Abstract
The photosynthetic picocyanobacteria Prochlorococcus and Synechococcus are models for dissecting how ecological niches are defined by environmental conditions, but how interactions with bacteriophages affect picocyanobacterial biogeography in open ocean biomes has rarely been assessed. We applied single-virus and single-cell infection approaches to quantify cyanophage abundance and infected picocyanobacteria in 87 surface water samples from five transects that traversed approximately 2,200 km in the North Pacific Ocean on three cruises, with a duration of 2–4 weeks, between 2015 and 2017. We detected a 550-km-wide hotspot of cyanophages and virus-infected picocyanobacteria in the transition zone between the North Pacific Subtropical and Subpolar gyres that was present in each transect. Notably, the hotspot occurred at a consistent temperature and displayed distinct cyanophage-lineage composition on all transects. On two of these transects, the levels of infection in the hotspot were estimated to be sufficient to substantially limit the geographical range of Prochlorococcus. Coincident with the detection of high levels of virally infected picocyanobacteria, we measured an increase of 10–100-fold in the Synechococcus populations in samples that are usually dominated by Prochlorococcus. We developed a multiple regression model of cyanophages, temperature and chlorophyll concentrations that inferred that the hotspot extended across the North Pacific Ocean, creating a biological boundary between gyres, with the potential to release organic matter comparable to that of the sevenfold-larger North Pacific Subtropical Gyre. Our results highlight the probable impact of viruses on large-scale phytoplankton biogeography and biogeochemistry in distinct regions of the oceans.
Subject terms: Biooceanography, Phage biology, Microbial ecology
High-resolution sampling across thousands of kilometres of open ocean reveals a hotspot of viruses at the boundary of major oceanic gyres that, at times, shaped the abundance and biogeography of marine picocyanobacteria.
Main
Shifts in microbial distributions occur along environmental gradients in the ocean, with changes in light, temperature and nutrient availability altering growth rates1–5. Viruses are the most abundant mortality agents on Earth and further shape microbial abundances, diversity and evolution6–8. The density and metabolic status of host cells impact infection by these obligate parasites, as viruses rely on the intracellular resources and cellular machinery of the host for their replication9,10. Thus, varying environmental conditions may shape virus populations and the degree to which they control host abundances and influence ecosystem functioning.
Marine picocyanobacteria of the genera Prochlorococcus and Synechococcus are the most abundant phototrophs on the planet and contribute a major fraction of global primary production11. Abiotic factors, particularly temperature, light and nutrient availability, are widely considered to control their geographical distribution12–16. Mortality factors, such as grazing and viral infection, are regarded as important regulators of picocyanobacterial abundance and diversity8,17 but are seldom considered as factors impacting cyanobacterial biogeography.
Picocyanobacteria are infected by several lineages of viruses belonging to the order Caudovirales18–20. Each lineage has distinct traits with infection characteristics that lie on a spectrum of virulence and host range, and differ in genome size, the core replication and morphogenesis genes they encode as well as the number and type of genes captured from their hosts10,18,19,21–24. Such traits affect their fitness and presumably influence their abundance under different environmental conditions9. Although viral diversity continues to be extensively characterized via global-scale genomic surveys25,26, the actual abundance and extent of infection by distinct virus lineages is largely unknown.
Results and discussion
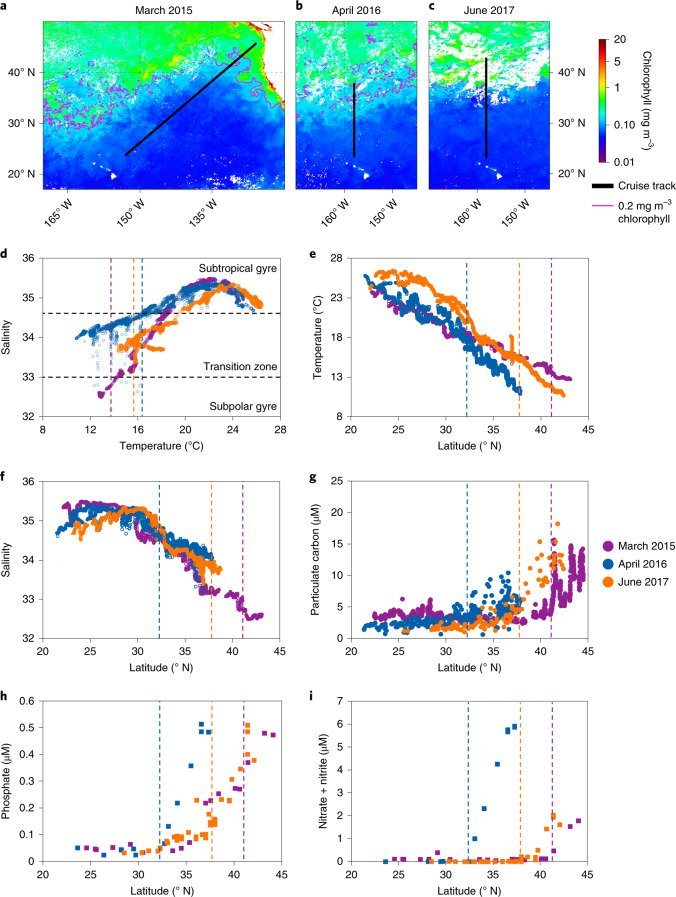
To explore how environmental gradients shape the distribution of cyanophages and picocyanobacteria, we conducted high-resolution surveys in surface waters along five oceanic transects on three cruises covering thousands of kilometres in the North Pacific Ocean in the spring or early summer of 2015, 2016 and 2017 (Fig. 1a–c). These cruises, two of which were out-and-back, passed through distinct regimes from warm, saline and nutrient-poor waters of the North Pacific Subtropical Gyre to cooler, less saline and nutrient-rich waters of higher latitudes influenced by the subpolar gyre (Fig. 1d–i)27. The shift between the two gyres was marked by abrupt changes in trophic indicators such as particulate carbon concentrations (Fig. 1g) and a chlorophyll front (defined as the 0.2 mg m−3 chlorophyll contour28; Fig. 1a–c). As such, the inter-gyre transition zone, defined by salinity and temperature thresholds29 (Fig. 1d), was distinct from both the subtropical and subpolar gyre ecosystems28.
Fig. 1. Gradients in environmental conditions across the North Pacific gyres.
a–c, Transects of three cruises overlaid on monthly averaged satellite-derived sea-surface chlorophyll in March 2015 (a), April 2016 (b) and June 2017 (c). d, Temperature–salinity diagram showing the boundaries of the subtropical and subpolar gyres (black dashed lines) based on the salinity thresholds reported by Roden29. e–i, Temperature (e), salinity (f) as well as the levels of particulate carbon (g), phosphate (h) and nitrate + nitrite (i) as a function of latitude. The coloured dashed lines show the position of the 0.2 mg m−3 chlorophyll contour. For environmental variables plotted against temperature, see Supplementary Fig. 3.
Unexpected Prochlorococcus decline
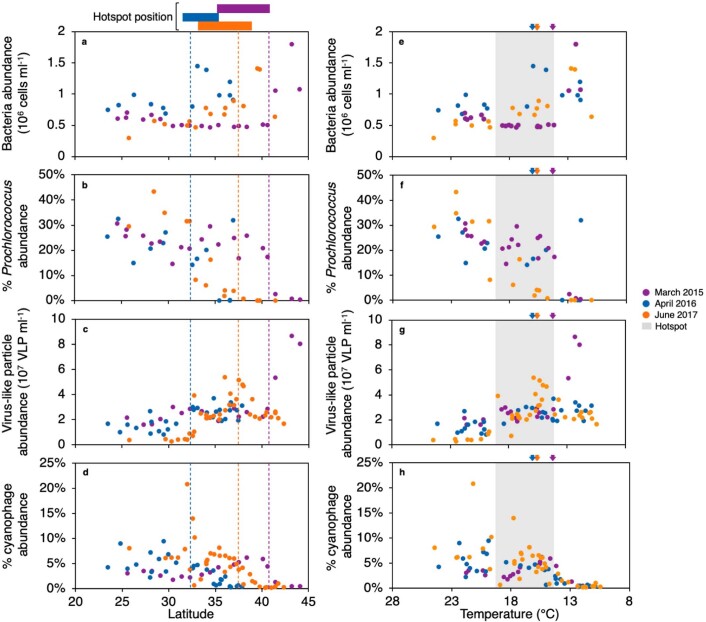
Prochlorococcus concentrations in the oligotrophic waters of the subtropical gyre were 1.5–3.0 × 105 cells ml−1, comprising an average of approximately 29% of the total bacteria (Extended Data Fig. 1) and numerically dominating the phytoplankton community in all three cruises (Extended Data Fig. 2). Prochlorococcus abundance remained high in the southern region of the transition zone in 2015 and 2016, decreasing precipitously to less than 2,000 cells ml−1 north of the chlorophyll front, generally constituting <1% of the total bacteria (Fig. 2 and Extended Data Figs. 1b,f and 2). This decline occurred at temperatures of about 12 °C (Fig. 2) and is consistent with the thermal limits on Prochlorococcus growth determined for cultures and in numerous field observations11,12,15,30 (see Supplementary Discussion). Conversely, Synechococcus was 10–100-fold more abundant in the transition zone relative to the subtropics and gradually decreased northwards towards the subpolar waters (Fig. 2 and Extended Data Fig. 2).
Extended Data Fig. 1. Bacteria and virus abundances across the transects.
Total bacteria (heterotrophic and picocyanobacteria) (a, e), percent Prochlorococcus of total bacteria (b, f), total virus-like particles (c, g), and percent cyanophages of total virus-like particles (d, h) in the surface waters of the North Pacific in March 2015 (purple), April 2016 (blue), and June 2017 (orange). Data are plotted against latitude (a-d) or temperature (e-h). Dashed lines (a-d) or arrows (e-h) show the latitude of the 0.2 mg·m−3 chlorophyll contour as in Fig. 1. Colour bars at the top (a-d) or shaded region (e-h) indicate the position of the virus hotspot.
Extended Data Fig. 2. Latitudinal distributions of picophytoplankton and cyanophages in the North Pacific Ocean.
Semicontinuous sampling of picophytoplankton across the transects. Note that the scale of picoeukaryote abundances is 2-fold lower than that of the picocyanobacteria. Cyanophage abundances are averages determined from 10,000 bootstrapping and resamplings of the phage-to-polony conversion efficiencies (see Methods). Error bars show 95% confidence intervals for cyanophage abundances which are the 95% quantiles of the bootstrap analyses.
Fig. 2. Shifts in the distributions of Prochlorococcus, Synechococcus and cyanophages in the North Pacific Ocean.
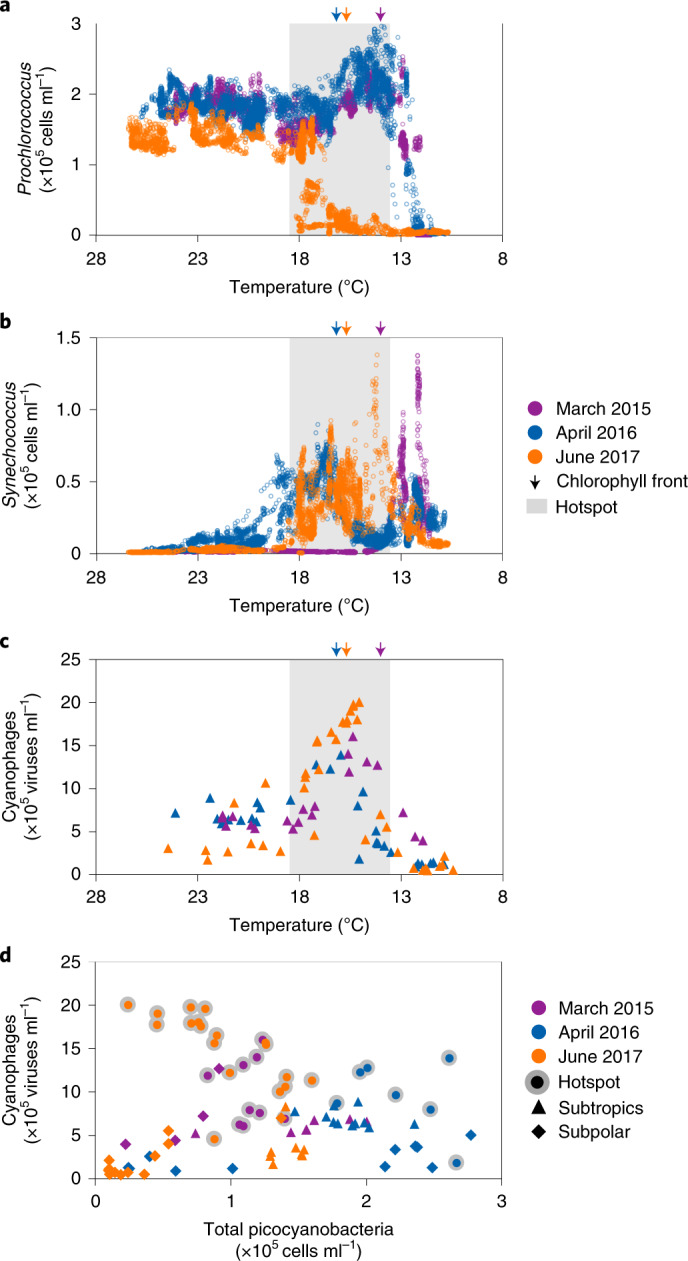
a–c, The distributions of Prochlorococcus (a), Synechococcus (b) and cyanophages (c) were measured at high resolution in the surface waters along the transects of the three cruises in this study and plotted as a function of temperature. d, The relationship between the total numbers of picocyanobacteria and cyanophages. There was no relationship across the data from all regimes (Pearson’s r = −0.008, two-sided P = 0.9, n = 87). Picocyanobacteria correlated positively with cyanophages in the subtropics (Pearson’s r = 0.54, two-sided P = 0.02, n = 26). In the hotspot, the picocyanobacteria abundance correlated negatively with that of cyanophages across all three cruises (Pearson’s r = −0.56, two-sided P = 0.0005, n = 34). There was no relationship found in the subpolar region (Pearson’s r = 0.2, two-sided P = 0.2, n = 27).
The picocyanobacterial abundance patterns differed dramatically in June 2017. The decline in Prochlorococcus occurred approximately two latitudinal degrees south (about 220 km) of the chlorophyll front (Extended Data Figs. 1b,f and 2), where the water temperatures were nearly 18 °C (Fig. 2a). This implicated factors other than temperature as responsible for substantially restricting the geographical distribution of Prochlorococcus. In contrast, the geographical range of Synechococcus was broader by approximately three degrees of latitude (about 330 km), and their integrated abundances across the transects were twofold higher than those observed in 2015 and 2016 (Fig. 2b and Extended Data Fig. 2). Thus, a more southern decline in Prochlorococcus and a broader distribution of Synechococcus was observed in 2017 relative to the 2015 and 2016 transects (Fig. 2 and Extended Data Fig. 2) as well as nine previous transects across this transition zone conducted in different years and seasons31–33 (Supplementary Discussion).
Total bacterial abundances were stable in the subtropics and increased 1.4–3.2-fold in the transition zone on all three cruises (Extended Data Fig. 1a,e). This increase occurred north of the chlorophyll front in 2015 and 2016, whereas abundances increased south of this feature in June 2017. Thus, the 2017 increase in total bacteria happened despite the anomalous loss of Prochlorococcus, which made up only 5% of the total bacteria south of the chlorophyll front relative to 20–30% in the equivalent region in 2015 and 2016 (Extended Data Fig. 1b,f).
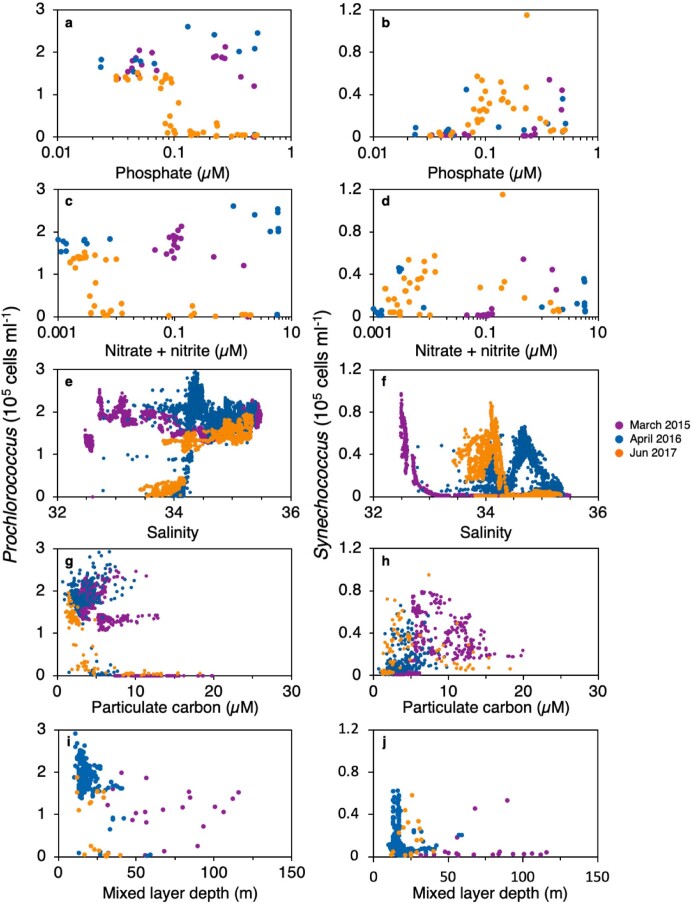
A suite of abiotic variables beyond temperature—many of which are considered important determinants for the biogeography of Prochlorococcus11–16,34—were assessed for their potential role in restricting the geographical distribution of Prochlorococcus on the 2017 transects (Extended Data Fig. 3). Prochlorococcus populations in 2017 were low compared with previous observations at similar macronutrient levels (phosphate and nitrate + nitrite; Extended Data Fig. 3a,c)11. Micronutrient (iron27) concentrations were within the range for optimal Prochlorococcus growth35 and lead concentrations27 were below levels toxic to Prochlorococcus36. Prochlorococcus populations in 2017 declined despite similar salinity (Extended Data Fig. 3e) and particulate carbon values (Extended Data Fig. 3g) to the 2016 transect; furthermore, Prochlorococcus populations thrived across a wider range of values for these variables in 2015. Prochlorococcus maintained high abundances across a range of mixed-layer depths of up to 100 m in 2015 and 2016 but declined at unusually shallow mixing depths in 2017 (Extended Data Fig. 3i). Collectively, the abiotic conditions observed in 2017 in the region of the Prochlorococcus decline supported large populations of Prochlorococcus in 2015 and 2016 (Extended Data Fig. 3). Thus, none of the physical or chemical factors investigated here can alone explain the unexpected decline in Prochlorococcus in 2017. However, we cannot rule out that a unique combination of these factors, or additional abiotic factors, led to the decline in Prochlorococcus.
Extended Data Fig. 3. Comparison of picocyanobacterial abundances to abiotic factors.
Prochlorococcus (left) and Synechococcus (right) abundances plotted against concentrations of phosphate (a,b), nitrate+nitrite (c,d), particulate carbon (e,f), salinity (g,h), and mixed layer depth (i,j) for the March 2015 (purple), April 2016 (blue), and June 2017 (orange) transects.
A virus hotspot affects picocyanobacterial distributions
The lack of an identifiable abiotic variable differentiating the 2017 transects from the other transects and the overall high abundances in total bacteria for all transects (Extended Data Fig. 1a,e) led us to hypothesize that a mortality factor specific to picocyanobacteria, such as infection by viruses, played a role in precipitating the observed shifts in picocyanobacterial geographical ranges. This was investigated through quantification of the abundances of cyanophages and the extent to which they infected Prochlorococcus and Synechococcus using single-virus37,38 and single-cell-infection39 polony methods in surface waters of the three different regimes across the cruise transects—that is, the subtropical and subpolar gyres as well as the transition zone between them. We targeted the T7-like clade A and clade B cyanopodoviruses and the T4-like cyanomyoviruses—three major cyanophage lineages based on isolation studies18–20,24,40, single-cell genomics41 and global metagenomic surveys25,26,42–44 (Supplementary Discussion)—as well as a more recently discovered group, the TIM5-like cyanomyoviruses42,44. Cyanophages from other lineages that are less common in metagenomic surveys39,42,43 were not investigated.
In the subtropical gyre, cyanophages were major components of the planktonic virus community, averaging 5.7 ± 1.9 × 105 cyanophages ml−1 and ranging between 0.7 and 21% of the total double-stranded DNA viruses (Fig. 2c and Extended Data Fig. 1d,h,2), consistent with earlier observations for this region38,39. T4-like cyanophages dominated the subtropical cyanophage community and were generally twofold more abundant than the T7-like clade B cyanophages, the second-most abundant group (Fig. 3 and Extended Data Fig. 4). Together, these two clades constituted >80% of cyanophages measured, with the remainder consisting of T7-like clade A and TIM5-like cyanophages (Fig. 3 and Extended Data Fig. 4). Cyanophage abundances correlated positively with total picocyanobacteria in the subtropical gyre (Pearson’s coefficient of multiple correlation (r) = 0.54, P = 0.02, n = 26; Fig. 2d), suggesting that cyanophages were limited by the availability of susceptible hosts in this region and were not regulating picocyanobacterial populations. On average, less than 1% of the cyanobacterial populations were infected (Fig. 4), with higher infection rates by T4-like cyanophages than T7-like cyanophages (Extended Data Figs. 5 and 6). These instantaneous measurements of infection were used to estimate the daily rates of mortality39 (Methods and Supplementary Discussion), which suggests that 0.5–6% of picocyanobacterial populations were lysed by viruses each day (Extended Data Fig. 7). This implicates other factors, such as grazing45, as the major causes of cyanobacterial mortality in the North Pacific Subtropical Gyre.
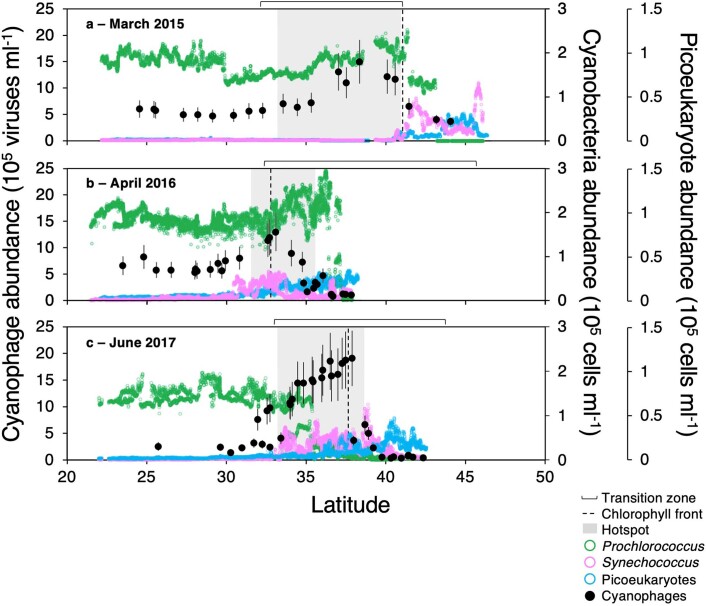
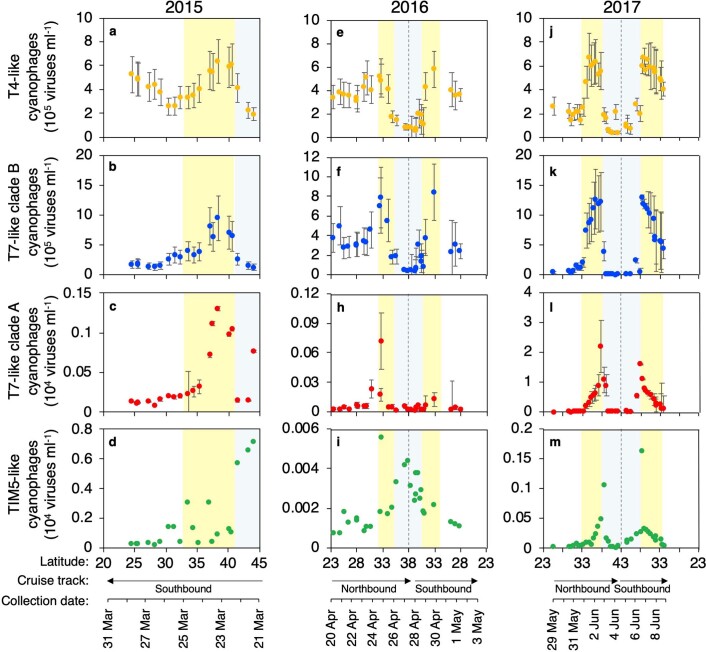
Fig. 3. Cyanophage community composition across the North Pacific gyres.
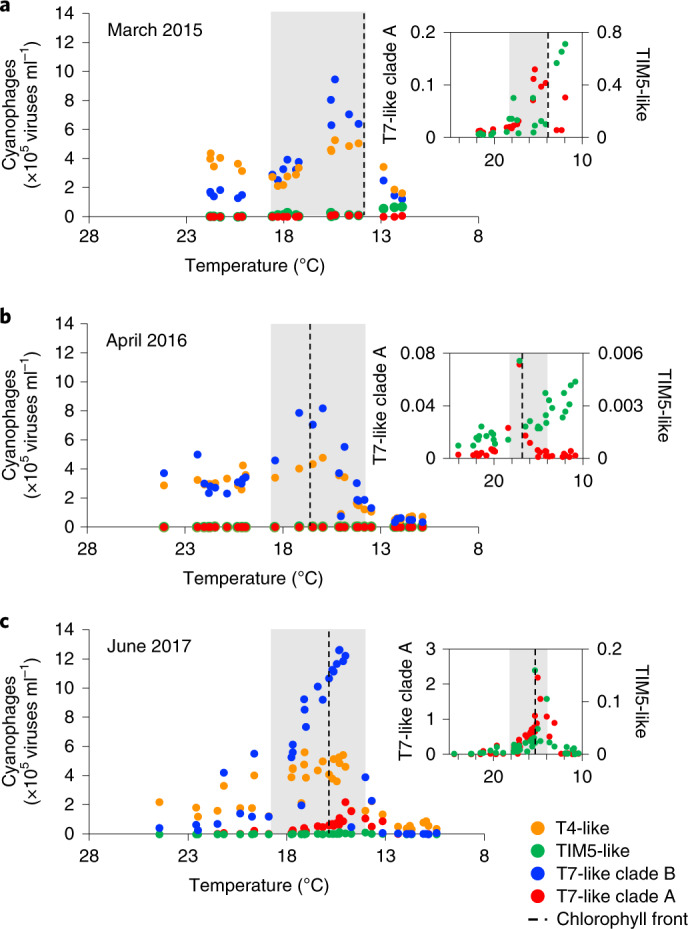
a–c, Cyanophage abundance for the March 2015 (a), April 2016 (b) and June 2017 (c) transects. Insets: T7-like clade A and TIM5-like cyanophage abundances on an expanded scale (similar to the main images, the units for the vertical axes are ×105 viruses ml−1). The grey shaded regions show the position of the virus hotspot. See Extended Data Fig. 4 for the confidence intervals and out-and-back reproducibility and Supplementary Fig. 4 for cyanophage lineages plotted against latitude.
Extended Data Fig. 4. Recurrent patterns in the abundances of different cyanophage lineages.
T4-like (orange), T7-like clade B (blue), T7-like clade A (red), and TIM5-like (green) cyanophage abundances are plotted for the 2015 (left panel, a-d), 2016 (middle panel, e-i), and 2017 (right panel, j-m) transects against latitude (primary x-axis). Northbound and southbound legs of the transects in 2016 and 2017, indicated below the axis, are unfolded on the x-axis as they overlap, and dashed lines indicate the switch in direction between the two transect legs. Date of sample collection is shown as a secondary x-axis. Cyanophage abundances are averages determined from 10,000 bootstrapping and resamplings of the phage-to-polony conversion efficiencies (see Methods). Error bars show 95% confidence intervals for cyanophage abundances which are the 95% quantiles of the bootstrap analyses. The location of the hotspot is indicated by yellow shading, sampling occurring northward of the hotspot is indicated by light blue shading. Note the change in the magnitude of the vertical axis for the different cyanophage lineages.
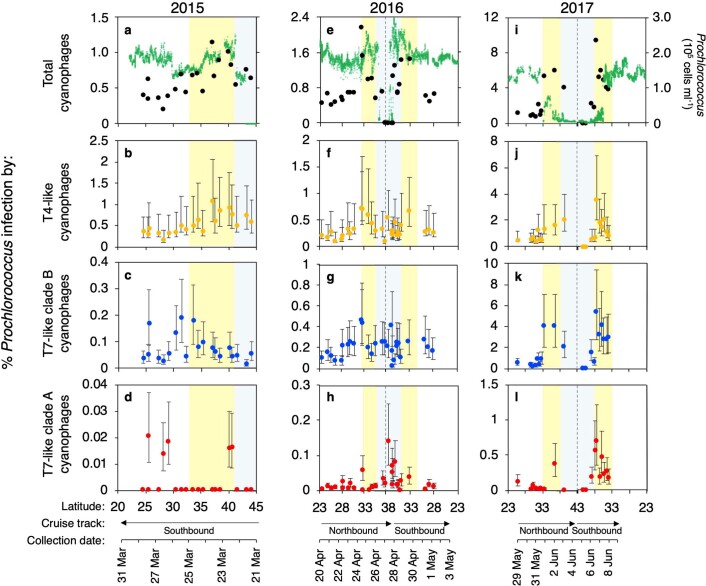
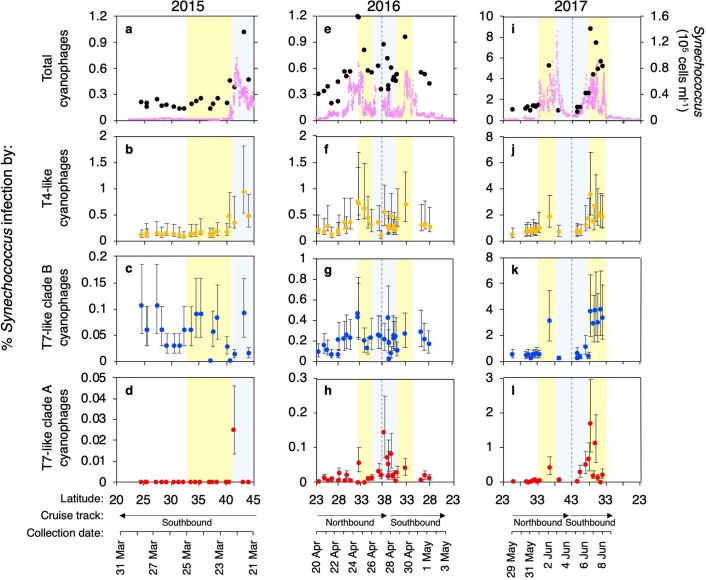
Fig. 4. Viral infection patterns of picocyanobacteria in the North Pacific Ocean.
a–f, Viral infection levels (black) of Prochlorococcus (a,c,e) and Synechococcus (b,d,f) plotted against temperature for the March 2015 (a,b), April 2016 (c,d) and June 2017 (e,f) transects. Insets: infection levels on an expanded scale. The solid lines show infection (red), Prochlorococcus (green) and Synechococcus (pink) averaged and plotted for every 0.5 °C. The dashed lines and shaded regions show the position of the chlorophyll front and the virus hotspot, respectively. For plots by latitude and the upper and lower bounds of infection, see Extended Data Figs. 5 and 6.
Extended Data Fig. 5. Recurrent patterns in the infection of Prochlorococcus by different cyanophage lineages.
Prochlorococcus abundances are shown in green (top row: a, e, i). Infection levels by all cyanophages combined (black: a, e, i), T4-like (orange: b, f, j), T7-like clade B (blue: c, g, k), and T7-like clade A (red: d, h, l) cyanophages are plotted against the latitudinal position of the cruise track. Northbound and southbound legs of the transects in 2016 and 2017, indicated below the axis, are unfolded on the x-axis as they overlap, and dashed lines indicate the switch in direction between the two transect legs. Date of sample collection is shown as a secondary x-axis. Infection levels are averages determined from 10,000 bootstrapping and resamplings of the cell-to-polony conversion efficiencies (see Methods). The maximum and minimum bounds of infection are represented by error bars. The limit of accurate detection of infection using this assay is <0.05% infection. The location of the hotspot is indicated by yellow shading, sampling occurring northward of the hotspot is indicated by blue shading.
Extended Data Fig. 6. Recurrent patterns in the infection of Synechococcus by different cyanophage lineages.
Synechococcus abundances are shown in pink (top row: a, e, i). Infection levels by all cyanophages combined (black: a, e, i), T4-like (orange: b, f, j), T7-like clade B (blue: c, g, k), and T7-like clade A (red: d, h, l) cyanophages are plotted against the latitudinal position of the cruise track. Northbound and southbound legs of the transects in 2016 and 2017, indicated below the axis, are unfolded on the x-axis as they overlap, and dashed lines indicate the switch in direction between the two transect legs. Date of sample collection is shown as a secondary x-axis. Infection levels are averages determined from 10,000 bootstrapping and resamplings of the cell-to-polony conversion efficiencies (see Methods). The maximum and minimum bounds of infection are represented by error bars. The limit of accurate detection of infection using this assay is <0.05% infection. The location of the hotspot is indicated by yellow shading, sampling occurring northward of the hotspot is indicated by blue shading.
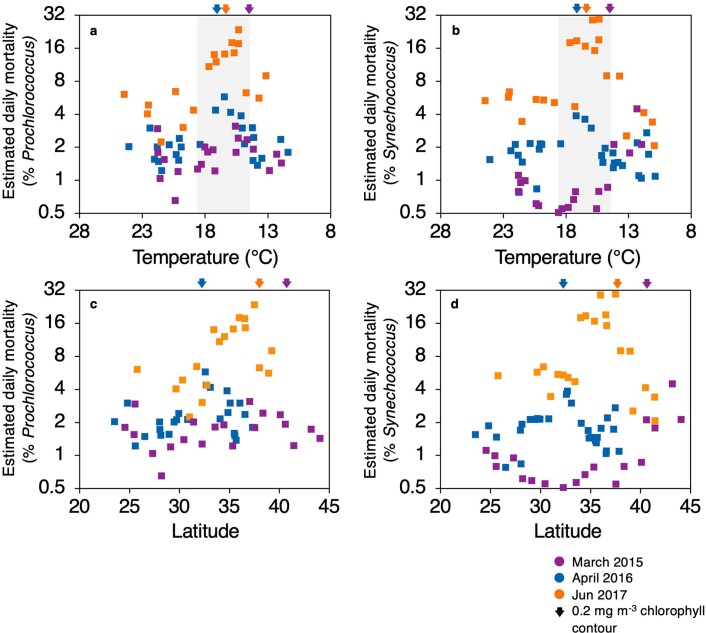
Extended Data Fig. 7. Estimated daily virus-mediated mortality of the picocyanobacterial in the North Pacific Ocean.
Mortality of Prochlorococcus (a, c) and Synechococcus (b, d) plotted against temperature (a, b) and latitude (c, d) was estimated by multiplying the instantaneous infection measurements by the number of infection cycles that could be completed in 24 hours based on temperature and light intensity adjusted average cyanophage latent periods (Supplementary Table 3).
Within the transition zone we observed a steep latitudinal increase in the abundance of cyanophages for every transect, which we define as a cyanophage hotspot (Fig. 2c and Extended Data Figs. 2 and 4). The cyanophage abundances in this hotspot were between three- and tenfold greater than in the subtropical gyre (Fig. 2c). Notably, cyanophages were approximately 25% more abundant (an increase of approximately 5 × 105 viruses ml−1) in the hotspot on the 2017 cruise relative to the other two cruises, reaching a maximum of 2 × 106 viruses ml−1. The hotspot peaked at temperatures of 15–16 °C on all transects, regardless of the geographical location, season or the exact pattern of the Prochlorococcus and Synechococcus distributions (Fig. 2c). Notably, the numbers of T7-like clade B cyanophages increased sharply in the transition zone to become the most abundant lineage, whereas T4-like cyanophages increased more modestly (Fig. 3 and Extended Data Fig. 4). The change in the cyanophage community structure was particularly pronounced in June 2017, when T7-like cyanophages were up to 2.3-fold more abundant than T4-like cyanophages (Fig. 3c). The switch in the relative abundance of T4-like and T7-like clade B cyanophages was diagnostic of the cyanophage hotspot compared with patterns in the subtropical and subpolar gyres.
To begin assessing whether cyanophages negatively affected cyanobacterial populations in the hotspot, we tested the relationship between the abundance of cyanophages and total cyanobacteria. This showed a significant negative correlation between cyanophage and cyanobacterial abundances across all three cruises (Pearson’s r = −0.56, two-sided P = 0.0005, n = 34). This relationship was particularly distinct in 2017, when cyanobacteria were at their overall lowest abundances and cyanophages at their highest (Pearson’s r = −0.65, two-sided P = 0.004, n = 18). This suggests that viruses are one of the key regulators of picocyanobacteria in the region of the hotspot. However, no significant correlation was found across all regimes and all years (Pearson’s r = −0.008, two-sided P = 0.9, n = 87; Fig. 2d), indicating that factors other than viruses are likely to be more important in regulating the abundances of cyanobacteria in other regimes.
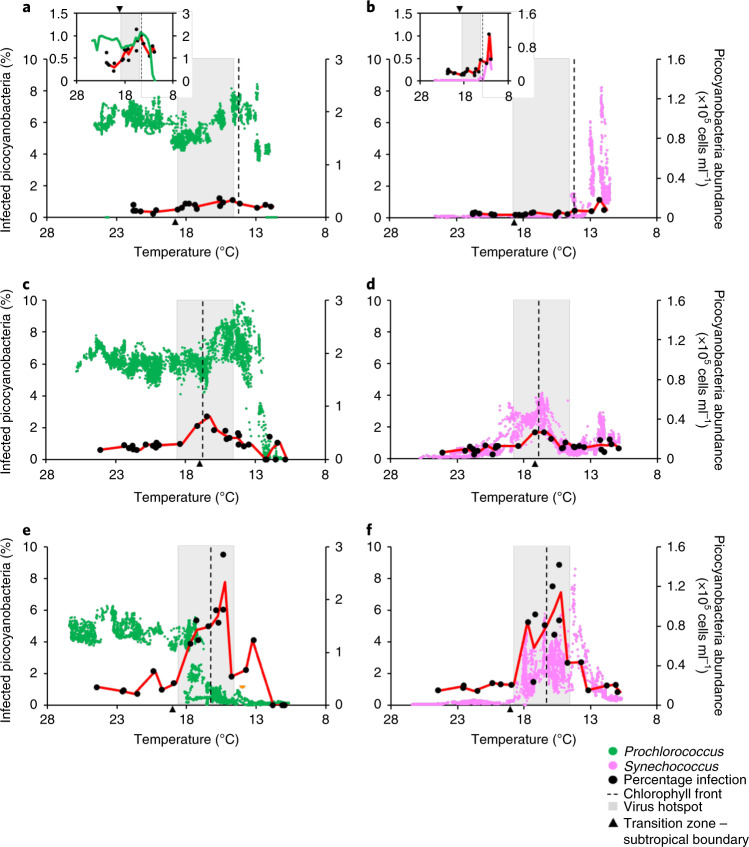
Our single-cell infection measurements allowed us to directly evaluate active viral infection and its impact on picocyanobacteria in the transition zone. Viral infection spiked in this region each year with infection levels that were an average of two- to ninefold higher than those in the subtropical gyre (Fig. 4 and Extended Data Figs. 5,6 and 8). Infection peaked within the temperature range of 12–18 °C and was associated with a concomitant dip in Prochlorococcus abundances in all three cruises (Fig. 4 and Extended Data Fig. 5). These findings provide independent support for the strong negative correlation between cell and virus abundances (Fig. 2d) being the result of virus-induced mortality.
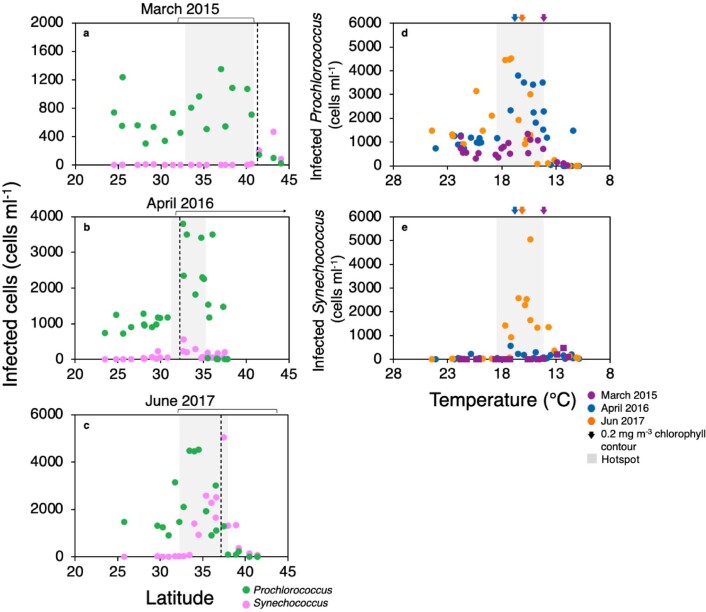
Extended Data Fig. 8. Changes in infected cell densities across latitude and temperature.
The density (cells·ml−1) of total infected Prochlorococcus (green) and Synechococcus (pink) are shown by latitude for the March 2015 (a), April 2016 (b), and June 2017 (c) transects. Prochlorococcus (d) and Synechococcus (e) infected cell abundances plotted against temperature across the March 2015 (purple), April 2016 (blue), and June 2017 (orange) transects. Shaded regions indicate the hotspot, dashed lines (a-c) or arrows (d, e) indicate the chlorophyll front, and brackets (a-c) indicate the transition zone.
Lineage-specific infection was also distinct in the transition zone relative to the subtropical gyre. Infection by T7-like clade B cyanophages generally increased to reach (2015 and 2016) or exceed (2017) those of T4-like cyanophages (Extended Data Figs. 5 and 6). In addition, the ratio of the abundances of T7-like clade B cyanophages to the number of cells they infected was 2.6-fold greater in the hotspot than the subtropics, whereas this ratio was similar in both regions for T4-like cyanophages. Together, these results indicate that, within the hotspot, the T4-like cyanophages displayed increased levels of infection, whereas the T7-like cyanophages displayed both increased levels of infection and produced more viruses per infection, suggesting that T7-like clade B cyanophages are better adapted to conditions in the transition zone (see below).
Of the three cruises, the highest levels of viral infection were observed in June 2017, with up to 9.5% and 8.9% of Prochlorococcus and Synechococcus infected, respectively (Fig. 4e,f). This dramatic increase in infection mirrored the massive decline in Prochlorococcus abundances (Fig. 4e and Extended Data Fig. 5i). We estimate that viruses killed 10–30% of Prochlorococcus and Synechococcus cells daily at these high instantaneous levels of infection (Extended Data Fig. 6) based on the expected number of infection cycles cyanophages were able to complete at the light and temperature conditions in the transition zone (Methods and Supplementary Discussion). Given that Prochlorococcus is estimated to double every 2.8 ± 0.8 d at the low temperatures in this region12, we estimate that 21–51% of the population was infected and killed in the interval before cell division. Synechococcus is expected to have faster growth rates at these temperatures, doubling every 1.1 ± 0.2 d (refs. 12,46). Thus, we estimate that less of the Synechococcus population (9–31%) was killed before division.
Under quasi-steady state conditions, abiotic controls on the growth rate of Prochlorococcus are balanced by mortality due to viral lysis, grazing and other mortality agents39,45,47. Based on the high levels of virus-mediated mortality, the parallel pattern between Prochlorococcus’ death and viral infection, and the negative correlation between cyanophage and picocyanobacterial abundances in the transition zone, we propose that enhanced viral infection in 2017 disrupted this balance, leading to the unexpected decline in Prochlorococcus populations. Grazing and other mortality agents not investigated here could also have contributed to additional mortality beyond the steady state, resulting in further losses of Prochlorococcus. In contrast to Prochlorococcus, Synechococcus maintained large populations despite high levels of infection (Fig. 4f), presumably due to their faster growth rates enabling them to maintain a positive net growth despite enhanced mortality. These findings suggest that virus-mediated mortality in 2017 was an important factor in limiting the geographic range of Prochlorococcus that resulted in a massive loss of habitat of approximately 550 km.
Cyanophage abundances and infection levels dropped sharply in the higher-latitude waters north of the hotspot (Figs. 2c, 4 and Extended Data Figs. 1d,h and 2). The abundances of both T7-like clade B and T4-like cyanophages declined precipitously, yet T4-like cyanophages were the dominant cyanophage lineage (Fig. 3). T7-like clade A cyanophages generally increased locally at the northern border of the hotspot and became the dominant T7-like lineage in two samples between 38 and 39.2° N in 2017 (Fig. 3c and Extended Data Fig. 4). In contrast to all other cyanophages, the abundances of TIM5-like cyanophages increased in waters north of the hotspot (Fig. 3 and Extended Data Fig. 4d,i,m) but remained a minor component of the cyanophage community. No relationship was found between cyanophage and cyanobacterial abundances (Fig. 2d), and less than 1.5% of picocyanobacteria were infected by all cyanophage lineages in these waters (Fig. 4).
The cyanophage hotspot in the transition zone is a ridge of high virus activity that separates the subtropical and subpolar gyres. The reproducibility of our observations, which were separated by days to weeks within each cruise (2016 and 2017) and by years among the three cruises (Extended Data Fig. 4), indicates that this virus hotspot is a recurrent feature at the boundary of these two major gyres in the North Pacific Ocean. This suggests that the hotspot forms due to the distinctive environment of the inter-gyre transition zone creating conditions that enhance infection of picocyanobacteria and proliferation of cyanophages. Prochlorococcus in the transition zone may be prone to stress due to being close to the limits of their temperature growth range5,6, which has the potential to increase susceptibility to viral infection. Alternatively, there may be temperature-dependent trade-offs between virus decay and production that lead to replication optima within a narrow temperature range48. Cyanophage infectivity has been observed to decay more slowly at colder temperatures49, which may allow for the accumulation of infective viruses, leading to increased infection. In addition, cyanophage infections may be more productive due to enhanced nutrient supply in the transition zone27 (Fig. 1h,i) relative to the subtropics, given that the cyanophages replicate in hosts with presumably greater intracellular nutrient quota and obtain more extracellular nutrients, both of which may increase progeny production9,10. The environmental factors influencing the production and removal of viruses probably vary in intensity at different times, leading to variability in cyanophage abundance and infection levels. Thus, the putative cyanophage replication optimum in the hotspot may reflect the combined effects of temperature and nutrient conditions that are intrinsically linked to the oceanographic forces that shape the transition zone itself.
Changes in the cyanophage community structure over environmental gradients are likely to reflect differences in host range, infection properties and genomic potential to remodel host metabolism9. Our data, together with previous measurements in the North Pacific Subtropical Gyre38,39, indicate that the T4-like cyanophages are the lineage best adapted to the low-nutrient waters of the subtropics (Fig. 2d–f). As these waters are inhabited by hundreds of genomically diverse subpopulations of Prochlorococcus50, the broad host range of many T4-like cyanophages18,19,22,51 may be advantageous for finding a suitable host. T4-like cyanophages also have a large and diverse repertoire of host-derived genes21,51—such as nutrient acquisition, photosynthesis and carbon-metabolism genes—that augment host metabolism52 and may increase fitness in nutrient-poor conditions in the subtropics51. In contrast, T7-like clade A and B cyanophages seem to be better adapted to conditions in the transition zone (Fig. 3). T7-like cyanophages have narrow host ranges19,22,40, with smaller genomes and fewer genes to manipulate the host metabolism23, which may allow them to replicate and produce more progeny in regions with elevated nutrient concentrations relative to subtropical conditions. The maximal abundances of TIM5-like cyanophages were found in the most productive waters at the northern end of the transects where the cyanobacterial abundances were lowest and Synechococcus was the dominant picocyanobacterium. This may be partially due to the narrow host range of TIM5-like cyanophages and their specificity for Synechococcus40,44. Our findings of reproducible lineage-specific responses to changing ocean regimes indicate that cyanophage lineages occupy distinct ecological niches.
Temperature and nutrient changes occurring in the transition zone are expected to result in shifts in picocyanobacterial diversity at the sub-genus level (Supplementary Discussion), which we speculate may affect community susceptibility to viral infection. One mechanism for this may be that the picocyanobacteria that thrive in the transition zone are intrinsically more susceptible to viral infection. Another scenario may be related to trade-offs associated with the evolution of resistance to viral infection. The horizontal advection of nutrient-rich waters to the transition zone28 may select for rapidly growing cells adapted for efficient resource utilization. Viral resistance in picocyanobacteria often incurs the cost of reduced growth rates53,54. Thus, competition for nutrients in this region may favour cells with faster growth rates but increased susceptibility to viral infection. Thus, it is probable that the cyanophage distributions do not always follow the cyanobacterial patterns (Extended Data Fig. 2) because of complex interactions between lineage-specific cyanophage traits, host community structure and environmental variables, which may vary seasonally or annually as a result of interannual variability in environmental conditions (see below).
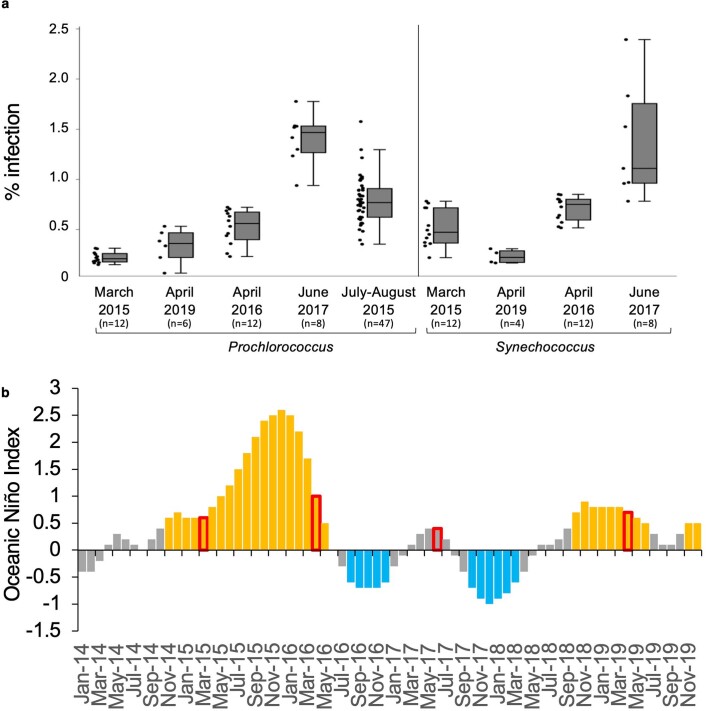
Despite consistent features in cyanophage distributions across the North Pacific Ocean, cyanophage infection was higher (Fig. 4 and Extended Data Fig. 7), whereas Prochlorococcus abundances were consistently lower (Fig. 2a), across the June 2017 transects relative to the March 2015 and April 2016 transects. Seasonality and/or climate variability could explain this interannual variability, although the data currently available to assess this are sparse. Viral infection of picocyanobacteria in the subtropical gyre increased from early spring to summer, suggesting a potential seasonal pattern that may extend across the transect (Extended Data Fig. 9a). In addition, the June 2017 transect occurred during a neutral-to-negative El Niño phase with lower sea-surface temperatures relative to the 2015 and 2016 transects, which were in years of a record marine heatwave, followed by a strong El Niño55 (Extended Data Fig. 9b). In 2015 and 2016, the Prochlorococcus abundances were found to be higher than usual in the North Pacific Ocean in this (Fig. 2a) and other studies56,57. Irrespective of the underlying drivers for the observed interannual variability, we speculate that an ecosystem tipping point was reached in the hotspot under the prevailing conditions in June 2017, aided by the higher cyanophage abundances yet smaller Prochlorococcus population sizes. In this scenario, picocyanobacterial populations were subjected to high infection levels that resulted in an accumulation of cyanophages, initiating a stronger than usual positive-feedback loop between infection and virus production, and precipitating the unexpected Prochlorococcus decline. Continued observations in the North Pacific Ocean are needed to evaluate the potential link between seasonality and/or large-scale climate forcing as ultimate drivers affecting virus–host interactions.
Extended Data Fig. 9. Seasonal and interannual infection dynamics.
(a) Seasonally increasing infection levels for Prochlorococcus (left) and Synechococcus (right) from cruises in March 2015, April 2019, April 2016, June 2017 (this study), and July-August 201539 in the subtropical gyre. Boxes show the median, 1st quartile, 3rd quartile, minimum and maximum are shown by box-and-whisker plots. Individual data points are shown to the left of each box. (b) Oceanic Niño Indices for 2014–2019 from the National Oceanographic and Atmospheric Administration89. Warm phases (>0.5) are indicated by orange, neutral phases (−0.5–0.5) are indicated by grey, and cool phases (<−0.5) are indicated by blue. Red boxes indicate the timing of the March 2015, April 2016, July 2017 and April 2019 cruises in this study. A record marine heatwave occurred in 2015 and 2016 when infection levels were low. The 2017 transects which had high viral infection occurred during a cool phase of the El Niño Oscillation. When El Niño conditions and the warm temperature anomaly returned in 2019, infection levels were low in the subtropical gyre in April, consistent with both seasonal and climatic hypotheses.
Predicting basin-scale virus dynamics
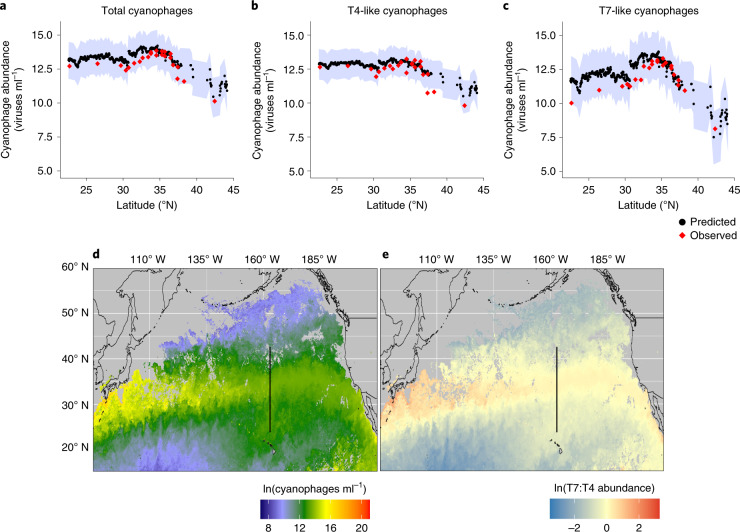
Measurements of cyanobacterial and cyanophage abundances rely on discrete sample collection from shipboard oceanographic expeditions, which limits the geographical and seasonal extent of available data. Therefore, we developed a multiple regression model based on high-resolution satellite data of temperature and chlorophyll to predict cyanophage abundances, a key proxy of cyanobacterial infection (Pearson’s r = 0.61, two-sided P = 1.7 × 10−8, degrees of freedom = 68, n = 70). We used the model to estimate the geographical extent of the virus hotspot. The model accurately predicted the location of the hotspot and cyanophage abundances along a fourth transect in April 2019 (Supplementary Table 1), with the majority of observations falling within the 95% confidence intervals of the model predictions (Fig. 5a–c). Application of the model to the larger region predicted that the virus hotspot formed a boundary extending across the North Pacific Ocean, with lower cyanophage abundances on both sides (Fig. 5d,e and Supplementary Fig. 1). This boundary had the hallmarks of the hotspot with a core that was dominated by T7-like cyanophages and the flanking gyre regions dominated by T4-like cyanophages. Thus, this feature may be more appropriately termed a ‘hot-zone’ due to its substantial projected aerial extent. Assuming the infection levels observed in the hotspot in June 2017 were similar throughout the hot-zone, the potential habitat loss for Prochlorococcus would be about 3.2 × 106 km2, approximately half of the cumulative area loss of the Amazonian rainforest to date58.
Fig. 5. Prediction of cyanophage abundances.
a–c, Model-based predictions of cyanophage abundances corresponding to the empirically measured total (a), T4-like (b) and T7-like clade B (c) cyanophage abundances along a transect in the North Pacific in April 2019. The shaded regions show the 95% confidence interval for the model predictions. d,e, Predicted total cyanophages (d) and the ratio of T4-like/T7-like clade B cyanophages (e) in June 2017 in the North Pacific Ocean. The black lines indicate the cruise track. The grey areas represent regions with no values due to cloud cover or that were beyond the limits of the predictive model. The hotspot peak corresponds to yellow regions in d and red regions in e.
Virus hotspot biogeochemistry
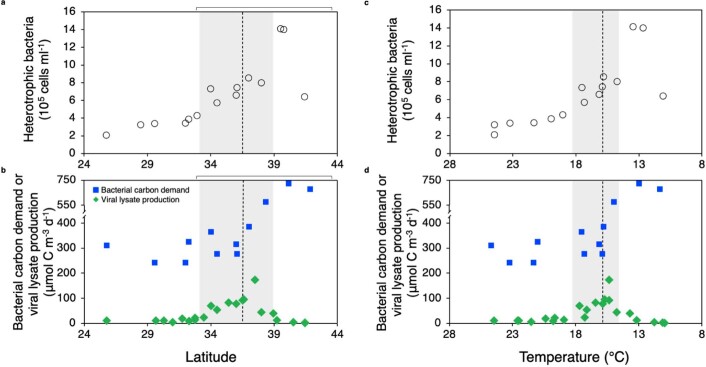
With the ability to predict biogeographic patterns of cyanophages, we evaluated the potential biogeochemical implications of virus-mediated picocyanobacterial lysis and release of organic material in sustaining the bacterial community6–9. The aerial extent of the hot-zone (approximately 4 × 106 km2) is only 14% of the size of the subtropical gyre (2.9 × 107 km2), and yet the total virus-mediated organic matter released from picocyanobacteria in the hot-zone in June 2017 was estimated to be on par with that for the entire North Pacific Subtropical Gyre (Methods and Supplementary Discussion). We estimate that viral lysate released from picocyanobacteria in the subtropical gyre could sustain 4.4 ± 0.8% of the calculated bacterial carbon demand there (Extended Data Fig. 10). In contrast, viral lysate released in the transition zone could sustain an average of 21 ± 12% of the bacterial carbon demand, reaching 33% in some regions (Extended Data Fig. 10), assuming that the bacterial assimilation and growth efficiencies were similar between the subtropical gyre and the hotspot. Thus, local generation of cyanobacterial viral lysate in the transition zone is likely to be an important source of carbon for the heterotrophic bacterial community that can rapidly utilize large molecular weight dissolved organic matter59 and may have contributed to the increase in their abundances south of the chlorophyll front in 2017 (Extended Data Fig. 1a,e).
Extended Data Fig. 10. Virus-mediated organic matter production and heterotrophic bacteria abundances across the transects in June 2017.
(a, c) The abundances of heterotrophic bacteria along the June 2017 transects are reproduced from Extended Data Fig. 1 for ease of comparison. (b, d) Estimated bacterial carbon demand (blue) calculated from 3H-Leucine incorporation and an assumed bacterial growth efficiency of 0.15 and virus-mediated lysate production (green) based on daily picocyanobacteria mortality. Dashed lines indicate the 0.2 mg·m−3 chlorophyll front. Shaded regions indicate the position of the cyanophage hotspot and brackets indicate the transition zone.
Conclusions
The oceans have few geographical boundaries, yet marine ecosystems exhibit clear changes in species distributions. Although the habitat of a species is constrained by a combination of abiotic and biotic interactions, the distributions of marine microbes have classically been linked only to abiotic conditions. Here we show that the region between the North Pacific Ocean gyres harbours a previously hidden virus hotspot with reproducible and distinct community composition and host–virus dynamics. This hotspot is superimposed on gradients in abiotic conditions, and together they influence important processes that shape the ecological succession of major marine primary producers and the cycling of organic matter in this region. The formation of the hotspot and the variation within was probably a result of distinct combinations of environmental conditions that ensued at different times, potentially having differential effects on virus diversity, infectivity and production as well as on host diversity and susceptibility to co-occurring viruses. Our modelling enabled predictions of viruses and their potential impact on picocyanobacterial distributions and biogeochemistry at a large geographical scale. Expansion of this model to other ocean regions, determination of population traits that lead to these ecosystem features and the development of population models for cyanophages and other autotroph-virus systems will allow us to gain a global view of the impacts of viruses on marine ecosystems in both present-day and future oceans11,12,14–16,30.
Methods
Sample collection
Samples were collected in the North Pacific Ocean during 21–29 March 2015 and 10–29 April 2019 aboard the RV Kilo Moana, 20 April–4 May 2016 aboard the RV Ka’imikai-O-Kanaloa and 26 May–13 June 2017 aboard the RV Marcus G. Langseth. The three cruises conducted in 2016–2019 were out-and-back along the 158° W longitudinal line, whereas the 2015 cruise transited unidirectionally from Oregon to Hawaii. Temperature and salinity measurements were collected continuously by a shipboard Sea-Bird Electronics SBE-21 thermosalinograph. Nutrients were collected into acid-cleaned high-density polyethylene bottles using a CTD rosette equipped with Niskin bottles closed at 15 m depth at latitude intervals of approximately 0.5°. The samples were immediately frozen at −20 °C until analysis in the laboratory. All samples used in the virus analyses (virus-like particles, cyanophage abundances, and infection) were collected every 4–8 h from the flow-through seawater system of the ship, which was located at depths between 5 m and 8 m.
For the analyses of viral abundances and infection levels, water was first filtered through a 20 µm mesh. Ten-millilitre samples were collected for iPolony infected-cell analysis, amended with glutaraldehyde (0.1% final concentration), incubated at 4 °C in the dark for 15–30 min, frozen in liquid nitrogen and stored at −80 °C. Forty-millilitre samples were filtered through a 0.2 µm syringe top filter to collect the virus-containing filtrate (Millipore, Durapore). For samples used in polony analyses of free cyanophages, the 0.2 µm filtrate was frozen as is at −80 °C. For samples used in the analyses of virus-like particles, formaldehyde (2% final concentration) was added to the filtrate, incubated for 15–30 min in the dark and stored at −80 °C.
Discrete samples used for the validation of picocyanobacteria abundances determined by SeaFlow (‘Picocyanobacteria and heterotrophic bacteria analysis’ section) and for enumeration of heterotrophic bacteria abundances were collected approximately every 8–12 h or at latitude intervals of approximately 0.5° and amended with glutaraldehyde (0.2% final concentration), incubated for 15–30 min in the dark and frozen as above.
Environmental conditions
The concentrations of soluble reactive phosphorus and nitrate + nitrite were measured as described by Foreman et al.60 using a segmented flow SEAL AutoAnalyzer III with high-resolution detectors and following the colorimetric reactions of Murphy and Riley61, and Strickland and Parsons62, respectively. The limit of quantification of these methods is approximately 30 nmol l−1 for soluble reactive phosphorus and 9 nmol l−1 for nitrate + nitrite, and the average precision is 0.2% and 0.4%, respectively. The accuracies, determined from daily analyses of Wako CSK standard phosphate and nitrate solutions, were within 2%. Low nitrate + nitrite concentrations (<0.5 µmol l−1) were determined using a high-sensitivity chemiluminiscent method63 with a detection limit of 1 nmol l−1. The precision of the high-sensitivity method ranges from 0.4% at 1,000 nmol l−1 to 7% at 2 nmol l−1. Underway particulate beam attenuation measurements were collected using a transmissometer (Wetlabs C-star), calibrated to discrete measurements of particulate organic carbon made via the standard high combustion method as described by White et al.64. Data were merged to an average resolution of 3 min for comparison to underway flow cytometry. Mixed-layer depth was calculated assuming the mixed-layer threshold is the depth at which the surface-referenced potential density is 0.1 kg m−3 greater than at the surface65 from CTD profiles taken across the 2016 and 2017 transects as well as from the March climatology based on Argo float profiles for the 2015 cruise given that no CTD profiles were taken on this transect. Sea-surface chlorophyll levels were derived from 4 km resolution, 32 d rolling composite MODIS-Aqua satellite products.
Virus analysis
Cyanophage abundances and virally infected picocyanobacteria were analysed using the polony37 and iPolony39 methods, respectively. Briefly, the virus-containing fraction of seawater or Prochlorococcus and Synechococcus that had been flow cytometrically sorted (‘Picocyanobacteria and heterotrophic bacteria analysis’ section) were mixed with 10% polyacrylamide and an acrydite-modified primer specific to the cyanophage group of interest. Gels were poured on 40 μm deep Blind-Silane pre-treated microscope slides (Thermo Fisher Scientific), polymerized for 30 min under argon gas to embed and spatially separate the viruses or cells, washed with sterile MilliQ water and 0.025% Tween-20 and dried. Polymerase chain reaction mixes containing 1×Taq buffer, 0.25 mM deoxyribonucleotide triphosphate mix, non-modified primer and 0.67 U µl−1 Jumpstart Taq polymerase (Sigma-Aldrich) were then diffused into the gels and the gels were covered with mineral oil. For a list of primers, their concentrations in reactions and the thermal cycling conditions refer to Supplementary Table 2 (refs. 37–39). After thermal cycling, the slides were washed with buffer E (10 mM Tris pH 7.5, 50 mM KCl, 2 mM EDTA pH 8 and 0.01% Triton X-100) to remove oil and the PCR reagents. The amplicons were denatured with heat and 70% formamide and then washed with buffer E to remove unbound template. A hybridization mix (900 mM NaCl, 60 mM NaH2PO4, 6 mM EDTA and 0.01% Triton X-100) containing degenerate probes specific to the virus family of interest and internal to the PCR primers were applied to the gels and hybridized. See Supplementary Table 2 for the probe sequences, concentrations and hybridization conditions37,38. After hybridization, excess probe was washed off with three rinses of buffer E. The slides were scanned using a Genepix 4000A or B microarray scanner and the Genepix Pro v5.0 software. All reactions were performed with a minimum of two technical replicates. Amplicons, also known as polonies, were enumerated using the ImageJ v1.0 software and normalized to the input volume of seawater for the free cyanophage analyses or to the number of cells in each reaction for the infected-cell analyses. The polony concentrations were then converted to cyanophage abundances or infected-cell percentages based on the previously determined efficiencies of detection37–39. In the case of infected cells, an additional correction for co-sorted free cyanophages was determined empirically for several samples or using a correction factor based on the number of free cyanophages39. For the free cyanophage analyses, a bootstrapping with resampling approach was used to determine the 95% confidence intervals based on the efficiencies of polony formation37. For infected cells, the upper and lower bounds were determined based on the efficiency of detection, assuming that all cells were in the early stages of infection when the detection efficiency is lowest or that all cells were in late-stage infections when detection was maximal39. The infected-cell abundances were derived from multiplying the per cent infection by the abundance of picocyanobacteria. Log-transformed cyanophage and infected-cell abundances were used to determine the linear correlation between the variables.
To convert instantaneous measurements of infection to daily virus-mediated picocyanobacteria mortality, we assumed that mortality is relative to the number of infection cycles that can occur in one day, as done previously39. The virus-mediated daily mortality rates were estimated by multiplying the instantaneous measurements of infection by the number of infection cycles that could be completed in 24 h based on the average latent period for cyanophage–cyanobacteria pairs from previously reported literature values (Supplementary Table 3). These culture-based values were typically determined in culture at approximately 21 °C at light intensities in the range of 10–50 µmol photons m−2 s−1—much dimmer than those in the upper mixed layer (see Supplementary Table 3 references). Thus, we further refined these estimates to be more conservative by adjusting for the impact of day length, light levels and temperature. First, we applied a correction for temperature, assuming that the latent period lengthened by 25% for every 3 °C decrease in temperature from 21 °C and similarly shortened for increasing temperatures (I. Pekarski and D. L., unpublished data). Second, cyanophage infections in high light intensities (210 µmol photons m−2 s−1) are 40% shorter than those conducted at low light intensities (15 µmol photons m−2 s−1)66. Third, we applied this light correction such that cyanophages had latent periods that were 60% shorter during daylight hours and that no lysis occurred during the night, as suggested previously39. These assumptions yielded estimates of about 3–4 cycles per day for the dominant cyanophage types in the subtropical gyre39 and 2–3 infection cycles per day considering the cooler temperatures and shorter day length in the transition zone. These assumptions yield conservative estimates in mortality. Using fewer assumptions, such as not adjusting for temperature or not considering day length and light levels, resulted in higher levels of mortality (a maximum of 66% versus the 51% reported here).
The viral-induced mortality per generation was calculated using the cumulative distribution function:
where M is the fraction of the total population that would be expected to be infected before division, I is the instantaneous infection level and µ is the estimated division rate of Prochlorococcus based on temperature using the relationships for high light I (strains MED4 and MIT9515) and high light II ecotypes67 (strains MIT9312 and MIT9215) or Synechococcus clades I (strain MVIR-16–2) and II (strain M16.1)46. We assumed that the doubling time of a natural population at any given temperature is equal to the division rate of the faster growing ecotype at that temperature. As such, Prochlorococcus high light II ecotypes were assumed to dominate at temperatures above 14.7 °C in the subtropics and the southern transition zone, whereas high light I ecotypes were assumed to dominate at temperatures below that threshold in the northern transition zone, consistent with empirical observations of Prochlorococcus community structure along thermal gradients in the field12,14,15. Similarly, Synechococcus clade II were assumed to dominate at temperatures above 23 °C in the subtropics, whereas clade I was assumed to dominate at temperatures below that threshold in the northern transition zone and subpolar waters.
The abundances of virus-like particles were enumerated after staining the formaldehyde-fixed 0.2 µm filtrate with 100× SYBR Green I, filtration onto a 0.02 µm 25 mm filter (Whatman, Anodisc) and visualization through epifluorescence microscopy using a Leica Application Suite X system68. At least 20 random fields of view per filter were captured. All slides were analysed with at least two technical replicates. The virus-like particles were enumerated using the ImageJ v1.0 software.
Picocyanobacteria and heterotrophic bacteria analysis
The optical properties of cyanobacteria cells were measured continuously using SeaFlow, an underway flow cytometer69. The cell abundance, size and carbon content of each cell were calculated as in Ribalet et al.70 using the R package popcycle v1.1.
Heterotrophic bacteria were analysed from discrete samples by staining each sample with SYBR Green (1×final concentration) for 15 min in the dark on ice. The stained samples were run on a BD Influx flow cytometer equipped with a small-particle detector and the cells were enumerated from a known volume of at least 50 µl. The unstained samples were also analysed to enumerate the picocyanobacteria. The concentration of picocyanobacteria was subtracted from the concentration of total bacteria to give the concentration of heterotrophic bacteria. The analyses were performed in triplicate and analysed using the FACSDiva v8 or Spigot software.
See Supplementary Fig. 2 for examples of the flow cytometry gating strategies.
Defining oceanic regimes and the virus hotspot
Oceanic regimes in the North Pacific Ocean were delineated based on definitions previously described in Roden29 where the subtropical gyre was limited to waters with salinity >34.6, the subpolar gyre was limited to waters with salinity <33 and the intermediate waters designated the North Pacific Transition Zone. These boundaries were based on shipboard salinity measurements or, in the absence of these measurements, satellite-based sea-surface salinity measurements derived from the NASA Soil Moisture Active Passive platform at 9 km2 resolution during the cruise periods71. In addition, we used chlorophyll concentrations from the MODIS-Aqua satellite at 4 km resolution over a rolling 32-d average during the cruise periods to serve as a biological marker of the different oceanic regimes (cloud cover substantially reduced the usefulness of satellite observations over shorter time scales)72. The 0.2 mg m−3 chlorophyll contour used to define the Transition Zone Chlorophyll Front is based on previous observations reported by Polovina and colleagues28.
The virus hotspot was defined as the position where the rates of change of the cyanophage abundances was most rapid. To remove the variability related to the seasonal migration of the transition zone, temperature was used as the independent variable against which to plot the cyanophage abundances and because the relationship was consistent between all four cruises (Fig. 2c). First, the cyanophage abundances (n = 119) were scaled to each cruise-wide maximum and then normalized to the average abundance in the subtropics to centre the data. The abundances were smoothed over a 2.5 °C sliding window. The second derivative of cyanophage abundances as a function of temperature was calculated and the maximum was used to define the temperature range of the hotspot (14.7−18.4 °C). This hotspot range was then translated to geospatial coordinates for each cruise independently according to the relationship between temperature and latitude.
Model projection of cyanophage abundances
We used a train-test splitting approach to identify the variables that best explained the observed T7-like, T4-like and total cyanophage abundances. The data from the 2015–2017 cruises (n = 87) were used as a training set and the data from the 2019 cruise (n = 24) were used as a testing set. The log-transformed abundances of Prochlorococcus, Synechococcus and total cyanobacteria; log-transformed chlorophyll concentration; the Prochlorococcus:Synechococcus ratio and their interaction with temperature were tested as predictors. The best model was chosen by comparison of the root-mean-square error (RMSE) that was calculated based on predicted (Pi) and observed (Oi) cyanophage abundances (equation (1))73. The RMSE is proportional to the scale of the dataset, with small values indicating high precision and large values indicating low precision. Although two models commonly had the best predictive value (Synechococcus × temperature and Prochlorococcus:Synechococcus × temperature), these models were not considerably better based on RMSE compared with models using chlorophyll (Supplementary Table 1). Furthermore, there are far fewer publicly available datasets that report global-scale Prochlorococcus and/or Synechococcus abundances than those that report chlorophyll. For these reasons, we employed the chlorophyll-based models for predictions. For all cyanophage groups, the interaction between temperature (T) and the chlorophyll (Chl) concentration (equations (2)–(4)) showed the relatively high predictive powers of the model (Supplementary Table 1). The fitted parameter values (a, b, c) are listed in Supplementary Table 4. All calculations were performed using the ‘stats’ package v3.6.2 in R.
| 1 |
| 2 |
| 3 |
| 4 |
For graphing maps of cyanophage distributions (Fig. 5 and Supplementary Fig. 1), we obtained values for the sea-surface chlorophyll concentration and sea-surface temperature from the MODIS-Aqua satellite at 4 km resolution over a 32-d rolling composite74. Data that covered a latitudinal and longitudinal range from 15° N to 70° N and 110° E to 100° W were extracted using SeaDAS v7.5.3. The cyanophage abundances were predicted using the above linear regression model for regions warmer than 5 °C as a bound on the thermal range of picocyanobacteria growth and where the chlorophyll concentrations were lower than 0.4 mg m−3. The maps were produced using the ‘mapdata’ v2.3.0 and ‘ggplot2’ v5.5.5 R packages75,76.
Organic matter turnover and bacterial production
Virus-mediated organic-matter production was estimated by subtracting the carbon in virus particles produced during infection from the total cellular carbon using the following equation:
where Lvir is the lysate generated per day, P is the abundance of Prochlorococcus, S is the abundance of Synechococcus, MPro and MSyn are the daily estimated mortality for Prochlorococcus and Synechococcus, respectively, CPro and CSyn are the daily average carbon quota per cell for Prochlorococcus and Synechococcus, respectively, derived from SeaFlow (‘Picocyanobacteria and heterotrophic bacteria analysis’ section), Cvir is the carbon content for a virus particle as reported in Jover et al.77 for either the T7-like cyanophage Syn5 or the coliphage T4 as representatives of T7-like and T4-like cyanophages, respectively, and B is the estimated burst size (Supplementary Table 3). Note that the proportion of total cellular carbon of a single virus particle is <0.5% and <0.1% for T4-like and T7-like cyanophages, respectively. Most reported burst-size measurements for cyanophages are different estimates of virus production (Supplementary Table 3). They measure infective viruses, total particles or free and packaged genomic DNA. Although these are not the same measure, we combined them in our use here. Nevertheless, our calculation was not strongly impacted by these methodological differences, both because the highest burst sizes of about 200 T7-like cyanophages cell−1 would be approximately 2% of the total carbon and these reports are for T7-like clade A viruses (I.M., I. Pekarski and D.L., unpublished data), which are minor in their contribution to infection in this dataset (Extended Data Figs. 3 and 4). The lysate production was calculated for both T4- and T7-like cyanophages and summed to estimate the total carbon released through the viral lysis of Prochlorococcus and Synechococcus daily. Average virus-mediated organic-matter production was determined for each of the three regimes, multiplied by the average surface area and average mixed-layer depth of the corresponding regime to estimate the cumulative virus-mediated production of organic matter per regime.
Prochlorococcus and Synechococcus cultured at 24 °C have elemental stoichiometries of 208 C:P and 6.4 N:P (ref. 78), and 130.7 C:P and 7.6 N:P (ref. 79), respectively. These ratios were used to convert cellular carbon to nitrogen and phosphorus production. We assumed that all cellular material released due to lysis, except for the progeny viruses, is biologically available to heterotrophic bacteria and that bacteria assimilate 100% of the organic matter. The average estimated virus-mediated organic-matter production was then divided by previously reported measurements of dissolved organic-matter production in the North Pacific Subtropical Gyre and other gyres. These values were 0.07 µmol C l−1 d−1 from Viviani et al.80, 1.8 nmol P l−1 d−1 from Björkman et al.81 and 33.6 nmol N l−1 d−1 from Sipler and Bronk82.
Bacterial production was determined through the measurement of 3H-leucine incorporation according to previously described methods83,84. Briefly, triplicate 30–40 ml samples were collected with Niskin bottles at a depth of 15 m pre-dawn with one sample as a killed control by the addition of trichloroacetic acid (1% final concentration). Each sample was spiked with approximately 3 GBq l-[3,4,5–3H(N)]-leucine (Perkin Elmer) to yield an added concentration of approximately 20 nM 3H-leucine. The samples were incubated for 1–3 h in the dark in on-deck flow-through incubators at in situ temperature. The samples were then filtered by vacuum onto 25 mm GF75 filters and the incorporation of 3H-leucine into protein (insoluble in cold 5% trichloroacetic acid) was quantified in 10 ml of Ultima Gold LLT liquid scintillation cocktail using a scintillation counter. Bacterial production was determined from leucine incorporation as previously described83, where the fraction of leucine in protein is 7.3% and ratio of cellular carbon per protein is 0.86. Bacterial carbon demand was estimated using the measured bacterial production values and an assumed bacterial growth efficiency of 0.15 that is typical of oligotrophic communities85–87.
Statistics
Pearson correlation analyses between cyanophage and cyanobacterial abundances were performed using RStudio v1.2.5019 and Python v3.8.2.
Reporting Summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Supplementary materials, discussion, Tables 1–4, Figs. 1–4 and refs. 90–108.
Multiple sequence alignment of environmental cyanophage sequences at the forward primer site of the T4-like cyanophage polony assay.
Multiple sequence alignment of environmental cyanophage sequences at the reverse primer site of the T4-like cyanophage polony assay.
Multiple sequence alignment of environmental cyanophage sequences at the probe site of the T4-like cyanophage polony assay.
Multiple sequence alignment of environmental cyanophage sequences at the forward primer site of the T7-like cyanophage polony assay.
Multiple sequence alignment of environmental cyanophage sequences at the reverse primer site of the T7-like cyanophage polony assay.
Multiple sequence alignment of environmental cyanophage sequences at the probe site of the T7-like cyanophage polony assay.
Acknowledgements
We thank the captains and crews of the RV Ka’imikai-O-Kanaloa, RV Kilo Moana and RV Marcus G. Langseth as well as O. Sosa for underway phosphate measurements and comments on the manuscript. In addition, we thank K. W. Brandt, R. Morales, M. Schatz, A. Hynes and A. Replogle for technical help and use of equipment and the Gradients team for discussions. Funding was provided by the Simons Foundation (Gradients grant no. 426570 and SCOPE grant no. 329108 to D.L., E.V.A., A.E.W. and D.M.K.; and LIFE grant nos. 529554 and 721254 to D.L.) and European Research Council (grant no. ERC-CoG 646868 to D.L.). M.C.G.C. was supported by a Fulbright Postdoctoral Fellowship. This manuscript is a contribution of the Simons Collaboration on Ocean Processes and Ecology (SCOPE).
Extended data
Source data
All data are provided in a single file due to the interconnected nature of the data, which will facilitate cross-referencing by readers.
Author contributions
M.C.G.C. and D.L. designed the study. M.C.G.C., F.R., I.M., B.P.D., Y.H., S.F., J.W., N.S., B.B.C., A.E.W. and E.V.A. participated in the sampling efforts. M.C.G.C., Y.H., S.G. and N.B. performed the virus analyses. M.C.G.C., F.R. and E.V.A. analysed the bacterial abundances. S.F., D.M.K. and A.E.W. contributed to the inorganic and organic water chemistry analyses. M.C.G.C., I.M. and B.B.C. conceptualized and developed the predictive models. B.P.D. performed and analysed the bacterial uptake experiments. M.C.G.C. and D.L. wrote the paper with contributions from all authors.
Peer review
Peer review information
Nature Microbiology thanks David Talmy, Zackary Johnson, Gianluca Meneghello and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Data availability
The data that support the findings of this study are available at https://simonscmap.com/catalog/cruises/ in the directories KM1502, KOK1606, MGL1704 and KM1906 (ref. 88). Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
is available for this paper at 10.1038/s41564-022-01088-x.
Supplementary information
The online version contains supplementary material available at 10.1038/s41564-022-01088-x.
References
- 1.Moore CM, et al. Processes and patterns of oceanic nutrient limitation. Nat. Geosci. 2013;6:701–710. [Google Scholar]
- 2.Cullen, J., Franks, P., Karl, D. M. & Longhurst, A. in The Sea Vol. 12 (eds Robinson, A. R. et al.) 297–335 (Wiley, 2002).
- 3.Thomas MK, Kremer CT, Klausmeier CA, Litchman E. A global pattern of thermal adaptation in marine phytoplankton. Science. 2012;338:1085–1088. doi: 10.1126/science.1224836. [DOI] [PubMed] [Google Scholar]
- 4.Browning TJ, et al. Nutrient co-limitation at the boundary of an oceanic gyre. Nature. 2017;551:242–246. doi: 10.1038/nature24063. [DOI] [PubMed] [Google Scholar]
- 5.Longhurst, A. R. Ecological Geography of the Sea (Academic Press, 2007).
- 6.Suttle CA. Viruses in the sea. Nature. 2005;437:356–361. doi: 10.1038/nature04160. [DOI] [PubMed] [Google Scholar]
- 7.Fuhrman JA. Marine viruses and their biogeochemical and ecological effects. Nature. 1999;399:541–548. doi: 10.1038/21119. [DOI] [PubMed] [Google Scholar]
- 8.Breitbart M, Bonnain C, Malki K, Sawaya NA. Phage puppet masters of the marine microbial realm. Nat. Microbiol. 2018;3:754–766. doi: 10.1038/s41564-018-0166-y. [DOI] [PubMed] [Google Scholar]
- 9.Zimmerman AE, et al. Metabolic and biogeochemical consequences of viral infection in aquatic ecosystems. Nat. Rev. Microbiol. 2020;18:21–34. doi: 10.1038/s41579-019-0270-x. [DOI] [PubMed] [Google Scholar]
- 10.Waldbauer JR, et al. Nitrogen sourcing during viral infection of marine cyanobacteria. Proc. Natl Acad. Sci. USA. 2019;116:15590–15595. doi: 10.1073/pnas.1901856116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flombaum P, et al. Present and future global distributions of the marine cyanobacteria Prochlorococcus and Synechococcus. Proc. Natl Acad. Sci. USA. 2013;110:9824–9829. doi: 10.1073/pnas.1307701110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zinser ER, et al. Influence of light and temperature on Prochlorococcus ecotype distributions in the Atlantic Ocean. Limnol. Oceanogr. 2007;52:2205–2220. [Google Scholar]
- 13.Biller SJ, Berube PM, Lindell D, Chisholm SW. Prochlorococcus: the structure and function of collective diversity. Nat. Rev. Microbiol. 2015;13:13–27. doi: 10.1038/nrmicro3378. [DOI] [PubMed] [Google Scholar]
- 14.Bouman HA, et al. Oceanographic basis of the global surface distribution of Prochlorococcus ecotypes. Science. 2006;312:918–921. doi: 10.1126/science.1122692. [DOI] [PubMed] [Google Scholar]
- 15.Johnson ZI, et al. Niche partitioning among Prochlorococcus ecotypes along ocean-scale environmental gradients. Science. 2006;311:1737–1741. doi: 10.1126/science.1118052. [DOI] [PubMed] [Google Scholar]
- 16.Ustick LJ, et al. Metagenomic analysis reveals global-scale patterns of ocean nutrient limitation. Science. 2021;372:287–291. doi: 10.1126/science.abe6301. [DOI] [PubMed] [Google Scholar]
- 17.Proctor LM, Fuhrman JA. Viral mortality of marine bacteria and cyanobacteria. Nature. 1990;343:60–62. [Google Scholar]
- 18.Waterbury JB, Valois FW. Resistance to co-occurring phages enables marine Synechococcus communities to coexist with cyanophages abundant in seawater. Appl. Environ. Microbiol. 1993;59:3393–3399. doi: 10.1128/aem.59.10.3393-3399.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sullivan MB, Waterbury JB, Chisholm SW. Cyanophages infecting the oceanic cyanobacterium Prochlorococcus. Nature. 2003;424:1047–1051. doi: 10.1038/nature01929. [DOI] [PubMed] [Google Scholar]
- 20.Mann NH. Phages of the marine cyanobacterial picophytoplankton. FEMS Microbiol. Rev. 2003;27:17–34. doi: 10.1016/S0168-6445(03)00016-0. [DOI] [PubMed] [Google Scholar]
- 21.Crummett LT, Puxty RJ, Weihe C, Marston MF, Martiny JBH. The genomic content and context of auxiliary metabolic genes in marine cyanomyoviruses. Virology. 2016;499:219–229. doi: 10.1016/j.virol.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 22.Zborowsky S, Lindell D. Resistance in marine cyanobacteria differs against specialist and generalist cyanophages. Proc. Natl Acad. Sci. USA. 2019;116:16899–16908. doi: 10.1073/pnas.1906897116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Labrie SJ, et al. Genomes of marine cyanopodoviruses reveal multiple origins of diversity. Environ. Microbiol. 2013;15:1356–1376. doi: 10.1111/1462-2920.12053. [DOI] [PubMed] [Google Scholar]
- 24.Suttle CA, Chan AM. Marine cyanophages infecting oceanic and coastal strains of Synechococcus—abundance, morphology, cross-infectivity and growth-characteristics. Mar. Ecol. Prog. Ser. 1993;92:99–109. [Google Scholar]
- 25.Gregory AC, et al. Marine DNA viral macro- and microdiversity from pole to pole. Cell. 2019;177:1109–1123. doi: 10.1016/j.cell.2019.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang S, Zhang S, Jiao N, Chen F. Marine cyanophages demonstrate biogeographic patterns throughout the global ocean. Appl. Environ. Microbiol. 2015;81:441–452. doi: 10.1128/AEM.02483-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pinedo-González P, et al. Anthropogenic Asian aerosols provide Fe to the North Pacific Ocean. Proc. Natl Acad. Sci. USA. 2020;117:27862–27868. doi: 10.1073/pnas.2010315117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Polovina JJ, Howell EA, Kobayashi DR, Seki MP. The transition zone chlorophyll front updated: advances from a decade of research. Prog. Oceanogr. 2017;150:79–85. [Google Scholar]
- 29.Roden, G. I. in Biology, Oceanography, and Fisheries of the North Pacific Transition Zone and Subarctic Frontal Zone Vol. 105 (ed. Wetherall, J. A.) 1–38 (NOAA, 1991).
- 30.Larkin AA, et al. Niche partitioning and biogeography of high light adapted Prochlorococcus across taxonomic ranks in the North Pacific. ISME J. 2016;10:1555–1567. doi: 10.1038/ismej.2015.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gainer PJ, et al. Contrasting seasonal drivers of virus abundance and production in the North Pacific Ocean. PLoS ONE. 2017;12:e0184371. doi: 10.1371/journal.pone.0184371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Juranek LW, et al. Biological production in the NE Pacific and its influence on air-sea CO2 flux: evidence from dissolved oxygen isotopes and O2/Ar. J. Geophys. Res. Oceans. 2012;117:c05022. [Google Scholar]
- 33.Sohm JA, et al. Co-occurring Synechococcus ecotypes occupy four major oceanic regimes defined by temperature, macronutrients and iron. ISME J. 2016;10:333–345. doi: 10.1038/ismej.2015.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saito MA, et al. Multiple nutrient stresses at intersecting Pacific Ocean biomes detected by protein biomarkers. Science. 2014;345:1173–1177. doi: 10.1126/science.1256450. [DOI] [PubMed] [Google Scholar]
- 35.Thompson AW, Huang K, Saito MA, Chisholm SW. Transcriptome response of high- and low-light-adapted Prochlorococcus strains to changing iron availability. ISME J. 2011;5:1580–1594. doi: 10.1038/ismej.2011.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Echeveste P, Agustí S, Tovar-Sánchez A. Toxic thresholds of cadmium and lead to oceanic phytoplankton: cell size and ocean basin-dependent effects. Environ. Toxicol. Chem. 2012;31:1887–1894. doi: 10.1002/etc.1893. [DOI] [PubMed] [Google Scholar]
- 37.Baran N, Goldin S, Maidanik I, Lindell D. Quantification of diverse virus populations in the environment using the polony method. Nat. Microbiol. 2018;3:62–72. doi: 10.1038/s41564-017-0045-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goldin S, Hulata Y, Baran N, Lindell D. Quantification of T4-like and T7-like cyanophages using the polony method show they are significant members of the virioplankton of the North Pacific Subtropical Gyre. Front. Microbiol. 2020;11:1210. doi: 10.3389/fmicb.2020.01210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mruwat N, et al. A single-cell polony method reveals low levels of infected Prochlorococcus in oligotrophic waters despite high cyanophage abundances. ISME J. 2021;15:41–54. doi: 10.1038/s41396-020-00752-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dekel-Bird NP, Sabehi G, Mosevitzky B, Lindell D. Host-dependent differences in abundance, composition and host range of cyanophages from the Red Sea. Environ. Microbiol. 2015;17:1286–1299. doi: 10.1111/1462-2920.12569. [DOI] [PubMed] [Google Scholar]
- 41.Berube PM, et al. Single cell genomes of Prochlorococcus, Synechococcus, and sympatric microbes from diverse marine environments. Sci. Data. 2018;5:180154. doi: 10.1038/sdata.2018.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aylward FO, et al. Diel cycling and long-term persistence of viruses in the ocean’s euphotic zone. Proc. Natl Acad. Sci. USA. 2017;114:11446–11451. doi: 10.1073/pnas.1714821114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nishimura Y, et al. Environmental viral genomes shed new light on virus-host interactions in the ocean. mSphere. 2017;2:e00359-16. doi: 10.1128/mSphere.00359-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sabehi G, et al. A novel lineage of myoviruses infecting cyanobacteria is widespread in the oceans. Proc. Natl Acad. Sci. USA. 2012;109:2037–2042. doi: 10.1073/pnas.1115467109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Connell PE, Ribalet F, Armbrust EV, White A, Caron DA. Diel oscillations in the feeding activity of heterotrophic and mixotrophic nanoplankton in the North Pacific Subtropical Gyre. Aquat. Microb. Ecol. 2020;85:167–181. [Google Scholar]
- 46.Pittera J, et al. Connecting thermal physiology and latitudinal niche partitioning in marine Synechococcus. ISME J. 2014;8:1221–1236. doi: 10.1038/ismej.2013.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ribalet F, et al. Light-driven synchrony of Prochlorococcus growth and mortality in the subtropical Pacific gyre. Proc. Natl Acad. Sci. USA. 2015;112:8008–8012. doi: 10.1073/pnas.1424279112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Demory D, et al. A thermal trade-off between viral production and degradation drives virus-phytoplankton population dynamics. Ecol. Lett. 2021;24:1133–1144. doi: 10.1111/ele.13722. [DOI] [PubMed] [Google Scholar]
- 49.Garza DR, Suttle CA. The effect of cyanophages on the mortality of Synechococcus spp. and selection for UV resistant viral communities. Microb. Ecol. 1998;36:281–292. doi: 10.1007/s002489900115. [DOI] [PubMed] [Google Scholar]
- 50.Kashtan N, et al. Single-cell genomics reveals hundreds of coexisting subpopulations in wild Prochlorococcus. Science. 2014;416:416–420. doi: 10.1126/science.1248575. [DOI] [PubMed] [Google Scholar]
- 51.Fuchsman CA, Carlson MCG, Garcia Prieto D, Hays MD, Rocap G. Cyanophage host-derived genes reflect contrasting selective pressures with depth in the oxic and anoxic water column of the Eastern Tropical North Pacific. Environ. Microbiol. 2020;23:2782–2800. doi: 10.1111/1462-2920.15219. [DOI] [PubMed] [Google Scholar]
- 52.Zeng Q, Chisholm SW. Marine viruses exploit their host’s two-component regulatory system in response to resource limitation. Curr. Biol. 2012;22:124–128. doi: 10.1016/j.cub.2011.11.055. [DOI] [PubMed] [Google Scholar]
- 53.Avrani S, Lindell D. Convergent evolution toward an improved growth rate and a reduced resistance range in Prochlorococcus strains resistant to phage. Proc. Natl Acad. Sci. USA. 2015;112:2191–2200. doi: 10.1073/pnas.1420347112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lennon JT, Khatana SAM, Marston MF, Martiny JBH. Is there a cost of virus resistance in marine cyanobacteria? ISME J. 2007;1:300–312. doi: 10.1038/ismej.2007.37. [DOI] [PubMed] [Google Scholar]
- 55.Di Lorenzo E, Mantua N. Multi-year persistence of the 2014/15 North Pacific marine heatwave. Nat. Clim. Change. 2016;6:1042–1047. [Google Scholar]
- 56.Larkin AA, et al. Persistent El Niño driven shifts in marine cyanobacteria populations. PLoS ONE. 2020;15:e0238405. doi: 10.1371/journal.pone.0238405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Peña MA, Nemcek N, Robert M. Phytoplankton responses to the 2014–2016 warming anomaly in the northeast subarctic Pacific Ocean. Limnol. Oceanogr. 2019;64:515–525. [Google Scholar]
- 58.da Cruz DC, Benayas JMR, Ferreira GC, Santos SR, Schwartz G. An overview of forest loss and restoration in the Brazilian Amazon. New For. 2021;52:1–16. [Google Scholar]
- 59.Amon RMW, Benner R. Rapid cycling of high-molecular-weight dissolved organic matter in the ocean. Nature. 1994;369:549–552. [Google Scholar]
- 60.Foreman RK, Björkman KM, Carlson CA, Opalk K, Karl DM. Improved ultraviolet photo-oxidation system yields estimates for deep-sea dissolved organic nitrogen and phosphorus. Limnol. Oceanogr. Methods. 2019;17:277–291. [Google Scholar]
- 61.Murphy J, Riley IP. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta. 1962;27:31–36. [Google Scholar]
- 62.Strickland, J. D. H. & Parsons, T. R. A Practical Handbook of Seawater Analysis Bulletin no. 167 (Fisheries Research Board of Canada, 1972).
- 63.Foreman RK, Segura-Noguera M, Karl DM. Validation of Ti(III) as a reducing agent in the chemiluminescent determination of nitrate and nitrite in seawater. Mar. Chem. 2016;186:83–89. [Google Scholar]
- 64.White AE, et al. Phenology of particle size distributions and primary productivity in the North Pacific subtropical gyre (Station ALOHA) J. Geophys. Res. Oceans. 2015;120:7381–7399. doi: 10.1002/2015JC010897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Holte J, Talley LD, Gilson J, Roemmich D. An Argo mixed layer climatology and database. Geophys. Res. Lett. 2017;44:5618–5626. [Google Scholar]
- 66.Puxty RJ, Evans DJ, Millard AD, Scanlan DJ. Energy limitation of cyanophage development: implications for marine carbon cycling. ISME J. 2018;12:1273–1286. doi: 10.1038/s41396-017-0043-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liang Y, et al. Estimating primary production of picophytoplankton using the carbon-based ocean productivity model: a preliminary study. Front. Microbiol. 2017;8:1926. doi: 10.3389/fmicb.2017.01926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Patel A, et al. Virus and prokaryote enumeration from planktonic aquatic environments by epifluorescence microscopy with SYBR Green I. Nat. Protoc. 2007;2:269–276. doi: 10.1038/nprot.2007.6. [DOI] [PubMed] [Google Scholar]
- 69.Swalwell JE, Ribalet F, Armbrust EV. SeaFlow: a novel underway flow-cytometer for continuous observations of phytoplankton in the ocean. Limnol. Oceanogr. Methods. 2011;9:466–477. [Google Scholar]
- 70.Ribalet F, et al. SeaFlow data v1, high-resolution abundance, size and biomass of small phytoplankton in the North Pacific. Sci. Data. 2019;6:277. doi: 10.1038/s41597-019-0292-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.JPL SMAP Level 3 CAP Sea Surface Salinity Standard Mapped Image Monthly V4.3 Validated Dataset (NASA PO.DAAC, accessed 23 November 2020); 10.5067/SMP43-3TMCS
- 72.NASA Goddard Space Flight Center, Ocean Ecology Laboratory, Ocean Biology Processing Group. MODIS Aqua Chlorophyll Data; 2018 Reprocessing (NASA OB.DAAC, accessed 19 February 2020); 10.5067/AQUA/MODIS/L3B/CHL/2018
- 73.Olden JD, Jackson DA. Torturing data for the sake of generality: How valid are our regression models? Ecoscience. 2000;7:501–510. [Google Scholar]
- 74.Moderate-resolution Imaging Spectroradiometer (MODIS) Aqua Global Level 3 Mapped Sea Surface Temperature. Ver. 2019.0 (NASA OBPG, accessed 19 February 2020); 10.5067/MODSA-MO4D9
- 75.Becker, R. A. & Wilks, A. R. mapdata: Extra MapDatabases. R version 2.3.0 (2018).
- 76.Wickham, H. ggplot2: Elegant Graphics for Data Analysis (Springer-Verlag, 2016)
- 77.Jover LF, Effler TC, Buchan A, Wilhelm SW, Weitz JS. The elemental composition of virus particles: implications for marine biogeochemical cycles. Nat. Rev. Microbiol. 2014;12:519–528. doi: 10.1038/nrmicro3289. [DOI] [PubMed] [Google Scholar]
- 78.Martiny AC, Ma L, Mouginot C, Chandler JW, Zinser ER. Interactions between thermal acclimation, growth rate, and phylogeny influence Prochlorococcus elemental stoichiometry. PLoS ONE. 2016;11:e0168291. doi: 10.1371/journal.pone.0168291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lopez JS, Garcia NS, Talmy D, Martiny AC. Diel variability in the elemental composition of the marine cyanobacterium Synechococcus. J. Plankton Res. 2016;38:1052–1061. [Google Scholar]
- 80.Viviani DA, Karl DM, Church MJ. Variability in photosynthetic production of dissolved and particulate organic carbon in the North Pacific Subtropical Gyre. Front. Mar. Sci. 2015;2:73. [Google Scholar]
- 81.Björkman K, Thomson-Bulldis AL, Karl DM. Phosphorus dynamics in the North Pacific subtropical gyre. Aquat. Microb. Ecol. 2000;22:185–198. [Google Scholar]
- 82.Sipler, R. E. & Bronk, D. A. in Biogeochemistry of Marine Dissolved Organic Matter 2nd edn (eds Hansell, D. A. & Carlson, C. A.) 127–232 (Academic Press, 2015).
- 83.Kirchman, D. in Methods in Microbiology Vol. 30 (ed. Paul, J. H.) 227–237 (Elsevier, 2001).
- 84.Kirchman D, K’nees E, Hodson R. Leucine incorporation and its potential as a measure of protein synthesis by bacteria in natural aquatic systems. Appl. Environ. Microbiol. 1985;49:599–607. doi: 10.1128/aem.49.3.599-607.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Carlson CA, Ducklow HW. Growth of bacterioplankton and consumption of dissolved organic carbon in the Sargasso Sea. Aquat. Microb. Ecol. 1996;10:69–85. [Google Scholar]
- 86.Anderson TR, Ducklow HW. Microbial loop carbon cycling in ocean environments studied using a simple steady-state model. Aquat. Microb. Ecol. 2001;26:37–49. [Google Scholar]
- 87.Giorgio PAD, Cole JJ. Bacteria growth efficiency in natural aquatic ecosystems. Annu. Rev. Ecol. Syst. 1998;29:503–541. [Google Scholar]
- 88.Ashkezari MD, et al. Simons Collaborative Marine Atlas Project (Simons CMAP): an open-source portal to share, visualize, and analyze ocean data. Limnol. Oceanogr. Methods. 2021;19:488–496. [Google Scholar]
- 89.Oceanic Niño Index (National Oceanographic and Atmospheric Administration, accessed 15 September 2020); https://origin.cpc.ncep.noaa.gov/products/analysis_monitoring/ensostuff/ONI_v5.php
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary materials, discussion, Tables 1–4, Figs. 1–4 and refs. 90–108.
Multiple sequence alignment of environmental cyanophage sequences at the forward primer site of the T4-like cyanophage polony assay.
Multiple sequence alignment of environmental cyanophage sequences at the reverse primer site of the T4-like cyanophage polony assay.
Multiple sequence alignment of environmental cyanophage sequences at the probe site of the T4-like cyanophage polony assay.
Multiple sequence alignment of environmental cyanophage sequences at the forward primer site of the T7-like cyanophage polony assay.
Multiple sequence alignment of environmental cyanophage sequences at the reverse primer site of the T7-like cyanophage polony assay.
Multiple sequence alignment of environmental cyanophage sequences at the probe site of the T7-like cyanophage polony assay.
Data Availability Statement
The data that support the findings of this study are available at https://simonscmap.com/catalog/cruises/ in the directories KM1502, KOK1606, MGL1704 and KM1906 (ref. 88). Source data are provided with this paper.