Abstract
Background
Acetaminophen (APAP) is a worldwide antipyretic as well as an analgesic medication. It has been extensively utilized during the outbreak of coronavirus 2019 (COVID-19). APAP misuse would lead to liver injury. Diacerein (DIA), an anthraquinone derivative, has antioxidant and inflammatory properties. Hence, this study attempted to evaluate the impact of DIA treatment on liver injury induced by APAP and its influence on nuclear factor-κB (NF-κB) /toll-like receptor 4 (TLR4)/high mobility group box-1(HMGB-1) signaling as well as the expression of peroxisome proliferator-activated receptor-gamma (PPAR-γ) expression.
Methods
Male albino rats received 25 as well as 50 mg/kg/day DIA orally for seven days. One hour after the last administration, rats received APAP (1gm/kg, orally). For histopathological analysis, liver tissues and blood were collected, immunohistochemical (IHC) assay, biochemical assay, as well as quantitative real-time polymerase chain reaction (qRT-PCR).
Results
DIA markedly reduced liver injury markers and ameliorated histopathological changes. Moreover, DIA dose-dependently alleviated oxidative stress status caused by APAP administration along with inflammatory markers, including the level of interleukin-1 beta (IL-1β), myeloperoxidase (MPO), tumor necrosis factor-alpha (TNF-α), and interleukin 6 (IL-6). Furthermore, DIA downregulated protein levels as well as mRNA of HMGB-1, TLR4, NF-κB p65 expression, and enhanced PPAR-γ expression. Moreover, DIA ameliorated apoptotic (Bax) and caspase-3 expressions and increased the anti-apoptotic (Bcl2) expression.
Conclusions
This study demonstrated that DIA exerts anti-apoptotic, anti-inflammatory, and antioxidant properties against liver injury induced by APAP that is attributed to inhibition of the HMGB1/TLR4/NF-κB pathway, besides upregulation of the expression of PPAR-γ.
Keywords: Diacerein (DIA), Acetaminophen (APAP), Hepatotoxicity, HMGB1/TLR4/NF-κB signaling, PPAR-γ, Bax/Bcl2/caspase-3
Introduction
Acetaminophen (APAP) is widely utilized as an over-the-counter drug for treating inflammation, fever, as well as pain [1]. APAP is currently the main antipyretic and analgesic drug recommended by several studies over non-steroidal anti-inflammatory drugs (NSAIDs) used to manage fever and pain associated with the pandemic infection of coronavirus 2019 (COVID-19). This assumption could cause a possible misuse of acetaminophen, which is considered the major cause behind drug-induced liver injury (DILI) worldwide. [1–3]. Although N-acetylcysteine (NAC) is the current drug used to protect against liver injury induced by APAP, it is only effective when given an early period of APAP ingestion and associated with adverse effects such as nausea, vomiting, allergic reactions, and headaches [4]. It is pivotal to find new therapeutic targets and effective agents.
Inflammation and elevated oxidative stress significantly contribute to APAP hepatotoxicity, resulting in hepatocyte necrosis and apoptosis [3, 5]. Consequently, necrotic hepatocytes induce damage-associated molecular patterns (DAMPs like high mobility group box 1(HMGB1 that is determined by toll-like receptor 4 (TLR, and activate the innate immune system and release excessive inflammatory mediators, including cytokines including nuclear factor-κB (NF-κB), tumor necrosis factor-alpha (TNF-α), as well as interleukin 1 beta (IL-1β), which eventually leads to severe hepatic injury [4, 6, 7]. Peroxisome proliferator-activated receptor-γ (PPAR-γ), which belongs to the family of the nuclear hormone receptors, was proven to enhance the antioxidant and anti-inflammatory gene transcription [8], modulate TLR4 activation [9], and regulate NF-κB-induced inflammation [10].
Diacerein (DIA), anthraquinone derivatives, is an anti-inflammatory, analgesic, and antipyretic drug used clinically to treat osteoarthritis. Furthermore, rhein, the active metabolite of DIA, was demonstrated to induce several pharmacological activities, such as anti-inflammatory, antioxidant, and antitumor [11]. DIA is an IL-1β inhibitor which evidenced to markedly protected against inflammatory damage via its ability to inhibit inflammatory cytokines like IL-1β and TNF-α [12], in addition to downregulating the TLR4/NF-κB-mediated signaling pathway activation, followed by suppressing the secretion of inflammatory cytokines [13]. It has been proven that DIA has a hepatoprotective impact against liver ischemia and reperfusion (I/R) via its antioxidant and anti-inflammatory activities [13]. However, its effect on liver injury induced by APAP remains unclear. Hence, this study attempted to examine the potential protective impact of DIA on hepatotoxicity induced by APAP and explore its impact on HMGB-1/TLR4/NF-κB, Bax/Bcl2/caspase-3 signaling pathway, and PPAR-γ expression.
Materials and methods
Chemicals and drugs
The chemicals utilized were highly analytical and purchased from Sigma-Aldrich (St. Louis, MO, USA). Paracetamol (Paramol ®) tablets (500 mg) were obtained from Misr Co. for Pharmaceutical Industries (Cairo, Egypt). They were dissolved in saline. DIA powder was purchased from Eva Pharma Company (Cairo, Egypt) and dissolved in 1% carboxymethyl cellulose (CMC).
Animals
Twenty-four male albino rats, with nearly 150 to 180 g, were purchased from the animal facility of The Nile Co. for Pharmaceuticals and Chemical Industries (Cairo, Egypt). Animals were kept in a controlled environment at a temperature between 20 to 25 °C, 55% humidity, as well as a 12- h light/dark cycle. They were supplied with free drinking and given a regular diet. One week prior to the experiment, animals were acclimatized. The experiment was carried out based on The Public Health Guide for the care and use of laboratory animals, as well as the Faculty of Pharmacy's Animal Ethics Committee, Al-Azhar University, Egypt.
Experimental design
The animals were randomized into four groups (n = 6 rats per group).
Control group; animals were administrated 1% CMC and saline for seven days. APAP group; rats were given a single APPA dose (1 g/kg, orally) [14] on the seventh day of the trial. DIA 25 + APPA and DIA 50 + APPA groups; rats received diacerein (25 and 50 mg/kg, orally) [15] respectively for a week, and subsequently were given APPA (1 g/kg, orally) on the last day of the experiment. Twenty-four hours after APAP exposure, in order to evaluate aspartate aminotransferase (AST), serum alanine aminotransferase (ALT), total bilirubin (TBB), lactate dehydrogenase (LDH), and blood samples were obtained. Afterward, rats were sacrificed, livers were dissected, washed in ice-cooled saline, and dried. Each dissected liver was subdivided into three sections: the first was preserved in a formalin solution of 10% for immunohistochemical and histopathological analysis. With regard to the second section, it was promptly frozen in liquid nitrogen and kept at − 80 °C for the analysis of gene expression. Finally, the third section was homogenized in saline for subsequent biochemical analysis.
Assessment of liver functions
Serum of rats used for assay of ALT, AST, and TBB using colorimetric kits (Abcam, UK), while LDH assayed using Randox (UK) kit following the instructions of the manufacturer.
Histopathological examination
The samples from the liver were preserved in a formalin of 10%, fixed in paraffin, dissected into 5-μm thickness sections, and stained with eosin and hematoxylin [16].
Assessment of lipid peroxidation and biomarkers of oxidative stress
The determination of lipid peroxidation in hepatic tissue homogenate was performed by measuring malondialdehyde (MDA) content using a commercial kit (Bio-diagnostic Co., Egypt). Diminished glutathione (GSH) level as well as glutathione peroxidase activity (GPx), superoxide dismutase (SOD), and catalase (CAT) activities were performed utilizing kits purchased from (Bio-diagnostic Co., Egypt). It should be noted that the procedures were carried out, and parameters were determined following the protocol of the manufacturer.
Enzyme-linked immunosorbent assay (ELISA) for hepatic HMGB1, TLR4, IL-1β, IL-6, TNF-α, and MPO levels
TLR4 and HMGB1 protein levels were determined using an ELISA kit (My BioSource, USA). IL-6, IL-1β, TNF-α, and MPO were quantified in hepatic tissue homogenate utilizing ELISA kits purchased from (RandD Systems, Inc, USA), (San Diego, California, USA), (Cloud-clone corp., USA), and (My BioSource, USA) respectively. Measurement of sample concentration as well as the measurement of all parameters was performed in accordance with the protocol of the manufacturer.
IHC assay for hepatic NF-κB (p65), PPAR-γ, Bax, caspase-3, and Bcl2 protein expressions
Paraffined blocks of hepatic tissue were sectioned and deparaffinized with xylene and subsequently rehydrated with alleviated concentrations of alcohol. The sections were left for 10 min in 3% H2O2 followed by 30 min in 0.1% trypsin at 37 °C, for antigen retrieval. They were subsequently incubated overnight at 4 °C with antibodies of polyclonal rabbit against Bcl2, NF-κB (p65), Bax, PPAR-γ, and caspase-3 (1:1000 dilution; Abcam, Cambridge, UK). Sections were treated at 37 °C for 30 min with biotinylated goat anti-rabbit (Invitrogen), the secondary antibody, and subsequently stained with 3, 3-diaminobenzidine and eventually counterstained with hematoxylin. Afterward, image analysis was carried out via ImageJ software (version 1.48) to assess the area percent (A%).
qRT-PCR analysis of HMGB-1 and TLR4 mRNA expression
Following the instructions of the manufacturer, the isolation of total RNA from the liver was performed utilizing a commercial kit (QIAzole and Qiagen RNeasy Mini Kits). The purity and the concentration of the isolated RNA were evaluated using the Nanodrop (ND-1000 USA). The primers used for assessment were obtained from (Invitrogen Life Technologies, Carlsbad, CA, USA). The primers' sequences utilized are like the following: HMGB-1 forward AGGCTGACAAGGCTCGTTATG; reverse TGTCATCCGCAGCAGTGTTG, TLR4 forward GGGGGGTATTTGACACACTCTA; reverse TCCTTTGGATGTCTCTATGCGA, β-actin forward GGAGATTACTGCCCTGGCTCCTAGC; reverse GGCCGGACTCATCGTACTCCTGCTT.
qRT-PCR was carried out utilizing QuantiTect® SYBR® Green RT-PCR master mix Kit (Qiagen, Hilden, Germany). Following a 10-min hot start at 95 °C, samples at 95 °C were exposed to 15 s of denaturation followed by 1 min of annealing for 45 cycles at 54–58 °C. Melting curve analysis was done from 60 °C to 95 °C, with temperature increments of 0.5 °C every 10 s to detect any nonspecific products. The Ct value was calculated using the thermal cycler's (Veriti®) software package, in which the ΔCt value denoted the number of the cycle when the fluorescence curve passed the baseline score. Based on the 2−ΔΔCt method, the target genes' relative expression of mRNA was determined via normalizing the control group and the housekeeping gene -actin.
Statistics
Statistical analysis of the experimental results was performed utilizing the GraphPad Prism (version 5.0, GraphPad Software Inc., San Diego, California) and expressed in the form of mean ± S.E.M. The one-way ANOVA was utilized to analyze data, whereas Tukey's post hoc tests were utilized for making comparisons. P < 0.05 was deemed significant.
Results
Impact of DIA on hepatic functions and histopathological changes in rats treated with APAP
As depicted in Table 1, administration of APAP significantly elevated ALT, AST, TBB, and LDH serum levels by 151%, 111.4%, 224%, and 132%, respectively, than controls. However, DIA (25 and 50 mg/kg) pre-treatment resulted in a substantial decline in AST, ALT, TBB, and LDH serum levels by (40%, 40.5%, 47%, and 37.5%) and (53%, 71%, 57%, and 42%), respectively, in a dose-related manner than the group treated with APAP. These results demonstrated that DIA induces a protective impact against liver damage induced by APAP.
Table 1.
Impact of DIA on hepatic functions in rats treated with APAP
| Parameters | Control | APAP | DIA 25 + APAP | DIA 50 + APAP |
|---|---|---|---|---|
| ALT (U/L) | 25.92 ± 0.55 | 65.10 ± 1.35a | 38.98 ± 0.80a,b | 30.78 ± 1.06a,b,c |
| AST (U/L) | 40.25 ± 0.61 | 85.14 ± 1.22a | 50.64 ± 0.57a,b | 24.67 ± 0.71a,b,c |
| TBB (U/L) | 17.45 ± 0.59 | 56.93 ± 0.85a | 30.06 ± 0.34a,b | 24.67 ± 0.71a,b,c |
| LDH (U/L) | 303.00 ± 1.38 | 704.00 ± 1.46a | 440.00 ± 2.30a,b | 410.00 ± 1.36a,b,c |
Data are expressed in the form of means ± S.E.M. (n = 6 per group), and one-way ANOVA was utilized for data analysis, followed by Tukey post hoc test. (a), (b), (c): P < 0.05 compared the controls, APAP, DIA 25 + APAP groups respectively
APAP acetaminophen, DIA diacerein, ALT alanine transaminase, AST aspartate transaminase, TBB total bilirubin
According to microscopic examination in Fig. 1, the histology of control rats' H&E-stained liver sections was normal (Fig. 1A). The dissected liver sections from the rats treated with APAP demonstrated diffuse hepatocellular vacuolation and congestion. In addition, the portal areas contained mononuclear inflammatory cells infiltration (Fig. 1B). Furthermore, portal fibroplasia was noticed along with the detected inflammatory reaction (Fig. 1C) and necrosis (Fig. 1D). However, pretreatment with DIA (25 and 50 mg/kg) ameliorated APAP-induced histopathological changes (Fig. 1E–H).
Fig. 1.
Effect of DIA pretreatment on histopathological changes in APAP-treated rats. Representative photomicrographs of A control sections show normal hepatocytes and portal area while APAP sections B–D show hepatocytes' diffuse vacuolation along with infiltration of portal mononuclear inflammatory cells (arrow), congestion (star) (B), portal fibroplasia (arrow) (C), and necrosis (D). The DIA 25 + APAP (E and F) sections display periportal hepatocellular vacuolation (E), congestion (star), and portal mononuclear inflammatory cells infiltration (F). The DIA 50 + APAP sections (G and H) show apparently normal hepatocytes normal portal area (G) with mild vacuolation in some hepatocytes (H). Magnifications: × 200 for all slides
Impact of DIA on hepatic oxidative stress in rats treated with APAP
As depicted in Table 2, administration of a single APAP dose induced a dramatic increase in the level of hepatic MDA reaching 768%, as well as marked depletion of GSH levels and activities of GPx, SOD, and CAT by 36%, 33%,41%, and 47% respectively, than the controls. Pretreatment with DIA (25 and 50 mg/kg) induced a substantial decline in the level of MDA reaching 66% and 78%, respectively, besides inhibition of the levels of GSH and GPx, SOD, and CAT activities by ([32%, 37%, 43.4%, and 32.4%] and [50%, 44%,58%, and 52%], respectively) in a dose-depended manner compared with animals treated with APAP. These findings verified that DIA ameliorates the oxidative damage induced by APAP and antioxidant conditions of the liver tissue.
Table 2.
Impact of DIA on the parameters of hepatic oxidative stress in rats treated with APAP
| Parameters | Control | APAP | DIA 25 + APAP | DIA 50 + APAP |
|---|---|---|---|---|
| MDA (nmol/g. tissue) | 0.41 ± 0.31 | 3.56 ± 0.18a | 1.22 ± 0.08a,b | 0.78 ± 0.01b,c |
| GSH (mmol/g. tissue) | 101.40 ± 0.43 | 64.99 ± 1.16a | 85.82 ± 1.12a,b | 97.31 ± 0.62a,b,c |
| GPx (U/g. tissue) | 152.90 ± 1.10 | 103.10 ± 0.77a | 141.30 ± 1.03a,b | 148.5 ± 0.43a,b,c |
| SOD (U/g. tissue) | 162.60 ± 1.10 | 95.90 ± 0.70a | 137.5 ± 0.91a,b | 152.1 ± 0.90a,b,c |
| CAT (U/g. tissue) | 199.80 ± 2.60 | 105.30 ± 1.50a | 139.10 ± 0.80a,b | 160 .40 ± 1.00a,b,c |
Data were expressed in the form of means ± S.E.M. (n = 6 per group), and then data analysis was performed utilizing the one-way ANOVA and then the Tukey post hoc test. (a), (b), (c): P < 0.05, compared to controls, APAP, DIA 25 + APAP groups, respectively
Effect of DIA on inflammation and neutrophils infiltration in APAP-treated in rats
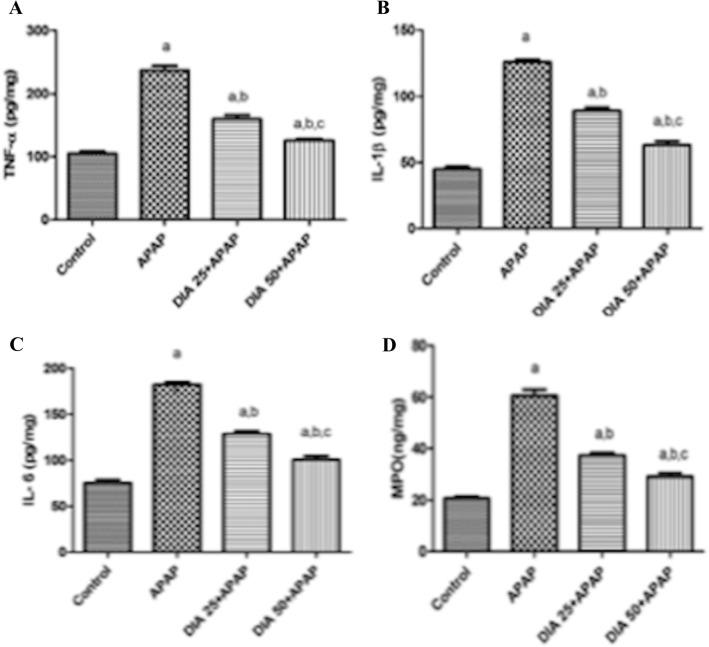
As depicted in Fig. 2A, B, C, D, administration of APAP markedly increased TNF-α, IL-1β, and IL-6 hepatic levels by 128%, 181%, and 143%, respectively than controls. In contrast, pretreatment with DIA (25 and 50 mg/kg) markedly reduced these changes by ([33%, 30%, and 29%) and (47%, 50%, and 45%] respectively) in a dose-depended manner than animals treated with APAP. These findings revealed that DIA could alleviate the inflammation induced by APAP.
Fig. 2.
Effect of DIA on hepatic TNF-α (A) IL-1β (B), IL-6 (C), and MPO (D) levels in APAP-treated rats. Results are expressed in the form of means ± S.E.M. (n = 6 per group), whereas data analysis was performed using one-way ANOVA and then the Tukey post hoc test. (a), (b), (c): P < 0.05, as compared to the control group, APAP, DIA 25 + APAP groups respectively
In Fig. 2D, neutrophil infiltration was assessed via the evaluation of MPO. Hepatic MPO level was increased in APAP-treated rats by 188% than controls. In comparison to rats treated with APAP, DIA pretreatment (25 and 50 mg/kg) reduced the increased MPO level by 39% and 52%, respectively. These findings verified that DIA could improve neutrophil infiltration in APAP-induced liver injury in a dose-dependent manner.
DIA impact on HMGB1/TLR4/ NF-κB (p65) signaling in rats treated with APAP
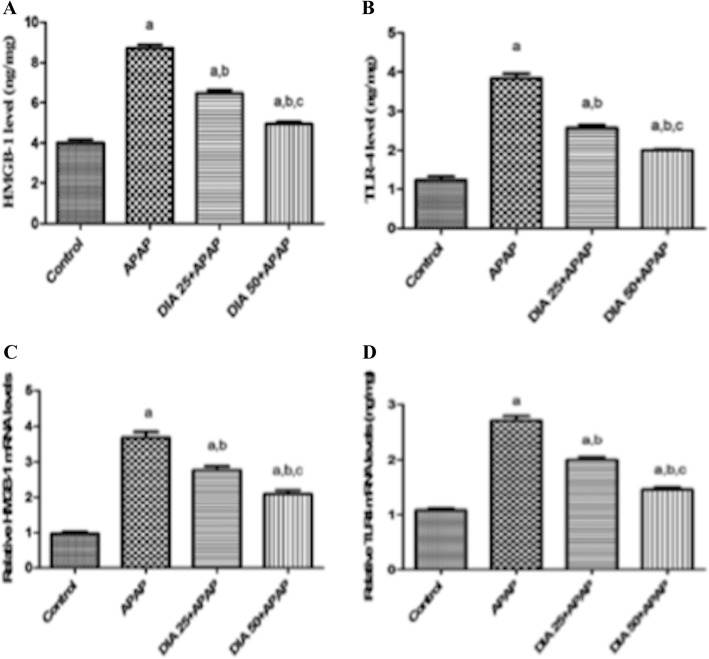
As demonstrated in Fig. 3A and B, administration of APAP substantially increased the mRNA expression of TLR4 HMGB1 by 2.7 and 1.7-fold, respectively, in comparison to controls. Consequently, APAP elevated HMGB1 and TLR4 protein levels by 117.5% and 214%, respectively, compared to control rats (Fig. 3C and D). DIA pretreatment (25 and 50 mg/kg) considerably downregulated the mRNA expression of TLR4 and HMGB1 by ([25% and 26%] and [43% and 39%], respectively) as well as decreased the protein levels of both by ([22% and 33%] and [34% and 48%] respectively), compared to the APAP-treated rats.
Fig. 3.
DIA effect on HMGB-1 (A) and TLR4 (B) gene expression, and their content of protein (C and D), in APAP-treated rats' liver tissues. Gene expression quantitation was performed utilizing real-time PCR. The mRNA levels were normalized to that of β-actin in each group and then expressed as relative quantification in comparison to controls. Gene values in the control cohort were defined as 1. (a), (b), (c): P < 0.05, compared to the control group, APAP, DIA 25 + APAP groups, respectively. Data are expressed as means ± S.E.M. (n = 6 per group), and data analysis was carried out utilizing the one-way ANOVA and then the Tukey post hoc test
In Fig. 4, the IHC examination of NF-κB (p65) expression displayed a strong positive reaction in the APAP-treated rats compared to the control group (Fig. 4B). Pretreatment with DIA (25 and 50 mg/kg) substantially decreased NF-κB (p65) expression in a dose-depended manner (Fig. 4C and D) compared to APAP-treated rats.
Fig. 4.
Representative photomicrograph of immunohistochemical staining of hepatic NF-κB (p65) positive cells in the liver (arrows). Control group (A), APAP-treated group (B), DIA 25 + APAP-treated group (C), DIA 50 + APAP-treated group (D) [× 200]
DIA effect on PPAR-γ expression in rats treated with APAP
As depicted in Fig. 5, immunostaining of PPAR-γ expression displayed weak expression in APAP- treated rats' liver tissues than controls (Fig. 5B). In contrast, pretreatment with DIA (25 and 50 mg/kg) displayed a strong positive reaction in a dose-depended manner in comparison to animals treated with rat APAP (Fig. 5C and D).
Fig. 5.
Representative photomicrograph of immunohistochemical of hepatic PPAR-γ positive cells in the liver (arrows). Control group (A), APAP -treated group (B), (C), DIA 25 + APAP-treated group (D), DIA 50 + APAP-treated group [× 200]
DIA impact on apoptosis in rats treated with APAP
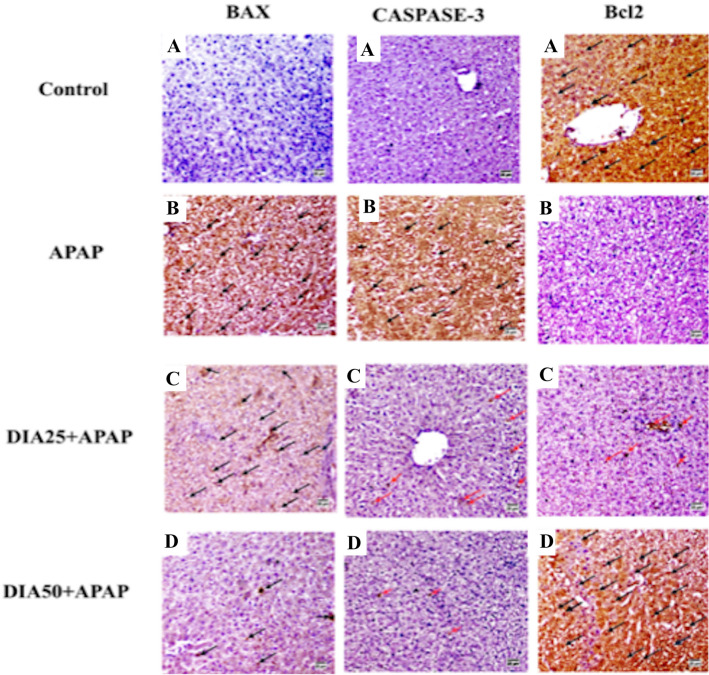
As displayed in Fig. 6A, B, C, D, and E, the IHC examination of Bax, active caspase-3, and Bcl2 in the liver tissue sections demonstrated an elevated expression of both Bax and caspase-3 accompanied by a weak expression of Bcl2 in the APAP-treated group. Pretreatment with DIA (25 and 50 mg/kg) showed a decreased expression of both caspase-3 and Bax accompanied by elevated Bcl-2 expression in a dose-dependent manner than APAP-treated rats. The present IHC analysis results suggest the ability of DIA to suppress apoptosis triggered by APAP in the rats' liver tissues.
Fig. 6.
Representative photomicrograph of immunohistochemical of hepatic BAX, caspase-3, and Bcl2 positive cells in the liver (arrows). Control group (A), APAP-treated group (B), DIA 25 + APAP-treated group (C), and DIA 50 + APAP-treated group (D) [× 200]
Discussion
Hepatoxicity triggered by an acute overdose of APAP is considered the main cause of DILI [2]. Several researchers illustrated that DIA has anti-inflammatory and antioxidant properties [12, 14, 15]. Studies have shown that DIA protects testicular injury, nephrotoxicity, and hepatotoxicity [13]. Hence, the present study investigated the hepatoprotective DIA impact against liver injury induced by APAP, denoting modulation of TLR4/ HMGB-1 as well as PPAR-γ signaling pathways.
Rats that received acute APAP overdose exhibited marked hepatocellular injury in rats verified by the significant increase in the liver index as well as serum levels of AST, ALT, TBB, and LDH. These enzymes are reliable indicators for liver injury, and assessing their serum levels helps identify hepatocellular damage. The histopathological analysis further confirmed the APAP-induced liver injury [14]. Pretreatment with DIA (25 and 50 mg/kg) markedly and dose-dependently protect against APAP-induced liver injury through a reduction in liver function markers, together with alleviating histopathological changes of hepatic tissue, which is in agreement with [10, 15].
Oxidative stress has been postulated to be directly involved in the development of APAP-induced toxicity by the formation of the reactive metabolite NAPQI that is causing lipid peroxidation, depletion in antioxidants, mitochondrial dysfunction, and ultimately leading to DNA damage and necrotic cell death [7]. GSH significantly contributes to the detoxification of NAPQI as well as scavenging of peroxynitrite in APAP hepatotoxicity. Nevertheless, excessive NAPQI induces prominent depletion of GSH and subsequently results in lipid peroxidation, which is indicated by MDA accumulation [17]. In addition, the decline of GSH levels, APAP decreases the other antioxidants in the body, such as GPx, CAT, and SOD which act as a defense against oxidative stress status [18]. In agreement with previous research, the current study displayed an increase in MDA content with a depletion in hepatic antioxidants GSH, GPX, SOD, and CAT in APAP-treated rats. These findings align with prior reports, which reported that oxidative stress induced by APAP occurs due to utilizing antioxidants to neutralize NAPQI metabolites, which induces severe oxidative stress [19–21]. The current study revealed that DIA (25 and 50 mg/kg) treatment caused a distinct elevation of hepatic GSH, GPX, SOD, and CAT associated with marked depletion of MDA content in a dose-depended manner. These findings are compatible with several studies that reported that DIA neutralizes ROS and has a potent antioxidant capacity in different body tissues [10, 12, 15, 18, 22–24].
APAP-induced oxidative stress results in hepatocellular necrosis, which elicits immune cell infiltration and a cascade of inflammatory responses [7]. Damage-correlated molecular pattern molecules (DAMPS) such as HMGB1 are released by necrotic hepatocytes, activating pattern recognition receptors (PPRs) such as TLR4 and TLR9 [25]. The stimulation of TLRs signaling releases chemokines and other cytokines like IL-1 β, IL-6, and TNF-α, which attracts inflammatory cells via the family members of TLR as well as correlated pathways as the NF-κB pathway [26]. In APAP-induced hepatotoxicity, the activated TLR4 receptor provokes a series of active mediators that dissociate and translocate NF-κB to the nucleus, a fundamental nuclear transcription factor composed of p50, p52, p65, c-Rel, to commence inflammatory mediators' transcription [23, 27–29]. The knockout or blockade of TLR4 was reported to protect from inflammation in APAP hepatotoxicity. In the current work, HMGB1, TLR4, as well as NF-κB expression (p-65) were reduced in the hepatic tissue of DIA-treated rats in a dose-depended manner. These results proved that DIA ameliorates inflammation by inhibiting the HMGB1/TLR4 pathway after APAP acute-over dose administration in rats. This finding aligns with previous studies in different animal disease models [13, 30].
In addition, TNF-α and IL-1β trigger immune cells and augment inflammatory response, which recruits monocytes and neutrophils in circulation to intensify inflammation and trigger a secondary inflammatory cascade. MPO activity is considered an early marker for inflammatory cell infiltration and predicts the incidence of inflammatory diseases [31].In the same context, this study displayed a marked elevation in pro-inflammatory cytokines (TNF-α, IL-1β, and IL-6) levels and MPO levels in the APAP-treated rats [18]. These findings were reversed significantly and dose-dependently by DIA treatment which can be mediated via inhibition of NF-κB (p65). In context, several studies reported the anti-inflammatory effect of DIA in various animal models of inflammation-induced disease [12, 30–32]. In addition, Saleh et al., (1999) illustrated that DIA inhibited MPO levels in the mice model of carrageenan-induced pleurisy [33].
PPAR-γ contributes to several cellular events, glucose homeostasis, and adipogenesis. It significantly contributes to regulating inflammation as a suppressor of inflammation caused by oxidative stress. This role can be due to the direct inhibitory activity of transcription of pro-inflammatory genes as NF-κB, which is involved in inflammation [34, 35]. The therapeutic effect of PPAR-γ agonist has been determined in APAP-induced hepatotoxicity in rats [36]. Also, agonists of PPAR-γ were manifested as potent TLR4 inhibitors [9]. This study demonstrated that the hepatotoxic impacts of APAP induced a decline in the expression of PPAR-γ [36]. Conversely, DIA treatment showed elevation of PPAR-γ expression associated with the decline in NF-κB (p65) expression in a dose-related manner. These findings are consistent with Wen et al. 2019, who illustrated that Rhein, the active metabolite of diacerein, exerts its anti-inflammatory effect by upregulating the PPAR-γ and downregulating NF-κB expressions in the inflammatory cell model using LPS-stimulated RAW264.7 cell [37].
In addition to oxidative stress, the contribution of apoptosis in the pathogenies of APAP-induced liver injury was confirmed [38]. Several studies emphasized that excessive ROS causes mitochondrial damage, which eventually activates the caspase cascade, leading to apoptosis. Caspase-3 has been proven to contribute to the pathway of hepatocellular apoptosis [33, 34, 39]. In addition, the balance between pro-apoptotic as well as anti-apoptotic Bcl-2 protein BAX displayed a pivotal role in regulating apoptosis during APAP hepatotoxicity [40]. Interestingly, promoting Bcl-2 protein expression can protect from APAP-induced apoptosis [41]. The present work demonstrated that acute dose of APAP caused a marked increase in the activity of caspase-3 as well as the expression of Bax with diminished expression of Bcl-2 [42], that DIA therapy restored these results in a dose-related manner. The anti-apoptotic effect of DIA has been reported previously in different tissues [12, 22, 23, 30, 43, 44].
The main limitations of our study were the failure to use immunoblotting analysis for NF-κB (p65) expression and apoptotic markers (procaspase-3 and cleaved caspase-3), along with TUNEL staining. Therefore, further investigation is suggested for these objectives.
Conclusion
DIA dose-dependently protects against liver damage induced by APAP via reducing oxidative stress, inflammatory process, as well as apoptosis. The anti-inflammatory activity attributed to the elevated expression of PPAR-γ, inducing suppression of HMGB-1/TLR4/NF-κB signaling pathway. Therefore, DIA represents an effective hepatoprotective therapy. Further research and clinical implications are needed.
Funding
There was no particular grant for this study from any funding agency in the public, private, or not-for-profit sectors.
Declarations
Conflict of interest
No conflict of interest to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Helal MG, Samra YA. Irbesartan mitigates acute liver injury, oxidative stress, and apoptosis induced by acetaminophen in mice. J Biochem Mol Toxicol. 2020 doi: 10.1002/jbt.22447. [DOI] [PubMed] [Google Scholar]
- 2.Thomas SHL. Paracetamol (acetaminophen) poisoning. Pharmacol Ther. 1993;60:91–120. doi: 10.1016/0163-7258(93)90023-7. [DOI] [PubMed] [Google Scholar]
- 3.Olry A, Meunier L, Délire B, Larrey D, Horsmans Y, Le H. Drug—induced liver injury and COVID—19 infection: the rules remain the same. Drug Saf. 2020;43(7):615–617. doi: 10.1007/s40264-020-00954-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hinson JA. Acetaminophen-induced hepatotoxicity. Drug Metab Dispos. 2003;31(12):1499–1506. doi: 10.1124/dmd.31.12.1499. [DOI] [PubMed] [Google Scholar]
- 5.Zhang R, Wang Q. Impact of liver functions by repurposed drugs for COVID-19 treatment. J Clin Transl Hepatol. 2022 doi: 10.14218/JCTH.2021.00368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lancaster EM, Hiatt JR, Zarrinpar A. Acetaminophen hepatotoxicity: an updated review. Arch Toxicol. 2015;89(2):193–199. doi: 10.1007/s00204-014-1432-2. [DOI] [PubMed] [Google Scholar]
- 7.Du K, Ramachandran A, Jaeschke H. Oxidative stress during acetaminophen hepatotoxicity: sources, pathophysiological role and therapeutic potential. Redox Biol. 2016;10:148–156. doi: 10.1016/j.redox.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di LH, Chen X, Yang Y, et al. Wogonin attenuates inflammation by activating PPAR-γ in alcoholic liver disease. Int Immunopharmacol. 2017;50(March):95–106. doi: 10.1016/j.intimp.2017.06.013. [DOI] [PubMed] [Google Scholar]
- 9.Wu Y, Zhao XD, Zhuang Z, et al. Peroxisome proliferator-activated receptor gamma agonist rosiglitazone attenuates oxyhemoglobin-induced Toll-like receptor 4 expression in vascular smooth muscle cells. Brain Res. 2010;1322:102–108. doi: 10.1016/j.brainres.2010.01.073. [DOI] [PubMed] [Google Scholar]
- 10.Lee KS, Kim SR, Park SJ, et al. Peroxisome proliferator activated receptor-γ modulates reactive oxygen species generation and activation of nuclear factor-κB and hypoxia-inducible factor 1α in allergic airway disease of mice. J Allergy Clin Immunol. 2006;118(1):120–127. doi: 10.1016/j.jaci.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 11.Moldovan F, Pelletier J, Jolicoeur FC, Cloutier JM, Martel-Pelletier J. Diacerhein and rhein reduce the ICE-induced IL-1β and IL-18 activation in human osteoarthritic cartilage. Osteoarthr Cartil. 2000;8(3):186–196. doi: 10.1053/joca.1999.0289. [DOI] [PubMed] [Google Scholar]
- 12.Falgarone G, Dougados M. Diacerein as a disease-modulating agent in osteoarthritis. Curr Rheumatol Rep. 2001;3(6):479–483. doi: 10.1007/s11926-001-0061-y. [DOI] [PubMed] [Google Scholar]
- 13.Ibrahim MA, Abdelzaher WY, Ibrahim YF, et al. Diacerein protects rats with liver ischemia/reperfusion damage: down-regulation of TLR4/ NFκ-B signaling pathway. Biomed Pharmacother. 2020;2021(134):111063. doi: 10.1016/j.biopha.2020.111063. [DOI] [PubMed] [Google Scholar]
- 14.Içer M, Zengin Y, Gunduz E, et al. Is montelukast as effective as N-acetylcysteine in hepatic injury due to acetaminophen intoxication in rats? Exp Toxicol Pathol. 2016;68(1):55–59. doi: 10.1016/j.etp.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 15.Abd-Ellatif RN, Hegab II, Atef MM, Sadek MT, Hafez YM. Diacerein protects against glycerol-induced acute kidney injury: modulating oxidative stress, inflammation, apoptosis and necroptosis. Chem Biol Interact. 2019;306(January):47–53. doi: 10.1016/j.cbi.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 16.Bancroft JD, Gamble M. Theory and practice of histological techniques. Amsterdam: Elsevier; 2008. [Google Scholar]
- 17.Yin H, Cheng L, Holt M, Hail N, MacLaren R, Ju C. Lactoferrin protects against acetaminophen-induced liver injury in mice. Hepatology. 2010;51(3):1007–1016. doi: 10.1002/hep.23476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sreevallabhan S, Mohanan R, Jose SP, et al. Hepatoprotective effect of essential phospholipids enriched with virgin coconut oil (Phoscoliv) on paracetamol-induced liver toxicity. J Food Biochem. 2021;45(2):1–9. doi: 10.1111/jfbc.13606. [DOI] [PubMed] [Google Scholar]
- 19.Bu T, Wang C, Meng Q, et al. Hepatoprotective effect of rhein against methotrexate-induced liver toxicity. Eur J Pharmacol. 2018;834:266–273. doi: 10.1016/j.ejphar.2018.07.031. [DOI] [PubMed] [Google Scholar]
- 20.Silva KLC, Camacho AP, Mittestainer FC, Carvalho BM, Santos A, Guadagnini D. Atorvastatin and diacerein reduce insulin resistance and increase disease tolerance in rats with sepsis. J Inflamm. 2018;15:1–11. doi: 10.1186/s12950-018-0184-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ibrahim YF, Refaie MMM, Kamel MY, et al. Molecular mechanisms underlying the effect of diacerein on trichloroacetic acid—induced hepatic pre-neoplastic lesions in rats. Hum Exp Toxicol. 2021;40:788–803. doi: 10.1177/09603271211056331. [DOI] [PubMed] [Google Scholar]
- 22.Ahmad MM, Rezk NA, Fawzy A, Sabry M. Protective effects of curcumin and silymarin against paracetamol induced hepatotoxicity in adult male albino rats. Gene. 2019;712(July):143966. doi: 10.1016/j.gene.2019.143966. [DOI] [PubMed] [Google Scholar]
- 23.Abdulrazzaq AM, Badr M, Gammoh O, et al. Hepatoprotective actions of ascorbic acid, alpha lipoic acid and silymarin or their combination against acetaminophen-induced hepatotoxicity in rats. Med. 2019 doi: 10.3390/medicina55050181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Refaie MMM, Amin EF, El-Tahawy NF, Abdelrahman AM. Possible protective effect of diacerein on doxorubicin-induced nephrotoxicity in rats. J Toxicol. 2016 doi: 10.1155/2016/9507563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mihm S. Danger-associated molecular patterns (DAMPs): molecular triggers for sterile inflammation in the liver. Int J Mol Sci. 2018;19(10):1–18. doi: 10.3390/ijms19103104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang S, Kuang G, Jiang R, et al. Geniposide protected hepatocytes from acetaminophen hepatotoxicity by down-regulating CYP 2E1 expression and inhibiting TLR 4/NF-κB signaling pathway. Int Immunopharmacol. 2019;74(May):105625. doi: 10.1016/j.intimp.2019.05.010. [DOI] [PubMed] [Google Scholar]
- 27.Abdel-Gaber SA, Mohammed RK, Refaie MMM. Mechanism mediating the protective effect of diacerein in ischemia-reperfusion-induced testicular injury in rats. Life Sci. 2018;209(August):57–62. doi: 10.1016/j.lfs.2018.07.060. [DOI] [PubMed] [Google Scholar]
- 28.Refaie MMM, El M, Wedad H. Diacerein ameliorates induced polycystic ovary in female rats via modulation of inflammasome/caspase1/IL1β and Bax/Bcl2 pathways. Naunyn Schmiedebergs Arch Pharmacol. 2022 doi: 10.1007/s00210-021-02175-2. [DOI] [PubMed] [Google Scholar]
- 29.Du YC, Lai L, Zhang H, et al. Kaempferol from: penthorum chinense Pursh suppresses HMGB1/TLR4/NF-?B signaling and NLRP3 inflammasome activation in acetaminophen-induced hepatotoxicity. Food Funct. 2020;11(9):7925–7934. doi: 10.1039/d0fo00724b. [DOI] [PubMed] [Google Scholar]
- 30.de Oliveira PG, Termini L, Durigon EL, Lepique AP, Sposito AC, Boccardo E. Diacerein: a potential multi-target therapeutic drug for COVID-19. Med Hypotheses. 2020;144(May):109920. doi: 10.1016/j.mehy.2020.109920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kubota K, Saiwai H, Kumamaru H, et al. Myeloperoxidase exacerbates secondary injury by generating highly reactive oxygen species and mediating neutrophil recruitment in experimental spinal cord injury. Spine. 2012;37(16):1363–1369. doi: 10.1097/BRS.0b013e31824b9e77. [DOI] [PubMed] [Google Scholar]
- 32.Aksentijevich I, Zhou Q. NF-κB pathway in autoinflammatory diseases: dysregulation of protein modifications by ubiquitin defines a new category of autoinflammatory diseases. Front Immunol. 2017 doi: 10.3389/fimmu.2017.00399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saleh TS, Calixto JB, Medeiros YS. Effects of anti-inflammatory drugs upon nitrate and myeloperoxidase levels in the mouse pleurisy induced by carrageenan. Peptides. 1999;20(8):949–956. doi: 10.1016/S0196-9781(99)00086-8. [DOI] [PubMed] [Google Scholar]
- 34.Simonin MA, Bordji K, Boyault S, et al. PPAR-γ ligands modulate effects of LPS in stimulated rat synovial fibroblasts. Am J Physiol Cell Physiol. 2002;282:125–133. doi: 10.1152/ajpcell.2002.282.1.c125. [DOI] [PubMed] [Google Scholar]
- 35.Martin H. Role of PPAR-gamma in inflammation. Prospects for therapeutic intervention by food components. Mutat Res Fundam Mol Mech Mutagen. 2010;690(1–2):57–63. doi: 10.1016/j.mrfmmm.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 36.Gupta G, Krishna G, Chellappan DK, Gubbiyappa KS, Candasamy M, Dua K. Protective effect of pioglitazone, a PPARγ agonist against acetaminophen-induced hepatotoxicity in rats. Mol Cell Biochem. 2014;393(1–2):223–228. doi: 10.1007/s11010-014-2064-9. [DOI] [PubMed] [Google Scholar]
- 37.Wen Q, Miao J, Lau N, et al. Rhein attenuates lipopolysaccharide-primed inflammation through NF-κB inhibition in RAW264.7 cells: targeting the PPAR-γ signal pathway. Can J Physiol Pharmacol. 2019;98(6):357–365. doi: 10.1139/cjpp-2019-0389. [DOI] [PubMed] [Google Scholar]
- 38.Hu B, Colletti LM. CXC receptor-2 knockout genotype increases X-linked inhibitor of apoptosis protein and protects mice from acetaminophen hepatotoxicity. Hepatology. 2010;52(2):691–702. doi: 10.1002/hep.23715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.El-sherbiny M, El-shafey M, El-agawy MSE. International Immunopharmacology Diacerein ameliorates testosterone-induced benign prostatic hyperplasia in rats: effect on oxidative stress, inflammation and apoptosis. Int Immunopharmacol. 2021;100(August):108082. doi: 10.1016/j.intimp.2021.108082. [DOI] [PubMed] [Google Scholar]
- 40.Hikita H, Takehara T, Kodama T, et al. Delayed-onset caspase-dependent massive hepatocyte apoptosis upon fas activation in bak/bax-deficient mice. Hepatology. 2011;54(1):240–251. doi: 10.1002/hep.24305. [DOI] [PubMed] [Google Scholar]
- 41.Zhou S, Wang Y, Zhu JJ. Simultaneous detection of tumor cell apoptosis regulators Bcl-2 and Bax through a dual-signal-marked electrochemical immunosensor. ACS Appl Mater Interfaces. 2016;8(12):7674–7682. doi: 10.1021/acsami.6b01010. [DOI] [PubMed] [Google Scholar]
- 42.Zhan X, Zhang J, Chen H, et al. Capsaicin alleviates acetaminophen-induced acute liver injury in mice. Clin Immunol. 2020;220(August):108578. doi: 10.1016/j.clim.2020.108578. [DOI] [PubMed] [Google Scholar]
- 43.Barakat N, Barakat LAA, Zakaria MM, Khirallah SM. Saudi Journal of Biological Sciences Diacerein ameliorates kidney injury induced by cisplatin in rats by activation of Nrf2/Ho-1 pathway and Bax down-regulation. Saudi J Biol Sci. 2021;28(12):7219–7226. doi: 10.1016/j.sjbs.2021.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fouad AA, Abdel-Aziz AM, Hamouda AAH. Diacerein downregulates NLRP3/caspase-1/IL-1β and IL-6/STAT3 pathways of inflammation and apoptosis in a rat model of cadmium testicular toxicity. Biol Trace Elem Res. 2020;195(2):499–505. doi: 10.1007/s12011-019-01865-6. [DOI] [PubMed] [Google Scholar]