Dear Editor,
The antibody response to SARS-CoV-2 is associated with outpatient outcomes; however, it is unknown whether specific immunoglobulin isotypes, i.e., IgG, IgA and IgM, binding to the receptor-binding domain (RBD) of the SARS-CoV-2 spike protein are associated with or alter mortality and use of ventilation or vasopressors in patients hospitalized with acute coronavirus disease 2019 (COVID-19).
This is a sub-study of ARBs CORONA I (NCT04510623), a multi-center observational cohort study of adults (n = 137) hospitalized with acute COVID-19. Plasma anti-SARS-CoV2 IgG, IgA, and IgM antibody isotypes were quantified, and changes in their concentrations from day 4 to 7 were investigated for association with mortality and use of ventilation or vasopressors. Antibody isotype concentrations were determined using the immunIQ COVID assay which is a quantitative assay that measures individual anti-SARS-CoV-2 isotypes (i.e., IgG, IgA, IgM, IgD, and IgE) and subtypes in plasma [1].
The ARBs CORONA I study included plasma collections at day 1, 4, 7, and 14 of hospital admission; from these time-points, we chose to analyze samples via the immunIQ assay at day 4 and day 7 only. The rationale for this selection was twofold: (i) given the likely average time from infection to admission, there were likely to be few measurable antibody responses at day 1, and (ii) we were interested in measurements early enough during admission that they could inform our outcomes of interest. Thus, we examined the association of anti-SARS-CoV-2 antibody isotype absolute concentrations and changes in concentrations in patients who survived to at least day 7 after admission for acute COVID-19.
The primary outcome was 28-day mortality. The secondary outcomes were in-hospital mortality and organ dysfunction, determined as invasion mechanical ventilation, vasopressors, and renal replacement therapy (RRT) that was initiation after day 4 or 7. RRT was not formally analyzed, because too few participants (n = 7) received this therapy. The estimated associations between antibody concentrations and binary outcomes were expressed as odds ratio (OR) and 95% confidence intervals (CI) around the estimated OR. As log2-concentrations were entered into these logistical regression models, the OR reflects the change in the odds of a given outcome for a one-unit difference in the log2 scale; this is equivalent to a doubling in the concentration in the raw scale.
Additional methodological details can be found in the Online Resource.
Figure 1 shows the individual- and group-level change for each of the anti-RBD antibody isotype concentrations from day 4 to day 7. There was a 2.5 × increase in IgG (95% CI 2.1–2.9), 2 × increase IgA (95% CI 1.7–2.3), and 1.7 × increase in IgM (95% CI 1.5–1.9) all p < 0.0001. Anti-RBD IgG, IgA, and IgM were above the lower limit of the measuring interval (LLMI) in 54.7%, 64.2%, and 62% of cases at day 4, respectively, and 80.3%, 83.9%, and 85%, respectively, at day 7.
Fig. 1.
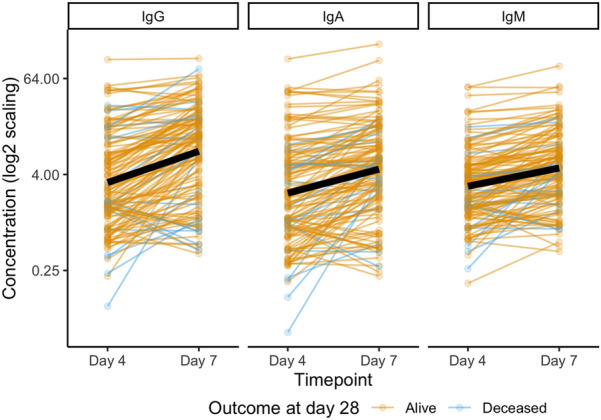
Plasma anti-SARS-CoV-2 RBD IgG, IgM, and IgA on days 4 and 7 of patients (n = 137) hospitalized for acute COVID-19. Line color denotes whether a patient was alive at day 28 (n = 120, yellow) or deceased (n = 17, blue), with the average trajectory noted as a thick black line
A doubling in anti-RBD IgG concentration from day 4 to 7 (i.e., a one-unit increase in log2 IgG concentration) was associated with a 44% decrease in the likelihood of death by day 28 (OR 0.56, 95% CI 0.32–0.93). A similar, though not statistically significant, association was observed between IgG change and in-hospital death (OR 0.68, 95% CI 0.44–1.03). A doubling in IgM concentration from day 4 to 7 was associated with reduced likelihood of invasive mechanical ventilation after day 7 (OR 0.15, 95% CI 0.02–0.67) and with reduced likelihood of vasopressor use after day 7 (OR 0.17, 95% CI 0.03–0.69). We did not observe an association between IgA change and these clinical outcomes.
Dexamethasone was used in 121 (88.3%) patients and use of dexamethasone soon after admission was associated with a higher antibody response on day 7. The antibody isotype concentrations at day 7 for those who received dexamethasone by day 1 were significantly higher than those who were not on dexamethasone for the first 7 days.
Additional results can be found in the Online Resource.
We believe that there are no prior publications associating anti-SARS-CoV-2-binding antibody isotype concentrations with acute organ dysfunction and need for ventilation or vasopressors in patients hospitalized with acute COVID-19. Ours is the first study, showing that the use of dexamethasone is associated with increased concentration of binding antibodies to SAR-CoV-2. The RBD of the spike protein is the strongest initial (within the first week) and lasting (more than 6 months) natural antibody epitope and prevents SARS-CoV-2 entry into human cells [2, 3]. Others found that a lack of IgG response 21 days after symptom onset was associated with progression to critical illness [4]. In another study, anti-RBD IgG was associated with survival among ICU-admitted patients [5]. Limitations of our study are that an association study cannot directly determine causation.
In conclusion, in acute COVID-19 hospitalized patients, the concentrations of IgG, IgA, and IgM binding antibodies against SARS-CoV-2 increased significantly from day 4 to day 7. Increases in anti-SARS-CoV-2 IgG and IgM were associated with lower mortality, and less use of ventilation and vasopressors, respectively.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
ARBs CORONA I Investigators: Taylor D. Pobran, Terry Lee, Matthew P. Cheng, Karen Tran, David Sweet, John Marshall, Arthur S. Slutsky, Srinivas Murthy, Joel Singer, David M. Patrick, Todd C. Lee, John H. Boyd, Keith R. Walley, Francois Lamontagne, Robert Fowler, Brent W. Winston, Greg Haljan, Donald C. Vinh, Alison McGeer, David Maslove, Puneet Mann, Kathryn Donohoe, Geraldine Hernandez, and Anita Palepu.
Funding
This study was funded by Michael Smith Foundation for Health Research (Grant nos. 16353, 2020-1199), Canadian Foundation for Innovation (Grant no. 40962), Canadian Institutes of Health Research (Grant no. 439993), and St. Paul’s Foundation and Mitacs.
Declarations
Conflicts of interest
MLD has received grants for COVI-19 research from the Michael Smith Foundation for Health Research and the Canadian Foundation for Innovation. JAR has received grants for COVID-19 research from the Canadian Institutes of Health Research and St. Paul's Foundation. JAR reports patents owned by the University of British Columbia that are related to the use of PCSK9 inhibitor(s) in sepsis, and related to the use of vasopressin in septic shock and a patent owned by Ferring for use of selepressin in septic shock. He is an inventor on these patents, was a founder, Director, and shareholder in Cyon Therapeutics Inc., and is a shareholder in Molecular You Corp. He reports receiving consulting fees in the last 3 years from: Asahi Kasei Pharmaceuticals of America (was developing recombinant thrombomodulin in sepsis), SIB Therapeutics LLC (developing a sepsis drug), and Ferring Pharmaceuticals (manufactures vasopressin and developing selepressin). He is no longer actively consulting for the following: La Jolla Pharmaceuticals (developing angiotensin II; he chaired the DSMB of a trial of angiotensin II from 2015-2017) and PAR Pharma (sells prepared bags of vasopressin). He reports having received an investigator-initiated grant from Grifols (entitled “Is HBP a mechanism of albumin’s efficacy in human septic shock?”) that was provided to and administered by the University of British Columbia. ASS reports having received a grant from Canadian Institutes of Health Research. He is a consultant to Apeiron Biologics (investigating the use of human recombinant ACE2 for COVID-19) and have received consulting fees from Apeiron Biologics. JM reports having received grants from the Canadian Institutes of Health Research and the Society of Critical Care Medicine. DCV reports salary funding from the Fonds de recherche en santé du Québec and having received grants from: the Canadian Institutes of Health Research, COVID Immunity Task Force, and the Jeffrey Modell Foundation. He reports having received consulting fees from Qu Biologics Inc. and UCB Biosciences GmbH. He reports having received educational payments from CSL Behring, Merck Canada Inc., Novartis Pharmaceuticals Canada Inc., and Shire Pharmaceuticals. He also reports having received travel reimbursements from CSL Behring. He reports a pending patent (PCT/US2021/042875) and report of invention from McGill University (2021-042). He is the Chair of the Association of Medical Microbiology and Infectious Disease Canada Guidelines Committee and a member of the Clinical Immunology Society Education Committee. AMG reports having received grants from Sanofi Pasteur, Pfizer Inc., and Merck & Co., Inc. He reports having received educational payments from GlaxoSmithKline, AstraZeneca, and Moderna Inc. He reports having participated on a Data Safety Monitoring Board or Advisory Board for AstraZeneca, Janssen Pharmaceuticals, Moderna, Medicago Inc., Merck & Co., Inc., Pfizer Inc., Seqirus. JS reports having licenses to the Inflammatory Bowel Disease Questionnaire (IBDQ) from the McMaster Industrial Liaison Office. He reports having participated on a Data Safety Monitoring Board or Advisory Board for the following: Canadian HIV Trials Network Data Safety Monitoring Board, Ontario Clinical Oncology Group, PREPARE Study, African-Canadian Study of HIV-Infected Adults and a Vaccine for Ebola (ACHIV-Ebola), Oral Probiotic Supplementation in Pregnancy to Reduce Group B Streptococcus Colonization (OPSiP), use of immune modulatory properties of ribavirin to enhance Hepatitis B Virus Nucleotide analog antiviral activity protocol, a randomized pilot trial of a cannabis harm reduction e-intervention for young adults with early psychosis who use cannabis (CHAMPS), and a randomized pilot trial of ICanChange—a Mobile Health Intervention to Reduce Cannabis use in Young Adults with Psychosis (iCC). TCL reports having received a grant from Fonds de recherche en santé du Québec. GH reports having received grants from the Canadian Institute for Health Research, Michael Smith Foundation for Health Research, Surrey Hospital Foundation, and the Fraser Health Authority, Department of Research and Evaluation Services. He is the site Medical Director at Surrey Memorial Hospital. AP reports having received a grant from the Canadian Institute for Health Research. She reports receiving consulting fees from Annals of Internal Medicine with the ACP Journals (as the Associate Editor). SM reports having received grants from the Canadian Institute for Health Research, Health Research Foundation, the Wellcome Trust, and Innovative Medicines Canada. All other authors state that they have no competing interests.
Footnotes
The members of the ARBs CORONA I Investigators are listed in the Acknowledgements section.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mari L. DeMarco, Email: mari.demarco@ubc.ca
the ARBs CORONA I Investigators:
Taylor D. Pobran, Terry Lee, Matthew P. Cheng, Karen Tran, David Sweet, John Marshall, Arthur S. Slutsky, Srinivas Murthy, Joel Singer, David M. Patrick, Todd C. Lee, John H. Boyd, Keith R. Walley, Francois Lamontagne, Robert Fowler, Brent W. Winston, Greg Haljan, Donald C. Vinh, Alison McGeer, David Maslove, Puneet Mann, Kathryn Donohoe, Geraldine Hernandez, and Anita Palep
References
- 1.Sherwood KR, Nicholl DDM, Fenninger F, Wu V, Wong P, Benedicto V, et al. Comprehensive immune profiling of a kidney transplant recipient with peri-operative SARS-CoV-2 infection: a case report. Front Immunol. 2021;12:753558. doi: 10.3389/fimmu.2021.753558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rydyznski Moderbacher C, Ramirez SI, Dan JM, Grifoni A, Hastie KM, Weiskopf D, et al. Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell. 2020;183(4):996–1012 e19. doi: 10.1016/j.cell.2020.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu J, Liang B, Chen C, Wang H, Fang Y, Shen S, et al. SARS-CoV-2 infection induces sustained humoral immune responses in convalescent patients following symptomatic COVID-19. Nat Commun. 2021;12(1):1813. doi: 10.1038/s41467-021-22034-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shang Y, Liu T, Li J, Kaweme NM, Wang X, Zhou F. Impact of treatment regimens on antibody response to the SARS-CoV-2 coronavirus. Front Immunol. 2021;12:580147. doi: 10.3389/fimmu.2021.580147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Secchi M, Bazzigaluppi E, Brigatti C, Marzinotto I, Tresoldi C, Rovere-Querini P, et al. COVID-19 survival associates with the immunoglobulin response to the SARS-CoV-2 spike receptor binding domain. J Clin Investig. 2020;130(12):6366–6378. doi: 10.1172/JCI142804. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


