Abstract
The indinavir dosage regimen currently used for human immunodeficiency virus (HIV)-infected children is not based on pharmacokinetic data obtained in the target patient population. The purpose of our study was to characterize indinavir pharmacokinetics and pharmacodynamics in HIV-infected children. Eleven children (age range, 9.0 to 13.6 years; weight range, 21.7 to 56.0 kg) receiving indinavir (500 mg/m2 every 8 h) in combination with lamivudine and stavudine were studied. The correlation of indinavir pharmacokinetic parameters and demographic parameters was evaluated. Also, the pharmacodynamic relationship between parameters of indinavir exposure and parameters of renal toxicity and immunologic recovery was studied. The area under the indinavir concentration-time curve (AUC) and patient body surface area (BSA) showed a significant negative correlation (r = 0.73; P = 0.012). Patients with smaller BSA had excessive indinavir AUC compared to adults. On the other hand, the median minimum drug concentration in plasma (Cmin) was lower than that reported for adults. The maximum indinavir concentration in serum was higher in patients with renal toxicity (5 out of 11 children), but the difference was not statistically significant (15.3 ± 8.2 versus 9.8 ± 4.4 mg/liter; P = 0.19). There was a trend toward higher immunologic efficacy in patients with greater indinavir exposure: the time-averaged AUC of the percentage of CD4+ lymphocytes over the baseline value for patients with indinavir Cmin > 95% inhibitory concentration (IC95) was higher than in patients with Cmin < IC95 (P = 0.068). Our study suggests that a dose reduction may be appropriate for children with small BSA and that a 6-h dosage regimen may be indicated for a substantial percentage of patients. Due to the low number of patients enrolled in this study, our results should be confirmed by a larger study.
Indinavir is a potent antiretroviral drug belonging to the family of protease inhibitors. Although the body of literature regarding the clinical efficacy and toxicity of this drug in adults is considerable, the usage in pediatric patients remains anecdotal (6, 7, 9, 10). An overview of the published experience with indinavir in pediatric patients indicates that the efficacy profile may be comparable to the one reported for adult patients, while renal toxicity occurs more frequently, with up to 44% of the patients experiencing renal side effects (6, 7, 10) compared to approximately 5 to 10% of adults.
In the studies cited above, indinavir was administered at a dose of 500 mg/m2 every 8 h (q8h). This dosage regimen was inferred from the one used for adults but is not based on pharmacokinetic (PK) data obtained with the target population. The purpose of our study was to characterize the PK profile of indinavir in human immunodeficiency type 1 (HIV-1)-positive children and to evaluate (i) the relationship of PK parameters with demographic parameters and (ii) the relationship of parameters of systemic exposure to indinavir with parameters of efficacy and toxicity.
Patients.
Eleven children vertically infected with HIV-1 were enrolled in the study after parental written informed consent was obtained. The patients enrolled in this study represent a subset of 25 patients enrolled in a pilot study of which the results regarding immune reconstitution and viral suppression have been recently presented (A. Vigano', M. Clerici, D. Bricalli, M. Sarasella, S. Difabio, N. Principi, and S. Vella, 12th World AIDS Conf., abstr. 12247).
All of the patients had been previously treated with a nucleoside analogue reverse transcriptase inhibitor(s) (zidovudine [AZT] alone or AZT in combination with dideoxyinosine) but were all protease inhibitor naive. The mean (standard deviation [SD]) durations of exposure to AZT and dideoxyinosine prior to study initiation were 44.1 (27.0) and 17.5 (12.1) months, respectively.
At the time of initiation of treatment with indinavir, the underlying therapy with nucleoside analogues was modified in accordance with current guidelines (1). Indinavir was administered at a dose of 500 mg/m2 q8h in association with lamivudine (4 mg/kg q12h) and stavudine (1 mg/kg q12h). Parents and children were told to maintain adequate hydration of their children throughout the observation period.
Virologic and immunologic assessment.
Plasma viral load was quantified using the branch DNA assay (Chiron, Emeryville, Calif.) with a lower limit of quantification of 400 copies/ml. The CD4+ lymphocyte count and the percentage of CD4+ lymphocytes with respect to the overall lymphocyte cell population (%CD4) were determined by means of flow cytometry with Coulter (Coulter Electronics, Inc., Miami Lakes, Fla.) or Ortho (Ortho Diagnostic System) flow cytometry. Viral load, CD4+ lymphocyte count, and %CD4 were measured at the baseline and at 1, 3, 6, 9, and 12 months after the beginning of indinavir treatment.
Toxicity evaluation.
Clinical (flank pain, renal colic) and laboratory (serum creatinine, hematuria, crystalluria, pyuria, casts) signs of renal toxicity possibly due to indinavir were recorded at each visit throughout the 12-month follow-up period. Additionally, total bilirubin was recorded at the baseline and at 1, 3, 6, 9, and 12 months following indinavir treatment initiation.
PK study.
Patient demographics at the time of PK study, as well as the baseline viral load, the CD4+ cell count, and %CD4 are shown in Table 1. The body mass index (BMI) was calculated as weight/height2. A complete medical history, a physical examination, and a panel of laboratory tests consisting of a chemistry screen and a complete blood cell count with differential and platelet counts were carried out within a week before the PK study. None of the patients had diarrhea at the time of the PK study. None of the patients had received drugs known or suspected to interact with indinavir PKs in the 2 weeks before the PK study began. None of the patients had signs of acute infection in the 1 month before the PK study began.
TABLE 1.
Patient demographics and indinavir PK parameters
| Patient | Dose/BSA (mg q8h/m2) | Age (yr) | Sexa | Wt (kg) | Ht (cm) | BSA (m2) | BMI | CDCb classification | Baseline
|
Indinavir PK parameters
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of CD4+ cells/μl | %CD4 | Viral load (log no. of copies/ml) | Cmax (mg/liter) | Tmaxc (h) | Cmin (mg/liter) | AUC (mg × h/liter) | CL/Fd (liters/h) | t1/2e (h) | |||||||||
| 1 | 556 | 9.6 | M | 31.0 | 137 | 1.08 | 17 | C2 | 602 | 21.5 | 4.18 | 8.3 | 1.5 | 0.051 | 25.2 | 23.8 | 0.84 |
| 2 | 441 | 10.0 | M | 47.5 | 145 | 1.36 | 23 | B2 | 669 | 27.0 | 3.08 | 14.1 | 1.5 | 0.050 | 30.7 | 19.6 | 0.87 |
| 3 | 550 | 11.6 | M | 30.0 | 142 | 1.09 | 15 | C3 | 60 | 2.8 | 3.87 | 11.5 | 1.0 | 0.046 | 45.3 | 13.3 | 0.56 |
| 4 | 455 | 10.0 | M | 46.2 | 144 | 1.32 | 22 | C2 | 547 | 21.3 | 3.96 | 12.7 | 1.0 | 0.056 | 26.0 | 23.1 | 0.87 |
| 5 | 455 | 10.2 | F | 22.1 | 128 | 0.88 | 14 | C3 | 10 | 0.9 | 3.97 | 16.6 | 2.0 | 0.095 | 55.1 | 7.3 | 0.66 |
| 6 | 522 | 11.6 | M | 33.9 | 141 | 1.15 | 17 | C2 | 470 | 26.9 | 2.46 | 7.1 | 2.0 | 0.073 | 25.0 | 24.1 | 0.65 |
| 7 | 465 | 9.0 | F | 21.7 | 123 | 0.86 | 14 | B2 | 472 | 29.0 | 4.92 | 28.8 | 2.0 | 0.800 | 117.0 | 3.4 | 0.90 |
| 8 | 522 | 11.0 | F | 33.0 | 145 | 1.15 | 16 | C2 | 300 | 15.0 | 3.88 | 14.8 | 2.0 | 0.130 | 49.0 | 12.3 | 0.68 |
| 9 | 488 | 13.6 | M | 56.0 | 171 | 1.64 | 19 | B3 | 25 | 1.4 | 4.23 | 7.3 | 2.0 | 0.071 | 19.1 | 42.0 | 0.89 |
| 10 | 496 | 12.1 | M | 36.4 | 145 | 1.21 | 17 | A2 | 365 | 18.0 | 3.74 | 11.0 | 2.0 | 0.095 | 41.6 | 14.4 | 0.71 |
| 11 | 533 | 12.1 | F | 47.2 | 167 | 1.50 | 17 | A2 | 336 | 28.0 | 4.43 | 3.2 | 4.0 | 0.113 | 15.9 | 50.3 | 0.83 |
| Mean | 498 | 11.0 | 36.8 | 144 | 1.20 | 17 | 351 | 17.4 | 3.88 | 12.3 | 1.9 | 0.144 | 40.9 | 21.2 | 0.77 | ||
| SD | 41 | 1.4 | 11.1 | 14 | 0.24 | 3 | 233 | 11.0 | 0.66 | 6.7 | 0.8 | 0.219 | 28.3 | 14.1 | 0.12 | ||
| %CVf | 8 | 12 | 30 | 10 | 20 | 17 | 66 | 63 | 17 | 55 | 42 | 153 | 69 | 67 | 15 | ||
M, male; F, female.
CDC, Centers for Disease Control and Prevention.
Tmax, time to Cmax.
CL/F, clearance/bioavailability.
t1/2, elimination rate constant.
%CV, Percent coefficient of variation.
Drug administration and sample collection.
The dose of indinavir (Merck Sharp & Dohme, Rome, Italy) administered to each patient was the closest approximation to the dose calculated based on the recommended dose of 500 mg/m2 of body surface area (BSA) q8h. The 200- or 400-mg capsules commercially available for use by adults were used. At the time of the PK study, the patients had been treated with the same indinavir dosage regimen for at least 3 months. On the day of the PK study, the patients fasted for at least 2 h before and at least 1 h after indinavir administration.
Blood samples (5 to 7 ml) were collected in preheparinized Vacutainer tubes at 0 (predose), 0.5, 1, 1.5, 2, 4, and 8 h following drug administration. Blood samples were placed on ice and centrifuged within 30 min after collection. The plasma was harvested and stored at −70°C until assayed for indinavir.
Analytical methods.
Indinavir concentrations were determined by means of a newly developed high-pressure liquid chromatography assay. Indinavir sulfate powder for laboratory use and an appropriate internal standard (L-707-943) were provided by Merck Sharp & Dohme. Briefly, indinavir extraction from plasma samples was done by addition of phosphate buffer to 0.5 ml of plasma (50:50, vol/vol), followed by elution of the mixture through Extrelut-1 columns (Merck, Darmstadt, Germany) using diethyl ether. Eluates were evaporated to dryness, reconstituted in the mobile phase, and injected onto a Supelcosil LC ABZ column (Supelco, Inc., Bellefonte, Pa.). UV detection was done at a wavelength of 220 nm.
The standard curve ranged from 0.025 to 15 mg/liter. The interday and intraday variabilities at concentrations of 0.085, 0.75, 3, and 8 mg/liter (quality controls) were less than 10%. Samples with concentrations above the upper limit of the standard curve were diluted 1:2 and reprocessed. Dilution did not affect quantitation at the quality control concentration of 30 mg/liter (n = 6).
PK analysis.
Indinavir PK parameters were obtained by noncompartmental methods using the program SIPHAR (Simed, Inc., Creteil, France). The area under the concentration-time curve (AUC) from time zero to 8 h following drug administration was calculated by use of the trapezoidal rule. The elimination rate constant was calculated as 0.693/lambda, where lambda was obtained by unweighted linear regression of the terminal linear portion of the concentration-versus-time curve. Clearance/bioavailability was calculated as the absolute dose administered divided by the AUC. Maximum concentration (Cmax), time to Cmax, and concentration at 8 h following administration (Cmin) were obtained by visual inspection of the data.
Statistical analysis was carried out on a MacIntosh computer using the program Staview II (Abacus Concepts Inc., Berkeley, Calif.). All demographic and PK data are reported as means ± SD. A value of P < 0.05 was considered the cutoff for significance. The Pearson correlation coefficient obtained by unweighted linear regression was used to evaluate the correlation between indinavir AUC and patient BSA.
Pharmacodynamic (PD) analysis. (i) Efficacy.
The relationship between the indinavir log-transformed AUC and parameters of immunologic response (CD4+ lymphocyte count and %CD4 recovery over a 12-month follow-up period) was studied by means of linear regression. %CD4 was considered because children may have physiologic lymphocytosis. Therefore, %CD4 may be a more reliable parameter of disease progression and treatment efficacy than the absolute CD4+ cells count in this particular patient population. The CD4+ cell count and %CD4 recovery were quantified in terms of the time-normalized AUC over the baseline and the difference between the maximum value observed over the follow-up period and the baseline value (Δmax). The following covariables were tested, besides the indinavir AUC, for effect on the CD4+ cell count and %CD4 recovery: patient age, BSA, BMI, baseline CD4+ cell count, %CD4, and viral load. Multivariate analysis was carried out by stepwise linear regression. Also, the relationship between indinavir Cmin and the parameters of CD4+ cell count and %CD4 recovery was analyzed by dichotomizing the patients depending on whether the Cmin was above or below the reported 95% inhibitory concentration of 0.075 mg/liter (ca. 100 nmol/liter) (3). Data were compared using the two-tailed unpaired t test.
A PD analysis for viral load could not be performed because all of the patients responded extremely well to therapy. In fact, at the first follow-up visit (1 month after the beginning of treatment), the viral load fell below the limit of quantification and remained undetectable (<400 copies/ml) for all of the follow-up period.
(ii) Toxicity.
The relationship between the parameters of indinavir exposure and the parameters of renal toxicity was evaluated by dichotomizing the patients into two groups depending on the presence or absence of signs of renal toxicity over the study period. The dependence of total bilirubin levels on the parameters of indinavir exposure was analyzed by parameterizing bilirubin as baseline normalized bilirubin AUC and Δmax.
Results of PK analysis.
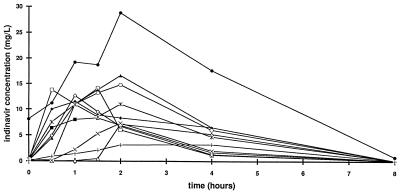
The PK parameters of indinavir in the 11 study patients are shown in Table 1. For seven of the plasma concentration-versus-time curves (Fig. 1), the half-life could be calculated only on the basis of two terminal points. Therefore, the confidence in this parameter estimate should be considered low. The median predose concentration and the concentration at 8 h following administrations were 0.081 and 0.073 mg/liter, respectively. The indinavir AUC was negatively correlated with BSA (r = 0.73; P = 0.012). None of the PK parameters was associated with the actual daily dose of indinavir.
FIG. 1.
PK profiles of indinavir in 11 HIV-infected children.
Results of PD analysis. (i) Efficacy.
All of the patients showed substantial immunologic recovery. The median (range) Δmax for CD4+ cell count and %CD4 were 317 (148 to 617) cells/μl and 11.3% (3.8 to 24.8%). Indinavir AUC and Cmax were tested after log transformation. The parameters of CD4+ cell count and %CD4 recovery appeared to be positively correlated with parameters of indinavir exposure, even though none of the relationships was statistically significant. It is noteworthy that the indinavir AUC showed a positive correlation with CD4+ Δmax (r = 0.49; P = 0.12) and %CD4 AUC (r = 0.45, P = 0.17) and Δmax (r = 0.43, P = 0.14). Patients with a Cmin lower than the IC95 had a lower %CD4 AUC (P = 0.068) and Δmax (P = 0.068).
A multivariate analysis was performed only for %CD4 Δmax, because for the other parameters of %CD4 recovery, none of the covariables tested resulted in a significant correlation. Baseline viral load was the better predictor of %CD4 Δmax, while indinavir AUC remained out of the model.
(ii) Toxicity.
Five (45.5%) of the 11 children had at least one episode of renal side effects over the 1-year follow up. Toxicity ranged from mild to severe: patients 4, 5, and 6 complained of flank or abdominal pain without evidence of kidney stones; patient 7 had renal colic, one 2-mm kidney stone, and a mild transient increase in serum creatinine (from 0.7 to 1.0 mg/dl) when the renal colic occurred. Patient 3 had one episode of renal colic with macrohematuria, had recurrent leukocyturia, and subsequently developed acute renal failure, defined as a persistent increase in the serum creatinine level to greater than 0.5 mg/dl above the baseline value. Indinavir treatment of patient 3 was discontinued, and his renal failure completely resolved within 3 months of indinavir withdrawal. All patients received indinavir for at least 1 year. All patients except patient 3 continued treatment with indinavir after the 1-year follow-up period.
There was a trend toward a higher indinavir AUC and Cmax in patients who had renal side effects. The indinavir AUC was 53.6 ± 37.7 mg × h/liter in patients with renal side effects versus 30.2 ± 12.9 mg × h/liter in patients with no side effects (P = 0.18). The Cmax was 15.3 ± 8.2 mg/liter in patients with renal side effects versus 9.78 ± 4.4 mg/liter in patients without side effects (P = 0.19). Notably, the patient with a higher indinavir Cmax and AUC (patient 7) had a kidney stone. None of indinavir exposure parameters correlated with parameters of bilirubin alteration. Bilirubin AUC and Δmax were highly correlated with baseline bilirubin (r = 0.84 and P = 0.001 and r = 0.77 and P = 0.005, respectively).
The dosage regimen of a drug to be administered to children is usually calculated with the goal of achieving parameters of drug exposure comparable to those of adults, with the assumption that similar drug exposure will result in comparable efficacy and toxicity. Our study indicates that the indinavir dosage regimen of 500 mg/m2 does not achieve this goal in HIV-infected children. In fact, in our study, the indinavir AUC and Cmax were higher, on average, than those reported in the literature for adult patients (8; E. P. Acosta, K. Henry, D. Weller, L. M. Page, L. Bacon, F. Rhame, I. Gilson, H. Rosenstein, T. Schacker, and C. V. Fletcher, Abstr. 37th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-15; G. Gatti, A. Di Biagio, C. De Pascalis, E. Pontali, M. Bassetti, M. Cruciani, and D. Bassetti, Abstr. 38th ICAAC, abstr. A66) treated with the standard dosage regimen of 800 mg q8h. For example, the average AUC reported for adults is 20 mg × h/liter. Also, it is noteworthy that the indinavir exposure parameters found in our study varied over a wide range, in agreement with another study (6), with some patients having particularly high values.
The negative correlation we found between indinavir AUC and BSA indicates that a dose of 300 to 400 mg/m2 q8h may be more appropriate for children with smaller BSA in order to avoid excessive drug exposure. A larger study should be conducted in order to estimate a precise BSA cutoff. Also, it is noteworthy that 6 of the 11 patients had a Cmin below the reported mean 95% inhibitory concentration of indinavir for HIV. This observation suggests that the total daily dose, particularly in the case of a dose reduction, as we advise, should be administered in four doses in order to obtain a higher Cmin and therefore reduce the risk of treatment failure. In a study, this approach was used for 75% of the patients (6) as an intervention based on individual Cmins observed following administration of the standard dosage regimen. To date, however, dosage regimen individualization based on drug concentrations in plasma is not applicable in everyday clinical practice because of the lack of a suitable assay for such a setting.
Monitoring of plasma drug concentrations and dosage adjustment are particularly relevant when a correlation between parameters of drug exposure and drug effect (efficacy and/or toxicity) can be demonstrated. It is a subject of debate whether the efficacy of indinavir may be predicted by parameters of drug exposure. Most researchers report that virologic response to indinavir correlates with parameters of drug exposure such as Cmin and AUC (11; Acosta et al., 37th ICAAC; D. M. Berger, R. M. W. Hoetelmans, J. W. Mulder, P. L. Meenhorst, P. W. H. Hugen, K. Brinkman, and P. P. Koopmans, 12th World AIDS Conf., abstr. 42275; R. Murphy, J.-P. Sommadossi, M. Lamson, P. Gagnier, D. Hall, M. Myers, and A. Dusek, 12th World AIDS Conf., abstr. 22404; M. Harris, C. Durakovic, S. Rae, S. Fransen, A. Shillington, B. Conway, and J. S. G. Montaner, Abstr. 37th ICAAC, abstr. I-173), while others cannot find such a relationship (J. Chodakewitz, P. Deitsch, R. Leavitt, J. McCrea, M. Nessly, A. Sterrett, and G. Winchell, 12th World AIDS Conf., abstr. 42266; L. Perello, C. Goujard, J. F. Delfraissy, and A. M. Taburet, 12th World AIDS Conf., abstr. 42272). Our study shows a trend toward a more favorable immunologic response in patients who had higher indinavir exposure. A classical maximum-effect (Emax) model was not used because preliminary analysis showed that such a model did not result in improvement of fit compared to linear regression. It has to be noted that parameters of indinavir immunologic efficacy were regressed versus log parameters of indinavir exposure, which is also considered a useful approach applied to pharmacodynamic analysis with the purpose of linearizing the central portion of the Emax relationship, i.e., the portion of the curve included between 20 and 80% of the Emax (5). It is noteworthy that the patients enrolled in this PK-PD substudy, as well as all of the patients in the main clinical study, responded extremely well both from a virologic standpoint, with prolonged and sustained suppression of viral replication, and in terms of immune reconstitution, with a major increase in CD4+ cell count, CD4+ naive cells, expansion of the TRCβ repertoire on CD4+ cells, and improvement of peripheral blood mononuclear cell proliferative response to recall antigens (Vigano' et al., 12th World AIDS Conf.). Also, a recovery of thymus dimension was observed (Vigano' et al., 12th World AIDS Conf.).
A significant relationship has been reported both for indinavir (2) and ritonavir (4) exposure versus renal toxicity (indinavir) and gastrointestinal and/or neurological side effects (ritonavir). In our study, indinavir AUC and Cmax showed only a trend toward higher values in patients with signs of renal toxicity compared with patients without renal toxicity. It is noteworthy that indinavir PKs were not studied on the day of occurrence of renal signs or symptoms. Therefore, intraindividual PK variability may have partially blunted the relationship between parameters of indinavir exposure and renal toxicity. Recently, a study with adults (2) reported that indinavir plasma concentrations were significantly higher in 12 out of 15 patients with renal side effects than those observed in a reference population without renal side effects. For five of these patients, the dose was reduced to 600 mg q8h.
In conclusion, our PK-PD study shows that (i) indinavir, used in combination with stavudine and lamivudine, has potent antiretroviral efficacy in pediatric patients and (ii) patients had higher-than-expected exposure, particularly those with smaller BSAs, and this may be the cause of the higher efficacy but also of a higher risk of renal toxicity, compared to that of adults. Our study suggests that a dose of 300 to 400 mg/m2 q8h would be more appropriate for patients with small BSAs. However, for a substantial percentage of patients, a q6h dosage regimen may be more strongly indicated. In this perspective, the development of an indinavir assay suitable for therapeutic drug monitoring in everyday clinical practice is needed in order to select patients for whom such a dosage regimen may be indicated. Our data should not be extrapolated to patients with BSAs outside the range observed in this study. Also, due to the low number of patients enrolled in this study, our results should be confirmed by a larger study.
Acknowledgments
This study was supported by grants 30A.0.07 and 30A.0.56 from the Istituto Superiore di Sanita' (Italian NIH).
We thank Franca Miletich for her skillful assistance with the indinavir high-pressure liquid chromatography assay.
REFERENCES
- 1.Carpenter C C J, Fischl M A, Hammer S M, Hirsch M S, Jacobsen D M, Katzestenstein D A, Montaner J S G, Richman D D, Saag M S, Schooley R T, Thompson M A, Vella S, Yeni P G, Volberding P A. Antiretroviral therapy for HIV infection in 1996. Updated recommendations of the International AIDS Society-USA panel. JAMA. 1997;277:1962–1969. [PubMed] [Google Scholar]
- 2.Dieleman J, Gyssens I C, van der Ende M E M, de Marie S, Burger D M. Urologic complaints in relation to indinavir plasma concentrations in HIV-infected patients. AIDS. 1999;13:473–478. doi: 10.1097/00002030-199903110-00005. [DOI] [PubMed] [Google Scholar]
- 3.Dorsey B D, Levin R B, McDaniel S L, Vacca J P, Guare J P, Darke P L, Zugay J A, Emini E A, Schleif W A, Quintero J C, Lin J H, Chen I W, Holoway M K, Fitzgerald P, Axel M G, Ostovic D, Anderson P S, Huff J R. L-735524: the design of a potent and orally bioavailable HIV protease inhibitor. J Med Chem. 1994;37:3443–3451. doi: 10.1021/jm00047a001. [DOI] [PubMed] [Google Scholar]
- 4.Gatti G, Di Biagio A, Casazza R, Bassetti R M, Cruciani M, Vella S, Bassetti D. The relationship of ritonavir plasma levels and side effects: implications for therapeutic drug monitoring. AIDS. 1999;13:2083–2089. doi: 10.1097/00002030-199910220-00011. [DOI] [PubMed] [Google Scholar]
- 5.Gibaldi M, Perrier D. Pharmacokinetics. 2nd ed. New York, N.Y: Marcel Dekker, Inc.; 1982. [Google Scholar]
- 6.Kline M W, Fletcher C V, Harris A T, Evans K D, Brundage R C, Remmel R P, Calles N R, Kirkpatrick S B, Simon C. A pilot study of combination therapy with indinavir, stavudine (D4T), and didanosine (DDI) in children infected with the human immunodeficiency virus. J Pediatr. 1998;132:543–546. doi: 10.1016/s0022-3476(98)70039-3. [DOI] [PubMed] [Google Scholar]
- 7.Melvin A J, Mohan K M, Manns Arcuino L A, Edelstein R E, Frenkel L M. Clinical, virologic and immunologic responses of children with advanced human immunodeficiency virus type 1 disease treated with protease inhibitors. Pediatr Infect Dis J. 1997;16:968–974. doi: 10.1097/00006454-199710000-00013. [DOI] [PubMed] [Google Scholar]
- 8.Merck Sharp & Dohme. Indinavir: technical informations. West Point, Pa: Merck Sharp & Dohme; 1998. [Google Scholar]
- 9.Monpoux F, Sirvent N, Cottalorda J, Mariani R, Lefbvre J C. Stavudine, lamivudine and indinavir in children with advanced HIV-1 infection: preliminary experience. AIDS. 1997;11:1523–1525. [PubMed] [Google Scholar]
- 10.Rutstein R M, Feingold A, Meislich D, Word B, Rudy B. Protease inhibitor therapy in children with perinatally acquired HIV infection. AIDS. 1997;11:F107–F111. doi: 10.1097/00002030-199712000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Stein D S, Fish D G, Bilello J A, Preston S L, Martineau G L, Drusano G L. A 24-week pen-label phase I/II evaluation of the HIV protease inhibitor MK-639 (indinavir) AIDS. 1996;10:485–492. doi: 10.1097/00002030-199605000-00006. [DOI] [PubMed] [Google Scholar]