Abstract
Introduction
The COVID-19 outbreak has affected care for non-COVID diseases like cancer. We evaluated the impact of the COVID-19 outbreak on prostate cancer care in the Netherlands.
Methods
Prostate cancer diagnoses per month in 2020–2021 versus 2018–2019 were compared based on preliminary data of the Netherlands Cancer Registry (NCR) and nationwide pathology network. Detailed data was retrieved from the NCR for the cohorts diagnosed from March-May 2020 (first COVID-19 wave) and March-May 2018–2019 (reference). Changes in number of diagnoses, age, disease stage and first-line treatment were compared.
Results
An initial decline of 17% in prostate cancer diagnoses during the first COVID-19 wave was observed. From May onwards the number of diagnoses started to restore to approximately 95% of the expected number by the end of 2020. Stage at diagnosis remainedstable over time. In low-risk localised prostate cancer radical prostatectomy was conducted more often in week 9–12 (21% versus 12% in the reference period; OR=1.9, 95% CI; 1.2–3.1) and less active surveillance was applied (67% versus 78%; OR=0.6, 95% CI; 0.4–0.9). In the intermediate-risk group, a similar change was observed in week 13–16. Radical prostatectomy volumes in 2020 were comparable to 2018–2019.
Conclusion
During the first COVID-19 wave the number of prostate cancer diagnoses declined. In the second half of 2020 this largely restored although the number remained lower than expected. Changes in treatment were temporary and compliant with adapted guidelines. Although delayed diagnoses could result in a less favourable stage distribution, possibly affecting survival, this seems not very likely.
Keywords: Coronavirus, SARS-CoV-2, Prostatic neoplasms, Healthcare, Treatment
Abbreviations
- ADT:
Androgen Deprivation Therapy
- ARTA:
Androgen Receptor Targeted Agents
- COVID-19:
Corona virus 2019
- EAU:
European Association of Urology
- EBRT:
External Beam Radiotherapy
- FMS:
Federation of Medical Specialists
- GP:
General Practitioner
- IKNL:
Netherlands Comprehensive Cancer Organization
- ISUP:
International Society of Urological Pathology
- NCR:
Netherlands Cancer Registry
- NVU:
Dutch Urological Association
- PALGA:
The nationwide network and registry of histo- and cytopathology in the Netherlands
- PSA:
Prostate Specific Antigen
- SES:
Socioe Economic Status
- TNM:
Tumour, Node, Metastasis
Introduction
Since the outbreak of the corona virus 2019 (COVID-19), the pandemic has continued to put a strain on society and healthcare. To date, over 220 million cases and over 4.5 million deaths have been registered worldwide [1]. In the Netherlands, the first COVID-19 case was diagnosed at the end of February 2020 in the Southern part of the country [2]. From the 23th of March, the Dutch government implemented a lockdown and strict social distancing measures to prevent the spread of COVID-19 [3,4]. As a result of the increasing number of infected patients and patients who needed to be admitted in the hospital, non-urgent procedures and treatments in hospitals were often postponed or cancelled [4,5]. Furthermore, appointments with the general practitioner (GP) were cancelled by patients who feared to become infected or who wanted to alleviate the healthcare system [5]. National screening programs, available in the Netherlands for breast, colorectal and cervical cancer, were temporary halted on the 16th of March. During this period, a substantial and alarming decline in het nationwide number of cancer diagnoses in the Netherlands was seen [6].
Preliminary data as presented by the Netherlands Comprehensive Cancer Organisation (Integraal Kankercentrum Nederland; IKNL) demonstrated a decline of 20–25% in cancer diagnoses of all sites, including a decline in urological cancer diagnoses [6]. During the first three months of the COVID-19 outbreak, at least 5000 less cancer diagnoses were made compared to 2019 [7]. To help clinicians prioritise diagnostic and surgical procedures in urological care during the first COVID-19 wave, adapted guidelines were published by several national and international associations, including the European Association of Urology (EAU) and the Federation of Medical Specialists (FMS). In low- and intermediate-risk localised prostate cancer, the advice was to avoid or postpone invasive procedures (e.g. prostatectomy and brachytherapy). In high-risk localized/locally advanced prostate cancer, it was recommended to consider external beam radiotherapy (EBRT) after an extended period of androgen deprivation therapy (ADT). In case of metastatic disease, it was recommended to avoid ADT combined with docetaxel and consider treatment with androgen receptor targeted agents (ARTA) instead [8], [9], [10]. The main considerations in drafting these adapted guidelines were the urgency of procedures, the risk of postponing elective care and the available capacity. In addition, the risk of adverse effects or events due to treatment, like risk of infection after chemotherapy were taken into account [8], [9], [10].
In the Netherlands, prostate cancer is the most common cancer amongst men with approximately 12.000 new diagnoses each year [11]. Prostate cancer is generally detected following opportunistic screening at the general practitioners’ (GP) office or because men visit the GP with complaints that may be related to prostate cancer. There is no formal population-based screening program for prostate cancer in the Netherlands. As the exact impact of the COVID-19 outbreak on prostate cancer care is largely unknown, and as data so far were incomplete, we aimed to report the impact of COVID-19 on 1) the number of prostate cancer diagnoses, 2) age and disease stage at diagnosis and 3) (time to) treatment strategies per disease stage in the Netherlands. Furthermore, the effect of the outbreak on biopsies and surgical capacity concerning radical prostatectomies will be evaluated.
Patients and methods
Patient selection
Data from the Netherlands Cancer Registry (NCR) were used for this historic cohort study. The NCR is a population based cancer registry maintained by IKNL and contains information on all newly diagnosed cancer patients in the Netherlands since 1989 [11]. Newly diagnosed cancers are identified through the nationwide Pathological Anatomical National Automated Archive (PALGA)[12] supplemented by the Dutch Hospital Data (DHD), which contains all hospital discharge diagnoses, to identify cancer diagnoses without histological confirmation. Patient- and tumour characteristics, disease stage and first-line treatment are routinely collected by trained data managers through consultation of the electronic health records. Vital status is updated annually by means of record linkage with the Personal Records Database.
To evaluate recent effects of the COVID-19 outbreak on the number of prostate cancer diagnoses and surgical volume of radical prostatectomies, we derived preliminary data from prostate cancer cases diagnosed in the period January 2018 - May 2021 from the NCR. These data are largely based on data from PALGA and included only date of diagnosis, gender, topography, morphology, and date of radical prostatectomy (if applicable). To evaluate the effects of the COVID-19 outbreak on biopsies performed, the number of biopsies and pathological results (i.e. malignant or non-malignant) were derived from the PALGA database from January 2015 to January 2021.
Additionally, to evaluate effects of the COVID-19 outbreak during the first wave, all patients newly diagnosed with or treated for prostate cancer (International Classification of Diseases for Oncology (ICD-O-3) topography code C61) between January-May (week 2–22) in 2020 and in 2018–2019 were identified in the NCR. We retrieved detailed data from the NCR on patient characteristics (age at diagnosis, postal code), tumour characteristics (Gleason grade, prostate specific antigen (PSA) at diagnosis, disease stage)), and primary treatment characteristics (type and date of treatment). Socioeconomic status (SES) was derived from Statistics Netherlands and was based on the patients’ postal code. Five geographical regions were determined, based on postal code. Disease stage was defined according to the eighth edition of the tumour, node and metastasis (TNM) classification [13].
Definitions
Patients diagnosed or treated between the 1st of March and the 31st of May 2020 were considered the COVID-cohort, and patients diagnosed or treated during the same period in 2018–2019 were considered the reference cohort. Both cohorts were divided into time periods based on COVID-19-related events occurring in 2020: week 9–12, week 13–16 and week 17–22. In week 9, the first Dutch COVID-19 patient was officially diagnosed [2]. In week 13, the Netherlands went into national lockdown[4] and in week 17 the first effects are to be expected from the national call to resume visiting the GP in case of any symptoms, as a decline in GP consultations was seen [14]. Week 2–8 was considered the pre-COVID period and week 9–22 the first COVID-19 wave. These time periods were used in the analyses as we considered the events to which the periods are related, to potentially have a significant effect on prostate cancer care. Due to the large difference in working days in week 1 of every year, week 1 was excluded from the period definitions. The reference cohort (2018–2019) was divided in similar periods.
Age at diagnosis was included in the analyses both as a continuous and categorical variable; <60, 60–70, 70–80 and >80 years. SES was categorised into low (first and second septile), medium (third, fourth and fifth septile) and high (sixth and seventh septile). PSA at diagnosis was divided into <10 ng/mL, 10–20 ng/mL and >20 ng/mL. Gleason grade was categorised using the International Society of Urological Pathology (ISUP) grade group system; ISUP 1 (Gleason score ≤6), ISUP 2 (Gleason score 3 + 4), ISUP 3 (Gleason score 4 + 3), ISUP 4 (Gleason score 8) and ISUP 5 (Gleason score 9–10) [15]. All prostate cancer diagnoses were stratified following the EAU risk group classification into low-risk localised, intermediate-risk localised, high-risk localised, high-risk/locally advanced and metastatic prostate cancer [16].
Primary treatment was divided in no active treatment, including active surveillance and watchful waiting as often no clear difference could be made based on information in electronic health records, radical prostatectomy, EBRT, brachytherapy, radiotherapy and ADT, ADT, taxane-based chemotherapy and treatment with ARTAs.
Biopsies were classified as malignant in case of adenocarcinoma, intraductal carcinoma, sarcomatoid carcinoma, neuroendocrine carcinoma, leiomyosarcoma, squamous cell carcinoma and lymphoma and as benign in case of benign prostatic hyperplasia, atypical cells, dysplasia, prostatic intraepithelial neoplasia and uncertain neoplasm.
Statistical analyses
Diagnosis
To evaluate changes over time, the absolute number of new prostate cancer diagnoses per month in the period January 2020-May 2021 was compared to 2018–2019 (averaged). In order to identify possible changes in the diagnostic process, we evaluated the number of biopsies in 2020 versus previous years (2015–2019) and the ratio malignant/non-malignant outcome was assessed. For a more detailed description of the effects of the first COVID-19 wave, we compared the number of prostate cancer diagnoses from week 2-22 of 2020 to the same period in 2018–2019 (averaged). The numbers were presented as three-week moving average, to smooth for variation. In addition, the relative change in number of diagnoses as observed in week 2-22 2020, was assessed by considering the three-week moving average in 2018–2019 as 100%. A correction for working days was applied in case a week consisted of less than five working days due to national holidays.
Descriptive statistics were used to compare patient- and tumour characteristics of patients diagnosed between week 2-22 of 2020 versus the same time period in 2018–2019. To evaluate the effects per age group and disease stage, the number of diagnoses per 100.000 person years was calculated for each time period and stratified by age and EAU risk group. The number of diagnoses of each period in 2020 was compared to the same period in 2018–2019 (averaged) using the iri command in STATA, considering p < 0.05 statistically significant. To evaluate whether a delay in diagnosis affected disease stage, the EAU risk group distribution in diagnosed patients before the outbreak (week 2–8) was compared to this distribution at the end of the first COVID-19 wave (week 17–22).
Treatment
To evaluated changes in treatment of patients diagnosed with prostate cancer between week 2–22 of 2020 compared to the same time period in 2018–2019, the average number of patients per treatment modality was calculated per week and per time period. A correction for the number of working days per week was applied. Logistic regression analyses were performed to evaluate the association between time periods in 2020 versus week 2–22 of 2018–2019 and the probability of receiving a certain treatment. Analyses were performed per disease stage and were adjusted for age at diagnosis.
In addition, we evaluated the effect of the outbreak on surgical volume of radical prostatectomies. We compared the number (three-week moving average) of radical prostatectomies in 2020 versus the average of 2018–2019, considering the three-week moving average in 2018–2019 as 100%. A correction for number of working days was applied. Time to prostatectomy, per time period, in patients treated between week 2–22 of 2020 and 2018–2019 was assessed as well.
All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, North Carolina, USA) and STATA version 16.1 software (StataCorp, College Station, Texas, USA). According to the Central Committee on Research involving Human Subjects (CCMO), this type of study does not require approval from an ethics committee in the Netherlands. This study was approved by the Netherlands Cancer Registry's Supervisory Committee (reference number K21.057).
Results
Number of prostate cancer diagnoses and biopsies in 2020
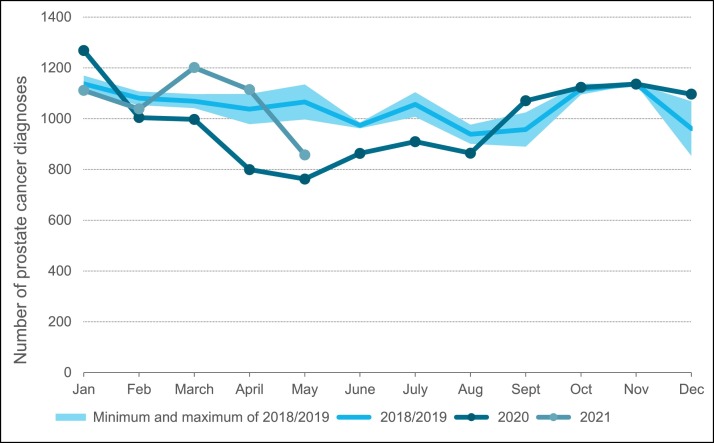
The effect of the COVID-19 outbreak on the number of prostate cancer diagnoses over time is shown in Fig. 1 . Following the COVID-19 outbreak, a large decline in the number of new prostate cancer diagnoses was observed. In May 2020 the lowest number of diagnoses was seen, representing a decrease of 28% (corresponding to approximately 300 diagnoses) compared to previous years. From May onwards the number of diagnoses started to restore to approximately 95% of the expected number by the end of 2020 based on previous years.
Fig. 1.
Number of new prostate cancer diagnoses per month in 2020 until May 2021, relative to the average number of new prostate cancer diagnoses in 2018–2019.
Concerning the number of prostate biopsies in 2020; approximately 18,500 biopsies were conducted in 2020 compared to an average of 21,500 biopsies in previous years (Table 1 ). The ratio malignant versus non-malignant gradually increased over time (from 51.3% in 2015 to 63.2% in 2019) with approximately 1–4% increase of malignant diagnoses each year. In 2020, this trend appears to be accelerated as the ratio of malignant biopsies increased from 63.2% in 2019 to 69.2% in 2020 (increase of 6%).
Table 1.
Number of biopsies with pathology results (i.e. malignant versus non-malignant) from 2015 to 2020. In total, 36 pathology results were unknown, which accounted for <ten results per year.
| 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | |
|---|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |
| Biopsies | 20,780 | 22,087 | 21,765 | 21,059 | 21,542 | 18,444 |
| Non-malignant | 10,113 (48.7%) | 10,551 (47.8%) | 9685 (44.5%) | 8450 (40.1%) | 7921 (36.8%) | 5679 (30.8%) |
| Malignant | 10,659 (51.3%) | 11,529 (52.2%) | 12,075 (55.5%) | 12,602 (59.8%) | 13,621 (63.2%) | 12,756 (69.2%) |
Prostate cancer diagnoses during the first COVID-19 wave
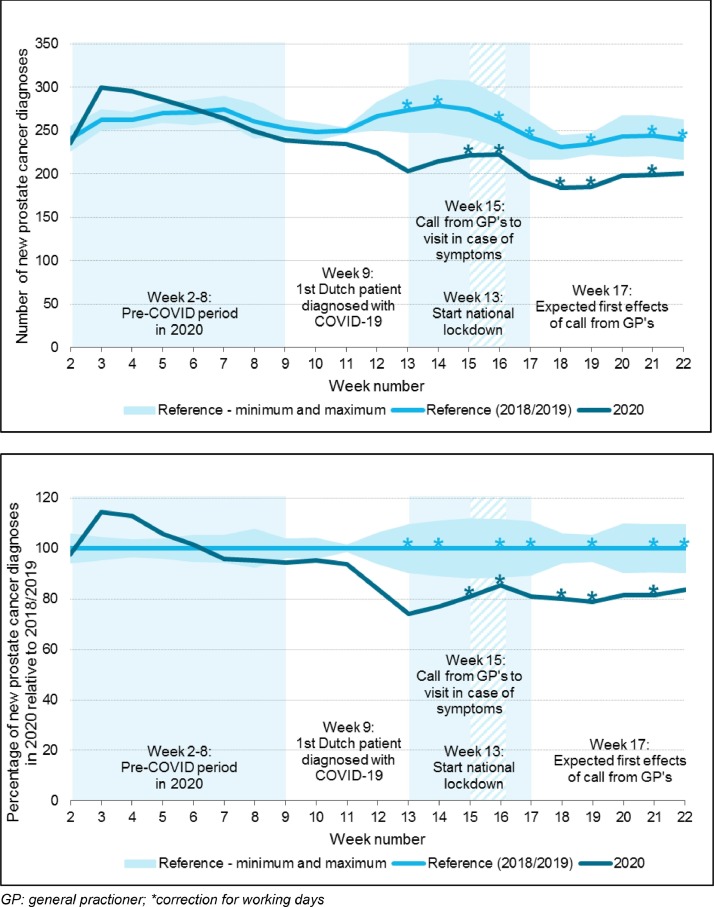
When focussing on the effects of the first wave corresponding with week 9–22 in 2020, we observed an average decline of 17%, accounting for approximately 580 diagnoses compared to the reference period (Fig. 2 ). In Table 2 , the baseline characteristics of patients diagnosed with prostate cancer between week 2–22 for 2020 and 2018–2019 are presented and stratified by time period. In Fig. 3 a, the effects of the outbreak on the number of diagnoses per time period for different age groups are presented. A non-significant drop in the number of diagnoses was seen from week 9 onwards in all age groups. After week 13, the observed decline was statistically significant in all age groups, except for patients younger than 60 years. The average decline in week 9–22 was 34%, 16% and 23% in patients aged 80+, patients aged 70–79 and 60–69, respectively.
Fig. 2.
Number of diagnoses in 2020 compared to 2018–2019 from January to May, presented with relevant dates and measures during the first COVID wave in the Netherlands. Fig. 2a: New prostate cancer diagnoses presented as three-week moving average. Fig. 2b: Percentage of new prostate cancer diagnoses in 2020, relative to the number of diagnoses in 2018–2019 (considered as 100%), presented as three-week moving average. GP: general practioner; *correction for working days.
Table 2.
Baseline characteristics of patients diagnosed with prostate cancer between January and May of 2020 (COVID-19 cohort) or 2018 and 2019 (reference cohort). For evaluation of differences during the first COVID wave, 2020 is split into four different periods of which the average per week is given. The weeks 2–22 of 2018–2019 are averaged. P-value was calculated using Chi-square for categorical variables and t-test for continuous variables. GP: general practitioner; IQR: interquartile range; ISUP: international society of urological pathology, PSA: prostate specific antigen, TNM: tumour, node, metastasis, EAU: European Association of Urology.
| Week 2–22 2018–2019 (averaged) (n = 5233) |
Week 2–22 2020 (n = 4753) |
Week 2–8 2020 (n = 1985) pre-COVID-19 period |
Week 9–12 2020 (n = 919) 1st Dutch patient diagnosed with COVID-19 |
Week 13–16 2020 (n = 794) start national lockdown |
Week 17–22 2020 (n = 1055) call to visit GP in case of symptoms |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | (%) | N | (%) | p-value | N | (%) | N | (%) | N | (%) | N | (%) | |
| Patient characteristics | |||||||||||||
| Age at diagnosis (median, IQR) | 70.5 | (65.0–75.5) | 71.0 | (65.0–75.0) | 0.74 | 71.0 | (66.0–75.0) | 70.0 | (65.0–75.0) | 71.0 | (65.0–75.0) | 71.0 | (66.0–75.0) |
| Age at diagnosis | 0.004 | ||||||||||||
| <60 years | 477 | (9.2) | 450 | (9.5) | 24 | (8.6) | 25 | (10.7) | 24 | (10.8) | 18 | (9.1) | |
| 60–69 years | 1873 | (35.9) | 1623 | (34.1) | 97 | (34.3) | 80 | (34.9) | 78 | (35.5) | 62 | (32.2) | |
| 70–79 years | 2330 | (44.5) | 2240 | (47.1) | 133 | (47.0) | 106 | (45.9) | 100 | (46.0) | 96 | (49.4) | |
| >=80 years | 554 | (10.6) | 440 | (9.3) | 29 | (10.2) | 20 | (8.5) | 17 | (7.7) | 18 | (9.3) | |
| Socioeconomic status | 0.07 | ||||||||||||
| Low | 1138 | (21.8) | 975 | (20.5) | 60 | (21.3) | 48 | (20.8) | 39 | (18.0) | 40 | (20.7) | |
| Middle | 2009 | (38.4) | 1806 | (38.0) | 103 | (36.2) | 85 | (36.8) | 86 | (39.5) | 80 | (41.3) | |
| High | 2084 | (39.9) | 1966 | (41.4) | 120 | (42.3) | 97 | (42.2) | 93 | (42.4) | 74 | (38.0) | |
| Unknown | 3 | (0.1) | 6 | (0.1) | 1 | (0.2) | 1 | (0.2) | 0 | 0 | 0 | 0 | |
| Geographical region | 0.43 | ||||||||||||
| East | 567 | (10.8) | 551 | (11.6) | 33 | (11.5) | 30 | (12.8) | 28 | (12.6) | 19 | (10.0) | |
| Middle | 1066 | (20.4) | 927 | (19.5) | 56 | (19.7) | 47 | (20.6) | 42 | (19.1) | 36 | (18.5) | |
| North | 421 | (8.1) | 362 | (7.6) | 22 | (7.6) | 20 | (8.6) | 14 | (6.5) | 15 | (7.6) | |
| South | 1161 | (22.2) | 1089 | (22.9) | 63 | (22.1) | 56 | (24.5) | 49 | (22.5) | 45 | (23.4) | |
| West | 2019 | (38.6) | 1824 | (38.4) | 111 | (39.1) | 77 | (33.5) | 86 | (39.2) | 78 | (40.6) | |
| Tumour characteristics | |||||||||||||
| ISUP Gleason grade group at diagnosis | <0.0001 | ||||||||||||
| ISUP 1 | 1668 | (31.9) | 1205 | (25.4) | 76 | (26.9) | 54 | (23.6) | 50 | (22.8) | 50 | (25.9) | |
| ISUP 2 | 1154 | (22.1) | 1202 | (25.3) | 68 | (24.0) | 63 | (27.4) | 55 | (25.3) | 50 | (25.9) | |
| ISUP 3 | 650 | (12.4) | 687 | (14.5) | 42 | (14.7) | 32 | (13.7) | 37 | (17.0) | 25 | (12.7) | |
| ISUP 4&5 | 1526 | (29.2) | 1398 | (29.4) | 85 | (29.8) | 69 | (30.0) | 62 | (28.2) | 56 | (29.0) | |
| Unknown | 237 | (4.5) | 261 | (5.5) | 13 | (4.6) | 12 | (5.2) | 15 | (6.7) | 13 | (6.5) | |
| PSA at diagnosis | <0.0001 | ||||||||||||
| PSA < 10 ng/Ml | 2400 | (45.9) | 2318 | (48.8) | 136 | (48.1) | 116 | (50.3) | 103 | (47.2) | 96 | (49.9) | |
| PSA 10–20 ng/mL | 1196 | (22.9) | 1100 | (23.1) | 68 | (24.1) | 50 | (21.7) | 54 | (24.6) | 42 | (21.5) | |
| PSA > 20 ng/mL | 1406 | (26.9) | 1194 | (25.1) | 69 | (24.4) | 59 | (25.6) | 57 | (26.1) | 49 | (25.3) | |
| Unknown | 231 | (4.4) | 141 | (3.0) | 9 | (3.3) | 6 | (2.5) | 5 | (2.1) | 6 | (3.3) | |
| Disease stage (cTNM) | <0.0001 | ||||||||||||
| cT0 | 80 | (1.5) | 73 | (1.5) | 3 | (1.1) | 4 | (1.5) | 5 | (2.1) | 4 | (2.0) | |
| cT1-cT2a | 2466 | (47.2) | 1980 | (41.7) | 121 | (42.6) | 95 | (41.1) | 84 | (38.7) | 82 | (42.6) | |
| cT2b | 134 | (2.6) | 149 | (3.1) | 10 | (3.5) | 8 | (3.5) | 6 | (2.9) | 4 | (2.3) | |
| cT2c | 532 | (10.2) | 626 | (13.2) | 35 | (12.2) | 31 | (13.6) | 32 | (14.6) | 26 | (13.5) | |
| cT3-cT4 and/or cN1 cM0 | 1091 | (20.8) | 1100 | (23.1) | 68 | (24.1) | 53 | (23.1) | 53 | (24.1) | 40 | (20.8) | |
| cM1 | 901 | (17.2) | 797 | (16.8) | 45 | (15.9) | 39 | (16.8) | 37 | (17.0) | 35 | (18.3) | |
| Unknown | 31 | (0.6) | 28 | (0.6) | 2 | (0.6) | 1 | (0.4) | 1 | (0.6) | 1 | (0.7) | |
| EAU prognostic risk group | <0.0001 | ||||||||||||
| Localised | |||||||||||||
| Low-risk | 916 | (17.5) | 636 | (13.4) | 41 | (14.5) | 28 | (12.2) | 27 | (12.2) | 25 | (13.2) | |
| Intermediate-risk | 1166 | (22.3) | 1085 | (22.8) | 64 | (22.6) | 55 | (23.9) | 46 | (21.0) | 46 | (23.7) | |
| High-risk | 928 | (17.8) | 978 | (20.6) | 56 | (19.8) | 49 | (21.2) | 49 | (22.4) | 39 | (20.1) | |
| Locally advanced | 1091 | (20.8) | 1100 | (23.1) | 68 | (24.1) | 53 | (23.1) | 53 | (24.1) | 40 | (20.8) | |
| Metastatic | 901 | (17.2) | 797 | (16.8) | 45 | (15.9) | 39 | (16.8) | 37 | (17.0) | 35 | (18.3) | |
| Unknown | 232 | (4.5) | 157 | (3.3) | 9 | (3.2) | 7 | (2.8) | 7 | (3.3) | 8 | (4.0) | |
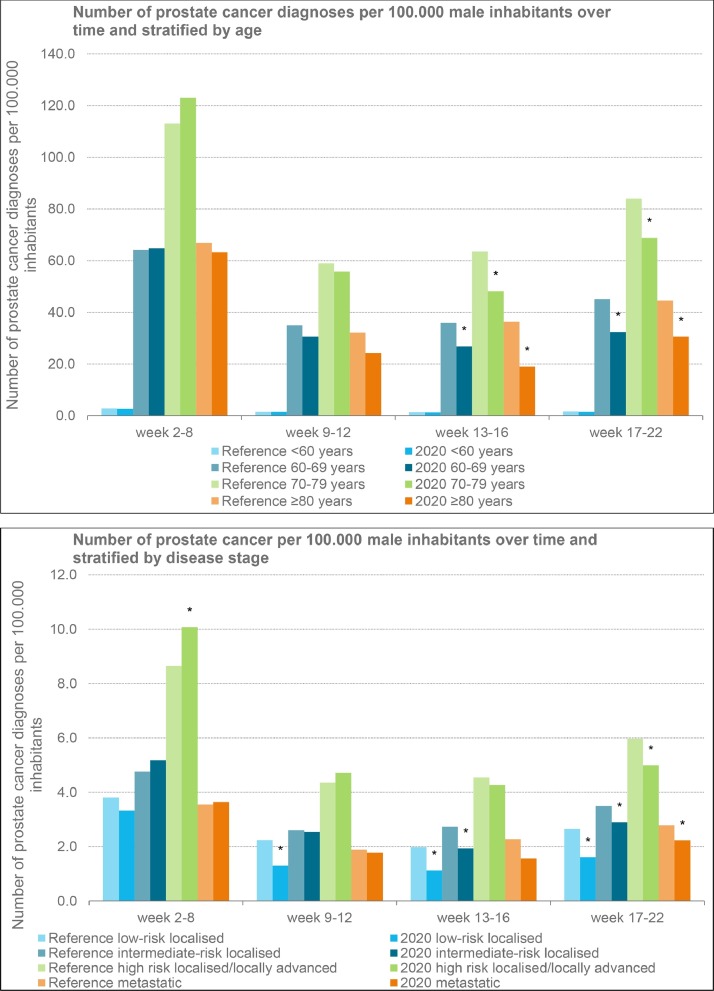
Fig. 3.
The number of new prostate cancer diagnoses per 100.000 male inhabitants over time in 2020, relative to new prostate cancer diagnoses in 2018–2019 (averaged). Fig. 3a: Diagnoses stratified by age. Fig. 3b: Diagnoses stratified by disease stage (EAU risk classification). *= significant difference.
Concerning the effect of the outbreak on the number of prostate cancer diagnoses by disease stage, a statistically significant decline was initially only detected in low-risk localised prostate cancer. From week 13, a statistically significant decline was also observed in intermediate-risk localised prostate cancer. From week 17, the observed decline in number of diagnoses was statistically significant in all risk groups (Fig. 3b). From week 9 through week 22, the average decline in number of diagnoses was most pronounced in low-risk localised prostate cancer with 41%, accounting for approximately 240 diagnoses. The observed declines in the other risk groups were 16%, 7% and 19% for intermediate-risk localised, high-risk localised/locally advanced and metastatic disease, respectively.
To evaluate whether the delay in diagnosis affected disease stage at diagnosis, the distribution of EAU risk groups by time period was assessed (supplementary data Figure A.1). Only small changes in the distribution were observed; the proportion of men diagnosed with low-risk prostate cancer was almost 2% less during the first COVID-19 wave (week 9–22) versus the pre-COVID period (week 2–8). In contrast, the proportion of patients diagnosed with metastatic castrate-sensitive prostate cancer was 1.5% higher during the first COVID-19 wave versus pre-COVID.
Treatment changes during the first COVID-19 wave
Next to effects on the number of diagnoses and disease stage, we evaluated the effect of the outbreak on treatment. In Table 3 , the applied treatment stratified by EAU risk group and by time period is presented and in Table 4 the odds ratios (OR) of specific treatments per EAU risk group for patients diagnosed in 2020 versus the reference period are presented. During the first COVID-19 wave, active surveillance was applied less frequently in low-risk localised prostate cancer as compared to the reference period. This decline was most prominent in week 9–12; 67.1% in 2020 versus 78% in 2018–2019 (OR = 0.62, 95% CI; 0.41–0.94). Instead patients underwent radical prostatectomy relatively often; 20.7% in 2020 versus 12.1% in 2018–2019 (OR=1.89, 95% CI; 1.15–3.11), and to a lesser extent radiation based treatment (4.6% and 7.1% versus 3.1% and 5.9%, for EBRT and brachytherapy,respectively). However, during the final weeks of the first wave, active surveillance was applied in approximately the same proportion of patients with low-risk prostate cancer as in the pre-COVID period.
Table 3.
Treatment strategies presented per risk group according to the EAU stratification, presented per time period in 2020 (COVID period) versus 2018–2019 (reference period). N = average per week (corrected for public holidays). therapy. * +/-androgen deprivation therapy and +/- local therapy of the prostate, +/- metastases directed therapy.
| week 2–22 |
Week 2–8 |
week 9–12 |
week 13–16 |
week 17–22 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ref | 2020 | Ref | 2020 | Ref | 2020 | Ref | 2020 | Ref | 2020 | |
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |
| Low-risk localised | ||||||||||
| No active treatment | 34.3 (76.2%) | 22.8 (72.4%) | 34.7 (74.8%) | 30.4 (74.3%) | 37.3 (78.0%) | 18.8 (67.1%) | 35.2 (77.5%) | 19.0 (71.2%) | 31.1 (76.2%) | 18.7 (73.9%) |
| Radical prostatectomy | 5.6 (12.4%) | 4.8 (15.2%) | 6.1 (13.1%) | 5.9 (14.4%) | 5.8 (12.1%) | 5.8 (20.7%) | 4.8 (10.6%) | 4.4 (16.5%) | 5.2 (12.7%) | 3.1 (12.3%) |
| External beam radiotherapy | 2.0 (4.4%) | 1.5 (4.8%) | 2.0 (4.3%) | 1.6 (3.9%) | 1.5 (3.1%) | 1.3 (4.6%) | 2.4 (5.3%) | 1.7 (6.4%) | 2.0 (4.9%) | 1.7 (6.7%) |
| Brachytherapy | 2.4 (5.3%) | 1.9 (6.0%) | 2.9 (6.3%) | 2.3 (5.6%) | 2.8 (5.9%) | 2.0 (7.1%) | 1.9 (4.2%) | 1.7 (6.4%) | 2.0 (4.9%) | 1.7 (6.7%) |
| Other | 0.6 (1.3%) | 0.3 (1.0%) | 0.7 (1.5%) | 0.7 (1.7%) | 0.4 (1.0%) | 0.3 (1.1%) | 0.8 (1.8%) | 0.0 (0.0%) | 0.5 (1.2%) | 0.2 (0.8%) |
| Intermediate-risk localised | ||||||||||
| No active treatment | 16.1 (28.0%) | 12.1 (22.4%) | 16.1 (27.8%) | 15.7 (24.6%) | 15.3 (27.6%) | 11.5 (20.9%) | 17.7 (28.1%) | 8.0 (17.5%) | 15.3 (28.3%) | 10.6 (23.1%) |
| Radical prostatectomy | 18.6 (32.4%) | 20.6 (38.2%) | 20.1 (34.7%) | 22.3 (34.9%) | 17.0 (30.6%) | 22.3 (40.5%) | 19.4 (30.8%) | 22.6 (49.5%) | 17.2 (31.9%) | 15.8 (34.5%) |
| External beam radiotherapy | 10.0 (17.4%) | 11.1 (20.6%) | 9.4 (16.2%) | 13.7 (21.4%) | 9.5 (17.1%) | 10.8 (19.6%) | 11.8 (18.8%) | 6.6 (14.4%) | 9.7 (18.0%) | 11.0 (24.0%) |
| Brachytherapy | 6.2 (10.8%) | 4.0 (7.4%) | 5.9 (10.2%) | 4.7 (7.4%) | 7.3 (13.2%) | 4.5 (8.2%) | 5.9 (9.4%) | 2.5 (5.5%) | 6.1 (11.3%) | 3.7 (8.1%) |
| Radiotherapy & Androgen deprivation therapy | 5.5 (9.6%) | 5.4 (10.0%) | 5.6 (9.7%) | 6.9 (10.8%) | 4.8 (8.6%) | 5.0 (9.1%) | 7.0 (11.1%) | 5.5 (12.0%) | 4.7 (8.7%) | 3.9 (8.5%) |
| Other | 0.9 (1.6%) | 0.7 (1.3%) | 0.7 (1.2%) | 0.6 (0.9%) | 1.3 (2.3%) | 1.0 (1.8%) | 0.8 (1.3%) | 0.6 (1.3%) | 0.7 (1.3%) | 0.9 (2.0%) |
| High-risk localised/locally advanced | ||||||||||
| No active treatment | 12.8 (12.9%) | 12.9 (12.5%) | 14.4 (13.6%) | 17.1 (13.8%) | 11.3 (12.2%) | 12.5 (12.3%) | 13.7 (13.1%) | 10.2 (10.1%) | 11.2 (12.2%) | 9.7 (12.4%) |
| Radical prostatectomy | 29.5 (29.7%) | 33.5 (32.5%) | 30.6 (28.9%) | 39.9 (32.1%) | 28.5 (30.6%) | 32.3 (31.7%) | 30.6 (29.3%) | 33.6 (33.2%) | 27.8 (30.3%) | 26.4 (33.7%) |
| External beam radiotherapy/ Brachytherapy | 11.1 (11.2%) | 11.3 (11.0%) | 11.4 (10.8%) | 11.7 (9.4%) | 10.3 (11.1%) | 11.5 (11.3%) | 12.1 (11.6%) | 12.1 (12.0%) | 10.5 (11.4%) | 10.3 (13.2%) |
| Radiotherapy & Androgen deprivation therapy | 35.6 (35.9%) | 38.2 (37.1%) | 37.6 (35.6%) | 46.6 (37.5%) | 33.5 (36.0%) | 37.8 (37.1%) | 36.6 (35.0%) | 39.9 (39.4%) | 33.9 (36.9%) | 26.8 (34.2%) |
| Androgen deprivation therapy | 8.9 (9.0%) | 6.2 (6.0%) | 9.9 (9.4%) | 7.7 (6.2%) | 7.5 (8.1%) | 7.0 (6.9%) | 10.5 (10.0%) | 4.7 (4.6%) | 7.4 (8.1%) | 4.6 (5.9%) |
| Other | 1.3 (1.3%) | 0.9 (0.9%) | 1.6 (1.5%) | 1.3 (1.0%) | 1.8 (1.9%) | 0.8 (0.8%) | 0.8 (0.8%) | 0.8 (0.8%) | 0.9 (1.0%) | 0.6 (0.8%) |
| Metastatic | ||||||||||
| No active treatment | 1.2 (2.7%) | 0.6 (1.5%) | 1.1 (2.5%) | 0.9 (2.0%) | 0.5 (1.2%) | 0.3 (0.8%) | 1.6 (3.1%) | 0.3 (0.8%) | 1.4 (3.2%) | 0.9 (2.6%) |
| Androgen deprivation therapy & radiotherapy | 3.8 (8.6%) | 6.9 (17.4%) | 2.9 (6.7%) | 7.6 (16.9%) | 3.5 (8.7%) | 7.3 (19.0%) | 4.6 (8.9%) | 7.2 (19.5%) | 4.3 (10.0%) | 5.5 (15.6%) |
| Androgen deprivation therapy | 21.3 (48.1%) | 18.7 (47.2%) | 20.1 (46.3%) | 22.7 (50.4%) | 20.0 (49.6%) | 18.3 (47.5%) | 25.8 (49.7%) | 15.7 (42.5%) | 20.4 (47.2%) | 15.8 (44.9%) |
| Taxanes* | 16.3 (36.8%) | 10.0 (25.3%) | 17.7 (40.8%) | 9.7 (21.6%) | 15.3 (38.0%) | 7.5 (19.5%) | 16.1 (31.0%) | 11.8 (32.0%) | 15.3 (35.4%) | 10.8 (30.7%) |
| Androgen receptor targeting agents* | 1.0 (2.3%) | 2.6 (6.6%) | 1.0 (2.3%) | 3.1 (6.9%) | 0.4 (1.0%) | 4.3 (11.2%) | 1.9 (3.7%) | 1.7 (4.6%) | 0.9 (2.1%) | 1.5 (4.3%) |
| Other | 0.7 (1.6%) | 0.8 (2.0%) | 0.4 (0.9%) | 1.0 (2.2%) | 0.5 (1.2%) | 1.0 (2.6%) | 1.3 (2.5%) | 0.3 (0.8%) | 0.5 (1.2%) | 0.7 (2.0%) |
Table 4.
Odds ratio's and 95% confidence intervals for different treatment strategies per risk group according to the EAU stratification, presented per time period in 2020 (COVID period) versus week 2–22 of 2018–2019 (reference period).
| Week 2–22 | Week 2–8 | Week 9–12 | Week 13–16 | Week 17–22 | |
|---|---|---|---|---|---|
| 2020 vs 2018–2019 | 2020 vs 2018–2019 | 2020 vs 2018–2019 | 2020 vs 2018–2019 | 2020 vs 2018–2019 | |
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Low-risk localised | |||||
| No active treatment | 0.80 (0.65–0.99) | 0.88 (0.65–1.17) | 0.62 (0.41–0.94) | 0.80 (0.51–1.27) | 0.86 (0.57–1.28) |
| Radical prostatectomy | 1.32 (1.01–1.72) | 1.25 (0.87–1.82) | 1.89 (1.15–3.11) | 1.30 (0.74–2.30) | 1.03 (0.60–1.77) |
| External beam radiotherapy | 1.09 (0.71–1.67) | 0.83 (0.44–1.58) | 0.99 (0.39–2.51) | 1.50 (0.63–3.54) | 1.45 (0.71–2.97) |
| Brachytherapy | 1.17 (0.80–1.72) | 1.07 (0.62–1.86) | 1.36 (0.64–2.89) | 1.11 (0.47–2.62) | 1.26 (0.62–2.57) |
| Intermediate-risk localised | |||||
| No active treatment | 0.76 (0.64–0.91) | 0.84 (0.66–1.07) | 0.74 (0.53–1.05) | 0.56 (0.37–0.85) | 0.77 (0.56–1.06) |
| Radical prostatectomy | 1.25 (1.07–1.47) | 1.10 (0.88–1.38) | 1.25 (0.93–1.70) | 2.04 (1.45–2.86) | 1.12 (0.84–1.50) |
| External beam radiotherapy | 1.26 (1.05–1.51) | 1.31 (1.02–1.68) | 1.24 (0.87–1.77) | 0.83 (0.53–1.30) | 1.50 (1.10–2.06) |
| Brachytherapy | 0.63 (0.49–0.82) | Week 2–8 | 0.67 (0.41–1.11) | 0.45 (0.22–0.89) | 0.71 (0.44–1.15) |
| Radiotherapy & Androgen deprivation therapy | 1.11 (0.87–1.41) | 1.16 (0.83–1.62) | 1.05 (0.65–1.71) | 1.39 (0.85–2.27) | 0.88 (0.55–1.41) |
| High-risk localised/locally advanced | |||||
| No active treatment | 0.98 (0.84–1.16) | 1.10 (0.88–1.36) | 0.95 (0.69–1.30) | 0.76 (0.53–1.08) | 1.00 (0.73–1.36) |
| Radical prostatectomy | 1.16 (1.02–1.31) | 1.15 (0.97–1.36) | 1.10 (0.87–1.40) | 1.22 (0.95–1.57) | 1.20 (0.95–1.51) |
| External beam radiotherapy /Brachytherapy | 0.99 (0.83–1.17) | 0.83 (0.65–1.06) | 1.02 (0.74–1.40) | 1.08 (0.78–1.50) | 1.20 (0.89–1.62) |
| Radiotherapy & Androgen deprivation therapy | 1.06 (0.95–1.18) | 1.07 (0.92–1.25) | 1.06 (0.86–1.31) | 1.16 (0.94–1.45) | 0.94 (0.76–1.16) |
| Androgen deprivation therapy | 0.66 (0.53–0.82) | 0.68 (0.50–0.92) | 0.73 (0.48–1.12) | 0.49 (0.29–0.82) | 0.69 (0.45–1.06) |
| Metastatic | |||||
| No active treatment | 0.58 (0.31–1.06) | 0.67 (0.28–1.56) | 0.23 (0.03–1.64) | 0.27 (0.04–1.94) | 0.92 (0.36–2.33) |
| Androgen deprivation therapy & radiotherapy | 2.27 (1.77–2.91) | 2.19 (1.56–3.08) | 2.51 (1.62–3.89) | 2.53 (1.60–4.02) | 2.01 (1.32–3.08) |
| Androgen deprivation therapy | 0.99 (0.83–1.19) | 1.14 (0.88–1.48) | 0.99 (0.70–1.42) | 0.92 (0.63–1.35) | 0.85 (0.61–1.17) |
| Taxanes* | 0.53 (0.43–0.64) | 0.42 (0.31–0.57) | 0.36 (0.24–0.56) | 0.69 (0.46–1.03) | 0.74 (0.53–1.05) |
| Androgen receptor targeting agents* | 2.98 (1.97–4.51) | 3.14 (1.85–5.33) | 5.19 (2.88–9.36) | 2.01 (0.84–4.82) | 1.82 (0.84–3.93) |
Significant results are presented in bold. * +/-androgen deprivation therapy and +/- local therapy of the prostate, +/- metastases directed therapy.
A similar treatment shift was seen in intermediate-risk localised prostate cancer in week 13–16; active surveillance was applied less frequently (17.5% versus 28.1%); OR=0.56, 95% CI; 0.37–0.85) and radical prostatectomy was applied relatively often (49.5% versus 30.8%; OR=2.04, 95% CI; 1.45–2.86). Men with intermediate-risk prostate cancer were treated with brachytherapy less often in 2020 compared to the reference period, especially in week 13–16 (5.5% versus 9.4%; OR=0.45, 95% CI; 0.22–0.89). However, this decline was also statistically significant in the pre-COVID period (7.4% versus 10.2%; OR=0.64, 95%CI; 0.44–0.93). In contrast, EBRT was applied more often; particularly in week 17–22 (24% versus 18%; OR=1.50, 95% CI; 1.10–2.06), as well as in week 2–8 (21.4% versus 16.2%; OR=1.31, 95% CI; 1.02–1.68).
In the high-risk localised/locally advanced disease group, radical prostatectomy was performed relatively often during the entire first wave, as well as in the pre-COVID period. In contrast, ADT monotherapy was applied less often in 2020 (6.0% versus 9.0%; OR=0.66, 95% CI; 0.53–0.82). In patients with metastatic castrate-sensitive prostate cancer, treatment with taxane-based chemotherapy was applied less often, which was most evident in week 9–12 (19.5% versus 38.0%; OR=0.36, 95% CI; 0.24–0.56). During the same period, patients received ARTAs (11.2% versus 1.0%; OR=5.19, 95% CI; 2.88–9.36) more often. From week 13 onwards, these differences were less evident. In 2020, more patients were treated with ADT and radiotherapy compared to the reference period (17.4% in 2020 versus 8.6% in 2018–2019; OR = 2.27, 95% CI; 1.77–2.91). This increase was seen during the entire first COVID-19 wave, as well as in the pre-COVID period.
Impact of the COVID-10 outbreak on surgical volume
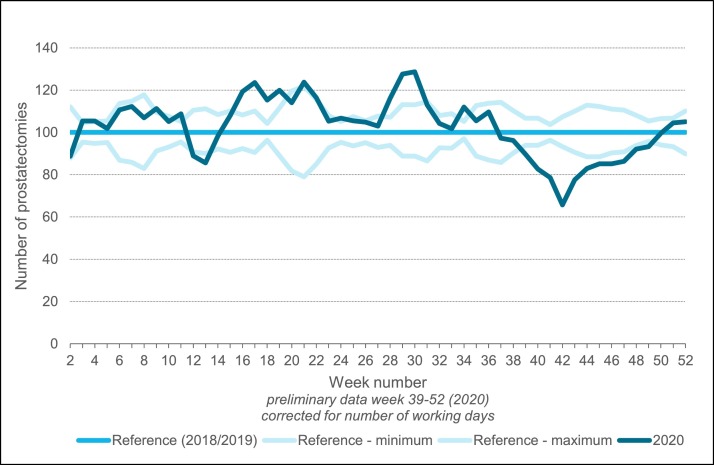
Finally, we also evaluated the effect of the COVID-19 outbreak on surgical volume in the Netherlands. In Fig. 4 , the number of radical prostatectomies by three-week moving averages of 2020, relative to 2018–2019 is presented. Overall, the number of prostatectomies was comparable to 2018–2019 (approximately 2575 in 2020 versus 2550 in 2018–2019). During the first COVID-19 wave, the number of prostatectomies was comparable to or slightly higher than in 2018–2019. From week 41 to 43 in 2020, a decrease is visible, which restored rather quickly.
Fig. 4.
Number of radical prostatectomies in 2020 relative to 2018–2019 presented as three-week moving averages.
For patients treated surgically during the first COVID-19 wave, time from diagnosis to prostatectomy was shorter compared to patients who received surgical treatment in the reference period. This effect was most pronounced in week 9–12 (median [IQR]: 60.0 [45.0–87.0] versus 80.0 [58.0–108.5] days). In week 13–22 in 2020, median time to prostatectomy was 64–72 days versus 76–78 days in 2018–2019.
Discussion
In this study, we evaluated the impact of the COVID-19 outbreak on the number of prostate cancer diagnoses, overall and by age and disease stage. We also assessed the effect on disease stage at diagnosis and treatment. In general we can conclude that after a substantial initial decline in the number of prostate cancer diagnoses of 17% during the first wave, this number largely restored during the rest of 2020 but remained lower than expected. The effect on disease stage at diagnosis was limited and changes in treatment appeared to be temporary. Also, surgical capacity concerning radical prostatectomies was not affected as the total number of prostatectomies performed in 2020 was comparable to 2018–2019.
The observed decline in prostate cancer diagnoses correlated with the detection of the first COVID-19 case in the Netherlands and the social lockdown by the Dutch government[4]. The decline was first seen and most prominent (41%) amongst patients with low-risk localised prostate cancer. We hypothesise that this is the result of less PSA testing in asymptomatic patients, due to less GP visits during the lockdown. Furthermore, urologists might have performed biopsies less often in this category of patients, according to the advice to postpone biopsies in patients at low risk of disease progression [8,10]. In addition, the use of mpMRI for the detection of prostate cancer as recommended in the guidelines since 2020, might have attributed to this as well [17]. Regarding different age groups, we observed that the number of diagnoses dropped in all age groups but the decline was largest in patients of 80 years and older (i.e. 34%). This is probably explained by hesitance to visit the GP for PSA-testing or possibly also in case of complaints, for fear of becoming infected. Also, GPs might have been more hesitant to refer these vulnerable patients to a hospital. The excess mortality due to COVID-19, accounting for approximately 9000 extra deaths in week 2–22 2020 (Source: CBS[18]) could also have had an effect as this would potentially deprive patients of being diagnosed with prostate cancer. We estimated the number of prostate cancer diagnoses that have been missed due to COVID-related excess mortality between March-May of 2020, using the age and gender-specific incidence of prostate cancer patients in our cohort. This resulted in an estimated number of 25 missed cases of prostate cancer in the Netherlands.
The extent of the decline in diagnoses during the first COVID-19 wave that we have observed in the Netherlands was smaller than what was observed in Sweden [19]. In Sweden the national cancer registry was also used to evaluate the effects of the first COVID-19 wave on prostate cancer care. They demonstrated an overall decline in diagnoses of 36% and found the largest decline (40%) in the low/intermediate-risk group. With respect to different age-groups, they also observed the largest decline amongst elderly patients with a 51% decline in patients aged above 75 years [19].
We must consider that in the Netherlands the absolute number of patients diagnosed with prostate cancer increases over the years. Based on the period 2015–2019, on average an annual increase of approximately 5.5% (which corresponds to approximately 650 patients) was observed [20]. On the other hand, as mentioned, the introduction of mpMRI for the detection of prostate cancer, might also have an effect as less biopsies might be performed resulting in less (low-risk) prostate cancer [17,21]. This was confirmed by the data we have presented in this study on the number of biopsies taken; namely less biopsies and a higher proportion of patients with malignant disease. Taking everything into account, the reported 5% decline in prostate cancer diagnoses is 2020 is probably a small underestimation.
No clear change in stage distribution was observed as a possible effect of delayed diagnosis. The observed small increase in high-risk and metastatic prostate cancer and small decrease in low-risk prostate cancer is most likely explained by the delay in GP visits and/or change in diagnostic strategies by urologists as described before and not yet due to delayed diagnosis. However, patients diagnosed in the second half of 2020, partly represent delayed diagnoses which were missed in the first half of 2020. These delayed diagnoses might have led to a shift towards higher stages. Unfortunately, data on disease stage of these patients were not available yet. Though, taken the biology of prostate cancer into account, the diagnostic delay of several months will probably not largely impact disease stage and subsequent survival. Based on a modelling study from the UK, evaluating the effect of a delay in cancer diagnoses, a three-month delay in prostate cancer diagnoses would result in a 0–4% reduction of ten-year survival [22].
Changes in treatment of prostate cancer appeared to be limited. Most striking observations were seen in low- and intermediate-risk prostate cancer. Shortly after the outbreak active surveillance was applied less often and radical prostatectomy was conducted more often in these patients, which was in contrast with the recommendations in the adapted guidelines for prostatic cancer care during COVID [8], [9], [10]. The EAU advised to postpone prostatectomies in patients with localised cancer and to consider androgen deprivation therapy and external beam radiotherapy as alternative to surgery for patients with high-risk localised cancer [10] Likewise, the FMS considered prostatectomies in patients with low-risk prostate carcinoma as procedures that could be postponed up to three months [9] Several explanations might have contributed to this temporally change in clinical practice. Possibly, surgical capacity remained available for oncological care during the first COVID-19 wave, due to downscaling of other regular care. Anticipating potential worsening of the COVID-19 situation, waiting lists for radical prostatectomies might have been caught up as much as possible. Secondly, the patients that were diagnosed after the outbreak, during the lockdown might represent a different patient population; patients who visit the GP or urologist during lockdown might be more worried and have strong preference for active treatment rather than active surveillance compared to the general prostate cancer population. Furthermore, patients or urologists could have chosen to avoid extensive (outpatient) follow-up during the lockdown, to prevent the spread of COVID-19.
Another change in treatment in low-risk localised prostate cancer, was a tendency towards more treatment with brachytherapy, which was in contrast with the advice by the FMS to postpone radiotherapy by six weeks up to three months in these patients [9] By contrast, brachytherapy was applied less often in patients with intermediate-risk localised prostate cancer, which is in accordance with the advice in the adapted guidelines to postpone brachytherapy [9,10,23]. Furthermore, external beam radiotherapy was applied more often in patients with intermediate-risk prostate cancer. However, these effects were already seen pre-COVID and therefore probably unrelated to the COVID-19 outbreak.
In patients with metastatic disease, we observed a temporary small increase in use of androgen receptor targeting agents and decrease in use of taxanes as systemic treatment. This corresponds with the adapted recommendations by the EAU to avoid androgen deprivation therapy combined with docetaxel and to consider abiraterone or prednisone in patients with metastatic hormone sensitive prostate carcinoma [10]. As the numbers were small and this trend seems to be present already in the pre-COVID period, this is not very likely to result from the COVID-19 outbreak. Finally, the combination of radiotherapy and androgen deprivation therapy was applied more often in the metastatic cancer group compared to the reference period. This is most likely the effect of published results of the STAMPEDE trial, which suggested that patients with a low metastatic burden could benefit from radiotherapy in addition to their hormonal therapy [24].
Interestingly, ADT has been suggested as potential treatment for COVID-19. Transmembrane serine protease 2 (TMPRSS2), which is associated with the development and progression of prostate cancer, facilitates entry of coronavirus into the host's cell [25]. Although it has been suggested that ADT decreases the risk of COVID-19 infection in patients with prostate cancer, [26] this was not supported by a recent meta-analysis and phase 2 trial [27,28].
Overall, the number of radical prostatectomies in 2020 was comparable to previous years and time to surgery in the COVID-19 period was shorter. This might be explained by the advice to postpone surgeries for benign diseases,[8,9] resulting in more surgical capacity for oncological surgery. A similar observation was made in the previously mentioned Swedish study which demonstrated no decline in the number of radical prostatectomies, which was also explained by prioritizing oncological surgery [19].
To our knowledge, next to the study of Fallara et al.,[19] detailed data on the effects of the COVID-19 outbreak in prostate cancer care, are scarce. We used up-to-date and high-quality data from the population-based nationwide NCR supplemented with data from PALGA, providing relevant insights into the effect of the COVID-19 pandemic on prostate cancer care.
One of the limitations of our study is that we could not yet evaluate a possible stage shift due to delayed diagnoses, as data of the second half of 2020 is still incomplete. However, as the number of diagnoses was largely resorted by the end of the year, we do not anticipate the impact on stage distribution to be substantial. Unfortunately, information on diagnostics performed by the GP like PSA assessments was not available as the NCR is mainly based on hospital data. In case these data would have been available, this could have supported our hypothesis that PSA assessments were performed less during the first wave, resulting in less low-risk localised prostate cancer diagnoses. Finally, some numbers, such as treatment with androgen receptor targeting agents in patients with metastatic disease, were too small to draw strong conclusions.
In conclusion, we observed a strong decline of prostate cancer diagnoses during the first COVID-19 wave, but this number was largely restored by the end of 2020 although at least 5% of the expected prostate cancers diagnoses were not observed. Changes in treatment were temporary and adherent to adapted guidelines. Although delayed diagnoses could result in a less favourable stage distribution, possibly affecting survival, this seems not very likely. Although it was expected that the COVID-19 pandemic would significantly impact cancer care, the magnitude of this problem in prostate cancer care was still unknown. The information gained by this evaluation can be used for future recommendations on clinical management in future periods of (unexpected restrictions)
Funding
This work was supported by the ZonMw [grant number: 10,430,022,010,014]. The funding sources had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report, and in the decision to submit the article for publication.
CRediT authorship contribution statement
Désirée van Deukeren: Conceptualization, Data curation, Formal analysis, Funding acquisition, Writing – original draft. Berdine L. Heesterman: Conceptualization, Data curation, Formal analysis, Funding acquisition, Writing – original draft. Lianne Roelofs: Data curation, Formal analysis, Funding acquisition, Writing – original draft. Lambertus A. Kiemeney: Conceptualization, Funding acquisition, Writing – original draft. J. Alfred Witjes: Conceptualization, Funding acquisition, Writing – original draft. Tineke J. Smilde: Funding acquisition, Writing – original draft. Geert J.L.H.van Leenders: Funding acquisition, Writing – original draft. Luca Incrocci: Funding acquisition, Writing – original draft. Ben G.L. Vanneste: Funding acquisition, Writing – original draft. Richard P. Meijer: Conceptualization, Funding acquisition, Writing – original draft. Sabine Siesling: Conceptualization, Funding acquisition, Writing – original draft. Bart P.J.van Bezooijen: Funding acquisition, Writing – original draft. Katja K.H. Aben: Conceptualization, Data curation, Funding acquisition, Writing – original draft.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. The authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Acknowledgements
The authors thank the registration team of the Netherlands Comprehensive Cancer Organisation (IKNL) for the collection of data for the Netherlands Cancer Registry as well as IKNL staff for scientific advice. We thank the Nationwide Network and Registry of Histo- and Cytopathology in the Netherlands (PALGA) for providing the data.
The members of the COVID and Cancer-NL consortium are: - Prof. Dr. S. Siesling: Dept of research and development, Netherlands Comprehensive Cancer Organisation (IKNL) Utrecht, the Netherlands; Technical Medical Centre, Dept Health Technology and Services Research, University of Twente, Enschede, the Netherlands - Dr. J.C. van Hoeve: Dept of research and development, Netherlands Comprehensive Cancer Organisation (IKNL) Utrecht, the Netherlands - Prof. dr. M.A.W. Merkx: Dept of research and development, Netherlands Comprehensive Cancer Organisation (IKNL) Utrecht, the Netherlands; dept of Oral and Maxillofacial Surgery, Radboud University Nijmegen Medical Centre, Nijmegen, The Netherlands - Prof. dr. N.J. de Wit: Dept of General Practice, Julius centre for Health Sciences and Primary Care, University Medical centre Utrecht (UMCU), Utrecht University, Utrecht, The Netherlands - M.Sc. I. Dingemans: Dutch Federation of Cancer Patient Organisations (NFK), Utrecht, The Netherlands - Prof. dr. I.D. Nagtegaal: Dept of Pathology, Radboud University Nijmegen Medical Centre, Nijmegen, The Netherlands, on behalf of the Automated Pathology Archive (PALGA) - Dr. A. Wilbrink: Dutch Hospital Data (DHD), Utrecht, The Netherlands - Prof. dr. C.H. van Gils: Dept of Epidemiology, Julius centre for Health Sciences and Primary Care, University Medical centre, Utrecht, The Netherlands. - Prof. dr. H.C.P.M. van Weert: Dept of General Practice, Amsterdam Public Health, Amsterdam UMC location AMC, Amsterdam, The Netherlands - Prof. dr. M. Verheij: Dept of Radiation Oncology, Radboud University Medical centre, Nijmegen, The Netherlands; on behalf of SONCOS (Dutch Multidisciplinary Oncology Foundation)
Footnotes
Microabstract: The number of new prostate cancer diagnoses declined substantially during the first COVID-19 wave (17%). Towards the end of 2020 the number of new diagnoses had largely recovered but remained lower than expected. Changes in treatment were limited and adherent to adapted guidelines. Although delayed diagnoses could result in a less favourable stage distribution, possibly affecting survival, this seems not very plausible.
Clinical practice points: In this study we have evaluated the impact of the COVID-19 outbreak on the number of prostate cancer diagnoses, disease stage and treatment in the Netherlands in 2020. Nationwide data on the effects of the COVID-19 pandemic on prostate cancer care are scarce. We have demonstrated that the COVID-19 outbreak resulted in a strong decline in prostate cancer diagnoses. This number largely restored during the second half of 2020 but remained lower than expected. Based on the currently available data, we do not observe an effect on disease stage due to delayed diagnosis. Observed treatment changes, i.e. less active surveillance and more radical prostatectomies in low and intermediate risk prostate cancer, were temporary. Other treatment changes were compliant with adapted recommendations in guidelines. The impact of the first COVID-19 wave on men with de novo prostate cancer appears to be limited, although long term effects have to be evaluated in the near future. Insight in short and long term impact of the COVID-19 outbreak can be used to define recommendations on clinical management to support optimal organisation of prostate cancer care in the future.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ctarc.2022.100553.
Appendix. Supplementary materials
References
- 1.JHU. COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) At John Hopkins University. Accessed September 16, 2021. https://gisanddata.maps.arcgis.com/apps/dashboards/bda7594740fd40299423467b48e9ecf6.
- 2.Government of the Netherlands. Man Diagnosed With Coronavirus (COVID-19) in the Netherlands. 02/2020. Accessed June 5, 2021. https://www.government.nl/latest/news/2020/02/27/man-diagnosed-with-coronavirus-covid-19-in-the-netherlands.
- 3.Government of the Netherlands. COVID-19: Additional measures in schools, the Hospitality Sector and sport. 03/2020. Accessed July 10, 2021. https://www.government.nl/latest/news/2020/03/15/additional-measures-in-schools-the-hospitality-sector-and-sport.
- 4.Rijksoverheid. Maart 2020: Maatregelen tegen Verspreiding coronavirus, Intelligente Lockdown [in Dutch]. 03/2020. Accessed July 10, 2021. https://www.rijksoverheid.nl/onderwerpen/coronavirus-tijdlijn/maart-2020-maatregelen-tegen-verspreiding-coronavirus.
- 5.National Institute for Public Health and the Environment. First Wave of COVID-19 Had Major Impact On Regular Healthcare and health. December 2020. Accessed June 1, 2021. https://www.rivm.nl/en/news/first-wave-of-covid-19-had-major-impact-on-regular-healthcare-and-health.
- 6.Dinmohamed A.G., Visser O., Verhoeven R.H.A., et al. Fewer cancer diagnoses during the COVID-19 epidemic in the Netherlands. Lancet Oncol. Published online April 2020. doi: 10.1016/S1470-2045(20)30265-5. [DOI] [PMC free article] [PubMed]
- 7.IKNL. COVID-19 En Kanker [in Dutch]. Published 2021. Accessed November 11, 2021. https://iknl.nl/COVID-19.
- 8.Amparore D., Campi R., Checcucci E., et al. Forecasting the future of urology practice: a comprehensive review of the recommendations by international and european associations on priority procedures during the COVID-19 pandemic. Eur. Urol. Focus. 2020;6(5):1032–1048. doi: 10.1016/j.euf.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.FMS. Prioritering Urologische Zorg [in Dutch]. 04/2020. Accessed November 1, 2021. https://www.nvu.nl/OverdeNVU/NieuwsDetails.aspx?p1=F61909D6-500E-4160-AF57-05F401F083C1.
- 10.Ribal M.J., Cornford P., Briganti A., et al. European association of urology guidelines office rapid reaction group: an organisation-wide collaborative effort to adapt the European association of urology guidelines recommendations to the coronavirus disease 2019 era. Eur. Urol. 2020;78(1):21–28. doi: 10.1016/j.eururo.2020.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.IKNL. Netherlands cancer registry (NCR). Accessed July 11, 2021. https://iknl.nl/en/ncr.
- 12.Casparie M., Tiebosch A.T.M.G., Burger G., et al. Pathology databanking and biobanking in The Netherlands, a central role for PALGA, the nationwide histopathology and cytopathology data network and archive. Cell Oncol. Off. J. Int. Soc. Cell Oncol. 2007;29(1):19–24. doi: 10.1155/2007/971816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brierley J.D., Gospodarowicz M.K., Wittekind C. TNM Classification of Malignant Tumours, 8th Edition. 8th ed. (Brierley JD, Gospodarowicz MK, Wittekind C, eds.). John Wiley & Sons; 2017.
- 14.KWF. Kankerorganisaties roepen op: zorgen over kanker? Blijf Er Niet Mee lopen, Bel Wél Je (huis)arts [in Dutch]. 10/04/2020. Accessed June 19, 2020. https://www.kwf.nl/pers/kankerorganisaties-roepen-op-zorgen-over-kanker-blijf-er-niet-mee-lopen-bel-wel-je-huisarts.
- 15.van Leenders G.J.L.H., van der Kwast T.H., Grignon D.J., et al. The 2019 international society of urological pathology (ISUP) consensus conference on grading of prostatic carcinoma. Am. J. Surg. Pathol. 2020;44(8):e87–e99. doi: 10.1097/PAS.0000000000001497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mottet N., van den Bergh R.C.N., Briers E., et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer-2020 update. part 1: screening, diagnosis, and local treatment with curative intent. Eur. Urol. 2021;79(2):243–262. doi: 10.1016/j.eururo.2020.09.042. [DOI] [PubMed] [Google Scholar]
- 17.FMS. Prostaatcarcinoom - Diagnostische prostaat MRI [in Dutch]. 30/01/2020. Accessed November 10, 2021. https://richtlijnendatabase.nl/richtlijn/prostaatcarcinoom/diagnostiek/beeldvormend_onderzoek/diagnostische_prostaat_mri_bij_prostaatcarcinoom.html.
- 18.CBS. Mortality in Times of corona. 29/05/2020. Accessed September 20, 2021. https://www.cbs.nl/en-gb/news/2020/22/mortality-in-times-of-corona.
- 19.Fallara G., Sandin F., Styrke J., et al. Prostate cancer diagnosis, staging, and treatment in Sweden during the first phase of the COVID-19 pandemic. Scand. J. Urol. 2021;55(3):184–191. doi: 10.1080/21681805.2021.1910341. [DOI] [PubMed] [Google Scholar]
- 20.IKNL. NKR cijfers; incidentie, prostaatkanker, Aantal [in Dutch]. Published 2021. Accessed November 11, 2021. https://cijfersoverkanker.nl.
- 21.van der Leest M., Cornel E., Israël B., et al. Head-to-head comparison of transrectal ultrasound-guided prostate biopsy versus multiparametric prostate resonance imaging with subsequent magnetic resonance-guided biopsy in biopsy-naïve men with elevated prostate-specific antigen: a large prospective Mu. Eur. Urol. 2019;75(4):570–578. doi: 10.1016/j.eururo.2018.11.023. [DOI] [PubMed] [Google Scholar]
- 22.Sud A., Torr B., Jones M.E., et al. Effect of delays in the 2-week-wait cancer referral pathway during the COVID-19 pandemic on cancer survival in the UK: a modelling study. Lancet Oncol. 2020;21(8):1035–1044. doi: 10.1016/S1470-2045(20)30392-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zaorsky N.G., Yu J.B., McBride S.M., et al. Prostate cancer radiation therapy recommendations in response to COVID-19. Adv. Radiat. Oncol. 2020;5(Suppl 1):26–32. doi: 10.1016/j.adro.2020.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parker C.C., James N.D., Brawley C.D., et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet. 2018;392(10162):2353–2366. doi: 10.1016/S0140-6736(18)32486-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mollica V., Rizzo A., Massari F. The pivotal role of TMPRSS2 in coronavirus disease 2019 and prostate cancer. Future Oncol. 2020;16(27):2029–2033. doi: 10.2217/fon-2020-0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bahmad H.F., Abou-Kheir W. Crosstalk between COVID-19 and prostate cancer. Prostate Cancer Prostatic Dis. 2020;23(4):561–563. doi: 10.1038/s41391-020-0262-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karimi A., Nowroozi A., Alilou S., Amini E. Effects of androgen deprivation therapy on COVID-19 in patients with prostate cancer: a systematic review and meta-analysis. Urol. J. 2021;18(6):577–584. doi: 10.22037/uj.v18i.6691. [DOI] [PubMed] [Google Scholar]
- 28.Welén K., Rosendal E., Gisslén M., et al. A phase 2 trial of the effect of antiandrogen therapy on COVID-19 outcome: no evidence of benefit, supported by epidemiology and in vitro data. Eur. Urol. 2022;81(3):285–293. doi: 10.1016/j.eururo.2021.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.