Abstract
Cannabinoid CB2 receptors (CB2R) are importantly involved in drug reward and addiction. However, the cellular mechanisms underlying CB2R action remain unclear. We have previously reported that cocaine self-administration upregulates CB2R expression in midbrain dopamine (DA) neurons. In the present study, we investigated whether cocaine or heroin also alters CB2R expression in striatal medium-spiny neurons that express dopamine D1 or D2 receptors (D1-MSNs, D2-MSNs) and microglia. Due to the concern of CB2R antibody specificity, we developed three mouse CB2-specific probes to detect CB2R mRNA using quantitative RT-PCR and RNAscope in situ hybridization (ISH) assays. We found that a single injection of cocaine failed to alter, while repeated cocaine injections or self-administration dose-dependently upregulated CB2R gene expression in both brain (cortex and striatum) and periphery (spleen). In contrast, repeated administration of heroin produced a dose-dependent reduction in striatal CB2 mRNA expression. RNAscope ISH assays detected CB2R mRNA in striatal D1- and D2-MSNs, not in microglia. We then used transgenic CX3CR1eGFP/+ microglia reporter mice and D1- or D2-Cre-RiboTag mice to purify striatal microglia or ribosome-associated mRNAs from CX3CR1eGFP/+, D1-MSNs, or D2-MSNs, respectively. We found that CB2R upregulation occurred mainly in D1-MSNs, not in D2-MSNs or microglia, in the nucleus accumbens rather than the dorsal striatum. These findings indicate that repeated cocaine exposure may upregulate CB2R expression in both brain and spleen, with regional and cell type-specific profiles. In the striatum, CB2R upregulation occurs mainly in D1-MSNs in the nucleus accumbens. Given the important role of D1-MSNs in brain reward function, the present findings provide new insight into mechanisms by which brain CB2Rs modulate cocaine action.
Keywords: cocaine, cannabinoid, CB2 receptor, microglia, self-administration, D1-MSNs
Introduction
The endocannabinoid system (ECS) is importantly involved in the development of dependence to many drugs of abuse, such as cannabis, cocaine, nicotine, opiates, and alcohol [1–5]. The ECS consists of cannabinoid receptors, endogenous ligands, and the enzymes involved in the synthesis and degradation of endocannabinoids. Two major subtypes of cannabinoid receptors, CB1 receptor (CB1R) and CB2 receptor (CB2R), have been cloned and characterized [6]. Because CB1Rs are highly expressed in the brain, while CB2Rs were initially found in peripheral immune cells, it has been generally believed that the psychoactive effects of cannabis are mediated by activation of brain CB1Rs instead of CB2Rs [7].
However, CB2Rs are found in the brains of healthy subjects, albeit at very low levels compared to brain CB1Rs [7–12]. Anatomically, CB2Rs are found in activated microglia during neuroinflammation and in many other CNS disorders [13] as well as in subpopulations of neurons in the brain of normal healthy subjects [14–17]. Functionally, brain CB2Rs have been shown to be involved in multiple dopamine (DA)-related CNS disorders, such as Parkinson’s disease [18, 19], schizophrenia [20], anxiety [21], depression [22], Huntington’s disease [23], and substance use disorders [8, 24]. In light of these findings, it has been proposed that brain CB2R expression is dynamic and can be upregulated in response to various insults as stated above.
In addition, we have recently reported that cocaine self-administration upregulates CB2R expression in midbrain DA neurons [11], indicating that inducible expression of CB2Rs is not restricted to microglia within the CNS. This CB2R upregulation may in part explain the antagonism of CB2R agonists against cocaine self-administration [9–11, 25–27], and of cocaine- or nicotine-induced hyperactivity and place preference [8, 24, 28–30], as activation of CB2Rs inhibits midbrain DA neurons and DA release in the nucleus accumbens (NAc) [26, 31]. These findings indicate that dynamic changes in CB2R expression outside microglia can critically shape behavior. Similarly, CB2R upregulation has also been reported in the NAc (shell) in rats after cocaine self-administration, followed by a reduction in CB2R expression in the prefrontal cortex (PFC) and NAc (core) after extinction from cocaine self-administration [32]. However, little is known about the cellular distributions of CB2Rs in the striatum and which type(s) of cells displaying CB2R upregulation after cocaine administration.
In the present study, we used multiple transgenic approaches to address these questions. We first used two custom-designed mouse CB2 mRNA-specific probes to examine whether acute or chronic cocaine or heroin administration alters CB2 mRNA expression in the PFC, striatum, and spleen by quantitative real-time polymerase chain reaction (qRT-PCR). We then used RNAscope in situ hybridization to examine the cellular distributions of CB2R mRNA in the ventral striatum (also called nucleus accumbens, NAc). Lastly, we used fluorescence-activated cell sorting (FACS) and transgenic CX3CR1eGFP/+ microglia reporter mice to isolate striatal microglia and D1- or D2-Cre-RiboTag mice to isolate ribosome-associated mRNAs from D1 or D2 receptor-expressing medium-spiny neurons (D1- or D2-MSNs) to examine CB2 mRNA expression in these specific cell populations. These data provide the first analysis of cell type-specific changes in CB2Rs in the striatum following cocaine or heroin exposure and reveal D1-MSNs as a key cell population in which dynamic changes in CB2R expression may be relevant for regulation of circuit function and behavior following drug exposure.
Materials and methods
Animals
Male wild-type (WT) and CB2R-knockout (CB2−/−) mice with C57BL/6J genetic backgrounds were used and bred at the National Institute on Drug Abuse (NIDA) Intramural Research Program (IRP). This strain of CB2−/− mice is C-terminal knocked out—only the last 341 base pairs on exon 3 are deleted that encode part of intracellular and extracellular third loops, transmembrane regions 6 and 7, and intracellular C-terminus region [33, 34]. This strain of CB2−/− mice was used as controls to determine the specificity of detected CB2 mRNA signals. Genotyping was performed in our laboratory according to protocol from Charles River Laboratories International Inc. (Wilmington, MA, USA). CX3CR1eGFP/+ breeders were originally obtained from Jackson labs (Stock # 005582) and bred at the NIDA, IRP. In these mice, eGFP is knocked into the CX3CR1 locus and eGFP expression in brain of these mice is specific to microglial cells [35]. All mice used for experiments were heterozygous (CX3CR1eGFP/+). D1-Cre hemizygote (line FK150) and D2-Cre hemizygote (line ER44) BAC transgenic mice with C57BL/6J background were purchased from GENSAT (www.gensat.org). Homozygous RiboTag mice with C57BL/6J background expressing Cre-inducible HA-Rpl22 were purchased from Jackson Laboratory [36–38]. These RiboTag mice were crossed to D1-Cre or D2-Cre mouse line to generate D1-Cre-RiboTag or D2-Cre-RiboTag transgenic mice for isolation of ribosome-associated mRNAs from cell type-specific D1-MSNs or D2-MSNs.
All mice used in the present experiments were matched for age (8–14 weeks) and weight (25–35 g). They were housed individually in a climate-controlled animal colony room on a reversed light-dark cycle (lights on at 7:00 PM, lights off at 7:00 AM) with free access to food and water. All experimental procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals of the US National Academy of Sciences, and were approved by the Animal Care and Use Committee of the NIDA of the US National Institutes of Health and the University of Maryland School of Medicine.
Single-dose cocaine treatment
To determine whether a single dose of cocaine injection alters brain CB2 gene expression, 28 drug naïve mice (WT) were randomly divided into four dose groups (10, 20, 30 mg/kg, i.p.) or vehicle (saline) (n = 7 per group). At 1 h after cocaine injection, all mice were deeply anaesthetized with 100 mg/kg pentobarbital and transcardially perfused with saline to remove all blood from the brain. Whole brains were then rapidly removed, and the PFC and striatum tissues were dissected on dry ice for qRT-PCR assays.
Repeated cocaine or heroin treatment
To determine whether repeated cocaine or heroin administration alters brain CB2 expression, additional 20 drug naïve WT mice were divided into five groups—repeated saline vs. repeated cocaine (10, 20 mg/kg, i.p., n = 4–8 per group) or heroin (2, 8 mg/kg, s.c.) (n = 4 each group). Before the drug injection, each animal was placed in a locomotor detection chamber (Accuscan Instruments, Inc., Columbus, OH, USA) for 3 days (3 h per day) for environmental habituation. Then, each animal received 2 days of saline injection before cocaine administration. On each test day, mice were placed in the chamber for 1 h of habituation, and then removed and given either saline (0.1 mL saline, i.p.), one dose of cocaine or heroin. Animals were then placed back into the locomotor chambers for 2 h to assess the effects of cocaine or heroin on locomotion. Each mouse received one injection per day for a total of 7 successive days. Data were collected in 10 min intervals using the VersaMax data analysis system (Accuscan Instruments, Inc., Columbus, OH, USA). Total distance traveled was used to compare saline- vs. the drug (cocaine or heroin)-induced changes in locomotion. At 24 h after the last injection, all mice were deeply anaesthetized with 100 mg/kg pentobarbital and transcardially perfused with saline to remove all blood from the brain. Whole brains were then rapidly removed, and the striatum, PFC or spleen tissues were dissected on dry ice for qRT-PCR assays.
Prolonged cocaine self-administration
We also examined whether prolonged cocaine self-administration alters CB2 expression in the brain. Intravenous (i.v.) catheterization surgery and cocaine self-administration procedures were identical to those we reported previously [26]. Briefly, after 5–7 days of recovery from surgery, each mouse was placed into a standard operant chamber and allowed to lever press for i.v. cocaine self-administration (1 mg/kg/infusion) under an FR1 reinforcement schedule. After 7 days of initial cocaine self-administration, the cocaine dose was changed from 1 mg/kg/infusion to 0.5 mg/kg/infusion with maximal 50 infusions for additional 3 weeks until stable self-administration was established. Stable self-administration was defined as: (1) at least 20 infusions per 3-h session; (2) less than 20% variability in daily cocaine infusions across two consecutive sessions; and (3) higher than 2:1 active/inactive lever press ratio [26, 39]. Methohexital sodium (Brevital) (an extremely short-acting barbiturate) was used to verify the patency of i.v. catheters during the cocaine self-administration experiment when necessary. At 24 h after the last cocaine self-administration session, animals were transcardially perfused with saline and the whole brain was either removed for RNAscope ISH assays or PFC and striatum were dissected for qRT-PCR.
Oral sucrose self-administration
To determine whether the changes in brain CB2 mRNA expression were cocaine-specific, we also examined brain CB2 mRNA expression in mice after the same duration of oral sucrose self-administration. The procedure for oral sucrose self-administration was identical to the comparable procedure for cocaine except, 1) no surgery was performed on the animals in the sucrose experiment; and 2) active lever press led to the delivery of 0.02 mL of 5% sucrose solution into a liquid food tray on the operant chamber wall. At 24 h after the last session of sucrose self-administration, animals were sacrificed for qRT-PCR and RNAscope ISH assays.
RNAscope in situ hybridization assay
To determine which type of cells in the striatum displayed CB2R upregulation, we used RNAscope ISH assays to examine CB2 mRNA expression in the striatum in naïve WT mice. After brain perfusion, whole brains were taken out and frozen on dry ice. The fresh frozen tissue sections (12 µm thick) were mounted on positively charged microscopic glass slides (Fisherbrand Superfrost Plus; Fisher Scientific, Pittsburgh, PA). A mouse CB2-specific RNA probe (RNAscope probe: Mm-Cnr2-O2, cat# 436091) that targets coding DNA sequence (291–719 bp) of the Mus Cnr2 mRNA sequence (NM_009924.3) (Fig. 4a) was designed and provided by the manufacturer (Advanced Cell Diagnostics division of Bio-Techne Corporation, Minneapolis, MN, USA) and well evaluated in a previous study [40]. RNAscope probes used to label microglia (Mm-Itgam-C2, cat # 311491-C2, targeting CD11b mRNA), D1-MSNs (Mm-Drd1a-C2, cat # 406491-C2, targeting Drd1a mRNA), or D2-MSN (Mm-Drd2-C3, cat # 406501-C3, targeting Drd2 mRNA) were provided by the manufacturer (Advanced Cell Diagnostics division of Bio-Techne Corporation, Minneapolis, MN, USA). RNAscope ISH assays for CB2 mRNA expression in each cell type were performed according to the manufacturer’s instructions [41]. Briefly, the slides were post-fixed at 4 °C for 15 min in pre-chilled 10% neutral buffered formalin (NBF, 23-245-684; Fisher Scientific International, Inc., Pittsburgh, PA, USA) and hybridized sequentially with target probes at 40 °C for 2 h, amplifier 1 at 40 °C for 30 min, amplifier 2 at 40 °C for 15 min, amplifier 3 at 40 °C for 40 min, amplifier 4 at 40 °C for 15 min, and counterstained with 4′,6-diamidino-2-phenylindole at room temperature for 10 s. Stained slides were coverslipped with fluorescent mounting medium (ProLong Gold Antifade Reagent P36930; Invitrogen division of Thermo Fisher Scientific Corporation, Waltham, MA, USA) and scanned into digital images using a Carl Zeiss microscope (Thornwood, NY, USA) at ×40 magnification using manufacturer-provided software. From each brain sample, three adjacent sections were stained using mouse CB2, ubiquitin C (UBC), and bacterial gene (dapB) probes, respectively. UBC was used as an endogenous positive control to assess RNA probe integrity. The dapB served as a negative control to assess background staining. The probe designs for UBC and dapB were as previously described [41]. ImageJ software (NIH) was used to quantify CB2 mRNA signals in each individual cell. Only cells with clear boundaries were subjected to quantification analysis.
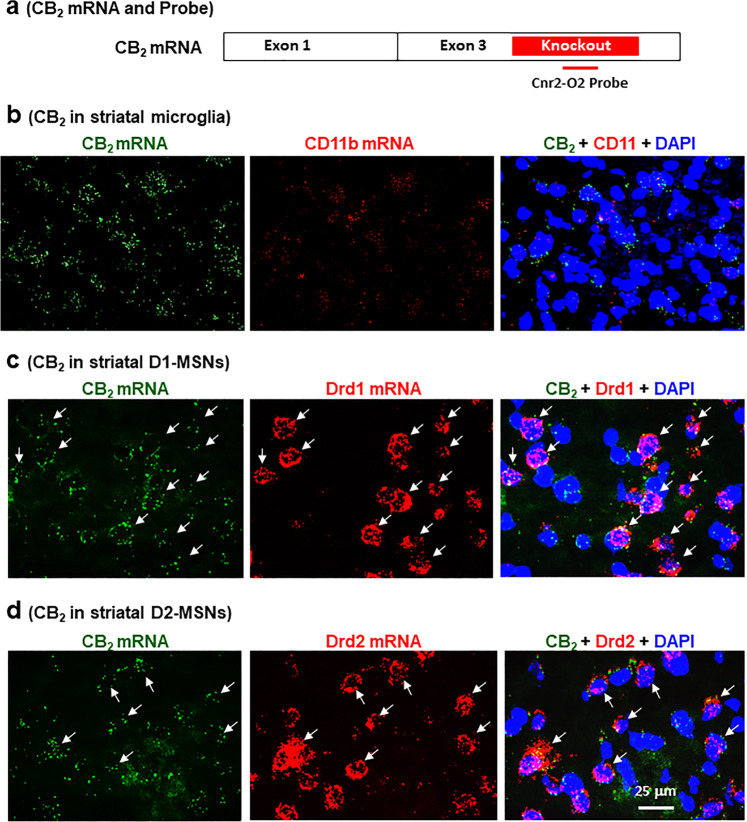
Fig. 4. Cellular distributions of CB2 mRNA in the NAc as assessed by RNAscope in situ hybridization.
a Diagram showing the transcript structure of the CB2A isoform and of another mCB2-specific probe (Cnr2-O2 probe) that targets the gene-deleted region in the CB2-KO mice. b CD11b mRNA is barely detectable in the NAc and no CB2 and CD11b co-localization was detected. CB2 mRNA was detected in Drd1-positive D1-MSNs (c) and Drd2-positive D2-MSNs (d).
Microdissection of nucleus accumbens and dorsal striatal tissues
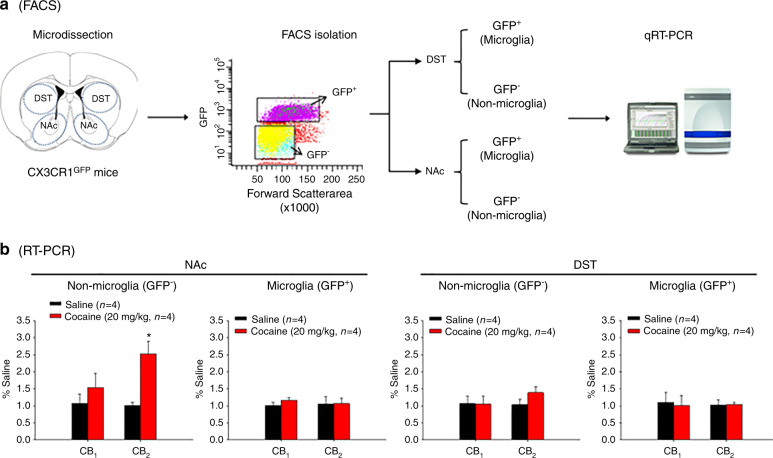
Heterozygous CX3CR1eGFP/+ reporter mice were used for the separation of microglia (GFP+) and non-microglia (GFP−) cell populations by FACS technologies. D1-Cre-RiboTag and D2-Cre-RiboTag mice were used to isolate ribosome-associated mRNAs from D1- or D2-MSNs. Before sacrificing, each phenotype of reporter mice was given either saline or cocaine (20 mg/kg, i.p., 7 days). At 24 h after the last injection, all mice were anesthetized with Euthasol (sodium pentobarbital 150 mg/kg and sodium phenytoin 19.2 mg/kg, Virbac) and perfused transcardially with 10 mL of oxygenated, ice-cold N-methyl-d-glucamine (NMDG)-based solution [42]. Brains were then rapidly dissected and coronal forebrain sections (300 μm thick) were prepared using a vibratome in ice-cold NMDG-based cutting solution bubbled continuously with 95% O2/5% CO2. NAc and DST were microdissected from striatal coronal sections (Bregma: 1.60–0.70 mm) using fine tipped forceps. Microdissected tissue was minced using a scalpel under the stereoscope before being transferred to Eppendorf tubes containing 1 mL Hibernate A solution (Brain Bits) stored on ice.
Microglia separation by FACS
Microdissected striatal tissues from WT and CX3CR1eGFP/+ mice were gently dissociated in Hibernate A solution using sequential trituration with fire-polished glass pipettes with openings of decreasing diameter (final pipette ~ 0.4 mm diameter opening). Resulting cell suspensions were spun down, re-suspended in 300 μL 1× PBS and filtered through a 40 µm mesh filter. Throughout the experiment, samples were kept at 4 °C on ice. Samples were sorted using a FACS Aria I cell sorter (BD Biosciences). The population of cells could be readily identified based on forward scattering (FSC) and side scattering (SSC) properties. A gating strategy based on FSC and SSC width and height was used to select only single cells. Microglial cells and non-microglia cells were then identified and sorted according to GFP expression. Cell suspensions from WT tissue were used as a GFP-negative control for the establishment of GFP-negative gates. GFP-positive and GFP-negative cells were collected from DST and NAc of CX3CR1eGFP/+ mice. The average collecting cells for each group are around 1000 [40, 42].
RNA extraction
RNA extraction from dissected tissues from WT mice
Total RNA was extracted using a RNeasy Mini kit (QIAGEN, Valencia, CA, USA), according to the kit instructions. Briefly, total less than 30 mg of brain tissue was disrupted and homogenized in 600 μL RLT buffer with 6 μL β-mercaptoethanol, and then centrifuged at 12,000 r/min for 10 min at room temperature. Following centrifugation, the supernatant was transferred to a new microcentrifuge tube, and then an equal amount of 70% ethanol was added and mixed by pipetting. The mixed sample was transferred to a RNeasy spin column and centrifuged for 30 s at 12,000 r/min. The RNA bound on the column membrane was washed out with RW1 buffer and RPE buffer. The RNA was then dissolved in 40 μL diethylpyrocarbonate-treated water. Purity and integrity of each extract were determined by absorbance at 260 nm using the Eppendorf BioPhotometer Plus (Eppendorf AG, Hamburg, Germany). The RNA was kept at −80 °C until assay.
RNA extraction from sorted microglial cell
Total RNA from sorted cells was isolated using the PicoPure RNA isolation kit (Arcturus Bioscience). All cells were sorted by FACS directly into Eppendorf tubes containing 50 µL PicoPure RNA extraction buffer and, following the sort, samples were incubated at 42 °C for 30 min and stored in RNase-free tubes at −80 °C until further processing. Column filtration, washing, and elution of RNA from the columns were performed according to the manufacturer’s instructions in section C of the PicoPure RNA isolation protocol. RNA samples were quantitated using the Agilent Bioanalyzer High Sensitivity kit (Agilent, Santa Clara, CA).
RNA extraction from RiboTag mice
NAc samples were pooled from four male and female D1-Cre-RiboTag and D2-Cre-RiboTag mice and RNA was isolated from immunoprecipitated polyribosomes using primary mouse anti-HA antibody (Covance Research Products, Cat# MMS101R) and secondary antibody coated magnetic Dynabeads (Dynabeads protein G, Invitrogen) to pulldown the MSN-specific RNA. Immunoprecipitated polyribosomes and non-immunoprecipitated input were prepared according to our previous studies [36, 37]. RNA from polyribosome immunoprecipitated samples or input was subsequently extracted using the Total RNA Kit (Omega) according to the manufacturer’s instructions.
cDNA synthesis and qRT-PCR assays
Single strand cDNAs were synthesized with Superscript III first strand cDNA synthesis kit (Invitrogen, Life Technologies), according to the manufacturer’s protocol. Procedures for qRT-PCR for detection of mCB2 mRNA were the same as we reported previously [10, 16]. In brief, a mCB2A probe and specific mCB2-KO Taqman probe (CB2-KO probe) were used, which targets the upstream sequence of intact gene or gene-deleted region in the exon 3 of mCB2 gene, respectively (Fig. 1a). Mouse glyceraldehyde 3-phosphate dehydrogenase (GAPDH)-mRNA was used as an endogenous control. All Taqman probe and primers were purchased from Applied Biosystems (Foster City, CA, USA). The qRT-PCR reactions were performed in a 7500 Fast qRT-PCR system using the program: 95 °C hold for 20 s followed by 40 cycles of 95 °C denaturation for 3 s and 60 °C annealing and extension for 30 s. qRT-PCR analyses of CB2-mRNA levels were performed using the 2−ΔΔCt method [43]. In the present study, data are presented as the fold change in mCB2 gene expression normalized first to the internal GAPDH gene or S18 gene and then relative to each vehicle group in each sample. The cycle threshold (Ct) was defined as the number of cycles required for the fluorescent signal to cross the threshold (i.e., exceeds background level). ΔCt was determined as [mean of the triplicate Ct values for the mCB2 gene] − [mean of the triplicate Ct values for GAPDH or S18]. ΔΔCt represented the difference between the paired tissue samples, as calculated by the formula ΔΔCt = [ΔCt of mCB2 in treated group tissue − ΔCt of mCB2 in vehicle group tissue]. The N-fold differential expression of the mCB2 gene in treated group tissues compared with vehicle group tissue was expressed as 2−ΔΔCt [10, 16, 43].
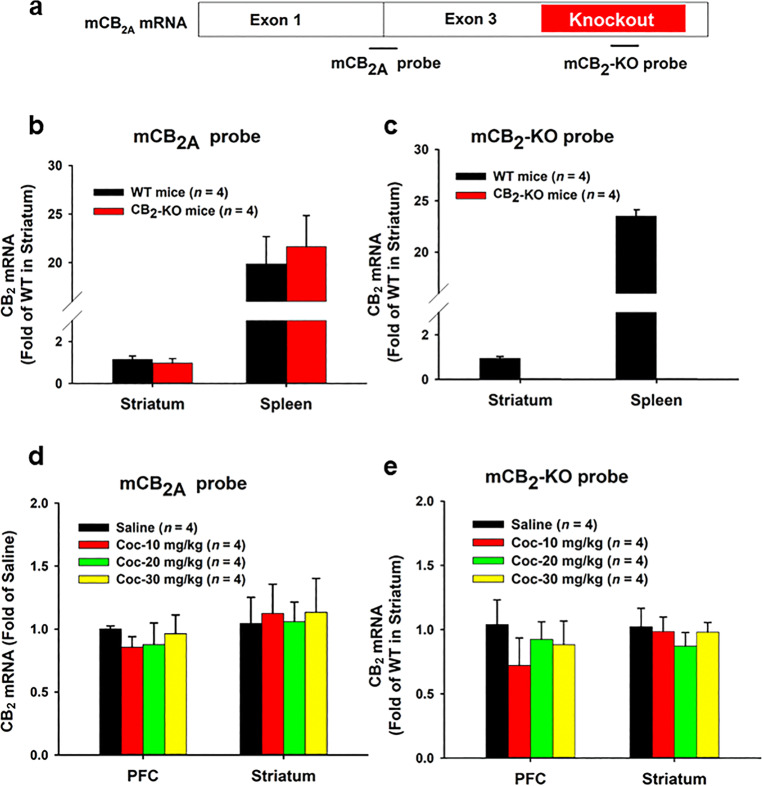
Fig. 1. Effects of acute cocaine administration on CB2 mRNA expression in the brain and spleen.
a A diagram showing the transcript structure of CB2A isoform and two Taqman probes that target either the upstream region (mCB2A probe) or the gene-deleted region (mCB2-KO probe) in the CB2-KO mice used in the present study. b, c qRT-PCR results, indicating that both the mCB2A and mCB2-KO probes detected similar levels of CB2 mRNA in the striatum and spleen in WT mice. While the mCB2A probe also detected similar levels of CB2 mRNA in CB2-KO mice, the mCB2-KO probe detected CB2 mRNA only in WT, not in CB2-KO mice. d, e qRT-PCR results: indicating that a single injection of cocaine (10, 20, 30 mg/kg, i.p.) did not alter CB2 mRNA expression, measured by both the mCB2 probes, in either the PFC or striatum in WT mice as measured 1 h after acute cocaine injection.
qRT-PCR from sorted microglia cells
The PreAmplification-RT-PCR method was used for FACS sorted samples [44]. TaqMan PreAmp Master Mix Kit was used for cDNA preamplification (Cat# 4391128; Applied Biosystems, Life Technologies) using pooled primer mixes of 20× dilution of TaqMan Gene Expression Assay. cDNAs were pre-amplified in an ABI 9700 Thermal Cycler using the program: 95 °C hold for 10 min, 14 cycles of 90 °C denaturation for 15 s, and 60 °C annealing and extension for 4 min. Pre-amplification PCR products were immediately diluted five times with molecular biology grade water and stored at −20 °C or immediately processed for RT-PCR. Duplex RT-PCR assays were performed on technical duplicates using a FAM-labeled probe for each target gene and a Vic-labeled probe for the endogenous control gene (gapdh) by using TaqMan Advanced Fast PCR Master Mix (Cat# 4444963; Life Technologies). RT-PCR reactions were run in a 7500 Fast qRT-PCR system using the program: 95 °C hold for 20 s followed by 40 cycles of 95 °C denaturation for 3 s and 60 °C annealing and extension for 30 s. qRT-PCR analyses of CB2-mRNA levels were also performed using the 2−ΔΔCt method as described above.
qRT-PCR from D1 or D2-MSNs
For cDNA synthesis, qRT-PCR and analysis we follow the steps as described previously [36, 37]. The list of primers used in this study is CB2-forward primer 5′-AGCTCGGATGCGGCTAGAC-3′, CB2-reverse 5′-AGGCTGTGGCCCATGAGA-3′ [10], Gapdh-forward 5′-AGGTCGGTGTGAACGGATTTG-3′ and Gapdh-Reverse 5′-TGTAGACCATGTAGTTGAGGTCA-3′ [36, 37]. The template cDNA sequence of CB2Rs was obtained from GenBank (ncbi.nlm.nih.gov/). Samples were run blinded to the condition. mRNA expression changes were measured using quantitative polymerase chain reaction with PerfeCTa SYBR Green FastMix (Quanta). Quantification of mRNA changes was performed using the −ΔΔCt method, using GAPDH as a housekeeping gene and immunoprecipitated samples were then normalized to the averaged input from the respective MSN subtype.
Data analysis
All the RT-PCR data were presented as means (±SEM). One-way analysis of variance (ANOVA) was used to analyze the difference in fold change of CB1 and CB2 mRNA among different groups. Individual group comparisons were carried out using the Student–Newman–Keuls method.
Results
Acute cocaine administration failed to alter CB2 mRNA expression
To explore whether cocaine exposure induces dynamic changes in CB2R expression, we designed two probes—one targets an exon junction of the CB2R gene (mCB2A probe) and another one targets the portion of exon 3 that is disrupted in CB2R-knockout mice (mCB2-KO probe) (Fig. 1a). In qRT-PCR analysis in WT mice, both mCB2A and mCB2-KO probes detected similar levels of CB2 mRNA signal in the striatum and spleen (Fig. 1b, c). Consistent with our previous findings [10, 16], we found that CB2 mRNA level is much higher (20–25-fold) in spleen than in striatum. In contrast, the mCB2-KO probe detected CB2 mRNA signal only in WT, not in CB2-KO mice, indicating that the detected signal is highly specific to the CB2R gene. We then used both probes to detect cocaine-induced changes in CB2 expression. We found that a single injection of cocaine at varying doses (10, 20, 30 mg/kg, i.p.) had no effect on CB2 mRNA expression in either the striatum or PFC at 1 h (Fig. 1d, e) or 3 h (data not shown) post injection.
Repeated cocaine administration increases CB2 mRNA expression in striatum and spleen
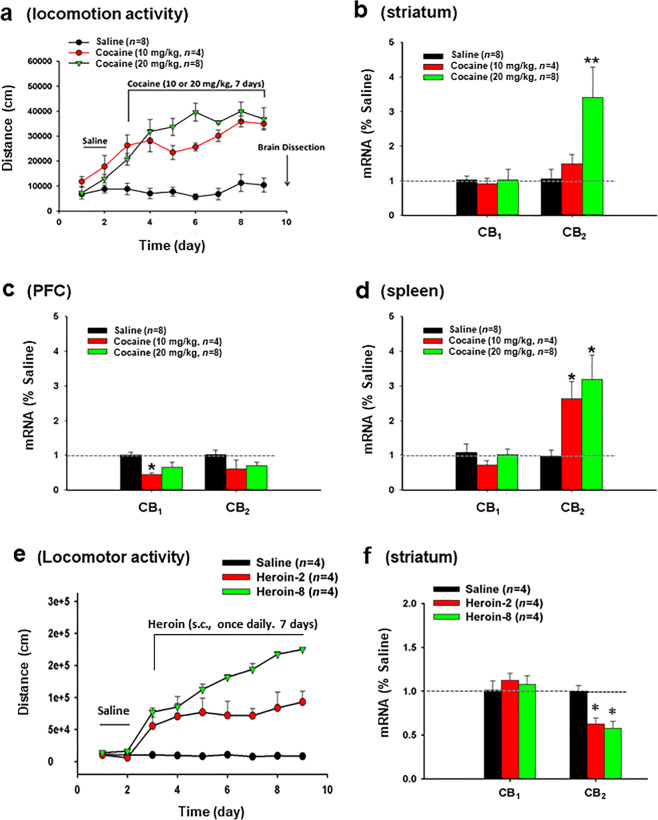
We then assessed whether repeated daily injections of cocaine similarly alter brain CB2 mRNA expression. Repeated injections of cocaine (10 or 20 mg/kg, i.p.) for 7 consecutive days produced robust locomotor sensitization (Fig. 2a), indicating circuit and behavior-level changes in response to drug exposure. At 24 h after the last cocaine injection, we measured CB1 and CB2 mRNA levels in striatum (Fig. 2b), PFC (Fig. 2c), and spleen (Fig. 2d) via qRT-PCR. We found that CB2 mRNA, as detected by mCB2-KO probe, was significantly increased in both the striatum (Fig. 2b, F2,11 = 7.03, P < 0.05) and spleen (Fig. 2d, F2,11 = 6.96, P < 0.05) in WT mice after repeated cocaine administration. In contrast, there was no change in CB2 mRNA expression in the PFC (Fig. 2c, F2,11 = 2.27, P > 0.05). Repeated cocaine injections also failed to significantly alter CB1 mRNA expression (Fig. 2b, d), with the exception of a small reduction in CB1 mRNA in the PFC following 10 mg/kg cocaine injections (Fig. 2c), suggesting that chronic cocaine treatment selectively upregulates CB2 mRNA expression in both the brain and periphery.
Fig. 2. Effects of repeated cocaine administration on CB1 and CB2 mRNA expression as measured by qRT-PCR.
a Repeated cocaine administration produced robust locomotor sensitization at both 10 and 20 mg/kg doses. b Cocaine, at 20 mg/kg only, significantly increased CB2 mRNA in the striatum as measured with the mCB2-KO probe; c cocaine, at 10 or 20 mg/kg, failed to alter CB2 mRNA expression in the PFC. d Cocaine, at both doses, significantly increased CB2 mRNA expression in spleen as measured at 24 h after the last cocaine injection. In contrast, repeated cocaine failed to alter CB1 mRNA expression except that cocaine, at 10 mg/kg, tended to decrease CB1 mRNA expression in the PFC (c). e Repeated heroin administration also produced robust locomotor sensitization in a dose-dependent manner. f Repeated heroin-treated mice displayed a reduction in CB2 mRNA expression in the striatum. *P < 0.05, compared to saline group.
Repeated heroin administration downregulates CB2 mRNA expression in striatum
To determine whether this effect can be generalized to other drugs of abuse, we also observed the effects of repeated heroin exposure on CB2 mRNA expression in the striatum. Figure 2e, f shows that repeated administration of heroin (2, 8 mg/kg, s.c., once daily for 7 days) produced robust locomotor sensitization in mice in a dose-dependent manner. Unexpectedly, a significant downregulation of CB2 mRNA expression was detected in the striatum as assessed by quantitative RT-PCR when measured at 24 h after the last heroin administration (Fig. 2f, F2,11 = 4.88, P < 0.05).
Cocaine self-administration upregulates CB2 mRNA expression
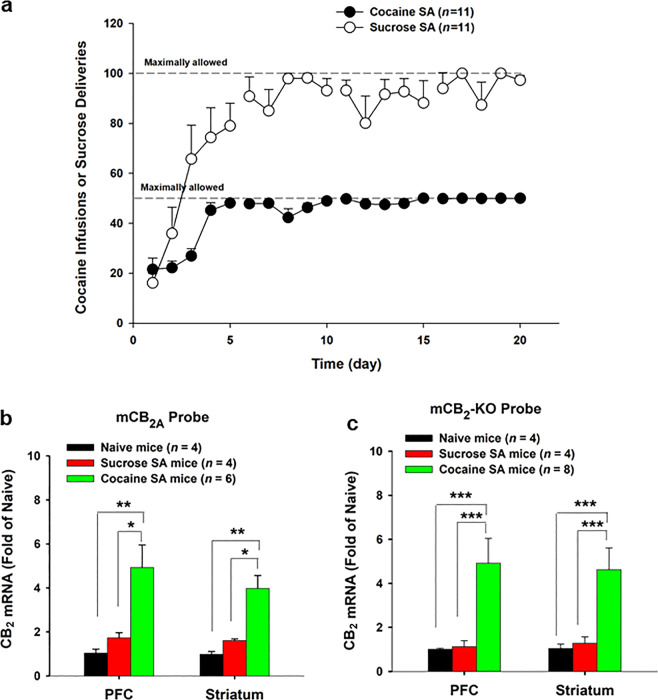
Molecular and circuit level changes following drug exposure can exhibit key differences depending on whether the drug is investigator administered or voluntarily consumed [45]. For this reason, we then examined whether chronic cocaine self-administration also upregulates CB2 mRNA expression in the brain. We compared these results of dynamic changes in CB2R expression following self-administration of palatable food (sucrose solution). Figure 3a shows the time courses of i.v. cocaine or oral sucrose self-administration; the majority of the animals reached the maximally allowed 50 cocaine infusions or 100 sucrose deliveries per session after ~1 week of self-administration training. At 24 h after the last cocaine self-administration, qRT-PCR analysis showed that CB2 mRNA levels were significantly increased in both the striatum and PFC (Fig. 3b, c) when compared with drug naïve mice or sucrose self-administration mice. One-way ANOVA revealed a statistically significant effect (Fig. 3b: PFC, F2,11 = 8.98, P < 0.01; ST: F2,11 = 16.35, P < 0.001; Fig. 3c: PFC, F2,13 = 7.14, P < 0.01; ST: F2,13 = 6.24, P < 0.05). In contrast, oral sucrose self-administration failed to alter brain CB2 mRNA expression when compared with drug naïve mice (P > 0.05).
Fig. 3. Effects of cocaine self-administration on CB1 and CB2 mRNA expression (by qRT-PCR).
a Mean numbers of cocaine self-administration (infusions) or oral sucrose deliveries during the 4 weeks of self-administration training; cocaine, but not sucrose, self-administration significantly upregulated CB2 mRNA expression in both the PFC and striatum in WT mice measured with the mCB2A (b) and mCB2-KO (c) probes, respectively. *P < 0.05, **P < 0.01, ***P < 0.001, compared to drug naïve mice or sucrose self-administration mice.
Cellular distributions of CB2 mRNA in the striatum
To determine which type of cells displays CB2 upregulation after cocaine exposure, we then used RNAscope ISH assays to examine CB2 mRNA expression in microglia, D1-MSNs, and D2-MSNs in the NAc. In this assay, we used another mouse CB2-specific RNA probe that targets the gene-deleted region in the CB2-KO mice that we used as control in this study (Fig. 4a). We detected low-to-moderate levels of CB2 mRNA in the NAc (Fig. 4b, left panel). However, when using a microglial-specific CD11b RNA probe, we failed to detect clear microglial cells (Fig. 4b, middle panel) nor co-localization of CB2 and CD11b (Fig, 4b, right panel). However, when using Drd1 or Drd2 RNA probes, we detected clear CB2 mRNA in ~50% Drd1-labled D1-MSNs (Fig. 4c) or Drd2-labeled D2-MSNs (Fig. 4d) in the NAc.
Repeated cocaine does not alter CB2 mRNA expression in purified microglia
Since CB2R-expressing D1-MSNs and D2-MSNs are mixedly distributed in the striatum, it is technically difficult for us to use RNAscope ISH methods to quantitatively measure CB2 mRNA density in each phenotype of cells. In addition, RNAscope ISH assays also failed to detect clear microglial cells in the NAc, which may be related to their extremely small cell sizes in normal healthy mice. Thus, to further determine which type of cells (neurons or microglia) in the NAc displays CB2 upregulation after cocaine exposure, we alternatively used three thansgenic mice and FACS technology to purify or isolate different phenotypes of cells. We first used CX3CR1eGFP/+ reporter mice to separate GFP+ microglia and GFP‒ non-microglial cells in the dorsal striatum (DST) and NAc (Fig. 5a). Surprisingly, repeated cocaine injection (20 mg/kg per day for 7 days) failed to alter CB2 mRNA expression in GFP+ microglia, but significantly increased CB2 mRNA expression in the GFP‒ non-microglial cell population (Fig. 5b, left panel, t = 2.24, P < 0.05) in the NAc. In contrast, no significant change in CB2 mRNA was observed in either GFP+ microglia or GFP‒ non-microglial cells in the DST after cocaine administration (Fig. 5b, right panels). Similarly, repeated cocaine administration did not alter CB1 mRNA expression in either cell population in the DST or NAc (Fig. 5b).
Fig. 5. Effects of repeated cocaine administration on microglial CB2 mRNA expression in the striatum.
a Flowchart of FACS and qRT-PCR techniques for separating GFP+ microglia and GFP− non-microglial cells from CX3CR1eGFP/+ mice. b qRT-PCR results, indicating that repeated cocaine administration (20 mg/kg, i.p. for 7 days) significantly increased CB2 mRNA expression in GFP− non-microglial cells, but not in GFP+ microglia, in the NAc. There was no change in CB2 mRNA expression in either cell population in the dorsal striatum (DST).
Repeated cocaine upregulates CB2 mRNA expression in D1-MSNs in the NAc
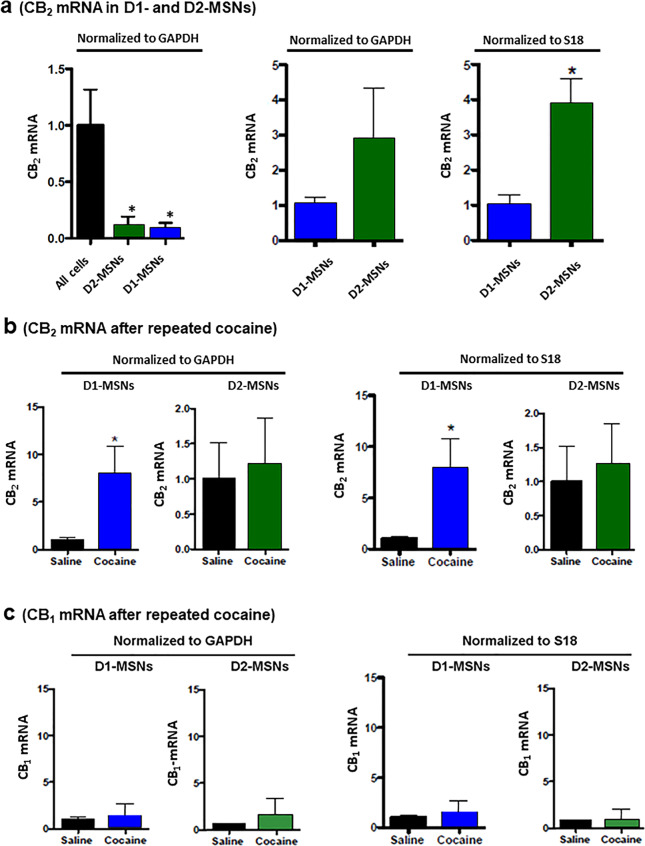
Given that cocaine upregulates CB2 mRNA expression in non-microglial cells within the NAc, we then sought to more clearly identify the cell population responsible for this dynamic change in CB2R expression. There are two major types of neurons in the striatum: D1- and D2-MSNs, which are differentially involved in motivated drug-taking and drug-seeking behavior [46, 47]. We then used Drd1a-Cre-RiboTag and Drd2-Cre-RiboTag mouse lines to isolate ribosome-associated RNAs from D1-MSNs and D2-MSNs, respectively. In drug naïve mice, CB2 mRNA levels in D1-MSNs or D2-MSNs were significantly lower than those in other RNA samples isolated from all the cells (Fig. 6a, left panel, F = 4.01, P < 0.05). However, when comparing D1- and D2-MSNs to one another, D2-MSNs displayed 3–4 folds higher basal levels of CB2 mRNA than D1-MSNs (Fig. 6a right two panels, t = 4.54, P < 0.05). Following repeated cocaine administration, D1-MSNs displayed significantly upregulated CB2 mRNA expression (Fig. 6b, right panel, t = 2.53, P < 0.05; left panel, t = 6.81, P < 0.05), while D2-MSNs did not (Fig. 6b, P < 0.05). CB1 mRNA expression was not altered by repeated cocaine administration in either D1-MSNs or D2-MSNs (Fig. 6c).
Fig. 6. Effects of repeated cocaine administration on CB2 mRNA expression in striatal D1-MSNs vs. D2-MSNs.
a qRT-PCR results, indicating the relative levels of CB2 mRNA in striatal D1-MSNs, D2-MSNs, and All (unsorted) cells, prepared by FACS from D1-Cre-RiboTag and D2-Cre-RiboTag mice. D2-MSNs displayed higher basal level of CB2 mRNA than D1-MSNs when normalized to the house-keep genes glyceraldehyde 3-phosphate dehydrogenase (GAPDH) or S18 protein; b repeated cocaine administration (20 mg/kg, i.p. for 7 days) selectively increased CB2 expression in D1-MSNs, not in D2-MSNs; c repeated cocaine administration failed to alter CB1R expression in the same samples. *P < 0.05, compared to “All cells” (a left panel), D1-MSNs (a right panel), or saline control mice (b).
Discussion
In this study, we used three mouse CB2R-specific probes and multiple (4) transgenic mouse lines to examine the impact of acute vs. repeated cocaine administration on brain CB2R expression. We found that a single injection of cocaine failed to alter CB2R expression, but repeated cocaine administration or self-administration caused a significant increase in CB2 mRNA expression in the striatum and spleen tissues. Unexpectedly, this CB2 upregulation does not occur in microglial cells, but occurs mainly in D1-MSNs in the NAc. In a series of parallel studies, we did not observe significant changes in CB1 mRNA expression. These findings suggest that neuronal CB2Rs are inducible and responsive to recurring cocaine use and abuse. The present finding that NAc CB2 was upregulated in D1-MSNs, combined with our previous finding that CB2 was upregulated in VTA DA neurons [11], at least in part explain how CB2R agonists attenuate cocaine self-administration and other addiction-related behaviors in rats and mice [25, 26, 48].
The expression of functional CB2Rs in the brain, particularly in neurons, has been the subject of debate for many years [7, 49]. Concerns have been raised regarding the CB2R signal specificity when using antibodies to detect brain CB2R by immunostaining. Therefore, in the present study, we chose to detect CB2 gene expression using three custom-designed mouse CB2-specific probes (mCB2A, mCB2-KO, Cnr2-O2) based on the CB2 gene structures in WT and CB2-KO mice, combined with the use of CB2-rich spleen tissues and CB2-KO mice as positive and negative controls. These probes have been confirmed to be mouse CB2-specific as assessed by the findings in CB2-KO mice in the present study (Fig. 1) and also in our previous studies [9, 10, 40]. Using qRT-PCR assays, we detected low levels of CB2 mRNA expression in the PFC and striatum in healthy, drug naïve mice.
An important finding in the present study was that a single injection of cocaine at a wide range of doses (10, 20, 30 mg/kg) failed to alter brain CB2 mRNA expression when measured at 1 or 3 h after cocaine injection. In contrast, repeated administration of high doses of cocaine (10, 20 mg/kg) for 7 consecutive days or cocaine self-administration (for 4 weeks) significantly upregulated CB2 mRNA expression in the striatum and the spleen. In addition, cocaine self-administration, but not repeated cocaine exposure for 7 days, also upregulated CB2 mRNA expression in the PFC, suggesting that prolonged cocaine exposure is required to alter CB2 expression in the cortex. It may also suggest that voluntary consumption of cocaine or the associative learning associated with self-administration is necessary to induce CB2 upregulation in the PFC. These findings are consistent with, but expand, previous findings that brain CB2Rs can be upregulated under chronic neuroinflammation as stated above.
Another important finding is that peripheral CB2R expression appears more sensitive to cocaine exposure than that in the brain since recurrent exposure to 10 or 20 mg/kg cocaine upregulates CB2 mRNA expression in spleen, while 20 mg/kg cocaine is required to upregulate CB2 expression in the brain. Cocaine is well known to suppress immune function and cause changes in phagocytic activity of macrophages and production of immunoregulatory cytokines [50–53] and cannabinoids are known to have anti-inflammatory and immunomodulatory properties by activation of CB2Rs [54, 55]. Thus, the present finding suggests that chronic exposure to cocaine may produce immunosuppressive effects by upregulation of CB2R expression in peripheral immune cells.
An unexpected finding is that repeated administration of opioids (heroin) produced an opposite reduction in NAc CB2 mRNA expression. This is consistent with a recent report indicating that repeated morphine administration caused a significant reduction in CB2, not CB1, mRNA expression in VTA [56], but is not fully consistent with an early report that repeated administration of morphine produces conditioned place preference, which is associated with a significant reduction in CB2 mRNA expression in brain stem and a significant increase in CB2 mRNA expression in the cerebral cortex and peripheral spleen and blood immune cells in rats [57]. These findings suggest that chronic administration of opioids may also differentially alter CB2 mRNA expression in different tissues or brain regions. Given that activation of CB2Rs is protective against various insults in the brain and periphery [6, 13, 58], the present finding suggests that CB2R upregulation may be similarly protective against chronic drug-induced changes or damage in the brain and periphery.
Another unexpected finding is that recurring cocaine use did not alter microglial CB2 mRNA expression. This finding is conflicted with a well-accepted view that brain CB2 upregulation occurs mainly or exclusively in microglial cells during chronic neuroinflammation [13]. This conclusion is largely based on early antibody-based findings that CB2-like immunostaining was found in neuritic plaque-associated astrocytes and microglia in hippocampus and entorhinal cortex in post-mortem brain tissues from patients with severe Alzeimor’s Disease [59], in CD3- or CD68-positive perivascular (microglial) cells in non-human primates with simian immunodeficiency virus-induced encephalitis [60], or in activated microglia and astrocytes in the spinal cord of mice with chronic neuropathic pain [61]. This is supported by the finding that CB2R-dependent-eGFP signal was also detected in microglia in areas of intense inflammation and amyloid deposition in the brain in transgenic CB2-Cre-reporter (CB2EGFP/f/f) mice co-expressing five familial Alzheimer’s disease mutations (5XFAD) [62]. However, there are two major concerns in these studies. First, it is unknown whether those CB2 antibodies are CB2-specific, and second, the CB2-like immunostaining was seen not only in microglia, but also in astrocytes [63]. In contrast to these findings, we have previously reported that cocaine self-administration upregulates CB2R expression in VTA DA neurons, not in glial cells [11]. Systemic administration of lipopolysaccharide (LPS), a bacterial endotoxin, caused significant microglial and astrocytic proliferation in the VTA, but failed to cause CB2R upregulation in microglia or astrocytes [10]. Together, these findings suggest that CB2R upregulation may occur in different phenotypes of cells, including microglia, astrocytes, or neurons depending upon different experimental or pathological conditions, which strongly argue the current prevailing view (dogma) that brain CB2R upregulation occurs mainly or exclusively in microglia.
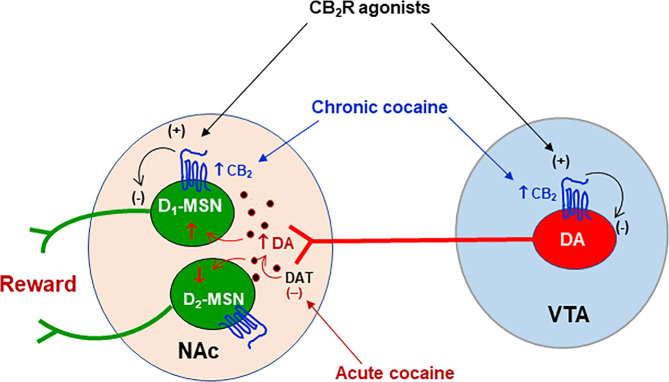
Perhaps the most important finding in this study is the discovery of CB2R upregulation in NAc D1-MSNs in mice after recurring cocaine use. Interestingly, D1-MSNs displayed lower basal level of CB2 mRNA expression than D2-MSNs, but displayed a higher CB2R response to repeated cocaine in the NAc, rather than in the DST. These findings suggest that neuronal CB2R upregulation in the striatum displays subregional- and cell type-specific profiles. As mentioned above, growing evidence indicates that brain CB2Rs modulate pharmacological action produced by cocaine [9, 10, 25, 26, 48], morphine [64, 65], nicotine [28], and alcohol [66] in a number of addiction- and pain-related behavioral models. However, the neural mechanisms underlying this CB2R-mediated action are poorly understood. First, it is unlikely that microglia CB2R are involved since there is little evidence supporting CB2 expression in microglia in normal healthy or in LPS-treated mice [10] or microglial CB2 upregulation in rats with cocaine self-administration history [11]. Second, as stated above, cocaine self-administration upregulates CB2 gene expression in VTA DA neurons [11], suggesting that a presynaptic CB2R mechanism in the mesolimbic DA system may be involved (Fig. 7). Lastly, in the present report, we found that chronic cocaine administration also upregulates CB2R expression in NAc D1-MSNs, suggesting that a postsynaptic CB2R mechanism may also play an important role in mediating CB2R modulation of cocaine action (Fig. 7).
Fig. 7. Schematic diagram showing the effects of cocaine on CB2R expression in the mesolimbic DA reward system.
This system originates from DA neurons in the VTA and projects to the NAc. Acute cocaine exposure blocks DA transporter (DAT) and elevates extracellular DA level in the NAc, which subsequently activates D1-MSNs via Gs-coupled D1Rs and inhibits D2-MSNs via Gi-coupled D2Rs, producing rewarding effects. Chronic cocaine administration increases CB2R expression in VTA DA neurons as we reported previously [11] and also in NAc D1-MSNs as shown in the present study. Accordingly, activation of CB2Rs by CB2R agonists would inhibit presynaptic VTA DA neurons and postsynaptic D1-MSNs in the NAc, producing therapeutic anti-cocaine effects. VTA ventral tegmental area, NAc nucleus accumbens, (+) activation, (−) inhibition.
It is unknown precisely how CB2Rs in D1-MSNs modulate cocaine action. Recent optogenetic studies indicate that activation of D1-MSNs in the NAc is critically associated with positive reinforcement, while activation of D2-MSNs are mostly associated with aversion [67, 68], although not all evidence supports this dichotomy [69, 70]. Accordingly, it is hypothesized that the acute rewarding effects of cocaine are most likely mediated by activation of D1-MSNs via Gs-coupled D1 receptors and inhibition of D2-MSNs via Gi-coupled D2 receptors [67, 71, 72] (Fig. 7). Since CB2Rs are Gi-coupled receptors, we further hypothesized that CB2R upregulation in VTA DA neurons or NAc D1-MSNs would increase neuronal response to endocannabinoids or CB2R agonists, producing enhanced inhibitory effects on both presynaptic DA neurons and postsynaptic D1-MSNs, which subsequently counteracts cocaine-induced increase in extracellular DA and cocaine’s action in postsynaptic D1-MSNs (Fig. 7). Thus, the present finding of CB2 upregulation in striatal D1-MSNs provides new mechanistic insight on how brain CB2Rs modulate pharmacological action produced not only by cocaine, but also by other drugs of abuse, such as opioids, nicotine and alcohol.
In conclusion, brain CB2R involvement has been recently reported in several DA-regulated CNS disorders, including substance abuse and addiction. The present findings of neuronal CB2R upregulation in NAc D1-MSNs not only challenges the well-accepted view that CB2R upregulation is mainly or exclusively expressed in activated microglia, but also provide additional evidence indicating that chronic cocaine use and abuse may upregulate CB2R expression in brain neurons and peripheral immune cells. These new findings suggest that CB2R upregulation may represent a general defense or protective mechanism in response to various insults including chronic psychostimulant abuse and addiction. Thus, brain CB2Rs may constitute a new therapeutic target in medication development for the treatment of cocaine use disorders as well as many other CNS disorders.
Supplementary information
Acknowledgements
This research was supported by the Intramural Research Program (IRP) of the National Institute on Drug Abuse (NIDA) (DA000633-01), National Institutes of Health (NIH), and grant support for MKL in UMB (NIH grant R01DA038613).
Author contributions
HYZ, ELG, MKL, and ZXX designed the experiments. HYZ, LDB, RC, HS, QRL, and MKL conducted the experiments. HYZ, LDB, RC, HS, MKL, and ZXX performed data analyses. HYZ and ZXX wrote the manuscript. LDB, ELG, and MKL revised the manuscript. All authors have approved the final version of this article.
Competing interests
The authors declare no competing interests.
Contributor Information
Hai-Ying Zhang, Email: haiying.zhang@nih.gov.
Zheng-Xiong Xi, Email: zxi@intra.nida.nih.gov.
Supplementary information
The online version contains supplementary material available at 10.1038/s41401-021-00712-6.
References
- 1.Fattore L, Spano MS, Deiana S, Melis V, Cossu G, Fadda P, et al. An endocannabinoid mechanism in relapse to drug seeking: a review of animal studies and clinical perspectives. Brain Res Rev. 2007;53:1–16. doi: 10.1016/j.brainresrev.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 2.Lupica CR, Riegel AC, Hoffman AF. Marijuana and cannabinoid regulation of brain reward circuits. Br J Pharmacol. 2004;143:227–34. doi: 10.1038/sj.bjp.0705931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maldonado R, Valverde O, Berrendero F. Involvement of the endocannabinoid system in drug addiction. Trends Neurosci. 2006;29:225–32. doi: 10.1016/j.tins.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 4.Parolaro D, Rubino T. The role of the endogenous cannabinoid system in drug addiction. Drug Dews Perspect. 2008;21:149–57. [PubMed] [Google Scholar]
- 5.Tanda G, Goldberg SR. Cannabinoids: reward, dependence, and underlying neurochemical mechanisms–a review of recent preclinical data. Psychopharmacology. 2003;169:115–34. doi: 10.1007/s00213-003-1485-z. [DOI] [PubMed] [Google Scholar]
- 6.Mechoulam R, Parker LA. The endocannabinoid system and the brain. Annu Rev Psychol. 2013;64:21–47. doi: 10.1146/annurev-psych-113011-143739. [DOI] [PubMed] [Google Scholar]
- 7.Atwood BK, Mackie K. CB2: a cannabinoid receptor with an identity crisis. Br J Pharmacol. 2010;160:467–79. doi: 10.1111/j.1476-5381.2010.00729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jordan CJ, Xi ZX. Progress in brain cannabinoid CB2 receptor research: from genes to behavior. Neurosci Biobehav Rev. 2019;98:208–20. doi: 10.1016/j.neubiorev.2018.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang HY, Bi GH, Li X, Li J, Qu H, Zhang SJ, et al. Species differences in cannabinoid receptor 2 and receptor responses to cocaine self-administration in mice and rats. Neuropsychopharmacology. 2015;40:1037–51. doi: 10.1038/npp.2014.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang HY, Gao M, Liu QR, Bi GH, Li X, Yang HJ, et al. Cannabinoid CB2 receptors modulate midbrain dopamine neuronal activity and dopamine-related behavior in mice. Proc Natl Acad Sci USA. 2014;111:E5007–15. doi: 10.1073/pnas.1413210111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang HY, Gao M, Shen H, Bi GH, Yang HJ, Liu QR, et al. Expression of functional cannabinoid CB2 receptor in VTA dopamine neurons in rats. Addiction Biol. 2017;22:752–65. doi: 10.1111/adb.12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang HY, Shen H, Jordan CJ, Liu QR, Gardner EL, Bonci A, et al. CB2 receptor antibody signal specificity: correlations with the use of partial CB2-knockout mice and anti-rat CB2 receptor antibodies. Acta Pharmacol Sin. 2019;40:398–409. [DOI] [PMC free article] [PubMed]
- 13.Benito C, Tolon RM, Pazos MR, Nunez E, Castillo AI, Romero J. Cannabinoid CB2 receptors in human brain inflammation. Br J Pharmacol. 2008;153:277–85. doi: 10.1038/sj.bjp.0707505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gong JP, Onaivi ES, Ishiguro H, Liu QR, Tagliaferro PA, Brusco A, et al. Cannabinoid CB2 receptors: immunohistochemical localization in rat brain. Brain Res. 2006;1071:10–23. doi: 10.1016/j.brainres.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 15.Lanciego JL, Barroso-Chinea P, Rico AJ, Conte-Perales L, Callen L, Roda E, et al. Expression of the mRNA coding the cannabinoid receptor 2 in the pallidal complex of Macaca fascicularis. J Psychopharmacol. 2011;25:97–104. doi: 10.1177/0269881110367732. [DOI] [PubMed] [Google Scholar]
- 16.Liu QR, Pan CH, Hishimoto A, Li CY, Xi ZX, Llorente-Berzal A, et al. Species differences in cannabinoid receptor 2 (CNR2 gene): identification of novel human and rodent CB2 isoforms, differential tissue expression and regulation by cannabinoid receptor ligands. Genes Brain Behav. 2009;8:519–30. doi: 10.1111/j.1601-183X.2009.00498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, et al. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005;310:329–32. doi: 10.1126/science.1115740. [DOI] [PubMed] [Google Scholar]
- 18.Garcia C, Palomo-Garo C, Garcia-Arencibia M, Ramos J, Pertwee R, Fernandez-Ruiz J. Symptom-relieving and neuroprotective effects of the phytocannabinoid Delta(9)-THCV in animal models of Parkinson’s disease. Br J Pharmacol. 2011;163:1495–506. doi: 10.1111/j.1476-5381.2011.01278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Price DA, Martinez AA, Seillier A, Koek W, Acosta Y, Fernandez E, et al. WIN55,212-2, a cannabinoid receptor agonist, protects against nigrostriatal cell loss in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease. Eur J Neurosci. 2009;29:2177–86. doi: 10.1111/j.1460-9568.2009.06764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ortega-Alvaro A, Aracil-Fernandez A, Garcia-Gutierrez MS, Navarrete F, Manzanares J. Deletion of CB2 cannabinoid receptor induces schizophrenia-related behaviors in mice. Neuropsychopharmacology. 2011;36:1489–504. doi: 10.1038/npp.2011.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia-Gutierrez MS, Manzanares J. Overexpression of CB2 cannabinoid receptors decreased vulnerability to anxiety and impaired anxiolytic action of alprazolam in mice. J Psychopharmacol. 2011;25:111–20. doi: 10.1177/0269881110379507. [DOI] [PubMed] [Google Scholar]
- 22.Garcia-Gutierrez MS, Perez-Ortiz JM, Gutierrez-Adan A, Manzanares J. Depression-resistant endophenotype in mice overexpressing cannabinoid CB2 receptors. Br J Pharmacol. 2010;160:1773–84. doi: 10.1111/j.1476-5381.2010.00819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palazuelos J, Aguado T, Pazos MR, Julien B, Carrasco C, Resel E, et al. Microglial CB2 cannabinoid receptors are neuroprotective in Huntington’s disease excitotoxicity. Brain. 2009;132:3152–64. doi: 10.1093/brain/awp239. [DOI] [PubMed] [Google Scholar]
- 24.Manzanares J, Cabanero D, Puente N, Garcia-Gutierrez MS, Grandes P, Maldonado R. Role of the endocannabinoid system in drug addiction. Biochem Pharmacol. 2018;157:108–21. doi: 10.1016/j.bcp.2018.09.013. [DOI] [PubMed] [Google Scholar]
- 25.Aracil-Fernandez A, Trigo JM, Garcia-Gutierrez MS, Ortega-Alvaro A, Ternianov A, Navarro D, et al. Decreased cocaine motor sensitization and self-administration in mice overexpressing cannabinoid CB2 receptors. Neuropsychopharmacology. 2012;37:1749–63. doi: 10.1038/npp.2012.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xi ZX, Peng XQ, Li X, Song R, Zhang HY, Liu QR, et al. Brain cannabinoid CB2 receptors modulate cocaine’s actions in mice. Nat Neurosci. 2011;14:1160–6. doi: 10.1038/nn.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adamczyk P, Miszkiel J, McCreary AC, Filip M, Papp M, Przegalinski E. The effects of cannabinoid CB1, CB2 and vanilloid TRPV1 receptor antagonists on cocaine addictive behavior in rats. Brain Res. 2012;1444:45–54. doi: 10.1016/j.brainres.2012.01.030. [DOI] [PubMed] [Google Scholar]
- 28.Ignatowska-Jankowska BM, Muldoon PP, Lichtman AH, Damaj MI. The cannabinoid CB2 receptor is necessary for nicotine-conditioned place preference, but not other behavioral effects of nicotine in mice. Psychopharmacology. 2013;229:591–601. doi: 10.1007/s00213-013-3117-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Canseco-Alba A, Schanz N, Sanabria B, Zhao J, Lin Z, Liu QR, et al. Behavioral effects of psychostimulants in mutant mice with cell-type specific deletion of CB2 cannabinoid receptors in dopamine neurons. Behav Brain Res. 2019;360:286–97. doi: 10.1016/j.bbr.2018.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu QR, Canseco-Alba A, Zhang HY, Tagliaferro P, Chung M, Dennis E, et al. Cannabinoid type 2 receptors in dopamine neurons inhibits psychomotor behaviors, alters anxiety, depression and alcohol preference. Sci Rep. 2017;7:17410. doi: 10.1038/s41598-017-17796-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Foster DJ, Wilson JM, Remke DH, Mahmood MS, Uddin MJ, Wess J, et al. Antipsychotic-like effects of M4 positive allosteric modulators are mediated by CB2 receptor-dependent inhibition of dopamine release. Neuron. 2016;91:1244–52. doi: 10.1016/j.neuron.2016.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bystrowska B, Frankowska M, Smaga I, Pomierny-Chamiolo L, Filip M. Effects of cocaine self-administration and its extinction on the rat brain cannabinoid CB1 and CB2 receptors. Neurotox Res. 2018;34:547–58. doi: 10.1007/s12640-018-9910-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buckley NE. The peripheral cannabinoid receptor knockout mice: an update. Br J Pharmacol. 2008;153:309–18. doi: 10.1038/sj.bjp.0707527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buckley NE, McCoy KL, Mezey E, Bonner T, Zimmer A, Felder CC, et al. Immunomodulation by cannabinoids is absent in mice deficient for the cannabinoid CB2 receptor. Eur J Pharmacol. 2000;396:141–9. doi: 10.1016/s0014-2999(00)00211-9. [DOI] [PubMed] [Google Scholar]
- 35.Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, et al. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol. 2000;20:4106–14. doi: 10.1128/mcb.20.11.4106-4114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chandra R, Engeln M, Francis TC, Konkalmatt P, Patel D, Lobo MK. A role for peroxisome proliferator-activated receptor gamma coactivator-1alpha in nucleus accumbens neuron subtypes in cocaine action. Biol Psychiatry. 2017;81:564–72. doi: 10.1016/j.biopsych.2016.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chandra R, Francis TC, Konkalmatt P, Amgalan A, Gancarz AM, Dietz DM, et al. Opposing role for Egr3 in nucleus accumbens cell subtypes in cocaine action. J Neurosci. 2015;35:7927–37. doi: 10.1523/JNEUROSCI.0548-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanz E, Yang L, Su T, Morris DR, McKnight GS, Amieux PS. Cell-type-specific isolation of ribosome-associated mRNA from complex tissues. Proc Natl Acad Sci USA. 2009;106:13939–44. doi: 10.1073/pnas.0907143106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song R, Zhang HY, Li X, Bi GH, Gardner EL, Xi ZX. Increased vulnerability to cocaine in mice lacking dopamine D3 receptors. Proc Natl Acad Sci USA. 2012;109:17675–80. doi: 10.1073/pnas.1205297109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stempel AV, Stumpf A, Zhang HY, Ozdogan T, Pannasch U, Theis AK, et al. Cannabinoid type 2 receptors mediate a cell type-specific plasticity in the hippocampus. Neuron. 2016;90:795–809. doi: 10.1016/j.neuron.2016.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang F, Flanagan J, Su N, Wang LC, Bui S, Nielson A, et al. RNAscope: a novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J Mol Diagnostics. 2012;14:22–9. doi: 10.1016/j.jmoldx.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Biase LM, Schuebel KE, Fusfeld ZH, Jair K, Hawes IA, Cimbro R, et al. Local cues establish and maintain region-specific phenotypes of basal ganglia microglia. Neuron. 2017;95:341–56 e6. doi: 10.1016/j.neuron.2017.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 44.Liu QR, Rubio FJ, Bossert JM, Marchant NJ, Fanous S, Hou X, et al. Detection of molecular alterations in methamphetamine-activated Fos-expressing neurons from a single rat dorsal striatum using fluorescence-activated cell sorting (FACS) J Neurochem. 2014;128:173–85. doi: 10.1111/jnc.12381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Venniro M, Caprioli D, Shaham Y. Animal models of drug relapse and craving: from drug priming-induced reinstatement to incubation of craving after voluntary abstinence. Prog Brain Res. 2016;224:25–52. doi: 10.1016/bs.pbr.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 46.DeLong MR, Wichmann T. Circuits and circuit disorders of the basal ganglia. Arch Neurol. 2007;64:20–4. doi: 10.1001/archneur.64.1.20. [DOI] [PubMed] [Google Scholar]
- 47.Graybiel AM. The basal ganglia. Curr Biol. 2000;10:R509–11. doi: 10.1016/s0960-9822(00)00593-5. [DOI] [PubMed] [Google Scholar]
- 48.Melis M, Frau R, Kalivas PW, Spencer S, Chioma V, Zamberletti E, et al. New vistas on cannabis use disorder. Neuropharmacology. 2017;124:62–72. doi: 10.1016/j.neuropharm.2017.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang HY, Shen H, Jordan CJ, Liu QR, Gardner EL, Bonci A, et al. CB2 receptor antibody signal specificity: correlations with the use of partial CB2-knockout mice and anti-rat CB2 receptor antibodies. Acta Pharm Sin. 2019;40:398–409. doi: 10.1038/s41401-018-0037-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pacifici R, Di Carlo S, Bacosi A, Zuccaro P. Macrophage functions in drugs of abuse-treated mice. Int J Immunopharmacol. 1993;15:711–6. doi: 10.1016/0192-0561(93)90143-m. [DOI] [PubMed] [Google Scholar]
- 51.Pacifici R, Fiaschi AI, Micheli L, Centini F, Giorgi G, Zuccaro P, et al. Immunosuppression and oxidative stress induced by acute and chronic exposure to cocaine in rat. Int Immunopharmacol. 2003;3:581–92. doi: 10.1016/S1567-5769(03)00050-X. [DOI] [PubMed] [Google Scholar]
- 52.Pellegrino T, Bayer BM. In vivo effects of cocaine on immune cell function. J Neuroimmunol. 1998;83:139–47. doi: 10.1016/s0165-5728(97)00230-0. [DOI] [PubMed] [Google Scholar]
- 53.Wang Y, Huang DS, Watson RR. In vivo and in vitro cocaine modulation on production of cytokines in C57BL/6 mice. Life Sci. 1994;54:401–11. doi: 10.1016/0024-3205(94)00698-9. [DOI] [PubMed] [Google Scholar]
- 54.Katchan V, David P, Shoenfeld Y. Cannabinoids and autoimmune diseases: a systematic review. Autoimmun Rev. 2016;15:513–28. doi: 10.1016/j.autrev.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 55.Robinson RH, Meissler JJ, Breslow-Deckman JM, Gaughan J, Adler MW, Eisenstein TK. Cannabinoids inhibit T-cells via cannabinoid receptor 2 in an in vitro assay for graft rejection, the mixed lymphocyte reaction. J Neuroimmune Pharmacol. 2013;8:1239–50. doi: 10.1007/s11481-013-9485-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang H, Lipinski AA, Liktor-Busa E, Smith AF, Moutal A, Khanna R, et al. The effects of repeated morphine treatment on the endogenous cannabinoid system in the ventral tegmental area. Front Pharmacol. 2021;12:632757. doi: 10.3389/fphar.2021.632757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang QY, Zhang M, Cao Y. Exposure to morphine affects the expression of endocannabinoid receptors and immune functions. J Neuroimmunol. 2012;247:52–8. doi: 10.1016/j.jneuroim.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 58.Pacher P, Mechoulam R. Is lipid signaling through cannabinoid 2 receptors part of a protective system? Prog Lipid Res. 2011;50:193–211. doi: 10.1016/j.plipres.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Benito C, Nunez E, Tolon RM, Carrier EJ, Rabano A, Hillard CJ, et al. Cannabinoid CB2 receptors and fatty acid amide hydrolase are selectively overexpressed in neuritic plaque-associated glia in Alzheimer’s disease brains. J Neurosci. 2003;23:11136–41. doi: 10.1523/JNEUROSCI.23-35-11136.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Benito C, Kim WK, Chavarria I, Hillard CJ, Mackie K, Tolon RM, et al. A glial endogenous cannabinoid system is upregulated in the brains of macaques with simian immunodeficiency virus-induced encephalitis. J Neurosci. 2005;25:2530–6. doi: 10.1523/JNEUROSCI.3923-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Beltramo M, Bernardini N, Bertorelli R, Campanella M, Nicolussi E, Fredduzzi S, et al. CB2 receptor-mediated antihyperalgesia: possible direct involvement of neural mechanisms. Eur J Neurosci. 2006;23:1530–8. doi: 10.1111/j.1460-9568.2006.04684.x. [DOI] [PubMed] [Google Scholar]
- 62.Lopez A, Aparicio N, Pazos MR, Grande MT, Barreda-Manso MA, Benito-Cuesta I, et al. Cannabinoid CB2 receptors in the mouse brain: relevance for Alzheimer’s disease. J Neuroinflammation. 2018;15:158. doi: 10.1186/s12974-018-1174-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Luongo L, Palazzo E, Tambaro S, Giordano C, Gatta L, Scafuro MA, et al. 1-(2’,4’-dichlorophenyl)-6-methyl-N-cyclohexylamine-1,4-dihydroindeno[1,2-c]pyraz ole-3-carboxamide, a novel CB2 agonist, alleviates neuropathic pain through functional microglial changes in mice. Neurobiol Dis. 2010;37:177–85. doi: 10.1016/j.nbd.2009.09.021. [DOI] [PubMed] [Google Scholar]
- 64.Iyer V, Slivicki RA, Thomaz AC, Crystal JD, Mackie K, Hohmann AG. The cannabinoid CB2 receptor agonist LY2828360 synergizes with morphine to suppress neuropathic nociception and attenuates morphine reward and physical dependence. Eur J Pharmacol. 2020;886:173544. doi: 10.1016/j.ejphar.2020.173544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li AL, Lin X, Dhopeshwarkar AS, Thomaz AC, Carey LM, Liu Y, et al. Cannabinoid CB2 agonist AM1710 differentially suppresses distinct pathological pain states and attenuates morphine tolerance and withdrawal. Mol Pharmacol. 2019;95:155–68. doi: 10.1124/mol.118.113233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Navarrete F, Garcia-Gutierrez MS, Manzanares J. Pharmacological regulation of cannabinoid CB2 receptor modulates the reinforcing and motivational actions of ethanol. Biochem Pharmacol. 2018;157:227–34. doi: 10.1016/j.bcp.2018.07.041. [DOI] [PubMed] [Google Scholar]
- 67.Kravitz AV, Tye LD, Kreitzer AC. Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nat Neurosci. 2012;15:816–8. doi: 10.1038/nn.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lobo MK, Covington HE, 3rd, Chaudhury D, Friedman AK, Sun H, Damez-Werno D, et al. Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science. 2010;330:385–90. doi: 10.1126/science.1188472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Soares-Cunha C, Coimbra B, David-Pereira A, Borges S, Pinto L, Costa P, et al. Activation of D2 dopamine receptor-expressing neurons in the nucleus accumbens increases motivation. Nat Commun. 2016;7:11829. doi: 10.1038/ncomms11829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Natsubori A, Tsutsui-Kimura I, Nishida H, Bouchekioua Y, Sekiya H, Uchigashima M, et al. Ventrolateral striatal medium spiny neurons positively regulate food-incentive, goal-directed behavior independently of D1 and D2 selectivity. J Neurosci. 2017;37:2723–33. doi: 10.1523/JNEUROSCI.3377-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yawata S, Yamaguchi T, Danjo T, Hikida T, Nakanishi S. Pathway-specific control of reward learning and its flexibility via selective dopamine receptors in the nucleus accumbens. Proc Natl Acad Sci USA. 2012;109:12764–9. doi: 10.1073/pnas.1210797109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Smith RJ, Lobo MK, Spencer S, Kalivas PW. Cocaine-induced adaptations in D1 and D2 accumbens projection neurons (a dichotomy not necessarily synonymous with direct and indirect pathways) Curr Opin Neurobiol. 2013;23:546–52. doi: 10.1016/j.conb.2013.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.