. This drug was optimized to overexpress VEGF-A while minimizing innate immune activation [76•]. AZD8601 is currently being evaluated in the EPICCURE (NCT03370887) Phase II clinical trial, a double-blind, randomized, placebo-controlled, multicenter, 6-month trial, with 24 patients scheduled for elective bypass surgery, including 3 groups of 8 patients who were randomized to receive either 3 mg AZD8601 (low dose), 30 mg AZD8601 (high dose), or placebo injection [76•]. During the first-in-human Phase I trial, the expression of functional VEGF-A was validated after AZD8601 administration [73]. AZD8601 also induced new blood vessel formation without an elevated innate immune response in human volunteers [76•]. The EPICCURE trial is designed to use quantitative 15O-water PET imaging to map ischemic but viable myocardium [76•]. However, this trial was limited to patients that undergo coronary artery bypass grafting; therefore, it is more difficult to assess the adverse events during drug administration or surgery [76•]. In summary, EPICCURE integrated innovative VEGF-A mRNA delivery with novel ischemia-guided administration to assess the safety and potentially beneficial angiogenic effects of AZD8601 on cardiac function and myocardial perfusion in patients with coronary artery dysfunction that require bypass surgery [76•].
Conclusions
The unquestionable success of the two mRNA vaccines for COVID-19 has shown the world the power and versatility of RNA-based therapeutics. This increased awareness has translated into an unparalleled surge of resources to develop new RNA medicines. However, with fewer than 40 ongoing or completed clinical trials evaluating different cardiovascular disease–targeting RNA drugs, RNA therapeutics remain a comparatively untapped source of treatments for these indications. The mRNA-based technologies described here amount to one of the most promising approaches for future drug development and can be applied to a broad range of potential applications.
Author Contribution
NB and TT contributed equally to this work and all authors acknowledge that both authors can list themselves as first author on career materials such as curriculum vitae. NB, TT, and DLK all performed the literature searches and wrote and revised sections of the manuscript. NB and TT created and edited the table and figure. DLK incorporated figure, table, and text revisions from all authors. All authors approved the final submitted manuscript.
Funding
This work was supported by a Houston Methodist Research Institution (HMRI) Career Cornerstone Award and a grant from the American Heart Association (20CDA35310329, both to DLK) and by grants from the Cancer Prevention and Research Institute of Texas (RP150611 and RP200619) to the RNA Core at the HMRI. The content presented here is solely the responsibility of the authors and does not represent the official views of the HMRI, CPRIT, or the American Heart Association.
Declarations
Conflict of Interest
Dr. Kiss runs an externally funded lab (American Heart Association [20CDA35310329], and NIH [R35GM137819-02, -02S1, -02S2]) that is actively designing and testing different candidate RNA therapeutics, including some with possible applications in cardiovascular disease. All authors anticipate seeking appropriate intellectual property protection for promising candidates that emerge from the lab’s work. Dr. Kiss has patents planned for circular RNA therapeutics and an inducible stable HUVEC cell line. Further, Dr. Kiss serves as a consultant for the RNA Core at the Houston Methodist Research Institute. In addition, Dr. Kiss reports private stock for BioLife Solutions Inc. Dr. Bejar is named as a co-inventor on a provisional patent filing for a candidate RNA therapeutic (EFS ID: 44070986 pending). Dr. Tat has a patent planned for circular RNA therapeutics.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Nada Bejar and Trinh T. Tat contributed equally
Contributor Information
Nada Bejar, Email: nbejar@houstonmethodist.org.
Trinh T. Tat, Email: ttat@houstonmethodist.org
Daniel L. Kiss, Email: dlkiss@houstonmethodist.org
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Stephenson ML, Zamecnik PC. Inhibition of Rous sarcoma viral RNA translation by a specific oligodeoxyribonucleotide. Proc Natl Acad Sci U S A. 1978;75(1):285–288. doi: 10.1073/pnas.75.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75(5):843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 3.Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391(6669):806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 4.Ellington AD, Szostak JW. In vitro selection of RNA molecules that bind specific ligands. Nature. 1990;346(6287):818–822. doi: 10.1038/346818a0. [DOI] [PubMed] [Google Scholar]
- 5.Tuerk C, Gold L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science. 1990;249(4968):505–510. doi: 10.1126/science.2200121. [DOI] [PubMed] [Google Scholar]
- 6.Wolff JA, Malone RW, Williams P, Chong W, Acsadi G, Jani A, et al. Direct gene transfer into mouse muscle in vivo. Science. 1990;247(4949 Pt 1):1465–1468. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- 7•.Jirikowski GF, Sanna PP, Maciejewski-Lenoir D, Bloom FE. Reversal of diabetes insipidus in Brattleboro rats: intrahypothalamic injection of vasopressin mRNA. Science. 1992;255(5047):996-998. doi:10.1126/science.1546298. First use of an exogenous mRNA to correct a medical condition in an animal model. [DOI] [PubMed]
- 8.Damase TR, Sukhovershin R, Boada C, Taraballi F, Pettigrew RI, Cooke JP. The limitless future of RNA therapeutics. Front Bioeng Biotechnol. 2021;9:628137. doi: 10.3389/fbioe.2021.628137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kulkarni JA, Witzigmann D, Thomson SB, Chen S, Leavitt BR, Cullis PR, et al. The current landscape of nucleic acid therapeutics. Nat Nanotechnol. 2021;16(6):630–643. doi: 10.1038/s41565-021-00898-0. [DOI] [PubMed] [Google Scholar]
- 10•.Kariko K, Buckstein M, Ni H, Weissman D. Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity. 2005;23(2):165-175. doi:10.1016/j.immuni.2005.06.008. The seminal paper which showed that incorporating modified nucleotides allowed synthetic mRNAs to evade the immune system. [DOI] [PubMed]
- 11.Sahin U, Kariko K, Tureci O. mRNA-based therapeutics--developing a new class of drugs. Nat Rev Drug Discov. 2014;13(10):759–780. doi: 10.1038/nrd4278. [DOI] [PubMed] [Google Scholar]
- 12••.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–15. 10.1056/NEJMoa2034577 undefined. [DOI] [PMC free article] [PubMed]
- 13••.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–16. 10.1056/NEJMoa2035389Findings from research established the efficacy and safety of one of the first mRNA-based vaccine/therapeutics for COVID-19 (Moderna).undefined. [DOI] [PMC free article] [PubMed]
- 14.Crooke ST, Baker BF, Crooke RM, Liang XH. Antisense technology: an overview and prospectus. Nat Rev Drug Discov. 2021;20(6):427–453. doi: 10.1038/s41573-021-00162-z. [DOI] [PubMed] [Google Scholar]
- 15.Crooke ST, Liang XH, Baker BF, Crooke RM. Antisense technology: a review. J Biol Chem. 2021;296:100416. doi: 10.1016/j.jbc.2021.100416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baker BF, Lot SS, Condon TP, Cheng-Flournoy S, Lesnik EA, Sasmor HM, et al. 2′-O-(2-Methoxy)ethyl-modified anti-intercellular adhesion molecule 1 (ICAM-1) oligonucleotides selectively increase the ICAM-1 mRNA level and inhibit formation of the ICAM-1 translation initiation complex in human umbilical vein endothelial cells. J Biol Chem. 1997;272(18):11994–12000. doi: 10.1074/jbc.272.18.11994. [DOI] [PubMed] [Google Scholar]
- 17.Hua Y, Vickers TA, Baker BF, Bennett CF, Krainer AR. Enhancement of SMN2 exon 7 inclusion by antisense oligonucleotides targeting the exon. PLoS Biol. 2007;5(4):e73. doi: 10.1371/journal.pbio.0050073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Minshull J, Hunt T. The use of single-stranded DNA and RNase H to promote quantitative ‘hybrid arrest of translation’ of mRNA/DNA hybrids in reticulocyte lysate cell-free translations. Nucleic Acids Res. 1986;14(16):6433–6451. doi: 10.1093/nar/14.16.6433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roberts TC, Langer R, Wood MJA. Advances in oligonucleotide drug delivery. Nat Rev Drug Discov. 2020;19(10):673–694. doi: 10.1038/s41573-020-0075-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goodchild J, Kim B, Zamecnik PC. The clearance and degradation of oligodeoxynucleotides following intravenous injection into rabbits. Antisense Res Dev. 1991;1(2):153–160. doi: 10.1089/ard.1991.1.153. [DOI] [PubMed] [Google Scholar]
- 21.Crooke ST, Seth PP, Vickers TA, Liang XH. The interaction of phosphorothioate-containing RNA targeted drugs with proteins is a critical determinant of the therapeutic effects of these agents. J Am Chem Soc. 2020;142(35):14754–14771. doi: 10.1021/jacs.0c04928. [DOI] [PubMed] [Google Scholar]
- 22.Prakash TP, Graham MJ, Yu J, Carty R, Low A, Chappell A, et al. Targeted delivery of antisense oligonucleotides to hepatocytes using triantennary N-acetyl galactosamine improves potency 10-fold in mice. Nucleic Acids Res. 2014;42(13):8796–8807. doi: 10.1093/nar/gku531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tavori H, Christian D, Minnier J, Plubell D, Shapiro MD, Yeang C, et al. PCSK9 association with lipoprotein(a) Circ Res. 2016;119(1):29–35. doi: 10.1161/CIRCRESAHA.116.308811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lim GB. Dyslipidaemia: ANGPTL3: a therapeutic target for atherosclerosis. Nat Rev Cardiol. 2017;14(7):381. doi: 10.1038/nrcardio.2017.91. [DOI] [PubMed] [Google Scholar]
- 25.Tsimikas S. A test in context: lipoprotein(a): diagnosis, prognosis, controversies, and emerging therapies. J Am Coll Cardiol. 2017;69(6):692–711. doi: 10.1016/j.jacc.2016.11.042. [DOI] [PubMed] [Google Scholar]
- 26.Raal FJ, Santos RD, Blom DJ, Marais AD, Charng MJ, Cromwell WC, et al. Mipomersen, an apolipoprotein B synthesis inhibitor, for lowering of LDL cholesterol concentrations in patients with homozygous familial hypercholesterolaemia: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;375(9719):998–1006. doi: 10.1016/S0140-6736(10)60284-X. [DOI] [PubMed] [Google Scholar]
- 27.Geary RS, Baker BF, Crooke ST. Clinical and preclinical pharmacokinetics and pharmacodynamics of mipomersen (kynamro((R))): a second-generation antisense oligonucleotide inhibitor of apolipoprotein B. Clin Pharmacokinet. 2015;54(2):133–146. doi: 10.1007/s40262-014-0224-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kastelein JJ, Wedel MK, Baker BF, Su J, Bradley JD, Yu RZ, et al. Potent reduction of apolipoprotein B and low-density lipoprotein cholesterol by short-term administration of an antisense inhibitor of apolipoprotein B. Circulation. 2006;114(16):1729–1735. doi: 10.1161/CIRCULATIONAHA.105.606442. [DOI] [PubMed] [Google Scholar]
- 29.Laina A, Gatsiou A, Georgiopoulos G, Stamatelopoulos K, Stellos K. RNA therapeutics in cardiovascular precision medicine. Front Physiol. 2018;9:953. doi: 10.3389/fphys.2018.00953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomas GS, Cromwell WC, Ali S, Chin W, Flaim JD, Davidson M. Mipomersen, an apolipoprotein B synthesis inhibitor, reduces atherogenic lipoproteins in patients with severe hypercholesterolemia at high cardiovascular risk: a randomized, double-blind, placebo-controlled trial. J Am Coll Cardiol. 2013;62(23):2178–2184. doi: 10.1016/j.jacc.2013.07.081. [DOI] [PubMed] [Google Scholar]
- 31.Swayze EE, Siwkowski AM, Wancewicz EV, Migawa MT, Wyrzykiewicz TK, Hung G, et al. Antisense oligonucleotides containing locked nucleic acid improve potency but cause significant hepatotoxicity in animals. Nucleic Acids Res. 2007;35(2):687–700. doi: 10.1093/nar/gkl1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Visser ME, Wagener G, Baker BF, Geary RS, Donovan JM, Beuers UH, et al. Mipomersen, an apolipoprotein B synthesis inhibitor, lowers low-density lipoprotein cholesterol in high-risk statin-intolerant patients: a randomized, double-blind, placebo-controlled trial. Eur Heart J. 2012;33(9):1142–1149. doi: 10.1093/eurheartj/ehs023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duell PB, Santos RD, Kirwan BA, Witztum JL, Tsimikas S, Kastelein JJP. Long-term mipomersen treatment is associated with a reduction in cardiovascular events in patients with familial hypercholesterolemia. J Clin Lipidol. 2016;10(4):1011–1021. doi: 10.1016/j.jacl.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 34•.Fogacci F, Ferri N, Toth PP, Ruscica M, Corsini A, Cicero AFG. Efficacy and safety of mipomersen: a systematic review and meta-analysis of randomized clinical trials. Drugs. 2019;79(7):751–66. 10.1007/s40265-019-01114-zThis paper assesses the efficacy and safety of the marketed ASO as lipid-lowering medicine mipomersen.undefined. [DOI] [PubMed]
- 35.Ioanna Gouni-Berthold VJA. Yang Q, Hurh E, Steinhagen-Thiessen E, Moriarty PM, Hughes SG, Gaudet D, Hegele RA, St L O’ Dea L, Stroes ESG, Tsimikas S, Witztum JL. Efficacy and safety of volanesorsen in patients with multifactorial chylomicronaemia (COMPASS): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Diabet Endocrinol. 2021;9(5):264–275. doi: 10.1016/S2213-8587(21)00046-2. [DOI] [PubMed] [Google Scholar]
- 36.Crooke ST, Witztum JL, Bennett CF, Baker BF. RNA-targeted therapeutics. Cell Metab. 2019;29(2):501. doi: 10.1016/j.cmet.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 37.Witztum JL, Gaudet D, Freedman SD, Alexander VJ, Digenio A, Williams KR, et al. Volanesorsen and triglyceride levels in familial chylomicronemia syndrome. N Engl J Med. 2019;381(6):531–542. doi: 10.1056/NEJMoa1715944. [DOI] [PubMed] [Google Scholar]
- 38.Gaudet AD D, Alexander V, Arca M, Jones A, Stroes E, Bergeron J, Civeira F, Hemphill L, Blom D, Flaim J, Hughes S, Geary R, Tsimikas S, Witztum J, Bruckert E. The approach study: a randomized, double-blind, placebo-controlled, phase 3 study of volanesorsen administered subcutaneously to patients with familial chylomicronemia syndrome (FCS) Atherosclerosis. 2017;263:e10-e. doi: 10.1016/j.atherosclerosis.2017.06.059. [DOI] [Google Scholar]
- 39.Karine Tremblay DBaDG Natural history and gene expression signature of platelet count in lipoprotein lipase deficiency. Atherosclerosis. 2017;263:e100. doi: 10.1016/j.atherosclerosis.2017.06.325. [DOI] [Google Scholar]
- 40.Paik J, Duggan S. Volanesorsen: first global approval. Drugs. 2019;79(12):1349–1354. doi: 10.1007/s40265-019-01168-z. [DOI] [PubMed] [Google Scholar]
- 41.Nusinersen (Spinraza) for spinal muscular atrophy. Med Lett Drugs Ther. 2017;59(1517):50–2. [PubMed]
- 42.Golodirsen (Vyondys 53) for Duchenne muscular dystrophy. Med Lett Drugs Ther. 2020;62(1603):119–20. [PubMed]
- 43.Keam SJ. Inotersen: first global approval. Drugs. 2018;78(13):1371–1376. doi: 10.1007/s40265-018-0968-5. [DOI] [PubMed] [Google Scholar]
- 44.Benson MD. Inotersen treatment for ATTR amyloidosis. Amyloid. 2019;26(sup1):27–28. doi: 10.1080/13506129.2019.1582497. [DOI] [PubMed] [Google Scholar]
- 45.Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411(6836):494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 46.Valencia-Sanchez MA, Liu J, Hannon GJ, Parker R. Control of translation and mRNA degradation by miRNAs and siRNAs. Genes Dev. 2006;20(5):515–524. doi: 10.1101/gad.1399806. [DOI] [PubMed] [Google Scholar]
- 47.Lam JK, Chow MY, Zhang Y, Leung SW. siRNA versus miRNA as therapeutics for gene silencing. Mol Ther Nucleic Acids. 2015;4:e252. doi: 10.1038/mtna.2015.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huntzinger E, Izaurralde E. Gene silencing by microRNAs: contributions of translational repression and mRNA decay. Nat Rev Genet. 2011;12(2):99–110. doi: 10.1038/nrg2936. [DOI] [PubMed] [Google Scholar]
- 49.Zhang H, Kolb FA, Jaskiewicz L, Westhof E, Filipowicz W. Single processing center models for human Dicer and bacterial RNase III. Cell. 2004;118(1):57–68. doi: 10.1016/j.cell.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 50.Bernstein E, Caudy AA, Hammond SM, Hannon GJ. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature. 2001;409(6818):363–366. doi: 10.1038/35053110. [DOI] [PubMed] [Google Scholar]
- 51.Scherer LJ, Rossi JJ. Approaches for the sequence-specific knockdown of mRNA. Nat Biotechnol. 2003;21(12):1457–1465. doi: 10.1038/nbt915. [DOI] [PubMed] [Google Scholar]
- 52.Meister G, Landthaler M, Patkaniowska A, Dorsett Y, Teng G, Tuschl T. Human Argonaute2 mediates RNA cleavage targeted by miRNAs and siRNAs. Mol Cell. 2004;15(2):185–197. doi: 10.1016/j.molcel.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 53•.Lamb YN. Inclisiran: first approval. Drugs. 2021;81(3):389–95. 10.1007/s40265-021-01473-6 undefined. [DOI] [PMC free article] [PubMed]
- 54••.Administration USFDA. FDA approves add-on therapy to lower cholesterol among certain high-risk adults. FDA Archive. 2021. doi:https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-add-therapy-lower-cholesterol-among-certain-high-risk-adults. Accessed 10 Jan 2022. FDA approves inclisiran, one of the first siRNAs shown to lower cholesterol.
- 55.Nair JK, Willoughby JL, Chan A, Charisse K, Alam MR, Wang Q, et al. Multivalent N-acetylgalactosamine-conjugated siRNA localizes in hepatocytes and elicits robust RNAi-mediated gene silencing. J Am Chem Soc. 2014;136(49):16958–16961. doi: 10.1021/ja505986a. [DOI] [PubMed] [Google Scholar]
- 56.Raal FJ, Kallend D, Ray KK, Turner T, Koenig W, Wright RS, et al. Inclisiran for the treatment of heterozygous familial hypercholesterolemia. N Engl J Med. 2020;382(16):1520–1530. doi: 10.1056/NEJMoa1913805. [DOI] [PubMed] [Google Scholar]
- 57.Rupaimoole R, Slack FJ. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov. 2017;16(3):203–222. doi: 10.1038/nrd.2016.246. [DOI] [PubMed] [Google Scholar]
- 58.Treiber T, Treiber N, Meister G. Regulation of microRNA biogenesis and its crosstalk with other cellular pathways. Nat Rev Mol Cell Biol. 2019;20(1):5–20. doi: 10.1038/s41580-018-0059-1. [DOI] [PubMed] [Google Scholar]
- 59.O’Brien J, Hayder H, Zayed Y, Peng C. Overview of microRNA biogenesis, mechanisms of actions, and circulation. Front Endocrinol (Lausanne) 2018;9:402. doi: 10.3389/fendo.2018.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vasudevan S. Posttranscriptional upregulation by microRNAs. Wiley Interdiscip Rev RNA. 2012;3(3):311–330. doi: 10.1002/wrna.121. [DOI] [PubMed] [Google Scholar]
- 61.Winkle M, El-Daly SM, Fabbri M, Calin GA. Noncoding RNA therapeutics - challenges and potential solutions. Nat Rev Drug Discov. 2021;20(8):629–651. doi: 10.1038/s41573-021-00219-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Krutzfeldt J, Rajewsky N, Braich R, Rajeev KG, Tuschl T, Manoharan M, et al. Silencing of microRNAs in vivo with ‘antagomirs’. Nature. 2005;438(7068):685–689. doi: 10.1038/nature04303. [DOI] [PubMed] [Google Scholar]
- 63.Orom UA, Kauppinen S, Lund AH. LNA-modified oligonucleotides mediate specific inhibition of microRNA function. Gene. 2006;372:137–141. doi: 10.1016/j.gene.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 64.Li Z, Rana TM. Therapeutic targeting of microRNAs: current status and future challenges. Nat Rev Drug Discov. 2014;13(8):622–638. doi: 10.1038/nrd4359. [DOI] [PubMed] [Google Scholar]
- 65.Bader AG, Brown D, Stoudemire J, Lammers P. Developing therapeutic microRNAs for cancer. Gene Ther. 2011;18(12):1121–1126. doi: 10.1038/gt.2011.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou LY, Qin Z, Zhu YH, He ZY, Xu T. Current RNA-based therapeutics in clinical trials. Curr Gene Ther. 2019;19(3):172–196. doi: 10.2174/1566523219666190719100526. [DOI] [PubMed] [Google Scholar]
- 67.Zhou J, Bobbin ML, Burnett JC, Rossi JJ. Current progress of RNA aptamer-based therapeutics. Front Genet. 2012;3:234. doi: 10.3389/fgene.2012.00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Talap J, Zhao J, Shen M, Song Z, Zhou H, Kang Y, et al. Recent advances in therapeutic nucleic acids and their analytical methods. J Pharm Biomed Anal. 2021;206:114368. doi: 10.1016/j.jpba.2021.114368. [DOI] [PubMed] [Google Scholar]
- 69.Keefe AD, Pai S, Ellington A. Aptamers as therapeutics. Nat Rev Drug Discov. 2010;9(7):537–550. doi: 10.1038/nrd3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vinores SA. Pegaptanib in the treatment of wet, age-related macular degeneration. Int J Nanomedicine. 2006;1(3):263–268. [PMC free article] [PubMed] [Google Scholar]
- 71.Odeh F, Nsairat H, Alshaer W, Ismail MA, Esawi E, Qaqish B, et al. Aptamers chemistry: chemical modifications and conjugation strategies. Molecules. 2019;25(1). 10.3390/molecules25010003. [DOI] [PMC free article] [PubMed]
- 72•.Kovacevic KD, Greisenegger S, Langer A, Gelbenegger G, Buchtele N, Pabinger I, et al. The aptamer BT200 blocks von Willebrand factor and platelet function in blood of stroke patients. Sci Rep. 2021;11(1):3092. 10.1038/s41598-021-82747-7This finding describes a new and interesting candidate RNA aptamer for preventing thrombosis.undefined. [DOI] [PMC free article] [PubMed]
- 73.Zangi L, Lui KO, von Gise A, Ma Q, Ebina W, Ptaszek LM, et al. Modified mRNA directs the fate of heart progenitor cells and induces vascular regeneration after myocardial infarction. Nat Biotechnol. 2013;31(10):898–907. doi: 10.1038/nbt.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zimmermann O, Homann JM, Bangert A, Muller AM, Hristov G, Goeser S, et al. Successful use of mRNA-nucleofection for overexpression of interleukin-10 in murine monocytes/macrophages for anti-inflammatory therapy in a murine model of autoimmune myocarditis. J Am Heart Assoc. 2012;1(6):e003293. doi: 10.1161/JAHA.112.003293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gan L-M, Lagerström-Fermér M, Carlsson LG, Arfvidsson C, Egnell A-C, Rudvik A, et al. Intradermal delivery of modified mRNA encoding VEGF-A in patients with type 2 diabetes. Nat Commun. 2019;10(1):871. doi: 10.1038/s41467-019-08852-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76•.Anttila V, Saraste A, Knuuti J, Jaakkola P, Hedman M, Svedlund S, et al. Synthetic mRNA encoding VEGF-A in patients undergoing coronary artery bypass grafting: design of a phase 2a clinical trial. Mol Ther Methods Clin Dev. 2020;18:464–72. 10.1016/j.omtm.2020.05.030The mechanism of action of the only synthetic mRNA therapeutic candidate (AZD8601) for cardiovascular diseases for expression of VEGF-A protein is described.undefined. [DOI] [PMC free article] [PubMed]