Abstract
Background
To highlight the clinical presentations and management outcomes of rhino-orbital mucormycosis during first wave of COVID-19 pandemic in North India.
Methods
A retrospective observational study. 15 patients with mucormycosis (orbital disease) who presented during short span of 3 months (October–December 2020) in a tertiary-care referral institution were analysed.
Results
At presentation, 13 of 15 patients had uncontrolled diabetes. Four had history of COVID-19 infection. All patients had advanced orbital disease with sinusitis; cavernous sinus involvement was in nine and intracranial spread in three patients. Liposomal amphotericin-B was started and prompt orbital exenteration with sinus surgery was performed in 12 patients. All 12 patients survived with an average follow-up of 4.8 months.
Conclusion
In the present series, cases with orbital spread of mucormycosis were mostly found in non-COVID uncontrolled diabetics. Exenteration was done in 80% of cases with advanced orbital disease. Prevention and early detection of infection at the stage of sino-nasal involvement might help to prevent spread and/or halt the orbital disease.
Keywords: Orbital mucormycosis, COVID-19, Aseptate fungus, Diabetes, Exenteration
Introduction
Orbital mucormycosis or zygomycosis is an aggressive and necrotising form of fungal infection that usually occurs secondary to the infection of the nasal cavity and paranasal sinuses. It is caused by mould fungus of order Mucorales, genera Rhizopus sp. and Mucor sp. commonly by the former. In the pre-COVID era, the reported overall incidence of orbital mucormycosis amongst diabetics was 0.15%.1 Immunocompromised condition such as severe neutropenia, haematological malignancies, and chronically debilitating disease can also predispose patients to fungal infection.2,3 Rhino-orbital–cerebral mucormycosis (ROM) has been the most frequent (55.6%) form of presentation.4
During the first wave of the COVID-19 pandemic, immediately after the surge in the number of cases in North India, we noticed a sudden increase in the number of cases admitted to our hospital with the orbital spread of mucormycosis. We hereby, report the clinical features, and management outcomes in 15 cases of ROM, managed at our institute, for 3 months (October–December, 2020). We also reviewed the literature on COVID-19, and ROM published in the literature.
Materials and methods
This is a retrospective observational study of patients with orbital spread of mucormycosis who presented at our institute from October to December, 2020, immediately after the peak of COVID-19 cases in North India. Written informed consent was obtained and the study was conducted following the Declaration of Helsinki. Institute ethics committee approval was obtained. All tissues were sent for microbiological and histopathological studies. On direct microscopy, patients who showed the presence of broad, aseptate hyphae with right angle branching and/or twisted, ribbon like hyphae were included in the study. Data was collected and analysed for age, gender, clinical presentation, history of COVID-19, imaging features, and management outcomes. A literature search was done on PubMed using keywords, orbital mucormycosis, ROM, COVID-19, corona infection.
Results
Basic demographics and clinical history
In the study period, 15 patients with proven ROM were recruited of which twelve patients were males, and three were females. The mean age of presentation was 43.9 + 18.1 years (range 14–66 years). Out of 15 patients, 13 had a history of diabetes mellitus of which two patients (cases 4 & 5) were diagnosed with diabetes mellitus on admission. All the 13 patients had uncontrolled diabetes with high levels of HbA1c (range 8.5–15.5%) with 2 patients (cases 8 & 10) also had diabetic ketoacidosis. Case 9 had acute lymphoblastic leukaemia and was on oral steroids for the past three years and the other patient (case 12) had a recent history of acute hepatitis A. Of all the patients four (cases 4, 11, 12, & 15) had a history of COVID-19 infection, following which they developed features of mucormycosis within 10–15 days of onset of infection. Of these patients, three (cases 4, 11, & 15) had a history of steroid use for the management of the COVID-19 infection. KOH-calcofluor white stain performed on the nasal biopsy was found to be positive for aseptate hyphae in 14 of 15 patients. In one patient (case 13), due to repeated negative nasal biopsies, the orbital biopsy was performed, which was positive for aseptate hyphae. In one patient (case 7), both septate and aseptate hyphae on KOH-calcofluor white stain was present, but culture had the growth of Aspergillus sp. only. Rhizopus was reported in one patient (case 1) and fungal culture report was negative in five cases.
Clinical features at presentation (Table 1)
Table 1.
Summary of clinical presentation, management, and outcomes of our cases.
| Case, No., age/sex | Visual acuity | Eye involved | Clinical features at presentation | Predisposing factors | Surgery performed | Survival outcome/last FU |
|---|---|---|---|---|---|---|
| Case 1 14/M | No PL | R | P, CC, TO, R - positive, CRAO | DM for 5 years | Exenteration, sinus debridement with maxillectomy | Survived, 5 months |
| Case 2 35/M | No PL | R | P, CC, TO, R- positive, CRAO | DM for 2 years | Exenteration, sinus debridement with maxillectomy | Survived, 6 months |
| Case 3 38/M | No PL | R | P, CC, TO, R- positive, CRAO | DM for 8 years | Exenteration with sinus debridement | Survived, 6 months |
| Case 4 45/F | No PL | R | P, CC, TO, R- positive, CRAO | DM for 10 days COVID-19 | Exenteration with sinus debridement | Survived, 5 months |
| Case 5 26/M | No PL | L | P, CC, TO, R- positive, CRAO | DM for 15 days | Exenteration, sinus debridement with ethmoidectomy | Survived, 5 months |
| Case 6 56/F | No PL | R | P, CC, TO, R- positive, CRAO | DM for 3 years | Exenteration, sinus debridement with maxillectomy | Survived, 5 months |
| Case 7 64/M | No PL | R | P, CC, TO, R- positive, pan ophthalmitis | DM for18 months | Exenteration, sinus debridement with maxillectomy | Survived, 4 months |
| Case 8 53/M | Vision could not be assessed | R | P, CC, R- positive, CRAO | DM for 8 years | Surgery deferred as the patient was on ventilator; Intra-orbital amphotericin B injections given | Death due to multi-organ failure on day 16 |
| Case 9 59/F | 20/80 | R | P, CC, R- positive | ALL on steroids for 4 years | Surgery deferred because of low platelet counts | Death on day 21 due to rapid progression of infection |
| Case 10 66/M | Vision could not be assessed | L | P, CC, Corneal haze, R-positive | DM for 8 years | Surgery deferred as patient was on ventilator | Death on day 7 |
| Case11 55/M | No PL | L | P, CC, TO, R- positive, CRAO | DM for 12 days, COVID-19 | Exenteration with sinus debridement | Survived, 4 months |
| Case 12 28/M | No PL | R | P, CC, TO, R- positive, CRAO | Hepatitis A, COVID-19 | Exenteration with sinus debridement | Survived, 6 months |
| Case13 32/M |
No PL | R | P, CC, TO, R- positive, CRAO | DM for 5years | Exenteration with sinus debridement | Survived, 4 months |
| Case 14 34/M |
No PL | R | P, CC, TO, R- positive, CRAO | DM for 4years | Exenteration with sinus debridement | Survived, 4 months |
| Case 15 61/F |
No PL | R | P, CC, TO, R- positive, CRAO | DM for 2years, COVID-19 | Exenteration with sinus debridement | Survived, 4 months |
M, male; F, female; PL, light perception; R, right; L, left; P, proptosis; CC, conjunctival congestion & chemosis; TO, total ophthalmoplegia; R, retropulsion test; CRAO, central retina artery occlusion; DM, diabetes mellitus; ALL, acute lymphoblastic leukaemia; FU, follow-up
The mean duration of ocular symptoms was 10.4 + 2.2 days (range 5–25 days). At presentation, 12 of 15 patients were systemically stable; two patients required ventilator support (cases 8 & 10) and one patient (case 7) had hemiplegia.
Out of 15 patients, twelve had a visual acuity of no perception of light. In one patient (case 9), visual acuity was 20/80. Moderate to severe proptosis, ptosis, total ophthalmoplegia, conjunctival congestion, and chemosis, were the presenting signs in all the patients except two patients with advanced disease who were on ventilator and assessment of ocular motility, ptosis, and visual acuity could not be done. The retropulsion test was significantly positive in all the patients. The cornea was clear in 13/15 patients. Central retinal artery occlusion was found in 12/15 patients. In two patients (cases 10 & 7), fundus could not be assessed because of corneal haze. Case 7 developed pan ophthalmitis, seven days following wound dehiscence of small incision cataract surgery, thereafter he developed proptosis.
Imaging at presentation
Contrast-enhanced imaging revealed the presence of proptosis with diffuse orbital soft tissue involvement till the apex in 14 patients. There was predominantly medial orbit involvement in one patient (case 8). Globe tenting was present in three patients (cases 1, 2, & 6) suggestive of extensive involvement of the orbital tissues. Cavernous sinus thrombosis was found in nine patients (cases 1, 2, 4, 7–10, 12, 15). Pansinusitis was present in 14/15 patients. Case 13 had maxillary and ethmoid sinusitis. Involvement of the central nervous system was present in three patients (Cases 7, 8, & 10). Fig. 1, Fig. 2B; shows the clinical presentation and imaging characteristics of some of our cases.
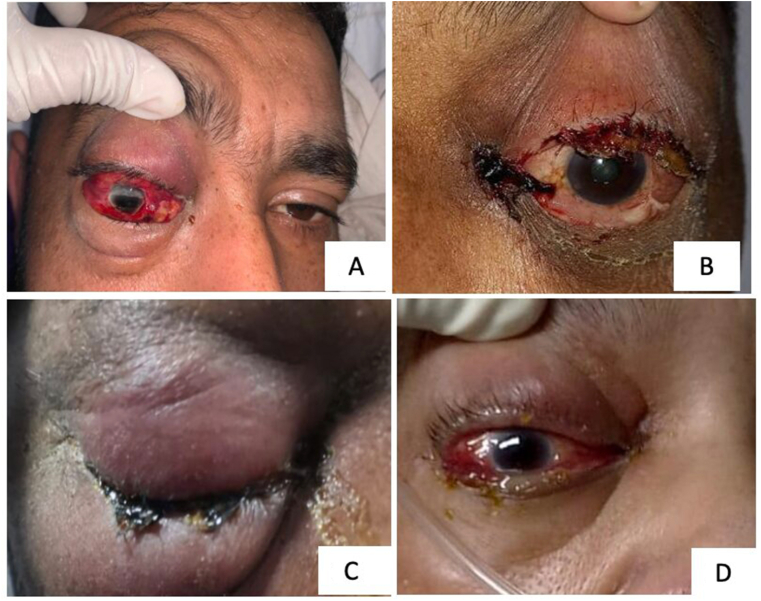
Fig. 1.
(A) Case 2, Right eye showing extensive inflammation with associated lid edema, erythema, subconjunctival hemorrhage, and conjunctival chemosis; (B) Case 11, Left eye shows complete ptosis, with conjunctival congestion and chemosis and necrosis near medial canthus. (C) Case 14, Right eye both upper and lower eyelid extensive erythema and induration with proptosis; (D) Case 15, Right eye shows proptosis and conjunctival chemosis with the tense eyelid.
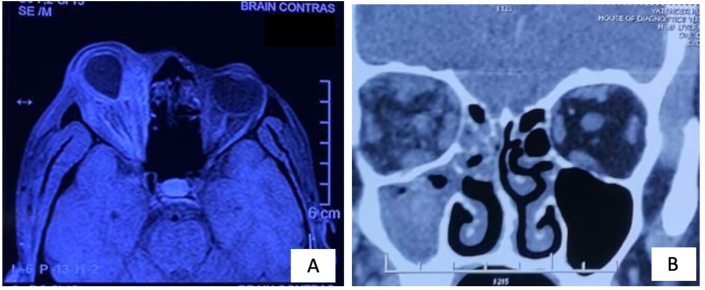
Fig. 2.
(A) Case 2, T1 weighted MRI scan (axial cut) shows diffuse orbital soft tissue inflammation reaching up to the apex associated with globe tenting. (B) Case 14, CT scan (coronal cut) shows heterogeneous opacities with diffuse fat stranding in the right orbit along with maxillary and ethmoid sinuses opacification.
Management
In view of proven ROM, all the patients were admitted and started with intravenous liposomal amphotericin B.5 A multidisciplinary surgical approach was planned in every case involving an otolaryngologist and an oculoplastic surgeon. Orbital exenteration along with sinus debridement was performed in 12 patients. In addition, maxillectomy was performed in four patients and ethmoidectomy in one patient, depending upon the extent of bone necrosis. Histopathology revealed extensive tissue necrosis, angioinvasion, presence of inflammatory cells, and fungal hyphae consistent with mucormycosis (Fig. 3A–D). In three patients, surgical intervention could not be performed as patients were systemically unstable, of which 1 patient (case 8) received injection amphotericin B in the medial orbit as the disease was predominantly in the medial orbit. Seven intra-orbital injections of amphotericin B in a dose of 3 ml (1 mg/ml) were given for 14 days.6
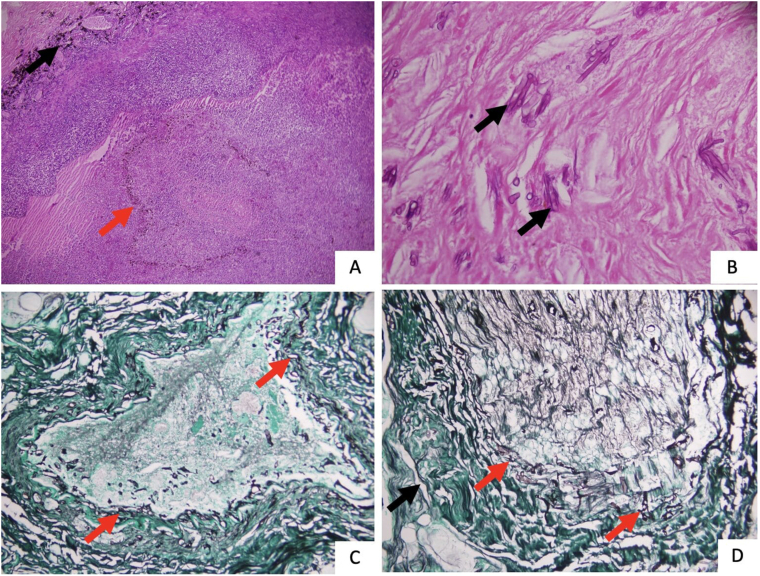
Fig. 3.
(A) Haematoxylin and Eosin (H&E) stain at 40x magnification shows acute suppurative exudative inflammatory cell infiltrate destroying the retina and choroid (red arrow shows retinal pigment epithelial layer. Black arrow shows choroid). (B) H&E stain at 400x magnifications, shows broad, aseptate fungal hyphae (black arrow) in a necrotic background within the orbital tissues. (C) Silver methenamine (SM) stain, at 200x magnification, shows broad, aseptate fungal hyphae (red arrow) invading the blood vessels. (D) Silver methenamine stain (SM), at 200x magnification, shows optic nerve head (black arrow) invasion by the fungal hyphae (red arrow).
Outcomes
All patients who underwent prompt and aggressive surgical intervention, with systemic control of the underlying condition survived till mean follow up of 4.8 ± 0.83 months (range 4–6 months). However, three of our patients (cases 8, 9, & 10) could not survive due to the rapidly progressive disease and succumbed to the infection. Intravenous liposomal amphotericin was administered for a period of approximately 3–4 weeks, depending on the systemic tolerance. Thereafter, patients were switched to oral posaconazole treatment after stabilisation of infection. Oral posaconazole 300 mg twice a day for two doses, then 300 mg once daily was started for 4–7 days till the serum levels of posaconazole reached > 0.7 ug/mL. Thereafter, the injection of amphotericin B was stopped. Patients were discharged on oral posaconazole.
Discussion
Through this communication, we would like to highlight the emergence of a new and fatal enemy of immunocompromised patients especially uncontrolled diabetics during the first wave of the COVID-19 pandemic from an Indian perspective. In a case series, reported from India during the pre-COVID era, 11 cases were reported over four years,7 on the contrary, in our case series, 15 cases with orbital spread were encountered within a short span of three months. In the present study, the mean age of patients was 43.9 ± 18.1 years with male predominance. The majority of our patients (86.7%) were uncontrolled diabetics. Four (26.7%) patients had a history of recent COVID-19 infection with the use of steroids in three (20%) patients for management. On PubMed search, we found three case series of 6, 10, and 31 patients, respectively, from cases presented during the first wave of COVID-19, reported in ophthalmic literature from India.8, 9, 10, 11 Sen et al. reported a series of six patients, with mean age, 60.5 ± 12 years, all were male patients. Of these, five patients had a recent history of uncontrolled diabetes with COVID infection, treated with steroids and one had concurrent COVID infection with diabetes, at presentation. Four patients had other co-morbidities also.8 Sarkar et al. reported a case series of 10 patients of ROM with COVID diagnosed on routine screening. The mean age was 45.5 years (range 23–67 years) with male predominance. All patients were known cases of diabetes mellitus with ketoacidosis in four patients. All except one patient, were on mechanical ventilation.9 Ravani et al. recently reported the largest case series of 31 patients with ROM. The mean age of patients was 56.3 years, with male predominance. The major risk factors reported were uncontrolled diabetes (96.7%), and COVID19 positivity (61.2%) with concomitant steroid use in 61.2% of patients.10 The clinical and/or imaging data of cases studied in the reported case series on ROM is limited. Further, in the largest reported case series, the outcome data related to ophthalmic features are not available.10 (Table 2).
Table 2.
Comparison of ophthalmic details of results of our study with 3 case series published in ophthalmic literature, on rhino-orbital mucormycosis cases presented during the first wave of COVID-19 in India.
| Parameters | Present study Total pts – 15; (n) |
Sen et al., 202110 Total pts – 6; (n) |
Sarkar et al., 202111 Total pts – 10; (n) |
Ravani et al., 202112 Total pts – 10; (n) |
|---|---|---|---|---|
| Vision (No PL) | 80% (12)a | 83.3% (5) | 50% (4 of 8) | 25.8% (8) |
| Proptosis | 100% (15) | 83.3% (5) | NA | 25.8% (8)b |
| Ophthalmoplegia | TO – 80% (12) | TO – 83.3% | NA | 77.4% (24)b |
| CRAO | 80% (12) | NA | 60% (6) | 6.45% (2) |
| Imaging findings of orbital disease | Diffuse orbital involvement till apex – 14; medial orbital involvement – 1; Globe tenting – 3 | Extraconal involvement – 50% (5)c | Orbital cellulitis – 61.3% (19)c; EOM involvement – 19.4% (6); Optic neuritis – 9.7% (3); Bony orbital wall erosion – 6.5% (2); |
|
| Orbital apex involvement | 93.3% (14) | 33.3% (2) | 50% (5) | NA |
| Cavernous sinus involvement | 60% (9) | 66.6% (4) | 10% (1) | 3.22% (1) |
| Pansinusitis | 93.3% (14) | Sinusitis present in all patients but details NA | 60% (6) | 77.4% (24) |
| Cerebral involvement | 20% (3) | 50% (3) | 10% (1) | 22.5% (7) |
| Surgery performed | Exenteration – 80% (12); Sinus debridement (12) with maxillectomy (4) & ethmoidectomy (1); not fit for surgery – 20% (3) | Exenteration – 33.3% (2); FESS – 100% (6) | Exenteration –10% (1); maxillectomy – 40% (4); FESS with debridement (site – NA)– 20% (2); not fit for surgery – 30% (3); on mechanical ventilation – 90% (9) | Exenteration – 12.9% (4); Sinus debridement – 100% Orbital debridement done but data on the number of patients and extent of debridement – NA |
| Clinical ophthalmic outcome | Socket healing present in all cases | Eye salvage – 66.7% (4); vision salvage – no patient | Unchanged status – 40% (4); improved – 10% (1) | NA |
| Survival | 80% (12) | 100% | 60% (6) | 90.32% (28) |
| Follow-up | 4–6 months | 0.3–3 months | 1 month | Minimum –75 days in all the patients |
(n), number of patients with feature present; TO, total ophthalmoplegia; NA, not available; PL, perception of light; CRAO, central retinal artery occlusion; pts, patients; FESS, functional endoscopic sinus surgery; EOM, extraocular movement
Two cases could not be assessed, case 9–20/80.
Severity not mentioned.
Location and extent not available.
In April 2021, two major series have been published on mucormycosis of faciomaxillary/paranasal sinuses, with a reported frequency of orbital involvement in 66.7% and 43.5% cases, respectively.11,12 In the former multi-centre study, 18 patients with maxillofacial/rhino-cerebro-orbital mucormycosis who were COVID positive and received steroids were analysed. Sixteen patients were poorly controlled diabetic. All the patients were started on liposomal amphotericin B. Loss of vision was noted in 12 of 18 patients (66.7%) out of whom 7 underwent exenteration. Ophthalmic details were not available in the study. Sinus debridement was done along with maxillectomy in 11 patients. Mortality was in six patients and one patient was lost to follow-up.11 In the latter series of 23 patients, with paranasal sinuses mucormycosis with concomitant COVID-19 infection, authors reported orbital involvement in ten patients (43.5%), none of them had vision loss indirectly suggestive of mild or subtle orbital involvement. The authors did not provide any other ophthalmic clinical and imaging details. None of the patients in their series underwent exenteration, though two patients were administered intra-orbital amphotericin B. All patients underwent surgical debridement. Twenty one patients were diabetic and all 23 patients had used steroids for control of COVID-19 infection. There were no mortalities. This study probably suggests that early detection and control of mucormycosis of paranasal sinuses and nasal cavity can help in the control of orbital infection thereby preventing its progression to an advanced stage of orbital disease and hence the need for exenteration and further spread to the brain.12
Patients with uncontrolled diabetes are, as such, prone to ROM due to impaired phagocytic activity of macrophages and neutrophils.13,14 Further, hyperglycemia, the release of iron, hypoxic environment, acidic pH and raised ketones, especially in patients with ketoacidosis are the factors that make the disease invasive and rapidly progressive.15, 16, 17
During the first wave of the COVID-19 pandemic, lack of non-COVID facilities in the hospital, non-compliance to medications, lack of physical activities, paucity in daily exercises and restricted travelling led to a surge in cases with uncontrolled diabetes.18, 19, 20, 21 These could have been the probable reasons for the recent onset of diabetes in two of our patients. Various mechanisms have been proposed for the increased risk of COVID-19 amongst diabetic patients, which include impaired macrophage and neutrophilic activity, and cytokine storm.22, 23, 24, 25 The most accepted mechanism is, however, the efficient entry of the virus into the cells through ACE 2 receptors expressed in a large number of organs, such as lungs, kidneys, and pancreas. The entry of the virus is facilitated in the pancreas, causes damage and an impaired insulin secretion leading to a state of hyperglycemia.26 Further, the use of steroids for the management of the lung infection in COVID-19, have led to a deterioration of diabetic status.27,28
All our patients had advanced orbital disease probably because of the fulminant and rapidly progressive nature of the disease in uncontrolled diabetics. Patients presented to us with a mean duration of ocular symptoms, 10.4 ± 2.2 days (range 5–25 days). Ours being a referral centre could be one of the reasons for the late presentation of the patients. Further, lockdown, fear of acquiring COVID infection and a major shift in the health care system for control of COVID-19 pandemic were probably some important factors for delayed presentation.29
The standard treatment for a necrotizing infection is wide surgical debridement i.e., removal of all necrotic tissues till healthy margins along with medical management. Most of the necrotizing infections, bacterial or fungal, are generally due to the highly virulent angio-invasive nature of the organism, which spreads fast leading to high morbidity and mortality, especially in immunocompromised individuals. Further, drug penetration is extremely poor within the necrosed tissues, so early and wide debridement is imperative to control the infection. Our decision for exenteration was made based on the extent of orbital involvement, the systemic status of the patients, and the overall prognosis.30 Clinically, presence of moderate to severe proptosis, significant resistance felt on retropulsion test, total ophthalmoplegia with no vision, supported by imaging findings of diffuse involvement of orbital tissues, extending till apex indicated advanced form of infection. We performed exenteration along with sinus surgery in 12 of 15 patients and all the patients survived till mean follow up of 4.8 + 0.83 months (4–6 months).
Depending on the extent of orbital disease, few reports are available on direct drug delivery methods of amphotericin B and/or selective debridement of the orbital necrotic tissue in patients with orbital disease in cases with ROM.6,10,31,32 Hirabayashi et al. in their study used retrobulbar injections of amphotericin B deoxycholate in conjunction with intravenous antifungals and endoscopic sinus debridement in a 68-year-old immunocompromised man with ROM, resulting in halted progression of orbital infection and restoring visual acuity with early hospital discharge.32 Seiff et al. in their study on seven consecutive patients with sino-orbital fungal infections, found conservative orbital debridement with local amphotericin B irrigations as an effective adjunct in the control of sino-orbital fungal infections.31 Close monitoring of disease status has to be done in such patients as mucormycosis is a rapidly progressive disease. Most oculoplastic surgeons remain in a dilemma as exenteration is facial disfiguring surgery and also there are no clear guidelines for exenteration.33 Intricate issues in management of these cases are known to vary depending on multiple factors. In patients with rapid progression of the orbital disease or advanced disease, as already mentioned, prompt exenteration along sinus surgery can be life-saving. The patient should be counselled about the need for exenteration, long-term treatment and the possibility of rehabilitation with a prosthesis to alleviate stress related to the disfiguring surgery.
Conclusion
Orbital spread of mucormycosis is a challenging and life-threatening condition. Its management requires a prompt multi-disciplinary, medical, and surgical approach because of its associated morbidity and mortality.
Disclosure of competing interest
The authors have none to declare.
References
- 1.Shinde R.V., Karande G.S., Mohite S.T., Patil S.R. Rhino-orbital mucormycosis in diabetes mellitus. J Clin Diagn Res. 2013 Jun;7(6):1145–1147. doi: 10.7860/JCDR/2013/5528.3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prakash H., Chakrabarti A. Global epidemiology of mucormycosis. J Fungi (Basel) 2019 Mar 21;5(1):26. doi: 10.3390/jof5010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pagano L., Offidani M., Fianchi L., et al. GIMEMA (gruppo italiano malattie EMatologiche dell'Adulto) infection program. Mucormycosis in hematologic patients. Haematologica. 2004 Feb;89(2):207–214. [PubMed] [Google Scholar]
- 4.Patel A.K., Patel K.K., Patel K., Gohel S., Chakrabarti A. Mucormycosis at a tertiary care centre in Gujarat, India. Mycoses. 2017 Jun;60(6):407–411. doi: 10.1111/myc.12610. [DOI] [PubMed] [Google Scholar]
- 5.Spellberg B., Ibrahim A.S. Recent advances in the treatment of mucormycosis. Curr Infect Dis Rep. 2010 Nov;12(6):423–429. doi: 10.1007/s11908-010-0129-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luna J.D., Ponssa X.S., Rodríguez S.D., Luna N.C., Juárez C.P. Intraconal amphotericin B for the treatment of rhino-orbital mucormycosis. Ophthalmic Surg Laser. 1996 Aug;27(8):706–708. PMID: 8858637. [PubMed] [Google Scholar]
- 7.Gupta S., Goyal R., Kaore N.M. Rhino-orbital-cerebral mucormycosis: battle with the deadly enemy. Indian J Otolaryngol Head Neck Surg. 2020 Mar;72(1):104–111. doi: 10.1007/s12070-019-01774-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sen M., Lahane S., Lahane T.P., Parekh R., Honavar S.G. Mucor in a viral land: a tale of two pathogens. Indian J Ophthalmol. 2021 Feb;69(2):244–252. doi: 10.4103/ijo.IJO_3774_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarkar S., Gokhale T., Choudhury S.S., Deb A.K. COVID-19 and orbital mucormycosis. Indian J Ophthalmol. 2021 Apr;69(4):1002–1004. doi: 10.4103/ijo.IJO_3763_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ravani S.A., Agrawal G.A., Leuva P.A., Modi P.H., Amin K.D. Rise of the phoenix: mucormycosis in COVID-19 times. Indian J Ophthalmol. 2021 Jun;69(6):1563–1568. doi: 10.4103/ijo.IJO_310_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moorthy A., Gaikwad R., Krishna S., et al. SARS-CoV-2, uncontrolled diabetes and corticosteroids-an unholy trinity in invasive fungal infections of the maxillofacial region? A retrospective, multi-centric analysis. J Maxillofac Oral Surg. 2021 Mar 6:1–8. doi: 10.1007/s12663-021-01532-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharma S., Grover M., Bhargava S., Samdani S., Kataria T. Post coronavirus disease mucormycosis: a deadly addition to the pandemic spectrum. J Laryngol Otol. 2021 Apr 8:1–6. doi: 10.1017/S0022215121000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pavlou S., Lindsay J., Ingram R., et al. Sustained high glucose exposure sensitizes macrophage responses to cytokine stimuli but reduces their phagocytic activity. BMC Immunol. 2018;19:24. doi: 10.1186/s12865-018-0261-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lecube A., Pachón G., Petriz J., Hernández C., Simó R. Phagocytic activity is impaired in type 2 diabetes mellitus and increases after metabolic improvement. PLoS One. 2011;6(8) doi: 10.1371/journal.pone.0023366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rammaert B., Lanternier F., Poirée S., Kania R., Lortholary O. Diabetes and mucormycosis: a complex interplay. Diabetes Metab. 2012 Jun;38(3):193–204. doi: 10.1016/j.diabet.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 16.Sivaramakrishnan H., Kannan S.M., Ravikumar C., Harishankar S., Chellamuthu S. A case of rhino-orbital cerebral mucormycosis with diabetic keto-acidosis. J Indian Med Assoc. 2008 Sep;106(9):600–601. 603. [PubMed] [Google Scholar]
- 17.Artis W.M., Fountain J.A., Delcher H.K., Jones H.E. A mechanism of susceptibility to mucormycosis in diabetic ketoacidosis: transferrin and iron availability. Diabetes. 1982 Dec;31(12):1109–1114. doi: 10.2337/diacare.31.12.1109. [DOI] [PubMed] [Google Scholar]
- 18.Füzéki E., Groneberg D.A., Banzer W. Physical activity during COVID-19 induced lockdown: recommendations. J Occup Med Toxicol. 2020 Aug 12;15:25. doi: 10.1186/s12995-020-00278-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marelli S., Castelnuovo A., Somma A., et al. Impact of COVID-19 lockdown on sleep quality in university students and administration staff. J Neurol. 2021 Jan;268(1):8–15. doi: 10.1007/s00415-020-10056-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang C., Pan R., Wan X., et al. Immediate psychological responses and associated factors during the initial stage of the 2019 coronavirus disease (COVID-19) epidemic among the general population in China. Int J Environ Res Public Health. 2020 Mar 6;17(5):1729. doi: 10.3390/ijerph17051729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cuschieri S. COVID-19 panic, solidarity and equity-the Malta exemplary experience. Z Gesundh Wiss. 2020 May 30:1–6. doi: 10.1007/s10389-020-01308-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muniyappa R., Gubbi S. COVID-19 pandemic, coronaviruses, and diabetes mellitus. Am J Physiol Endocrinol Metab. 2020 May 1;318(5):E736–E741. doi: 10.1152/ajpendo.00124.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abdi A., Jalilian M., Sarbarzeh P.A., Vlaisavljevic Z. Diabetes and COVID-19: a systematic review on the current evidences. Diabetes Res Clin Pract. 2020 Aug;166:108347. doi: 10.1016/j.diabres.2020.108347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar A., Arora A., Sharma P., et al. Is diabetes mellitus associated with mortality and severity of COVID-19? A meta-analysis. Diabetes Metab Syndr. 2020 Jul-Aug;14(4):535–545. doi: 10.1016/j.dsx.2020.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Orioli L., Hermans M.P., Thissen J.P., Maiter D., Vandeleene B., Yombi J.C. COVID-19 in diabetic patients: related risks and specifics of management. Ann Endocrinol (Paris) 2020 Jun;81(2-3):101–109. doi: 10.1016/j.ando.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yan Y., Yang Y., Wang F., et al. Clinical characteristics and outcomes of patients with severe covid-19 with diabetes. BMJ Open Diabetes Res Care. 2020 Apr;8(1) doi: 10.1136/bmjdrc-2020-001343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mehta S., Pandey A. Rhino-orbital mucormycosis associated with COVID-19. Cureus. 2020 Sep 30;12(9) doi: 10.7759/cureus.10726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mekonnen Z.K., Ashraf D.C., Jankowski T., et al. Acute invasive rhino-orbital mucormycosis in a patient with COVID-19-associated acute respiratory distress syndrome. Ophthalmic Plast Reconstr Surg. 2021 Mar-Apr 01;37(2):e40–e80. doi: 10.1097/IOP.0000000000001889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poyser A., Deol S.S., Osman L., et al. Impact of COVID-19 pandemic and lockdown on eye emergencies. Eur J Ophthalmol. 2020 Nov 19 doi: 10.1177/1120672120974944. 1120672120974944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cornely O.A., Alastruey-Izquierdo A., Arnez D., et al. Mucormycosis: ECMM MSG global guidelines writing group. Global guidelines for the diagnosis and management of mucormycosis: an initiative of the European confederation of medical mycology in co-operation with the mycosis study group education and research consortium. Lancet Infect Dis. 2019 Dec 19;12:e405–e421. doi: 10.1016/S1473-3099(19)30312-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seiff S.R., Choo P.H., Carter S.R. Role of local amphotericin B therapy for sino-orbital fungal infections. Ophthalmic Plast Reconstr Surg. 1999 Jan;15(1):28–31. doi: 10.1097/00002341-199901000-00007. [DOI] [PubMed] [Google Scholar]
- 32.Hirabayashi K.E., Kalin-Hajdu E., Brodie F.L., Kersten R.C., Russell M.S., Vagefi M.R. Retrobulbar injection of amphotericin B for orbital mucormycosis. Ophthalmic Plast Reconstr Surg. 2017 Jul/Aug;33(4):e94–e97. doi: 10.1097/IOP.0000000000000806. [DOI] [PubMed] [Google Scholar]
- 33.Songu M., Unlu H.H., Gunhan K., Ilker S.S., Nese N. Orbital exenteration: a dilemma in mucormycosis presented with orbital apex syndrome. Am J Rhinol. 2008 Jan-Feb;22(1):98–103. doi: 10.2500/ajr.2008.22.3121. PMID: 18284868. [DOI] [PubMed] [Google Scholar]