Abstract
Immunotherapy based on natural killer (NK) cells is a promising approach for treating a variety of cancers. Unlike T cells, NK cells recognize target cells via a major histocompatibility complex (MHC)-independent mechanism and, without being sensitized, kill the cells directly. Several strategies for obtaining large quantities of NK cells with high purity and high cytotoxicity have been developed. These strategies include the use of cytokine−antibody fusions, feeder cells or membrane particles to stimulate the proliferation of NK cells and enhance their cytotoxicity. Various materials, including peripheral blood mononuclear cells (PBMCs), umbilical cord blood (UCB), induced pluripotent stem cells (iPSCs) and NK cell lines, have been used as sources to generate NK cells for immunotherapy. Moreover, genetic modification technologies to improve the proliferation of NK cells have also been developed to enhance the functions of NK cells. Here, we summarize the recent advances in expansion strategies with or without genetic manipulation of NK cells derived from various cellular sources. We also discuss the closed, automated and GMP-controlled large-scale expansion systems used for NK cells and possible future NK cell-based immunotherapy products.
Keywords: NK cells, PBMCs, UCB, iPSCs, NK cell lines, Genetic manipulation
Subject terms: Immunotherapy, Cancer therapy
Introduction
In the last decade, cancer immunotherapy has made great progress and has become a promising approach for treating many cancers. T cell-based immunotherapy has been developed and successfully applied in the clinical treatment of various cancers. However, T cell-based immunotherapy has some limitations, such as the risk of graft-versus-host disease (GVHD), cytokine release syndrome (CRS), and neurotoxicity [1, 2]. Investigating the uses of antitumor effector cells and immunotherapy strategies other than those involving T cells is urgent.
Natural killer (NK) cells, as innate immune cells with the ability to directly kill malignant cells, are increasingly being considered a promising immunotherapy tool and are currently being tested in clinical trials [3]. Various sources of NK cells, such as peripheral blood (PB) mononuclear cells (PBMCs), umbilical cord blood (UCB) cells, induced pluripotent stem cells (iPSCs), and NK cell lines, have been employed to generate NK cells in vitro [4, 5]. Despite the great progress and developments that have been made to obtain NK cells with high purity and potent cytotoxic capacity by using various strategies, including feeder-free systems, feeder cells, and some NK cell products that have been tested in clinical trials [6], NK cell-based immunotherapy is still facing several challenges. The major challenge has been obtaining large quantities of NK cells for clinical use. This issue, however, can be circumvented by the promising ability to genetically modify NK cells to generate forms that display increased cytotoxicity and that can specifically target specified types of cells [7]. Therefore, to enhance the efficacy of clinical therapy using NK cells, gene manipulation of NK cells is being rapidly developed. Due to the relatively high safety and high feasibility of off-the-shelf manufacturing, genetically modified NK cells may be the next great cancer immunotherapy [2, 8]. In this review, we focus on recent progress made in expansion protocols using various sources, genetic engineering, and good manufacturing practice (GMP) production of NK cells and discuss the prospects of immunotherapy based on NK cells.
Technical limitations for NK cell expansion
Challenges of NK cell immunotherapy compared with T cell
The number of NK cells is lower in tumor samples
Studies have reported that the number of NK cells in PB and tumor tissues is lower than that of T cells. NK cells account for only 5–15% of lymphocytes in normal PB, while T cells account for over 50% of PB lymphocytes. However, approximately 90% of NK cells in the circulation are CD56dim cells, which exhibit a stronger cytolytic function without antigen priming [9, 10]. However, the T cells in PB are mainly composed of CD4+ T cells (25% to 60% of cells) and naïve CD8+ T cells (5% to 30% of cells). Therefore, as NK cells have direct killing function, nearly all of them in the PB will enact killing when they recognize a malignant disease or infected cells. However, only some of the T cells in the PB will act as cytotoxic T lymphocytes (CTLs) to enact killing. Moreover, naïve NK cells are in a responsive state compared to naïve CD8+ T cells.
However, the correlation of NK cell number and proportion with disease prognosis varies across tumor types [11, 12]. A higher proportion of infiltrating NK cells in tumors such as breast tumors, pulmonary adenocarcinoma, and gastrointestinal stromal tumors is associated with a lower relapse rate [13, 14]. The different functions of NK cells in tumors may be due to the level of activation or exhaustion of NK cells, as well as differences in the types and abundances of cytokines present in the tumor. Exploration of the causes and mechanisms governing changes in NK cell type and abundance can help optimize NK cell-based immunotherapy plans.
NK cell surveillance is in only the early stage
NK cells with the capacity of spontaneous cytotoxicity are considered to serve as superior surveillance cells. Early studies have found that NK cells play an important role in inhibiting tumor initiation and tumor metastasis [15]. Therefore, some researchers believe that NK cells play an immune surveillance role in only the early stage of the disease. When the tumor burden increases, NK cells have limited antitumor effects. Therefore, NK cell therapy may be used in the early stages of tumor initiation or after surgery, radiotherapy, and chemotherapy, which reduce the tumor burden. NK cell therapy should be used as early as possible to make it more effective. However, NK cells can still be useful for the treatment of advanced tumors. In addition to their direct tumor cell killing effect, NK cells also have immunomodulatory effects, such as weakening immune suppressor cells and recruiting immune cells to enhance antitumor functions [16, 17]. Therefore, the application of NK cell therapy should consider tumor burden, and the outcome of NK cell therapy may be correlated with tumor burden.
NK cells decrease with age and metabolism
Studies have shown that the proportion of NK cells in the blood, bone marrow, lungs, spleen, and other organs shows a downward trend with age [10]. Although the ability to proliferate among aged NK cells [18], the capacity for IFN-γ production in the CD56bright subset and the expression of CD107a, perforin, and granzyme B in the CD56dim subset are comparable to those of young NK cells, the killing ability of individual aged NK cells is decreased [19–21]. People with infectious diseases, cancer, and chronic inflammatory disease show abnormal metabolism. This metabolic environment may lead to NK cell dysfunction and differentiation into other cell types, such as regulatory NK cells or innate lymphoid cells [22]. Age- or metabolism-related immune dysfunctions influence lifespan and tumor incidence. Although the frequency of CD8+ T cells is reduced in the periphery and lymphoid organs in old age, the natural cytotoxic activity of PB lymphocytes is negatively correlated with the probability of tumor occurrence [20, 23, 24]. Moreover, studies in most hematological malignancies and certain solid tumors have shown that the number and activity of peripheral NK cells are positively correlated with progression, relapse and the overall survival rate [25–30].
However, the lifespan of activated NK cells in vivo is approximately 7 days [31], and transferred in vitro-activated NK cells can persist for 7 to 22 days in vivo [32], both of which are still lower than the lifespan of effector CD8+ T cells and memory CD8+ T cells in vivo (approximately 2–3 weeks to months) [33, 34]. Moreover, the doubling time of NK cells in response to stimulation is approximately 1.5 days [31]; in contrast, naïve T cell can rapidly divide, undergoing 15 divisions within 7 days and having a doubling time of approximately 10 h in response to stimulation [35]. These characteristics of NK cells highlight that the optimal regimens and indications for NK cell therapy are different from those of T cell therapy.
NK cells kill leukemia but not solid tumor cells
The prominent antitumor effect of and prevention of GVHD by NK cells in allogeneic bone marrow transplantation for the treatment of leukemia suggest that NK cells are an important tool in the treatment of hematological malignancies [36–39]. However, whether NK cells can be used to treat solid tumors is not clear. Conflicting data published previously about the function of NK cells in solid organ transplantation have resulted in a controversial understanding of the role of NK cells in the treatment of solid tumors [36]. In the transplantation of different organs, the differentiated cellular functions displayed by NK cells and the different microenvironments result in different functions: transplant rejection or protection.
Due to the effects of the tumor microenvironment and the various immune escape mechanisms employed by tumor cells, current strategies employing not only NK cells but also CTLs or tumor-infiltrating lymphocytes in the treatment of solid tumors have not achieved significant effects [40].
The sensitivity of NK cells versus CD8+ T cells to chemotherapeutic drugs or radiotherapy
After the first course of treatment with most chemotherapeutic drugs, such as docetaxel, 5-FU, CPT-11, fludarabine, and cyclophosphamide, NK cells and CD8+ T cells decrease slightly or significantly, but these two types of cells do not show a difference in sensitivity to chemotherapeutic drugs [41–43]. However, after the second course of docetaxel administration, a further decrease in the absolute number of CD8+ T cells but not NK cells is observed [43]. Therefore, the sensitivity of NK cells and T cells to chemotherapy drugs is not significantly different in the early stage of chemotherapy. However, radiotherapy causes a more significant decrease in the number of T cells than in the number of NK cells. T cells and their precursors are relatively radiosensitive. Mature NK cells are relatively radioresistant; however, their precursors are radiosensitive [44]. Therefore, in the combination of radiotherapy and chemotherapy for tumor treatment, NK cell therapy may be a relatively better choice. NK cells have relatively better resistance to radiotherapy and chemotherapy, and multiple infusions of cells can also reduce the side effect of reduced cell numbers caused by radiotherapy and chemotherapy.
Advantages of NK cells over iNKT cells
iNKT cells, γδT cells, and mucosa-associated invariant T (MAIT) cells, due to their expression of NK lineage receptors and semi-invariantly rearranged T cell receptors, are considered to have the functions of both NK cells and T cells. Although it is believed that NK cells can be replaced by iNKT cells to a certain extent, NK cells still have advantages over iNKT cells.
iNKT cells account for only 0.01–0.1% of T cells and can be divided into many different functional subgroups based on differences in cytokine release and function, such as NKT1, NKT2, NKT17, and NKT10, similar to how T cells are categorized. NKT1 cells are the only subgroup expressing cytotoxic molecules [45]. Moreover, 3–5 days after activation, iNKT cells undergo massive apoptosis, and their lifespan is not long [46]. Although iNKT cells can also kill cells in a ligand-dependent manner by releasing granules and death receptors, CD1d-negative tumor cells show resistance to iNKT cell therapy alone [46, 47]. Therefore, the target cell population killed by NK cells is different from that of iNKT cells.
In human PB or lymphoid tissues, γδT cells account for 0.5–16% of CD3+ T cells. γδT cells can be classified into different groups based on expression of the γ chain and δ chain. Vγ9Vδ2 T cells are a subgroup that has been found mainly in PB and has a strong antitumor effect. Regulatory or inhibitory γδT cell subgroups can maintain immune tolerance and, in some conditions, promote tumor development. γδT cells mainly recognize small, phosphorylated metabolite antigens. Although γδT cells can directly kill tumor cells via the mechanisms as NK cells, including granule release, death receptor ligand-dependent mechanisms, and antibody-dependent cellular cytotoxicity (ADCC), the killing ability of γδT cells is weaker than that of NK cells, which may be due to their lower levels of granules and CD16 expression [47–49].
MAIT cells are enriched in mucosal layers but also present in PB and comprise 1–10% of all CD3+ cells. MAIT cells can recognize vitamin B derivatives of microbial origin presented by MHC-related protein 1. MAIT cells have the following advantages as antitumor therapy cells: 1. They have advantages in location and advantages in the treatment of mucosal-associated tumors. 2. MAIT cells expresses high levels of multidrug resistance protein 1 and are thus more tolerant to chemotherapeutics. 3. Many MAIT cells have memory-like characteristics and can be quickly activated. However, both antitumor and tumor-promoting effects of MAIT cells have been reported, partly because there are different subgroups of MAIT cells, which have different effects in response to different stimuli and different tumor microenvironments. These subgroups have not been studied in depth. Therefore, the development of MAIT cells remains to be assessed [47, 50].
Advantages and disadvantages: NK cells over T cells
Types and affinity of ligands recognized by NK cells or T cell receptors
T cells specifically recognize antigens in the form of peptides presented on MHCs (pMHCs) on antigen-presenting cells (APCs) [51, 52]. A single peptide/MHC pair can activate T cells as an early activation signal (calcium signaling), three peptides/MHC pairs can trigger CD8+ T cell killing effects, and ten peptides/MHC pairs can form a mature synapse [53–55]. The affinity of most functional TCRs for pMHC ranges from 1000 to 100,000 nM (KD values) [56, 57]. However, NK cells recognize receptor proteins on cells in a nonspecific manner that is not restricted by MHC molecules [58]. The activation-initiating signal of NK cells is strictly regulated by the integration of activating and inhibitory receptor signals. NK cells fail to elicit a killing response when a single activating NK cell receptor, such as NKp46, NKG2D, DNAM1, or 2B4, is engaged [59]. Even if the target cell upregulates multiple activation ligands, inhibitory ligand upregulation may prevent NK cells from being activated [53]. Therefore, the exact threshold at which NK cells are triggered to kill target cells is not clear. In addition, the affinity of NK cell receptors for ligands ranges from KD values of 1.4 to 51 nM [60–62]. Overall, compared with T cells, NK cells recognize a wider range of antigens, but the sensitivity and affinity of NK cell recognition are not as good as those of T cell recognition.
Cytotoxic killing
Resting T cells store low levels of perforin and granzyme B. After T cells are stimulated by antigen, transcription and translation, the levels of perforin and granzyme B are upregulated, and the whole process requires 18–24 h [63, 64]. T cells can kill an average of 2.4 tumor cells per hour. Conversely, mature NK cells store high levels of perforin and granzyme B, over 200 lytic granules worth, which help NK cells trigger lytic responses within minutes [65]. Only approximately 2–4 granules released by NK cells in a single degranulation event are sufficient to kill a target cell [66]. Generally, one NK cell can kill three to four tumor cells before its cytotoxicity is lost. There are a small number of NK cells that can kill more than 30 tumor cells [8].
In addition, both CD8+ CTLs and NK cells have been suggested to depend on cytotoxic molecules (including granzymes and perforin), on Fas—Fas ligand (FasL) interactions, and on soluble factors such as tumor necrosis factor (TNF)-α [9, 67]. NK cells can also kill target cells through natural mechanisms and ADCC, while T cells cannot [68, 69]. NK cells, overall, are superior to T cells in terms of killing response time and diversity of killing pathways, but the total killing ability of CTLs is stronger than that of NK cells, partly because CTLs proliferate faster and survive longer in vivo than NK cells.
The priming of NK cells and T cells cannot be compared at present. The number of activating receptors that need to engage NK cells to induce degranulation has not yet been determined.
Cytokine release
T cells are divided into multiple cell subsets, and they secrete multiple types of cytokines to perform different functions. For example, CD8+ T, CD4+ Th1 and CD4+ Th17 cells secrete proinflammatory cytokines (tumor necrosis factor (TNF), interferon-γ (IFNγ), IL-4, IL-5, etc.), which play an important antiviral and antitumor role by mediating humoral and cell immunity [34, 70]. Conversely, CD4+ Th2 cells and Tregs are reported to secrete IL-10 and TGF-β and suppress the immune response [70–72]. In contrast to T cell grouping, NK cell grouping is relatively simple (CD56dim and CD56bright). All the cytokines mainly secreted from CD56bright NK cells are proinflammatory cytokines, and no immunosuppression-related cytokines have been reported [70]. The types of cytokines secreted by NK cells are not as abundant as those secreted by T cells, which reduces the risk of developing cytokine release syndrome after NK cell treatment.
Proliferation in vivo
Approximately 5.1% of CD8+ cells proliferate per day, resulting in an average doubling time of 14 days in the resting state. After being activated, T cells enter the rapid proliferation phase, and approximately 46.8% of CD8+ T cells proliferate per day, which is equivalent to a doubling time of 1.5 days. The proliferation rate is approximately 10 times higher than that of resting T cells [73, 74]. However, the mean percentage of proliferative NK cells in healthy people is 4.3 ± 2.4% per day (equivalent to a doubling time of 16 days), and proliferation rates were approximately 5.7%/day after infection [75, 76]. Studies have also identified numerous NK and T cell receptors and downstream signaling molecules, demonstrating that NK cell expansion is limited to approximately 1000-fold and that T cell expansion is greater than 50,000-fold for certain epitopes [77]. The proliferation efficiency of NK cells is indeed weaker than that of T cells.
Recall response
When exposed to certain antigens, CD8+ T cells are activated and perform their killing function. After that, approximately 5–10% of CD8+ T cells acquire immunological memory and show a quantitatively and qualitatively enhanced recall response after exposure to specific antigens again [63]. These memory CD8+ T cells can be distinguished from naïve CD8+ T cells by CD3, CD45RA, CCR7, and CD62L marker expression and maintain memory for several years [78, 79]. Some studies have indicated that priming with cytokines (IL-12, IL-15, and IL-18) or stimulation by viral infection [80] can enhance the responsiveness of a group of NK cells to cytokines and activate receptor stimulation that persists for at least 4 months [81–83]. Although these NK cells are also called memory or memory-like NK cells, the specific markers that define this group of cells have not yet been defined [80]. At present, the mechanism by which NK cells acquire memory still needs to be further studied.
CRS, neurotoxicity, and GVHD
Irrespective of whether the chimeric antigen receptor (CAR)-T cells used for treatment are autologous or allogeneic, serious side effects are commonly observed, such as the release of a wide variety of cytokines including interleukin-6 (IL-6), IFN-γ, TNF, IL-2, IL-8, and IL-10, which may lead to the development of CRS [84, 85]; in addition, the massive amounts of cytokines can spread into the central nervous system and lead to the development of immune effector cell-associated neurologic syndrome (ICANS) [84–87]. In addition, GVHD occurs when donor-derived T cells recognize recipient (host) cells as foreign. It has been reported that of patients receiving CAR T cells, 37%–93% develop CRS, 23%-67% develop ICANS [86, 87], 30–50% develop acute GVHD (grade I–IV) and 14% experience severe acute GVHD (grade III–IV) [88].
In contrast to T cell treatment, NK cell treatment has not been reported to result in CRS or ICANS, regardless of whether autologous or allogeneic NK cells were used. This result may be because the proliferation and cytokine secretion abilities of NK cells in vivo are not as good as those of T cells [89]. Approximately 7% of patients receiving KIR ligand mismatched NK cell treatment experienced GVHD. However, this increased GVHD occurrence was positively correlated with higher CD3 chimerism, suggesting that the cell preparations include mixed T cells and trigger a GVHD response [90, 91]. Overall, NK cell treatment has higher safety than T cell treatment.
Universal/Allogeneic use
T cell receptor recognition of antigen is MHC-restricted. Donor T cell recognition of a mismatch in polymorphic MHC class I or II molecules, which are expressed on APCs, induces acute GVHD [88, 92]. Safety issues severely limit the application of allogeneic T cells [93]. In contrast, NK cells have no MHC restriction, and binding of allogeneic MHC class I molecules to NK cells does not lead to their excessive activation [94]. Therefore, instead of causing serious side effects, allogeneic NK cell treatment has a better antitumor effect than autologous NK cell treatment [95, 96]. Therefore, for universal use, NK cells show a significantly greater advantage than T cells.
Residual tumors and tumor relapse
When residual tumor is present, tumor cells may exit the primary site and enter the PB as circulating tumor cells (CTCs), causing tumor metastasis and relapse [97]. CTCs express FasL, which binds to its receptor Fas on cytotoxic CD8+ T or CD4+ T helper cells and induces T cell apoptosis. Conversely, CTCs lose inhibitory ligand expression or acquire expression of NK cell-activating ligands that enable NK cells to eliminate the CTCs. NK cells can kill approximately 80% of metastatic cells via the perforin exocytosis pathway [98]. In addition, cancer stem cells (CSCs) have been proven to cause tumor relapse and metastasis. These cells lose the expression of MHC class I molecules, which helps CSCs escape the killing of T cells [99, 100]. However, MHC class I molecules can pair with inhibitory receptors of NK cells to maintain NK cell tolerance. When the expression of MHC class I molecules is lost, CSCs are more easily recognized and killed by NK cells. In addition, CSCs upregulate ligands, such as MICA/B, Fas, and DR5, which makes them more susceptible to NK cell-induced apoptosis [101]. A large number of studies have confirmed that, compared with non-CSCs CSCs (identified by multiple CSC markers: CD24+/CD44+, CD133+, and aldehyde dehydrogenasebright) are preferentially killed by NK cells [101, 102]. Compared with T cells, NK cells have great advantages in the treatment of residual tumors and control relapse.
Technical manipulations of NK cell immunotherapy compared with T cell therapy
Expansion
Currently, commercial microbeads (Dynabeads) with activating antibodies against CD3 as a TCR stimulus and CD28 as a costimulatory cue are commonly used to expand T cells ex vivo [103]. Currently, the manufacturing of CAR-T cell products is relatively mature, and many studies have reported rapid and closed expansion systems for the production of CAR-T cells under GMP conditions. It usually takes approximately 10 days of cell culture to achieve the numbers necessary for a clinical dose [104, 105]. Recently, one study established a rapid expansion system to manufacture CD19 CAR-T cell products from PBMCs in only 6 days to achieve large numbers that are sufficient for patient treatment [106]. However, compared to T cell expansion, NK cell expansion is more time-consuming and difficult. The numbers of infused NK cells range from 5 to 100 × 106 cells/kg in clinical trials, and current in vitro expansion systems of NK cells usually take 14 to 28 days to achieve the numbers necessary for a clinical dose [107]. Feeder-free and feeder cell systems have been developed for the expansion of NK cells in vitro. NK cells expanded using a feeder-free system usually have a limited expansion fold. Although expanded NK cells expanded with feeder cell systems have high purity and expansion fold, they have safety issues when used in the clinic. The expansion systems for NK cells are still being optimized. Although expansion systems for NK cells are not as mature as those of T cells, great progress has recently been made in in vitro NK cell expansion. For example, K562-mbIL21-41BBL-derived membrane particles were used to expand PBMC-derived NK cells over 105-fold with high purity over 28 days [108]. This technology is a cell-free system that has evolved from a previous platform that used genetically modified K562 cells as feeder cells to expand NK cells.
Transfection
Two types of gene modification methods, viral and nonviral methods, have been applied for NK and T cell transfection. The retrovirus or lentivirus transfection efficiency of NK cells is usually only 30–40% [109]. A possible reason for this low efficiency is that NK cells inherently have antiviral properties. Compared with that of NK cells, the transfection efficiency of T cells is higher. The retroviral vector transfection efficiency of anti-CD19 CAR-T cells can reach almost 80% [106]. The electroporation efficiency of CAR-T cells generated from PBMCs can reach as high as 88% [110]. Therefore, T cells have attracted much attention for TCR or CAR engineering due to their relatively high transfection efficiency. Although NK cells have low viral transfection efficiency, there are strategies to increase NK cell transfection, such as using nonviral methods. The electroporation transduction efficiency of primary NK cells was over 70% [111]. Moreover, the transfection efficiency levels of NK cell lines and iPSC-NK cells are higher than those of primary NK cells. The efficiency of electroporation-based transfection of the NK-92 cell line has been shown to reach as high as approximately 80% [112]. It is well established that using lentivirus vectors can yield relatively high transduction efficiencies (>70%) in iPSC-NK cells [113]. Because most genetic modifications of iPSC-NK cells are concentrated in the pluripotent stem cell (PSC) stage, in which the cells have not yet differentiated into NK cells, iPSC-derived NK cells can provide a convenient platform for gene modification.
Cryopreservation and recovery
To realize off-the-shelf use, cryopreservation of expanded NK or T cell products is key. To date, the cryopreservation protocol for NK cells is not well defined, and many studies are still optimizing protocols to retain NK cell viability and function after thawing. The mean NK cell viability ranges from 83% to 93% after cryopreservation [114]. The expression of surface receptors of cryopreserved NK cells, such as NKp46, decreases, and the same dose of frozen NK cells has weaker antitumor activity than that of fresh NK cells [115]. For these reasons, cryopreservation of NK cells is rarely used for clinical trials. Although several clinical trials have used cryopreserved NK cells, there is no mention of freezing medium formulation or freezing protocols. Similar to the case for NK cells, cryopreservation also has negative effects on T cell products. The viability of cryopreserved CAR-T cells ranges from 73.7% to 98.4% [116]. Although there are some well-studied commercial freezing media for CAR-T cell products, gene expression related to mitochondrial dysfunction, cell cycle damage pathways, and apoptosis signaling was found to be increased in cryopreserved CAR-T cell products compared with fresh CAR-T cells [117]. Based on these studies, cryopreservation does impact NK cells and CAR-T cells, and more in-depth research is needed to optimize the freezing and thawing methods and study the effect of cryopreserved NK or T cell products on clinical outcomes.
Persistence after infusion
Clinical trials have shown that IL-2 or IL-15 injection can induce NK cell expansion in vivo in humans, but allogeneic NK cells usually survive only a few weeks after infusion [32, 118, 119]. The limited proliferation in vivo and short inpatient persistence of adoptively transferred primary NK cells have resulted in poor clinical outcomes in the treatment of solid tumors [120]. However, unlike NK cells, CAR-T cells can persist in vivo and remain functional for several months or even years [121]. The major reason for the persistent difference between NK and T cells is that T cells can form memory T cells in the body and can persist in vivo for a long time to provide continued immune protection [122]. Due to the short persistence of NK cells in vivo, many studies have focused on strategies to increase their persistence in vivo and antitumor activity. Modification of genes related to NK cell metabolism and replication, such as CISH deletion by CRISPR-CAS9 technology and TERT overexpression by retrovirus infection, can promote NK cell in vivo persistence and enhance antitumor activity [123, 124]. Furthermore, another strategy endows NK cells with a memory-like phenotype by preactivating them with a cytokine cocktail (IL-12, IL-15, and IL-18) to improve their persistence [125].
Receptor modification
TCR recognizes peptides bound to MHC on the surface of tumor cells to initiate the activation of T cells. In T cell-based immunotherapy, T cells can be genetically engineered to express high-affinity TCRs targeting tumor antigen epitopes. Currently, the major challenge of TCR T cell therapy is to design and engineer high-affinity TCRs that specifically recognize particular tumor neoantigen epitopes rather than shared antigens. CARs are fusion proteins containing an extracellular region derived from an antibody that target proteins or glycans on the cell surface in an MHC-independent manner, and most CARs have been built based on high-affinity therapeutic antibodies [126, 127]. Due to the MHC-independent recognition mechanism, CAR-T cells have a fundamental antitumor advantage compared with engineered TCR T cells. Unlike that of T cells, the activation of NK cells is regulated by germline-encoded activating and inhibitory NK cell surface receptors. NK cells perform functions in an antigen-independent and non-MHC-restricted manner [128]. Therefore, NK cell receptors can be modified through genetic manipulation, which enhances the ability of NK cells to recognize tumors, although it is a panspecific recognition. NKG2D is one of activation receptors of NK cells. The interaction between it and its ligands, which are commonly detected on tumor cells, is stronger than that of most NK cell receptors and ligand and than many TCR interactions with MHC proteins [129]. Due to the high affinity of NKG2D, an engineered NKG2D CAR has been developed to improve the NK cell tumor response in clinical trials in metastatic colorectal cancer [128]. Therefore, NK cells are a promising alternative to T cells for immunotherapy due to their unique recognition and activation mechanisms, which are activated through an array of endogenous activating receptors. Even if a tumor antigen that CAR targets is downregulated on tumor cells, CAR NK cells can still function, unlike CAR-T cells [130].
Synthetic function
Immune cells can be genetically modified to express a receptor (CAR) that recognizes specific proteins on the target cell surface. The manufacturing CARs for T cells is well developed, and three generations of CARs have been developed with different structures [56]. The efficiency of T cell transfection can reach more than 80%, as mentioned above, so T cells have attracted much attention for CAR engineering. Although the transfection efficiency of primary NK cells is low (<70%), iPSC-NK cells and the NK-92 cell line provide relatively good and convenient platforms for CAR manufacturing. Currently, most clinical trials utilizing CARs are designed for T cells and are not optimal for NK cell signaling. Therefore, researchers have focused on designing CARs specifically for NK cell signaling. For instance, a CAR containing the transmembrane domain of NKG2D, the 2B4 costimulatory domain, and the CD3ζ signaling domain, termed NKG2D-2B4ζ, was designed for iPSC-NK cells to activate NK cell-specific signaling. The expression of such an NK-CAR construct strongly improved antigen-specific NK cell signaling and had similar antitumor activity to T-CAR (CD28-41BBζ)-expressing T cells in an ovarian cancer xenograft model [131]. Therefore, NK cell-specific CARs are worth exploring to enhance NK cell signaling and cytotoxic function.
In addition to conventional CARs, novel single-chain variable fragment (scFv) recombinant reagents termed bispecific killer engagers (BiKEs) and trispecific killer engagers (TriKEs) have been developed to enhance NK cell cytotoxic function via ADCC (Fig. 1). BiKEs and TriKEs are usually designed with an anti-CD16 component considering that the ADCC activity of NK cells has significant potency in clinical trials [132]. These novel reagents are designed to form an antigen-specific immunological synapse between NK cells and tumor cells. Therefore, the selection of a target of tumor cells is key in designing these constructs. The selected targets are usually tumor antigens on the cell surface that are highly and selectively expressed. Currently, CD20, CD19, CD33, CD30, EGF-R, HER2, and MOV19 have been selected as targets in different types of tumors [132]. Since BiKEs do not induce the proliferation of NK cells, TriKEs are more popular because they feature IL-15 added into the BiKE platform to enhance NK cell proliferation, and some studies have shown great potency of TriKEs [133–135]. In addition to IL-15, other cytokines that modulate NK cell function can also be included in the platform, which means that BikEs/TriKEs are quite flexible. For T cells, bispecific T cell engagers (BiTEs), which include one binding site targeting tumor cells and another targeting CD3, have been designed [134]. The most representative BiTE, blinatumomab, which targets CD19, has been proven to be highly efficient in acute lymphoblastic leukemia (ALL) [136]. The surface targets of tumor cells are also key for designing BiTEs, similar to the case for BiKEs. To date, other targets, such as CD33 or BCMA, have been reported in BiTE constructs to treat acute myeloid leukemia and relapsed/refractory multiple myeloma, and the efficiency of these BiTEs has been tested in phase I clinical trials [137, 138].
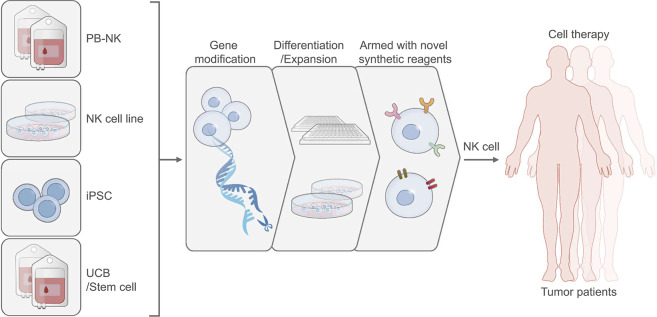
Fig. 1.
The sources of NK cells for immunotherapy and strategies of capacity enhancement. Various type of cells, including PBMCs, UCB, iPSCs, and NK cell lines, have been used as the sources to generate NK cells for immunotherapy. Gene modification technologies, differentiation protocols, and novel synthetic reagents have been developed to improve NK cell function
Expansion of NK cells from PBMCs
PBMCs constitute one of the main sources of NK cells due to their advantages of being relatively easy to collect and amenable to in vitro expansion and lacking toxic side effects. However, the proportion of NK cells in PBMCs is only 10–15%. Methods for expanding PBMC-derived NK cells, such as the use of a combination of cytokines and feeder cells or membrane particles to stimulate NK cell expansion in vitro, have been developed. These different expansion systems show considerably different NK cell expansion efficiency levels.
Feeder cell-free systems for PBMC-derived NK cell expansion
Cytokines are critical components for maintaining NK cells survival and support proliferation of NK cells in vitro without the use of feeder cells. However, different expansion efficiencies have been found when using different cytokines, combinations of cytokines, and culture conditions. The expansion of NK cells using cytokines such as IL-15 or IL-2 alone is relatively modest (Table 1). An approximately 200-fold greater NK cell expansion efficiency in a period of 14 days was achieved when NK cells were cultured in KBM-501 medium supplemented with 0.5% normal human AB serum and 2813 IU/ml IL-2 [139]. Although the resulting number of expanded cells could satisfy clinical needs, a multicytokine combination can induce NK cell proliferation more effectively than single cytokines [140]. Purified NK cells expanded ex vivo with 10 ng/mL IL-15 and 100 IU/ml IL-2 have shown higher-fold expansion than NK cells expanded using IL-2, IL-15, or IL-21 alone. Furthermore, the use of high levels of IL-2 (1000 IU/ml) alone was associated with a decline in the average expansion rates of NK cells over the course of 6 days, but simultaneous cultivation with IL-15 and IL-21 was found to maintain viability and expansion of NK cells over a period of 4–6 weeks [140, 141]. Currently, the optimal combination of cytokines is considered to be IL-2 with IL-18. CD3-depleted PBMCs stimulated with IL-2 and IL-18 for 14 days have shown 496.5-fold ± 55.0-fold NK cell expansion efficiencies with purities of 99.0% ± 0.6% [142]. Although the cytokine combinations used to expand NK cells result in a sufficient number of highly cytotoxic NK cells, the overall expansion fold is still less than ideal. Therefore, methods involving the combination of cytokines with antibodies or OK432 (a penicillin-killed, lyophilized formulation of a low-toxicity strain (Su) of Streptococcus pyogenes) have been applied to enhance ex vivo NK cell expansion. NK cells from nonpurified PBMCs showed an average expansion of 637.5-fold (ranging from 167.1-fold to 1613-fold) after 21 days of cultivation in 700 U/mL IL-2 supplemented with 0.01 KE/mL OK432 and anti-CD16 (Table 1); in this case, the purity of the expanded NK cells was 84.3 ± 14.9% [143, 144]. This expansion efficiency was much better than that resulting from the expansion system involving just cytokine combinations [145].
Table 1.
Current expansion system of PBMC-derived NK cells
| Initial cell | Stimulation substance | Medium | Culture time | Expansion fold | Purity of NK cells | Ref. |
|---|---|---|---|---|---|---|
| Cytokine alone | ||||||
| Purified allo-NK cells (>91% NK cells) | IL-15 | RPMI 1640 + 10 ng/ml IL-15 | 9 days | 2.5-fold (1.8–3.5-fold) | Not mentioned | [219] |
| CD3-depleted allo-NK cells | IL-2 | KBM-501 medium + 0.5% human AB serum + 2813 IU/ml IL-2 | 14 days | 200-fold | >90% | [220–222] |
| Cytokine in combinations | ||||||
| CD3-depleted allo-NK cells | IL-2+ IL-18 | AIM-V medium +5% auto-plasma + 3000 IU/mL IL-2 + IL-18 | 14 days | 496.5 ± 55.0-fold | 99.0% ± 0.6% | [142] |
| Purified allo-PB-NK cells (> 95% NK cells) | IL-15 +IL-21 | NK MACS medium + 1% NK MACS supplements + 5% human AB plasma + 1% Pen/ Strep + 10 ng/mL IL-15 + 25 ng/mL IL-21 | 10–12 days | 7.5-fold (2.7–16.2-fold) | 94.6% (86.1~97.8%) | [141] |
| Purified allo- NK cells (>85% NK cells) | rhIL-2+IL-15 | X-VIVO 10 medium + 5% human plasma + 1000 U/ml rhIL-2 + 10 ng/ml IL-15 | 12 days | 30-fold | 94% | [139, 142, 222] |
| Purified allo-NK cells (> 95% NK cells) | IL-15+IL-21+ IL-18 | SCGM + 10% human serum + 10 ng/ml IL-15 + 10 ng/ml IL-21 + 10 ng/ml IL-18 | 21 days | 17.19 ± 4.85-fold | 96.34 ± 2.41% | [223] |
| Allo-NK cells | IL-2+IL-15+IL-18 | NK MACS medium + 5% human AB plasma+1% NK MACS supplements + 1% Pen/ Strep + IL-2 + IL-15 + IL-18 | 3~5 days | 30-fold | 45% | [145, 151] |
| Cytokine combine with antibody or other reagents | ||||||
| Allo-NK cells | IL-2 + IL-12 + IL-18 + anti-CD56 + anti-CD16 + anti-CD355 | KBM502 medium + 0.5% auto-plasma+200 ng/mL of IL-2 + 10 ng/mL of IL-12 + 100 ng/mL of IL-18 + 2.5 μg anti-human antibodies (CD56 + CD16 + CD355) | 14 days | 140-fold | 64.7 ± 9.6% | [224] |
| Auto-NK cells | IL-2 + OK432 + anti-CD16 | BINKIT medium + 5% auto-plasma + 700 U/mL IL-2 + 0.01 KE/mL OK432 + anti-CD16 | 21 days | 637.5-fold (167.1–1613-fold) | 84.3 ± 14.9% (54–96.7%) | [143, 144] |
| Cytokine combine with feeder cells | ||||||
| Allo-NK cells | K562-41BBL-mbIL21 -CD137L +IL-2 | RPMI 1640 + 10% FBS + 1% Glu + 1% Pen/ Strep+50 IU/mL IL-2 | 14 days | 2621-fold | >90% | [151] |
| AIM-V medium+5%Immune Cell Serum Replacement+ TexMACS +OpTmizer,+SCGM, + ABS-001+ StemXVivo + 50 IU/mL IL-2 | 5448-fold | |||||
| Allo-NK cells | K562-mbIL15-CD137L +IL-2 | X-VIVO 10 medium + 10% human AB serum + Glu + 100 IU/mL IL-2 | 12 days | 100-fold | >90% | [225] |
| Auto-NK cells | K562-mb15-41BBL +IL-2 | RPMI 1640 + 10% FBS + 1% Pen/ Strep + IL-2 | 14 days | 270.3 ± 84.3-fold | 86.1 ± 5.3% | [226] |
| Allo-NK cells | K562-41BBL-mbIL15 +IL-2 | RPMI-GlutaMax + 10% human AB serum+IL-2 | 14 days | 34.64–57.74-fold | 79.16 ± 20.53% | [227–231] |
| Allo-NK cells | K562-41BBL-mbIL21 + IL-2 | RPMI 1640 + auto-serum + 1000 IU/mL IL-2 | 14 days | 1024-fold (823–1950-fold) | 81% (73.6–91.6%) | [226] |
| Allo-NK cells | K562-41BBL-CD19-mbIL21 + IL-2 | RPMI 1640 + 10% FBS + 50 IU/ml rhIL-2 | 14 days | >2000-fold | >95% | [227, 232] |
| Purified allo-NK cells (> 95% NK cells) | K562-41BBL-CD19-mbIL21 + IL-2 | RPMI 1640 + 10% FBS + 2 mmol/L l-Glu + 100 U/mL Pen/ Strep +50 IU/ml rhIL-2 | 21 days | 47,967-fold | >95% | [152, 228, 232, 233] |
| K562-41BBL-CD19-mbIL15 + IL-2 | 21 days | 825-fold | ||||
| Allo-NK cells | K562-OX40L + IL-2 + IL-15 + IL-21 | RPMI 1640 + 10% FBS + 100 U/mL Pen+100 µg/mL Strep+ 4 mmol/L l-Glu+100 IU/ml rhIL-2 + 5 ng/mL IL-15 + 100 ng/mL IL-21 | 35 days | 1987.5-fold (301.3–7093.7-fold) | 80% | [146] |
| Allo-NK cells | PC3PSCA-IL-2-4-1BBL-mIL-15mutDAP12 + IL-2 + IL-21 | NK MACS medium + 1% NK MACS supplement + 5% human AB serum+1000 IU/mL rhIL-2 + 20 ng/mL IL-21 | 18 days | 86.2 ± 38.8-fold | 64.3% | [146] |
| Allo-NK cells | Wilms tumor cell line + IL-2 | RHAM· medium+5% Auto-plasma+200 IU/mL rhIL-2 | 14 days | 112.9-fold | 96.0% | [147] |
| Allo-NK cells | Jurkat + rhIL-2 | RPMI 1640 + 10% FBS + 500 IU/mL rhIL-2 | 14 days | 100-fold | 90% | [234] |
| CD3-depleted auto-NK cells | Auto-PBMCs + IL-2 | CellGro SCGM medium + 1% Auto- plasma + 500 IU/ml IL-2 | 14 days | 15,000-fold | 98.2 ± 0.3% | [115] |
| Purified allo-NK cells | Allo-PBMC + IL-2 + IL-15 | SCGM + 1% V/V PHA + 500 IU/ml IL-2 + 10 ng/ml IL-15 | 14 days | 100-fold | 97 ± 1.7% | [235] |
| Purified allo-NK cells (> 95% NK cells) | HLA-I negative EBV-LCL-721.221 + IL-15 + IL2 + IFN-α | 1640 + 10%FBS + 25 IU/ml IL-15 + 100 IU/ml IL-2 + 100 IU/ml IFN-α | 20 days | 60-fold (21–144-fold) | > 90% | [236] |
| Purified allo-NK cells (> 95% NK cells) | EBV-LCL + IL-2 | TexMACS medium + 5% human AB serum+500 IU/mL IL-2 | 14 days | 3000-fold | Not mentioned | [237] |
| Allo-NK cells | RN-T + IL-2 + OK432 | GT-T507α culture medium + 1% Auto-plasma+IL-2 +OK432 | 21 days | 4720-fold (1372–14,116-fold) | 90.96 % | [149] |
| CD3-depleted allo-NK cells (94%) | MSCs +IL-2 | RPMI 1640 + 10% FBS + 1% Pen/ Strep +1% Glu +100 IU/mL IL-2 | 21 days | 16-fold (6.2–24.5-fold) | 91% (89–94%) | [237, 238] |
| Membrane particle | ||||||
| Auto-NK cells | K562-mbIL15-41BBL | SCGM + 10% FBS + 2 mmol/L Glutamax + 100 U/mL IL-2 + 200 μg/mL Membrane particle | 14 days | 424-fold (290–570-fold) | >90% | [108] |
Pen penicillin, Strep streptomycin, EBV Epstein-Barr viral, FBS fetal bovine serum, Glu glutamine, Allo allogeneic, Auto autologous, SCGM stem cell growth medium, EBV-LCL Epstein-Barr virus transformed lymphoblastoid cell line, MSCs bone marrow mesenchymal stem cells, PBMC peripheral blood mononuclear cell, RN-T RetroNectin FN-CH296 stimulated T cells
Feeder cell systems for PBMC-derived NK cell expansion
In addition to strategies employing cytokines, other options including irradiated tumor cells, allogeneic PBMCs and lymphoblastoid derivatives as feeder cells to enhance ex vivo NK cell expansion have been explored. K562 cells, which lack HLA antigen expression, enhance the expansion of NK cells through activated costimulatory signals. A greater than-300-fold NK cell expansion has been reported when using K562 cells as feeder cells combined with IL-2 and IL-15 [146]. However, an only approximately 100-fold expansion was achieved when using cells from other tumor cell lines to stimulate NK cells [147–148]. Feeder cells have been genetically modified with expressed specific costimulatory ligands or membrane-bound interleukins to stimulate the expansion of NK cells. This method was found to be more effective at expanding NK cells than the methods using the soluble form of a cytokine or an unmodified tumor cell line. A membrane-bound form of interleukin (IL)-21 (mbIL21) or mbIL15 and CD137 ligand have been artificially expressed in K562 cells, and this strategy has been reported to mediate NK cell proliferation and generate highly cytotoxic NK cells. However, Fernandez and colleagues reported achieving a higher-fold NK cell expansion and purity by using PBMCs exposed to K562-mbIL21-41BBL cells than by using PBMCs exposed to K562-mbIL15-41BBL cells [150]. An approximately 823–2800-fold expansion of NK cells was achieved by K562-mb21-41BBL cells with 73.6–99% NK cell purity during a 14-day culture, and a corresponding 903-84308-fold expansion with over 90% purity was achieved over the course of 21 days (Table 1). Note that the medium applied to K562-feeder cell expansion systems is always supplemented with fetal bovine serum, human AB serum or human plasma, which bring about concerns of infection risk. Moseman and colleagues demonstrated a twofold-greater expansion of NK cells when using AIM-V medium supplemented with TexMACS, OpTmizer, SCGM, ABS-001, and StemXVivo than when using RPMI 1640 medium supplemented with 10% FBS and 1% GlutaMAX [151]. The expansion system for K562 cell-expanded NK cells still needs to be optimized.
Despite these developments, the available strategies involving the use of tumor cell lines to expand NK cells still present safety hazards in clinical use. Therefore, feeder cell-based systems for expanding NK cells with irradiated cells, such as PBMCs, RetroNectin FN-CH296-stimulated T (RN-T) cells, cells of the Epstein-Barr lymphoblastoid cell line (EBV-LCL), and BM-mesenchymal stem cells, have been developed (Table 1). Of these cells, autologous PBMCs used as feeder cells have been shown to be most advantageous, yielding on average a 15000-fold expansion of NK cells by Day 14 with 98.2 ± 0.3% purity [115].
Membrane particles for PBMC-derived NK cell expansion
In addition, tumor cell membrane particles are also effective alternatives for tumor cell lines for expanding NK cells. Membrane particles harvested as described previously [108]—specifically harvested from K562-mbIL15-41BBL or K562-mbIL21-41BBL cells through cell lysis using nitrogen cavitation at 300 psi and purified using sucrose gradient centrifugation—not only maintained the activation efficiency of the membrane receptor that induced the NK cell expansion but also avoided the risk of tumor cells being mixed in with the amplified product. Compared with K562-mbIL15-41BBL-derived membrane particles, K562-mbIL21-41BBL-derived membrane particles induced NK cells with twice the fold expansion (424-fold vs. 825-fold) by Day 14 of culture with the same purity of expanded NK cells [108]. Interestingly, the proliferation and viability of NK cells were decreased at 22 days of expansion with K562-mbIL15-41BBL-derived membrane particles. However, culture with K562-mbIL21-41BBL-derived membrane particles yielded an over 100,000-fold expansion by Day 28. The purity and cytotoxicity of these NK cells were similar on days 28 and 14 [108].
Gene manipulation of PBMC-derived NK cells
At present, PB-NK cells can be expanded for up to 15 weeks in vitro, and the maximum output of expanded PB-NK cells can reach 1 × 1013 cells [152, 153]. However, a better amplification effect can be achieved through genetic modification of PB-NK cells. With the help of genetic modification, the ex vivo expansion time can be prolonged, a larger amount of cells can be produced, and the dosage of cytokine cocktail can be reduced.
Fujisaki et al. showed that TERT, the gene encoding human telomerase reverse transcriptase, can affect the amplification time of PB-NK cells. TERT was transduced into 7 day-expanded PB-NK cells, which recovered NK cell proliferation. and the NK cells continue to expand for 150 weeks in vitro [153]. In another investigation, the proliferation ability of inducible MyD88/CD40 (iMC)-modified NK cells was stronger. After 8 days of expansion by irradiated K562 cells, the number of iMC-modified NK cells was three times more than that of unmodified NK cells. Moreover, data shows that treatment with rimiducid (Rim), a small molecule reagent that can activate iMC in NK cells, during the expansion process increased the number of iMC-NK cells by 2 times [154]. In addition, it has been reported that the low IL-2 requirement can be achieved by editing PB-NK cells to express cytokines or cytokine receptors. Imamura et al. generated mbIL15-PB-NK cells via retroviral particle infection. In the absence of IL-2, these mbIL15-PB-NK cells were detected up to 75 days in vitro, compared to the mock-PB-NK cells, which persisted only 2 weeks [155]. Overexpression of thrombopoietin (TPO) receptor (c-MPL) in NK cells can cause the cells to expand at a low IL-2 concentration (25 U/ml) in response to TPO (50 ng/ml), and the expansion fold is the same as that achieved with the 500 U/ml IL-2 culture (approximately 34-fold) [156].
Expansion of NK cells from cord blood
Two different methods have been used to obtain large numbers of NK cells from cord blood. One method involves expanding NK cells in UCB, and another is to induce cord blood-derived CD34+ hematopoietic stem and progenitor cells (HSPCs) to differentiate into NK cells, which are then expanded. Although UCB-NK cells express lower levels of KIR, NKG2C, DNAM1, adhesion molecules, and IL-2Ra and higher levels of CD94/NKG2A and CXCR4 than peripheral blood (PB)-derived NK cells [157, 158], the frequency of NK cells in UCB is approximately 15–20%, slightly higher than that in PB [158]. Moreover, the cytotoxicity of ex vivo-expanded UCB-NK cells under some conditions has been shown to be similar to that of PB-NK cells [159].
Expansion systems for cord blood CD56+ cell- or MNC-derived NK cells
Various methods for expanding UCB-NK cells or UCB-mononuclear cells (MNCs) have been investigated in several studies. Most of the studies employed feeder cells in the expansion systems. However, the expansion folds of the different systems have differed considerably. Coculture of CD3-depleted UCB-MNCs with bone marrow stem cells as feeder cells (at a ratio of 8:1) and in the presence of IL-2, IL-15, IL-3, and FLT-3L expanded NK cells 104 ± 15-fold over the course of 2 weeks but resulted in an NK cell purity of only 65 ± 10% [160], which is relatively low for an expansion system using purified NK cells as the starting material. Another group reported coculturing CD3-depleted UCB-MNCs with cells of the EBV-transformed HLA-I and B lymphoblastoid cell line PLH (at a ratio of 1:4) and in the presence of IL-2 and IL-15 for approximately 3 weeks, at which point the UCB-NK cells showed an average 700-fold expansion with a purity of 94.28 ± 2.08% [161]. The Katy group reported a large-scale UCB-NK cell expansion system using feeder cells. They cocultured UCB-MNCs with K562 cells expressing mbIL21, 4-1BBL, CD64 and CD86 (at a ratio of 1:2) and in the presence of IL-2. Depletion of CD3+ cells was performed every 7 days during the cultivation process. This system can expand UCB-NK cells 3000-fold on average in approximately 2 weeks with an NK cell purity above 95% [162].
Single or simple combinations of cytokines such as IL-2, IL-5, and IL-21 have been used to expand UCB-NK cells. However, due to the limited expansion seen with these conditions, new feeder-free expansion systems are being developed. The Wu group reported that UCB-MNCs stimulated with IL-2, group A streptococcus, and zoledronate for 3 weeks can yield NK cells with an average purity of 95% and a 1561-fold expansion [163]. This feeder-free method reduces the risk of introducing other types of cells into the final product, but the purity of NK cells still needs to be improved.
Differentiation and expansion systems for NK cells derived from cord blood CD34+ cells
Although CD34+ HSPCs make up only approximately 1% of nucleated cord blood cells, the convenience and stability of cryopreserved UCB cells have resulted in UCB CD34+ cells becoming a relatively common source of NK cells.
Previously, major studies in this area employed stromal cells such as M2-10B4, AFT024, EL08.1D2, and OP9-DLL4 cells in the differentiation and expansion systems. These stromal cells combined with the cytokines IL-3, IL-7, IL-15, SCF, and FLT-3L can induce the differentiation of CD34+ cells into NK cells. After approximately 4–5 weeks, up to approximately 4000-fold-expanded cell products can be obtained, with NK cell purity levels greater than 90% [159, 164–166]. However, these stromal cells are not of human origin, and this issue has created a bottleneck in the clinical application of the resulting products because of the use of heterogeneous materials.
The Dolstra and Spanholtz group in The Netherlands reported the largest cytokine cocktail expansion system to date, using UCB CD34+ cells as the starting cells. They used GMP-grade serum-free medium, namely, Glycostem basal growth medium, combined with 3 different cytokine combinations (SCF, IL-7, FLT-3L, TPO, G-CSF, GM-CSF, and IL-6 in the first 9 days; SCF, IL-7, FLT-3L, IL-15, G-CSF, GM-CSF and IL-6 during Days 9–14; and SCF, IL-7, IL-15, IL-2, G-CSF, GM-CSF, and IL-6 starting at Day 14) at different stages of expansion and differentiation to induce CD34+ cells to differentiate into NK cells and expand the NK cells. After approximately 5 weeks, the cells were found to have expanded by more than 15000-fold (ranging from 16,991-fold to 73,666-fold), with a CD56+ cell purity of over 95%. Later, the researchers also developed a closed-system culture process. This culture process has made it possible to provide off-the-shelf NK cell products for immunotherapy [167–169].
Regardless of whether the induction system contains stromal cells or a cytokine cocktail, the UCB CD34+ cell-derived NK cells expressed high levels of NKG2D, 2B4, CD161, NKp46, NKp44, and NKp30. KIRs, CD16, and NKG2C were expressed at low levels or barely expressed [159, 165, 166, 168, 170]. Grzywacz and colleagues showed that most cells acquired receptors, such as NKG2D, NKp30, and NKp46, during the differentiation process; however, the vast majority of iNK cells consistently expressed CD161 and NKp44 [166].
Genetic manipulation of cord blood-derived NK cells
Genetic modification of CB-NK cells to achieve better persistence in vivo mainly focuses on the TGFβR and IL-15 signaling pathways. Studies have reported that CB-NK cells expressing a dominant-negative TGFβR can continue to exert their antitumor function in the presence of TGF-β [171, 172]. Droplet digital PCR (ddPCR) detection results show that CB-NK cells overexpressing the truncated TGFβRII domain can persist in vivo for approximately 2 months [173]. However, more intuitive evidence for prolonged persistence of NK cells can be obtained by flow cytometry results. IL-15-overexpressing CB-NK cells can be detected in peripheral blood and many tissues at high frequencies, more than 80%, at approximately 49 days, and these CAR NK cells still exist in vivo for approximately ten weeks [174]. CISH gene knockout has been reported to prolong the in vivo persistence duration of CB-NK cells. At approximately 1 month, iC9/CAR19/IL-15 CISH−/− CB-NK cells in peripheral blood can be detected by flow cytometry, and the percentage of these modified cells is 40%, which is 2-fold higher than that of iC9/CAR19/IL-15 CB-NK cells [175].
Expansion of NK cells from iPSCs or ESCs
In addition to obtaining NK cells from PB and UCB as mentioned above or using NK cell lines, NK cells can also be induced from PSCs, allowing for off-the-shelf production (Fig. 1). Studies have shown that PSC-derived NK cells not only have the advantages of primary NK cells and NK cell lines but also lack their problems [176]. PB-NK and UCB-NK cells are easily accessible, but the number and purity of expanded NK cells are greatly affected by the donor, and the products are usually heterogeneous [158]. Cells of NK cell lines are homogeneous, and it is often necessary to inhibit the proliferation of such cells in the treatment of patients; however, such inhibition also affects the antitumor activity [177]. Although studies have suggested that NK cells differentiated from PSCs show immature phenotypes, such as higher levels of NKG2A expression, studies have also shown that PSCs can be induced to differentiate into NK cells resembling primary NK cells, such as iPSC-NK cells expressing multiple NK receptors (NKG2D, TRAIL, CD16, and so on) [178]. In brief, iPSC- or embryonic stem cell (ESC)-derived NK cells have attracted widespread attention because they can provide homogeneous, highly cytotoxic NK cell products.
Differentiation system for iPSC- or ESC-derived NK cells
The differentiation of PSCs into NK cells is currently thought to proceed in two stages, with PSCs differentiating into hematopoietic progenitor cells (HPCs) in the first stage and HPCs differentiating into NK cells in the second stage (Fig. 2) [178]. The cultivation time for such differentiation experiments is approximately 5–8 weeks, and such cultivation has resulted in a high purity of NK cells (>90%) (Table 2) [113, 123, 159, 179, 180]. The system established by the Kaufman group to induce the differentiation of PSCs into NK cells is relatively mature and includes a feeder system and a feeder-free system [178, 181, 182].
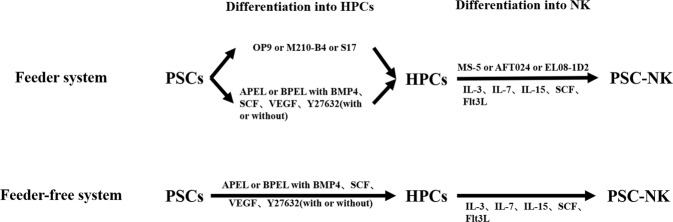
Fig. 2.
At present, the differentiation stage of PSC-NK can be divided into hematopoietic progenitor cell differentiation stage and NK differentiation stage, and the differentiation system can be divided into two systems according to the existence of feeder cells. For feeder system (up panel), some xenogeneic stromal cell lines, such as OP9, M210-B4, or S17, were used to form hematopoietic progenitor cells (HPCs). However, HPCs can also be obtain through spin embryoid bodies (EBs) with the help of the combination of cytokines (BMP4, SCF, and VEGF) and small molecules (Y-27632). Subsequently, HPCs were co-incubated with MS-5, AFT024 or EL08-1D2, accompanied by the presence of cytokines (IL-7, IL-15, SCF, Flt3L, and IL-3 only first week), finally to generate PSC-NK. For feeder-free system (bottom panel), two differentiation stage did not involve xenogeneic stromal cell lines, and the induction was completed under the combination of cytokines
Table 2.
Differentiation system of NK cell derived from iPSC or EC
| Source | Differentiation system | Medium | Culture time | Expansion fold | Purity of NK | Ref. | |
|---|---|---|---|---|---|---|---|
| Phase I | Phase II | ||||||
| Differentiation system based on feeder cells | |||||||
| hESC (H1 and H9) | OP9 + MS-5 | α-MEM + 10% FBS + 100 μM MTG | α-MEM + 10% FBS + 100 μM MTG + 50 ng/mL SCF + 50 ng/mL Flt3L + 10 ng/mL IL-3(first week) + 20 ng/mL IL-7 + 20 ng/mL IL-15 | 4 weeks | 10-fold | 11.3 ± 2.4% | [239] |
| hESC (H9) | S17 + AFT024 | RPMI 1640 + 15% FBS + 2 mM l-glutamine + 0.1 mM β-ME + 1% MEM-nonessential amino acids + 1% P/S | DMEM: HAMS/F12(2:1) + 20% heat-inactivated human AB serum + 5 ng/ml sodium selenite + 50 μM ethanolamine + 20 mg/L ascorbic acid + 25 μM β-ME + 1% P/S + 10 ng/ml IL-15 + 5 ng/ml IL-3(first week) + 20 ng/ml IL-7 + 20 ng/ml SCF + 10 ng/ml Flt3L | 3–9 weeks | 40-fold | Not mentioned | [240] |
| hESC (H9) | M210-B4 + AFT024 | RPMI 1640 + 15% FBS + 2 mM l-glutamine + 0.1 mM β-ME + 1% MEM-nonessential amino acids + 1% P/S | DMEM: HAMS/F12(2:1) + 20% heat-inactivated human AB serum + 5 ng/ml sodium selenite + 50 μM ethanolamine + 20 mg/L ascorbic acid + 25 μM β-ME + 1% P/S + 10 ng/ml IL-15 + 5 ng/ml IL-3(first week) + 20 ng/ml IL-7 + 20 ng/ml SCF + 10 ng/ml Flt3L | 7–8 weeks | 100-fold | 98.5% | [181] |
| hESC (H9) iPSC (BJ1-iPS12) | M210-B4 + AFT024 | RPMI 1640 + 15% FBS + 2 mM l-glutamine + 0.1 mM β-ME + 1% MEM-nonessential amino acids + 1% P/S | DMEM: HAMS/F12(2:1) + 20% heat-inactivated human AB serum + 5 ng/ml sodium selenite + 50 μM ethanolamine + 20 mg/L ascorbic acid + 25 μM β-ME + 1% P/S + 10 ng/ml IL-15 + 5 ng/ml IL-3(first week) + 20 ng/ml IL-7 + 20 ng/ml SCF + 10 ng/ml Flt3L | 7–8 weeks | Not mentioned | Not mentioned | [182] |
| hESC (H9 and H1) iPSC (UCBiPS7, NHDFiPS, BJ1-iPS) | M210-B4 + EL08-1D2 | RPMI 1640 + 15% defined FBS + 2 mM l-glutamine + 1% nonessential amino acids + 1% P/S + 0.1 mM β-ME | IL-3 + IL-7 + IL-15 + SCF + Flt3L | 7~8 weeks | Not mentioned | Not mentioned | [178] |
| iPSC (PBC) | OP9 + OP9-DLL1 | a-MEM + 20% FBS | a-MEM + 20% FBS + 10 ng/mL SCF + 5 ng/mL Flt3L + 5 ng/mL IL-7 + 10 ng/mL IL-15 | 6–7 weeks | 5-fold | 99% | [179] |
| hESC (H9 and H1) iPSC (DF19-9-7T, IISH2i-BM9) | OP9-DLL4 | IF9S + 50 ng/mL FGF2 + 50 ng/mL BMP4 + 15 ng/mL Activin A + 2 mM LiCl + 10 μM TGF-β inhibitor (day 2: 50 ng/ml FGF + 50 ng/ml VEGF; day 4 and 6: 50 ng/ml FGF2 + VEGF + TPO + SCF + IL-6 + 10 ng/ml IL-3) | α-MEM + 20% FBS + 100 ng/ml Flt3L + 40 ng/ml SCF + 5 ng/ml IL-7 + 35 nM UM171 α-MEM media + 10 ng/ml IL-2 + 5 ng/ml IL-15 | 4–5 weeks | Not mentioned | >90% | [180] |
| iPSCs (UCBiPS7) | STEMdiff Hematopoietic Kit + AFT024 | STEMdiff Hematopoietic Kit | DMEM: HAMS/F12(2:1) + 20% heat-inactivated human AB serum + 5 ng/ml sodium selenite + 50 μM ethanolamine + 20 mg/L ascorbic acid + 25 μM 2-ME + 1% P/S + 10 ng/ml IL-15 + 5 ng/ml IL-3(first week) + 20 ng/ml IL-7 + 20 ng/ml SCF + 10 ng/ml Flt3L | 7–8 weeks | Not mentioned | Not mentioned | [189] |
| hESC (H1) | Spin EB + MS-5/SP-HOXB4 + MS-5 | IMDM + 15% FCS + 1 mM l-glutamine + 1% P/S + 0,1 mM β-ME + 1% nonessential amino acids + 10 ng/mL FGF + 100 ng/mL SCF + 100 ng/mL Flt3 + 10 ng/mL IL-3 + 10 ng/mL IL-6 + 50 ng/mL G-CSF + 10 ng/mL BMP4 + 10 ng/mL VEGF | Standard complete H5100 human long-term culture medium RPMI 1640 + 5% FCS + 10% human AB serum + 1 mM l-glutamine + 1% P/S + 0.1 mM β-ME + 50 ng/mL SCF + 50 ng/mL Flt3L + 5 ng/mL IL-2 + 20 ng/mL IL-15 + 20 ng/ mL IL-7 | 8 weeks | 32.2 ± 22.3-fold | 66.2 ± 24.4% | [241] |
| hESC iPSC | Spin EB + EL08-1D2 | BPEL + 40 ng/ml SCF + 20 ng/ml + VEGF + 20 ng/ml BMP4 | IL-3(first week) + IL7 + IL-15 + SCF + Flt3L | 6 weeks | 56–111-fold | Not mentioned | [242] |
| hESCs (H9) iPSCs (UCBiP7) | Spin EB + EL08-1D2 | BPEL + 40 ng/ml SCF + 20 ng/ml + VEGF + 20 ng/ml BMP4 | IL-3 + IL7 + IL-15 + SCF + Flt3L | 6 weeks | 56.8-fold | 96.7% | [178] |
| hESCs (H9) iPSCs (UCBiP7) | Spin EB + EL08-1D2 | BPEL + 40 ng/ml SCF + 20 ng/ml + VEGF + 20 ng/ml BMP4 | IL-3 + IL7 + IL-15 + SCF + Flt3L | 6–7 weeks | Not mentioned | >90% | [186] |
| hESC (H9 and H1) iPSC (CD34+ UCB, fibroblasts, PBC) | Spin EB + EL08-1D2 | BPEL + 40 ng/ml SCF + 20 ng/ml + VEGF + 20 ng/ml BMP4 | DMEM: HAMS/F12(2:1) + 15 % heat-inactivated human AB serum + 2 mM l-glutamine + 1 μM β-ME + 5 ng/mL sodium selenite + 50 μM ethanolamine + 20 mg/L ascorbic acid + 1 % P/S + 5 ng/mL IL-3(first week) + 20 ng/mL SCF + 20 ng/mL IL-7 + 10 ng/mL IL-15 + 10 ng/mL Flt3L | 5–7 weeks | 3.47–11.57-fold | Not mentioned | [243] |
| hESCs (H9) iPSC (CD34+ UCB) | Spin EB + OP9-DLL1 | BPEL + 40 ng/ml SCF + 20 ng/ml + VEGF + 20 ng/ml BMP4 | NK differentiation media + SCF + IL-15 + IL-7 + Flt3L + IL-3(first week) | 6 weeks | 10–15-fold | 50.6% | [192] |
| iPSC (Fibroblasts) | Spin EB + EL08-1D2 | DMEM: HAMS/F12(2:1) + 4.5 g/l glucose + l-glutamine + 24 M β-ME + 50 M ethanolamine + 20 mg/l ascorbic acid + 50 g/l sodium selenite + 1% P/S + 20% heat-inactivated human AB serum + 10 ng/ml IL-15 + 5 ng/ml IL-3 + 20 ng/ml IL-7 + 20 ng/ml SCF + 10 ng/ml Flt3L | 5 weeks | 105–106-fold (from iPSC) | 40–92.9% | [187] | |
| Differentiation system based on cytokines | |||||||
| hESCs (H9) iPSCs (UCBiP7) | Spin EB | BPEL + 40 ng/ml SCF + 20 ng/ml + VEGF + 20 ng/ml BMP4 | IL-3(first week) + IL7 + IL-15 + SCF + Flt3L | 6 weeks | 40.4-fold | 76.4% | [178] |
| hESCs iPSCs | Spin EB | BPEL + 40 ng/ml SCF + 20 ng/ml + VEGF + 20 ng/ml BMP4 | IL-3(first week) + IL7 + IL-15 + SCF + Flt3L | 6 weeks | 56–111-fold | Not mentioned | [242] |
| hESCs (H9) | Spin EB | BPEL + 40 ng/ml SCF + 20 ng/ml + VEGF + 20 ng/ml BMP4 | IL-3(first week) + IL7 + IL-15 + SCF + Flt3L | 6–7 weeks | Not mentioned | Not mentioned | [244] |
| hESCs (H9) iPSCs (UCBiP7) | Spin EB | BPEL + 40 ng/ml SCF + 20 ng/ml + VEGF + 20 ng/ml BMP4 | IL-3(first week) + IL7 + IL-15 + SCF + Flt3L | 6–7 weeks | Not mentioned | >90% | [186] |
| hESC (H9 and H1) iPSC (CD34+ UCB, fibroblasts, PBC) | Spin EB | BPEL + 40 ng/ml SCF + 20 ng/ml + VEGF + 20 ng/ml BMP4 | DMEM: HAMS/F12(2:1) + 15 % heat-inactivated human AB serum + 2 mM l-glutamine + 1 μM β-ME + 5 ng/mL sodium selenite + 50 μM ethanolamine + 20 mg/L ascorbic acid + 1 % P/S + 5 ng/mL IL-3 + 20 ng/mL SCF + 20 ng/mL IL-7 + 10 ng/mL IL-15 + 10 ng/mL Flt3L | 6–7 weeks | 3.47–11.57-fold | Not mentioned | [240, 243] |
| iPSCs (UCBiP7) | Spin EB | BPEL + 40 ng/ml SCF + 20 ng/ml + VEGF + 20 ng/ml BMP4 | IL-3(first week) + IL7 + IL-15 + SCF + Flt3L | 6–7 weeks | Not mentioned | >97% (98.3%) | [184] |
| iPSCs (UCBiP7) | Spin EB | APEL + 40 ng/ml SCF + 20 ng/ml + VEGF + 20 ng/ml BMP4 | 5 ng/mL IL-3(first week) + 10 ng/mL IL-15 + 20 ng/mL IL-7 + 20 ng/mL SCF + 10 ng/mL Flt3L | 6–7 weeks | Not mentioned | Not mentioned | [131] |
| hESC (H9 and H1) iPSC (CD34+ UCB, fibroblasts, PBC) | Spin EB | APEL + 10 μM Y-27632 + 40 ng/ml SCF + 20 ng/ml + VEGF + 20 ng/ml BMP4 | DMEM: HAMS/F12(2:1) + GlutaMAX™-I + 15 % heat-inactivated human AB serum + 1 % P/S + 2 mM l-glutamine + 25 μM β-ME + 5 ng/mL sodium selenite + 50 μM ethanolamine + 20 mg/L ascorbic acid + 5 ng/mL IL-3(first week) + 10 ng/mL IL-15 + 20 ng/mL IL-7 + 20 ng/mL SCF + 10 ng/mL Flt3L | 4-5 weeks | 4.2–42-fold | >90% | [164, 182] |
| iPSC (CD34+ UCB cells) | Spin EB | APEL + 10 μM Y-27632 + 40 ng/ml SCF + 20 ng/ml + VEGF + 20 ng/ml BMP4 | DMEM: HAMS/F12(2:1) + GlutaMAX™-I + 15 % heat-inactivated human AB serum + 1 % P/S + 2 mM l-glutamine + 25 μM β-ME + 5 ng/mL sodium selenite + 50 μM ethanolamine + 20 mg/L ascorbic acid + 5 ng/mL IL-3(first week) + 10 ng/mL IL-15 + 20 ng/mL IL-7 + 20 ng/mL SCF + 10 ng/mL Flt3L | 5–6 weeks | 10-fold | >90% | [123] |
| hiPSC (hnCD16-iPSC) | Spin EB | APEL + 10 μM Y-27632 + 40 ng/ml SCF + 20 ng/ml + VEGF + 20 ng/ml BMP4 | DMEM: HAMS/F12(2:1) + 2 mM l-glutamine + 1% P/S + 25 μM β-ME + 20% heat-inactivated human serum AB + 5 ng/mL sodium selenite + 50 μM ethanolamine + 20 mg/mL ascorbic acid + 5 ng/mL IL-3(first week) + 10 ng/mL IL-15 + 20 ng/mL IL-7 + 20 ng/mL SCF + 10 ng/mL Flt3L | 5–6 weeks | Not mentioned | 96% | [245] |
| iPSC | Spin EB | APEL2 + 40 ng/ml SCF + 20 ng/ml + VEGF + 20 ng/ml BMP4 | 5 ng/mL IL-3(first week) + 10 ng/mL IL-15 + 20 ng/mL IL-7 + 20 ng/mL SCF + 10 ng/mL Flt3L | 5–6 weeks | Not mentioned | Not mentioned | [159] |
| iPSC (PBC) | Spin EB | IMDM + 20% FBS + 100 ng/mL SCF + 10 ng/mL IL-3 + 10 ng/mL IL-6 + 20 ng/mL Flt3L + 20 ng/mL BMP4 | 5 ng/mL IL-3(first week) + 10 ng/mL IL-15 + 20 ng/mL IL-7 + 20 ng/mL SCF + 10 ng/mL Flt3L | 7 weeks | Not mentioned | 76.2% | [185] |
| QHJI-iPSC (PBC) | Spin EB | StemPro-34 + 2 mmol/L l-glutamine + 400 μM monothioglycerol + 50 μg/mL ascorbic acid-2- phosphate + insulin-transferrin-selenium supplements + 40 ng/mL hBMP-4 + 10 ng/mL FGF + 50 ng/mL VEGF | 50 ng/mL SCF + 20 ng/mL Flt3L + 20 ng/mL IL-3 + 30 ng/mL TPO FcDLL4-coated plates + 10 ng/mL Flt3L + 5 ng/mL IL-7 | 6 weeks | 164-fold | 96.9% | [113] |
β-ME β-mercaptoethanol, BMP4 bone morphogenetic protein 4, FGF fibroblast growth factor, Flt3L Fms-related Tyrosine Kinase 3 Ligand, G-CSF granulocyte colony stimulating factor, LiCl Lithium chloride, MTG monothioglycerol, P/S penicillin–streptomycin, PBC peripheral blood cells, SCF stem cell factor, Spin EB Spin embryoid bodies, TPO thrombopoietin, UCB umbilical cord blood, VEGF vascular endothelial growth factor
Some xenogeneic stromal cell lines, such as S17, OP9, and M210-B4, have been used to form HPCs, and the percentages of CD34+ or CD34+CD45+ cells in the HPCs were relatively low (mostly 10–20%) (Table 2) [181, 182]. In these processes, the CD34+ or CD34+CD45+ cells were then sorted and subjected to NK cell differentiation for approximately 4–5 weeks. Subsequently, the HPCs were cocultured with MS-5, AFT024, or EL08-1D2 cells; a wide range of purity levels of NK cells, between 10% and 90% (Table 2), resulted from these experiments [181, 182]. In experiments carried out in 2005, S17 and AFT024 cells were used to generate HPCs and NK cells, respectively, but only 29.0–37.5% of the cells obtained were CD45+CD56+ cells, and most of the other cells expressed CD33, indicating that the cells were in the early stages of differentiation. The Kaufman group then set out to optimize the system. They sorted CD34+CD45+ cells by cocultivating H9 human embryonic stem cells (hESCs) with M210-B4 cells (but not with S17 cells) and then culturing the resulting cells with irradiated AFT204 cells for 30–35 days. As a result of this optimization, 97.8% of the cells they obtained were CD56+ cells [181]. However, other studies have shown that HPCs can be generated using spin embryoid bodies (EBs) without the need to sort CD34+ cells for NK cell differentiation, providing a new way to obtain HPCs [183]. In that study, CD56+ cell purity levels of approximately 40% to 92.9% were obtained.
The use of xenogeneic cells may cause safety concerns, so a feeder-free system was established for the sake of clinical production efforts. This system was designed to be based on spin EBs without stromal cells used during culture, with the spin EBs seeded in 24-well plates only cultured with IL-3 for the first week; subsequently, SCF, IL-7, IL-15, and Flt3L were used in the NK differentiation medium for NK cell formation for the next 4–5 weeks. This process has been found to yield a high purity of CD56+ cells (>90%) at the final harvest (Table 2) [159, 178, 184]. The Kaufman group provided an improved protocol on the basis of this protocol. PSCs were made to experience feeder-free adaptation for only 1–2 passages. HPCs could be formed by using spin EBs after only approximately 1–2 weeks, which greatly shortened the culture time [123, 185]. However, the cell numbers increased from an initial 3000/well to 8000/well. In addition, several studies have established other differentiation systems. Wang and colleagues differentiated PBC-iPSCs (iPSCs derived from peripheral blood cells) into NK cells in a two-stage culture. OP9 and OP9-DLL1 cells were used in the first and second stages, respectively, and 99% of the cells obtained were NK cells [179]. Kaneko and colleagues reported the differentiation of iPSCs into NK cells in three steps: they made iPSCs differentiate into spin EBs first and then let those spin EBs form lymphocyte progenitor cells (LCPs) (CD7+CD45+ cells); finally, PHA was used to differentiate the LCPs into NK cells [113]. In this system, the purity levels of CD34+CD45+ cells and CD7+CD45+ cells reached as high as 86.8% and 96.9%, respectively [113]. NK cells induced by all of these systems were mature, expressing high levels of CD16 and other NK cell receptors. They exhibited cytotoxic functions against tumor cells in vitro and in vivo [113, 123, 159, 186].
Expansion system for iPSC- or ESC-derived NK cells
Studies have shown only a small amount of amplification, approximately 5–40-fold, for PSC-NK cells in the differentiation stage (Table 2) [152, 178]. Therefore, it is necessary to take additional steps to expand NK cells to obtain enough NK cells for preclinical or clinical treatments. Currently, feeder systems are mainly used to expand PSC-NK cells, and feeder cells include genetically modified, irradiated K562 cells and irradiated PBMCs.
K562 cells transduced to express CD64, CD86, CD137L, truncated CD19, and mbIL21 (K562-41BBL-mbIL21) were generated by Lee and are the most common feeder cells used in NK cell expansion, yielding 102- to 103-fold PSC-NK cell expansion [159]. Kaufman and colleagues used irradiated mbIL21-CD137L-K562 cells for iPSC-NK (iNK) cell expansion, and this process yielded a total expansion of 105–106-fold [187]. Note that the total expansion “fold” values here each refer to the number of iNK cells resulting after 3 weeks relative to the number of iPSC cells on Day 0. PSC-NK cells can be expanded for more than 60 days, and the cytotoxic activity is not decreased during this expansion time. Other feeder cells are also used to expand PSC-NK cells, such as irradiated PBMCs [113]. In contrast to the system established by Kaufman, the system used by Kaneko and colleagues allowed HPCs to differentiate into LPCs, as mentioned above, and this differentiation was accompanied by a 164-fold expansion. Subsequently, the number of LPCs stimulated with irradiated PBMCs and PHA increased from 5 × 107 cells to 3.3 × 109 cells during the two-week expansion period (Table 3) [113]. In brief, NK cells induced by PSCs can be expanded in vitro to obtain a larger number of cells, specifically at least 109 cells, when starting with 106 cells.
Table 3.
Expansion system of NK cell derived from iPSC or ESC
| Feeder cells NK: feeder cells | Medium | Culture time | Expansion fold | Purity of NK | Ref. | |
|---|---|---|---|---|---|---|
| initial | Expanded | |||||
| K562-CD64-CD86-41BBL-CD19-mbIL21 D0 1:2; Weekly 1:1 | RPMI 1640 + 10% FBS + 50 IU/ml rhIL-2 + l-glutamine + P/S | 6 weeks | >108-fold | ~10% | ~80% | [159] |
| K562-CD64-CD86-41BBL-CD19-mbIL21 D0 1:2; Weekly 1:1 | RPMI 1640 + 15% FBS + 1% P/S + 50 U/ml IL-2 | >6 weeks | 102~103-fold | 70-95% | >99% | [183] |
| K562-CD64-CD86-41BBL-CD19-mbIL21 D0 1:2; Weekly 1:1 | RPMI 1640 + 10 % FBS + 2 mM l-glutamine + 1 % P/S + 50 U/mL IL-2 | >6 weeks | >103-fold | No mentioned | No mentioned | [240, 243] |
| K562-CD64-CD86-41BBL-CD19-mbIL21 D0 1:2; Weekly 1:1 | RPMI 1640 + 10 % FBS + 2 mM l-glutamine + 1 % P/S + 50 U/mL IL-2 | No mentioned | a several log-fold | >97% | >97% | [184] |
| K562-CD64-CD86-41BBL-CD19-mbIL21 D0 1:2; Weekly 1:1 | 60% DMEM + 30% Ham’s F12 + 10% human AB serum + 20 µM β-ME + 50 µM ethanolamine + 20 µg/ml ascorbic Acid + 5 ng/ml sodium selenite + 10 mM HEPES + and 100–250 IU/ml IL-2 | No mentioned | No mentioned | No mentioned | No mentioned | [189] |
| K562-CD64-CD86-41BBL-CD19-mbIL21 D0 1:2; Weekly 1:1 | RPMI 1640 + 10 % FBS + 2 mM l-glutamine + 1 % P/S + 50 U/mL IL-2 | 4 weeks | >103-fold | 95% | No mentioned | [164, 182] |
| K562-CD64-CD86-41BBL-CD19-mbIL21 D0 1:2; Weekly 1:1 | RPMI 1640 + 15% FBS + 1% P/S + 50 U/ml IL-2 | No mentioned | No mentioned | No mentioned | No mentioned | [131, 145] |
| K562-CD64-CD86-41BBL-CD19-mbIL21 D0 1:2; Weekly 1:1 | RPMI 1640 + 2 mM l-glutamine + 1% P/S + 1% nonessential amino acids + 10% standard FBS or 10% human serum AB + 50-100 U/mL IL2 | No mentioned | No mentioned | No mentioned | No mentioned | [245] |
| K562-mbIL15-41BBL cells 1:10 | No mentioned | 2 weeks | 74-fold | No mentioned | No mentioned | [179] |
| irradiated PBMC No mentioned | No mentioned | 2 weeks | 66-fold | No mentioned | No mentioned | [113] |
mbIL21 membrane-bound IL-21, P/S penicillin–streptomycin, β-ME β-mercaptoethanol.
Genetic manipulation of iPSC- or ESC-derived NK cells
Studies have reported that genetically modified iPSCs can maintain good pluripotency [186, 188, 189]. iPSC- or ESC-derived NK cells provide a convenient gene manipulation platform for genetic modifications at the PSC stage.
A series of studies have shown that the modification of IL-15 and CIS can improve the proliferation ability of PSC-NK cells by lowering cytokine requirements. Zhu et al. used the CRISPR system to knock out the CIS protein, which can block the JAK/STAT signaling pathway downstream of IL-15, such that the modified iPSC-NK cells could survive at a low IL-15 concentration (1 ng/ml) [152]. Fate Therapeutics has developed iPSC-NK cell products, such as FT576, in which the IL-15RF (IL-15/IL-15 Rα fusion) protein is knocked in, and they can live for long periods in vitro without additional cytokines [190, 191]. Bjordahl et al. reported that FT576 could induce approximately 61-fold expansion ex vivo without cytokines in the presence of IL-15RF, while WT iPSC-NK cells only expanded fourfold [190].
During the process of NK cell differentiation from PSCs, the production of NK cells can also be increased by gene modifications. Angelos et al. used the CRISPR/Cas9 system to knock out aryl hydrocarbon receptor (AHR) in hESCs (AHR-/- hESCs), and this strategy produced more hematopoietic progenitor cells than a strategy employing WT hESCs during the early stage of differentiation (CD34+CD45+ 35.5% vs. 12.9%; CD34+CD43+ 38.2% vs. 22.3%), and the differentiation efficiency of mature hematopoietic lineages (CD34−CD45+) was better than that of WT hESCs (87.7% vs. 51.3%) [192].
In addition, CIS deficiency can also extend the persistence of iPSC-NK cells in vivo. Two weeks after NK cell reinfusion treatment in a mouse xenograft tumor model, the percentage of CISH−/− iPSC-NK cells in peripheral blood was approximately 13-fold higher than that of WT iPSC-NK cells (3.75% vs. 0.29%), which means that the absence of CISH prolonged the persistence of NK cells in the mice.
Expansion of NK cells from cell lines
Human NK cell lines
The very first NK/T cell line suggested to be used for adoptive transfer was TALL-104 in the early 90 s by Cesano A et al. Currently, several NK cell lines, including NK-92, NKG, NKL, KHYG-1, YT, NK-YS, SNK-6, and IMC-1, are available. Although all of these cell lines are derived from patients with leukemia or lymphoma, it is convenient to acquire, culture, and genetically manipulate these cell lines, and for these reasons, they remain a source for NK cell therapy.
Different cell lines differ with regard to molecular expression and cytotoxicity. NK-92 cells were established by Hans Klingemann at the Terry Fox Laboratory in Vancouver, Canada. The NK-92 cell line was established from a male patient with non-Hodgkin’s lymphoma. NK-92 cells are positive for the surface markers CD56 and CD25 and negative for CD3, CD4, CD8, CD16, CD32, CD64, and HLA-DR. Therefore, the growth of NK-92 cells is IL-2-dependent and cannot cause ADCC [193]. Further research showed that NK-92 cells lack most of the KIRs and only express KIR2DL4, which can bind to HLA-G and mediate inhibitory effects. However, the cells express high levels of activating molecules and cytotoxic effector molecules, including NKG2D, NKp30, NKp46, 2B4, TRAIL, FasL, and TNF alpha. This expression pattern indicates that NK-92 cells display high cytotoxic activity [194]. The very first clinical application of unmodified NK-92 cells was reported by Tonn, T et al. in J Hematotherapy and Stem Cell Research in 2001.
The NKG cell line was also established from a male patient with non-Hodgkin’s lymphoma. NKG cells are positive for the cell surface markers CD56 and CD25 and negative for CD3, CD4, CD8, and CD16. A detailed cell surface molecular analysis showed that NKG cells express high levels of the activating molecules NKp30, NKp44, NKp46, NKG2D, and NKG2C and the cytotoxic effector molecules IFN gamma, TRAIL, FasL, granzyme B, and perforin and low levels of KIR2DL2/L3. This expression pattern indicates that NKG cells also display high cytotoxic activity. However, blocking NKG2D and NKp30 can completely abolish the cytotoxicity of NKG cells toward tumor cells [195].
The KHYG-1 cell line was established from a female patient with NK cell leukemia. KHYG-1 cells are positive for the cell surface markers CD8, CD33, CD56, CD158a, CD158b, and HLA-DR but negative for CD3 and CD25 and have a low level of CD16. KHYG-1 cells do show high cytotoxicity against K562 cells and other cell lines in vitro, but their expression of CD8, a T cell-associated marker, and their expression of CD33, a myeloid antigen, have made their characterization as an NK cell line controversial [196].
The NK-YS and SNK-6 cell lines were both established in Japan. The former was established from a patient with leukemic-state nasal angiocentric NK cell lymphoma. The latter was established from a patient with nasal T/NK cell lymphoma. Both cell lines are EBV-positive cell lines with a CD3-CD56+CD16-CD25+HLA-DR+ phenotype. Cells of both cell lines have been shown to display IL-2-dependent proliferation. NK-YS cells but not SNK-6 cells display an LGL morphology [197, 198]. The IMC-1 cell line was established from a patient with NK leukemia. IMC-1 cells were shown to be negative for EBV, to have a CD3-CD56+HLA-DR+ phenotype, and to express very low levels of CD16—but also to express the CD34 stem and progenitor cell antigen at a low level [199].
Although these NK cell lines have been developed, only the NK-92 cell line has been used for NK cell-based immunotherapy clinical research. The other NK cell lines are currently in the preclinical research stage.
Expansion systems for NK cell lines
Cells of all of these NK cell lines can be expanded in medium containing IL-2. Therefore, the major expansion system for NK cell lines is still based on cytokine-dependent culture methods. When NK-92 cells were established, they were cultured in alpha MEM supplemented with bovine serum, horse serum, hydrocortisone, inositol, folic acid and IL-2. The cells doubled in approximately 24 h and could continuously proliferate for more than 18 months ex vivo, with approximately twice-weekly medium changes. However, due to this expansion system containing animal-derived ingredients, it is not suitable as a clinical-grade amplification system. The Tonn group reported a clinical-grade system for manufacturing NK-92 cells. The cells were cultured in X-Vivo 10 medium containing recombinant transferrin supplemented with 500 U/mL IL-2 and 5% human plasma. The cells were γ-irradiated with 10 Gy before being used [200].
The use of human plasma increases, to a certain extent, production costs and the risk of use due to endotoxins in the plasma. In contrast, serum-free expansion systems have advantages in terms of production cost and safety. The Alici group reported a serum-free NK-92 cell expansion system. By gradually adapting NK-92 cells to a serum-free culture system, the researchers were able to culture the cells in stem cell growth medium (SCGM) supplemented with 1000 U/mL IL-2. The viability, proliferation, receptor expression, and expression of cytotoxic molecules of these cells were similar to those of NK-92 cells cultured in medium supplemented with 20% FBS. However, the expression of some genes associated with the regulation of the immune response and the protein and cholesterol biosynthesis pathways was changed. Moreover, NK-92 cells cultured in a serum-free expansion system show reduced cytotoxic capacity, although the cytotoxic capacity can be recovered by adding serum [201].
Genetic manipulation of NK cell lines
To reduce the cytokine requirement during expansion, IL-15, SCF, EPOR (EPO receptor) and c-MPL (TPO receptor) have been transduced into NK cell lines. Jiang et al. pointed out that the proliferation rate of IL-15-expressing NKL cells cultured with 40 U/mL IL-2 is similar to that of control NKL cells cultured with 100 U/mL IL-2 [202]. In addition, in another study, IL-15-NK92 cells expanded approximately 20-fold after a week in culture with 10 U/mL IL2 or IL-15, while NK92 cells did not proliferate [203]. Similarly, NK92 cells expressing SCF expanded 100-fold when cultured with 100 U/mL IL-2 for 7 days, which was 1.7-fold higher than the expansion of unmodified NK92 [204]. Chanswangphuwana et al. confirmed that, compared with the expansion fold of 1 U/mL IL-2 for 8 days, the expansion fold of TPO or EPO plus low-dose IL-2 for EPOR+ NK92 cells and c-MPL+ NK92 cells was increased by 33-60 times [156]. In addition, Xu et al. showed that the specific costimulatory domain in the CAR structure may also affect the proliferation ability of NK cells. The relative proliferation increase of 2B4.z-NK92 cells can reach approximately ninefold at the third day after selection, while that of mock-NK92 cells was only 4.5-fold [205].
Good manufacturing practice (GMP)-compliant production of NK cells
NK cell lines
NK-92 cells, due to their IL-2-dependent proliferation, can be easily expanded in culture, and GMP-compliant proliferation of NK-92 cells in gas-permeable bags, cell flasks, or bioreactors is straightforward [206]. Researchers have optimized and validated the processes related to the GMP-compliant manufacture of genetically modified CAR-expressing NK-92 cells. GMP-grade X-Vivo 10 media supplemented with human plasma and IL-2 have been shown to enable efficient expansion of NK-92 cells and to maintain specific cytotoxicity and stable CAR expression (>98%) for a long time. However, to our knowledge, no detailed information about the final cell numbers and production devices of these GMP-compliant production systems has been made available. Nevertheless, large numbers of NK-92 cells cultured in VueLife 750-C1 bags prefilled with X-Vivo 10 medium and then transported over long distances were shown to retain high cytotoxicity toward K562 cells (>90%) and high viability (>90%) [207].
iPSC-NK cells
Although hESCs, UCB CD34+ cell-derived iPSCs, and fibroblast-derived iPSCs can be used to generate NK cells, in the early years, these hPSC sources were not widely used in the production and treatment of NK cells because they are difficult to obtain and complex to produce [179]. As reported in recent years, Fate Therapeutics is a company that focuses on applying iPSCs in immune cell therapy. The GMP-compliant manufacturing facility of the company was designed for the manufacture of off-the-shelf cell products using clonal master iPSC lines as the starting source, which are a renewable source for routine cGMP mass production of NK cell products. Several of their iPSC-derived NK cell products have been applied in clinical trials. The FT516 product of Fate Therapeutics was the first genetically engineered iPSC-derived NK cell therapy product to receive FDA approval for clinical trials [208]. Another NK cell product of theirs, namely, FT538, which was approved by the FDA as an investigational new drug (IND) in a multidose phase I clinical trial, was derived from a clonal master iPSC line that was made by reprogramming and engineering donor-derived human fibroblasts. The GMP process was able to produce 4.5 × 1012 FT538 NK cells [209]. To date, apart from the progress made by Fate Therapeutics, GMP production of NK cell products has been very limited because most studies related to iPSCs are still in the scientific research stage, and hence, any preliminary products tend not to meet the requirements of clinical applications.
UCB CD34+ NK cells
The Spanholtz group reported a closed-system culture process to expand NK cells derived from UCB CD34+ cells. The researchers used the CliniMACS system to optimize the enrichment of CD34+ cells from UCB, and then, UCB CD34+ cells were differentiated and amplified into NK cell products in an automated bioreactor. During the course of approximately 6 weeks, the cells expanded more than 2000-fold, with over 90% of the cells produced being CD56+ cells [168]. Furthermore, the Netherlands-based company Glycostem Therapeutics was the first company in the world to set up a completely closed-system, GMP-compliant platform for manufacturing allogeneic NK cells from fresh UCB stem cells [210]. On the basis of the system developed by the Spanholtz group, CD34+ cells derived from fresh UCB were selected using a fully automated CliniMACS Prodigy apparatus and further differentiated into mature NK cells in Xuri bioreactors. The NK cell product was shown to be scalable, resulting in 2000-3000-fold expansion and production of up to 1.1 × 1010 NK cells from a single UCB unit over the course of 40 days. The PVC Sterile Connection Device and Sealer as well as the C-Flex Biowelder and Sealer were used in the GMP clean room, and the system was kept closed [210]. Such a closed system, in which cells are never exposed to the open environment, was designed to avoid risk of contamination.
PB-NK cells
PBMC collection by leukapheresis has been commonly used for GMP-compliant expansion of NK cells [211]. Lim and colleagues established a simplified and efficient method for the large-scale expansion and activation of NK cells from PBMCs under GMP conditions. The researchers used several automated devices to realize GMP-compliant production. In their work, PBMCs from healthy donors were isolated using leukapheresis, and T cell-depleted PBMCs were separated by using a VarioMACS apparatus and then cocultured with irradiated autologous PBMCs in the presence of OKT3 and IL-2 for 14 days in A-350N culture bags in a GMP-compliant facility, resulting in a highly (>98%) pure population of CD3−CD16+CD56+ NK cells and an expansion fold ranging from 520 to 860 [212]. Furthermore, feeder cell-free scalable production of NK cells using membrane particles that express IL-21 and 41BB ligand has also been used in a GMP-compliant facility [213]. In this system, a few different closed devices were used to manufacture NK cells on standard industry platforms. A MACS Prodigy system was used to deplete CD3+ T cells, and a Prodigy CCU was used to stimulate and initially expand NK cells using membrane particles. Then, NK cells were expanded in a Xuri perfusion bioreactor. Finally, NK cells were harvested by a LOVO apparatus and cryopreserved using a Custom Biogenics System 2101 freezer. The GMP-compliant process was shown to yield a robust 1000-fold expansion of PB-NK cells in less than 2 weeks, and this platform can be scaled to produce 108-fold expansion of NK cells over 40 days while maintaining cytotoxic potency [213]. This cell-free system has evolved from a previous platform that used genetically modified K562 cells as feeder cells to expand NK cells. More recently, to achieve GMP-compliant production of genetically engineered NK cells and facilitate the clinical application of CAR NK cells, an increasing number of studies have used CliniMACS Prodigy devices to integrate multiple steps, including NK cell purification, transduction, and cultivation, in a closed system. Apart from the Miltenyi Biotec CliniMACS Prodigy device, other special culture platforms, such as the Zellwerk ZRP platform and its bioreactor types, are available. Expansion of NK cells based on this Zellwerk platform results in mass production under GMP conditions and allows for cell culture and isolation within a functionally closed environment [214].
Future prospects
Automatic and closed systems for NK cell production
Large-scale production of NK cells typically takes 14 to 28 days, and the traditional manual production process requires frequent replenishment of fresh medium, cytokines, and growth factors to ensure that NK cells grow in an optimal environment, which undoubtedly increases the risk of introducing foreign contaminants and requires significant labor and financial resources. Therefore, it is necessary to develop a fully automated and closed NK cell production system that not only makes the entire production process GMP compliant but also provides significant cost savings in the long run. Progress has recently been made in closed culture systems for large-scale expansion of NK cells, such as the permeable cell culture flask G-Rex (Wilson Wolf Manufacturing, New Brighton, MN, USA) and the wave bioreactor (GE Healthcare Life Sciences, Chicago, USA) [107, 114]; these culture systems do not require repeated fluid changes or manipulation of cells, greatly reducing the risk of contamination. Moreover, the closed environment and automation of culture flasks produce more active NK cells in larger quantities than are produced by strategies with frequent manual operations [215]. Recently, Miltenyi Biotec invented a fully automated cell processing platform, i.e., CliniMACS Prodigy®; this platform has been applied in CAR-T cell product manufacturing and combines cell sorting, cell centrifugation, washing and cell cultivation under GMP conditions. Coupling of this system with the Xuri W25 apparatus (GE Healthcare) for automated closed-system production of NK cells has been reported to be not only effective but also more GMP compliant [216]. The CliniMACS Prodigy system has also been reportedly used in combination with Xuri bioreactors to differentiate and bulk expand NK cells from UCB CD34+ stem cells [210]. Most of the NK cells used in clinical practice to date have been produced in limited quantities, which barely meet the needs of patients. With the highly unmet need to treat large cohorts of patients and to do so with multiple doses, fully automated and closed production of NK cells on a large scale with high efficiency is necessary. Moreover, automated closed systems could reduce costs and increase the quality control of NK cell production. However, the development of automated closed production systems is challenging due to the different sources of NK cells and different methods used to expand them.
Off-the-shelf supplies of NK cell products
NK cells do not require strict HLA matching and hence can be used as an allogeneic therapeutic without the risk of causing GVHD. In recent clinical trials, the numbers of infused NK cells ranged from 5 to 100 × 106 cells/kg [107]. Traditional NK cell-based therapy involves one donor for one patient, and mass production of expanded allogeneic NK cells usually takes several weeks. Therefore, an off-the-shelf NK cell product, if made available, could simply be given directly to the cancer patient, saving much time and costs. Moreover, the supervision and quality control of off-the-shelf NK cell products could be realized relatively easily. The NK cell line NK-92 has been used in the clinic and is known as an ‘off-the-shelf therapeutic’ for adoptive NK cell-based immunotherapy [217]. NK-92 cells can be easily expanded with doubling times between 24 and 36 h without relying on feeder cells and require only minimal gene modification [206]. However, before being infused into patients, NK-92 cells should be irradiated because of their risk of undergoing malignant transformation, which limits the application of NK-92 cells [218]. iPSCs could be another good source of off-the-shelf NK cell products. Recent studies have indicated similar killing capacities displayed by iPSC-NK cells and PB-NK cells against tumor cells such as ovarian cancer tumor cells [184], suggesting that iPSC-NK cells are a practicable resource for immunotherapy. One study reported a system for manufacturing KIR-negative NK cells from standardizable PBC-iPSCs that could expand cells on a large scale to produce off-the-shelf NK cells that may serve a wide range of patients [179]. Recent experiments demonstrated that CAR-iPSC-NK cells not only have high homogeneity due to their production from a standardized cell population but also show enhanced NK cell activity compared to iPSC-NK or PB-NK cells [219]. Therefore, CAR-iPSC-NK cells could, as off-the-shelf NK cells, be produced on a large scale and used to treat large numbers of patients with multiple doses. Very large amounts of NK cells expanded from PB or CB cells in one production batch could also be shipped for off-the-shelf use. One study reported that K562-mbIL21-41BBL-derived membrane particles expanded PBMC-derived NK cells over 105-fold over the course of 28 days of expansion [108]. The Spanholtz group reported the largest cytokine cocktail expansion system, which could expand NK cells over 1.5 × 103-fold using UCB CD34+ cells as the starting cells [167]. These production systems make it possible for PB- or CB-derived NK cells to become off-the-shelf products. However, most current expansion systems expand NK cells in limited quantities to make more types of off-the-shelf NK products. Hence, developing new production systems to improve efficiency and reduce expansion costs is crucial.
Artificial intelligence-based cell manufacturing
In the future, the manufacture of NK cells will consist of a process involving cell source selection, production system selection, quality control, product storage and product delivery (Fig. 3). The manufacture of NK cells mainly includes several systems: cell storage systems, which involve the storage of expansion-starting cells and the storage of the off-the-shelf products after expansion; intelligent screening systems, which accomplish screening of suitable expansion systems for NK cells from different sources or individuals, maximize the yield and activity of NK cells, and select the most suitable NK cell products for different patient (Fig. 4); production systems, which enable fully enclosed and automated NK cell production; and quality control systems, which involve automatic sampling, test result evaluation and intelligent adjustment of the production process. Artificial intelligence technology, due to its advantages of automatic and intelligent planning, searching, and control, can connect the above systems through intelligent robots and intelligent systems to form an artificial intelligence-based cell factory. Such integration will greatly improve the expansion efficiency of NK cells, make full use of the existing developed NK cell expansion systems and cell resources, and promote applications of NK cell therapy.
Fig. 3.
“Unattended” intelligent NK cell factory with “off-the-shelf/customized/self-supporting” capabilities. It is expected that, the NK cells manufacturing just likes an assemble-line, composed of three steps: (1) Starting cell processing and storage, includes starting cell selection, separation and cryopreservation; (2) Expansion and function improvement, including NK cell culture, gene engineering, quality control system and packaging; (3) Storage, the products are cryopreserved. During the manufacturing process, an quality control system involves automatic sampling, test result evaluation and intelligence adjustment of the production process is interacted with the monitor and control system. All the information of the samples, tests and processes running parameters are monitored and controlled by the Monitor and control system
Fig. 4.
Donor and recipient matching system. The NK cell biomarkers from each donor and the characteristic from each recipient were assessed. The matching search engine selects the best matching donor NK cells for an recipient by comparing the NK cell biomarkers from donors and the tumor biomarkers from recipient, and provides an NK cell manufacture process by selecting the available enhancing strategies such as gene modification and arming, to achieve a best predicted efficacy
Acknowledgements
This work was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (Grant no. XDB29030202) and the Ministry of Science and Technology of China (Grant no. 2016YFC1303503).
Author contributions
FF, SQX, WHX and ZGT conceived and designed the work. FF, SQX, MHC, and YTL wrote the paper. JJY, JM, XS, and YGH reviewed the literature. FF and SQX provided equal contributions to this work.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Fang Fang, Siqi Xie.
Contributor Information
Weihua Xiao, Email: xiaow@ustc.edu.cn.
Zhigang Tian, Email: tzg@ustc.edu.cn.
References
- 1.Sonpavde G. PD-1 and PD-L1 inhibitors as salvage therapy for urothelial carcinoma. N Engl J Med. 2017;376:1073–4. doi: 10.1056/NEJMe1701182. [DOI] [PubMed] [Google Scholar]
- 2.Xie G, Dong H, Liang Y, Ham JD, Rizwan R, Chen J. CAR-NK cells: a promising cellular immunotherapy for cancer. EBioMedicine. 2020;59:10. doi: 10.1016/j.ebiom.2020.102975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng M, Chen Y, Xiao W, Sun R, Tian Z. NK cell-based immunotherapy for malignant diseases. Cell Mol Immunol. 2013;10:230–52. doi: 10.1038/cmi.2013.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fang F, Wang W, Chen M, Tian Z, Xiao W. Technical advances in NK cell-based cellular immunotherapy. Cancer Biol Med. 2019;16:647–54. doi: 10.20892/j.issn.2095-3941.2019.0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shimasaki N, Jain A, Campana D. NK cells for cancer immunotherapy. Nat Rev Drug Discov. 2020;19:200–18. doi: 10.1038/s41573-019-0052-1. [DOI] [PubMed] [Google Scholar]
- 6.Fang F, Xiao W, Tian Z. NK cell-based immunotherapy for cancer. Semin Immunol. 2017;31:37–54. doi: 10.1016/j.smim.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 7.Fang F, Xiao W, Tian Z. Challenges of NK cell-based immunotherapy in the new era. Front Med. 2018;12:440–50. doi: 10.1007/s11684-018-0653-9. [DOI] [PubMed] [Google Scholar]
- 8.Huntington ND, Cursons J, Rautela J. The cancer-natural killer cell immunity cycle. Nat Rev Cancer. 2020;20:437–54. doi: 10.1038/s41568-020-0272-z. [DOI] [PubMed] [Google Scholar]
- 9.Del Vecchio F, Martinez-Rodriguez V, Schukking M, Cocks A, Broseghini E, Fabbri M. Professional killers: The role of extracellular vesicles in the reciprocal interactions between natural killer, CD8+ cytotoxic T-cells and tumour cells. J Extracell Vesicles. 2021;10:e12075. doi: 10.1002/jev2.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dogra P, Rancan C, Ma W, Toth M, Senda T, Carpenter DJ, et al. Tissue determinants of human NK cell development, function, and residence. Cell. 2020;180:749–63.e713. doi: 10.1016/j.cell.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang C, Cheng H, Zhang Y, Fan K, Luo G, Fan Z, et al. Anergic natural killer cells educated by tumor cells are associated with a poor prognosis in patients with advanced pancreatic ductal adenocarcinoma. Cancer Immunol Immunother. 2018;67:1815–23. doi: 10.1007/s00262-018-2235-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun H, Liu L, Huang Q, Liu H, Huang M, Wang J, et al. Accumulation of tumor-infiltrating CD49a(+) NK cells correlates with poor prognosis for human hepatocellular carcinoma. Cancer Immunol Res. 2019;7:1535–46. doi: 10.1158/2326-6066.CIR-18-0757. [DOI] [PubMed] [Google Scholar]
- 13.Nayyar G, Chu Y, Cairo MS. Overcoming resistance to natural killer cell based immunotherapies for solid tumors. Front Oncol. 2019;9:51. doi: 10.3389/fonc.2019.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang Q, Huang M, Meng F, Sun R. Activated pancreatic stellate cells inhibit NK cell function in the human pancreatic cancer microenvironment. Cell Mol Immunol. 2019;16:87–89. doi: 10.1038/s41423-018-0014-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lopez-Soto A, Gonzalez S, Smyth MJ, Galluzzi L. Control of metastasis by NK cells. Cancer Cell. 2017;32:135–54. doi: 10.1016/j.ccell.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 16.Overacre-Delgoffe AE, Chikina M, Dadey RE, Yano H, Brunazzi EA, Shayan G, et al. Interferon-gamma drives Treg fragility to promote anti-tumor immunity. Cell. 2017;169:1130–41.e1111. doi: 10.1016/j.cell.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barry KC, Hsu J, Broz ML, Cueto FJ, Binnewies M, Combes AJ, et al. A natural killer-dendritic cell axis defines checkpoint therapy-responsive tumor microenvironments. Nat Med. 2018;24:1178–91. doi: 10.1038/s41591-018-0085-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pinti M, Appay V, Campisi J, Frasca D, Fülöp T, Sauce D, et al. Aging of the immune system: Focus on inflammation and vaccination. Eur J Immunol. 2016;46:2286–301. doi: 10.1002/eji.201546178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaszubowska L, Foerster J, Kaczor JJ, Schetz D, Ślebioda TJ, Kmieć Z. Expression of cellular protective proteins SIRT1, HSP70 and SOD2 correlates with age and is significantly higher in NK cells of the oldest seniors. Immun Ageing. 2017;14:3. doi: 10.1186/s12979-017-0085-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manser AR, Uhrberg M. Age-related changes in natural killer cell repertoires: impact on NK cell function and immune surveillance. Cancer Immunol Immunother. 2016;65:417–26. doi: 10.1007/s00262-015-1750-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naumova E, Pawelec G, Mihaylova A. Natural killer cells, ageing and cancer. Cancer Immunol Immunother. 2016;65:367–70. doi: 10.1007/s00262-016-1817-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kobayashi T, Mattarollo SR. Natural killer cell metabolism. Mol Immunol. 2019;115:3–11. doi: 10.1016/j.molimm.2017.11.021. [DOI] [PubMed] [Google Scholar]
- 23.Pangrazzi L, Weinberger B. T cells, aging and senescence. Exp Gerontol. 2020;134:110887. doi: 10.1016/j.exger.2020.110887. [DOI] [PubMed] [Google Scholar]
- 24.Crespo J, Sun H, Welling TH, Tian Z, Zou W. T cell anergy, exhaustion, senescence, and stemness in the tumor microenvironment. Curr Opin Immunol. 2013;25:214–21. doi: 10.1016/j.coi.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carlsten M, Jaras M. Natural killer cells in myeloid malignancies: immune surveillance, NK cell dysfunction, and pharmacological opportunities to bolster the endogenous NK cells. Front Immunol. 2019;10:2357. doi: 10.3389/fimmu.2019.02357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang WT, Zhu HY, Wu YJ, Xia Y, Wu JZ, Wu W, et al. Elevated absolute NK cell counts in peripheral blood predict good prognosis in chronic lymphocytic leukemia. J Cancer Res Clin Oncol. 2018;144:449–57. doi: 10.1007/s00432-017-2568-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martner A, Rydström A, Riise RE, Aurelius J, Brune M, Foà R, et al. NK cell expression of natural cytotoxicity receptors may determine relapse risk in older AML patients undergoing immunotherapy for remission maintenance. Oncotarget. 2015;6:42569–74. doi: 10.18632/oncotarget.5559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chretien A-S, Granjeaud S, Gondois-Rey F, Harbi S, Orlanducci F, Blaise D, et al. Increased NK cell maturation in patients with acute myeloid leukemia. Front Immunol. 2015;6:564. doi: 10.3389/fimmu.2015.00564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gonzalez-Rodriguez AP, Villa-Álvarez M, Sordo-Bahamonde C, Lorenzo-Herrero S, Gonzalez S. NK Cells in the Treatment of Hematological Malignancies. J Clin Med. 2019;8:1557. doi: 10.3390/jcm8101557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moretta A, Bottino C, Vitale M, Pende D, Cantoni C, Mingari MC, et al. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Ann Rev Immunol. 2001;19:197–223. doi: 10.1146/annurev.immunol.19.1.197. [DOI] [PubMed] [Google Scholar]
- 31.Sun JC, Lanier LL. NK cell development, homeostasis and function: parallels with CD8(+) T cells. Nat Rev Immunol. 2011;11:645–57. doi: 10.1038/nri3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perera Molligoda Arachchige AS. Human NK cells: from development to effector functions. Innate Immun. 2021;27:212–29. doi: 10.1177/17534259211001512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Westera L, Drylewicz J, den Braber I, Mugwagwa T, van der Maas I, Kwast L, et al. Closing the gap between T-cell life span estimates from stable isotope-labeling studies in mice and humans. Blood. 2013;122:2205–12. doi: 10.1182/blood-2013-03-488411. [DOI] [PubMed] [Google Scholar]
- 34.Farhood B, Najafi M, Mortezaee K. CD8(+) cytotoxic T lymphocytes in cancer immunotherapy: A review. J Cell Physiol. 2019;234:8509–21. doi: 10.1002/jcp.27782. [DOI] [PubMed] [Google Scholar]
- 35.Bevan MJ. Helping the CD8(+) T-cell response. Nat Rev Immunol. 2004;4:595–602. doi: 10.1038/nri1413. [DOI] [PubMed] [Google Scholar]
- 36.Villard J. The role of natural killer cells in human solid organ and tissue transplantation. J Innate Immun. 2011;3:395–402. doi: 10.1159/000324400. [DOI] [PubMed] [Google Scholar]
- 37.Cooley S, Weisdorf DJ, Guethlein LA, Klein JP, Wang T, Le CT, et al. Donor selection for natural killer cell receptor genes leads to superior survival after unrelated transplantation for acute myelogenous leukemia. Blood. 2010;116:2411–9. doi: 10.1182/blood-2010-05-283051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiang YZ, Barrett AJ, Goldman JM, Mavroudis DA. Association of natural killer cell immune recovery with a graft-versus-leukemia effect independent of graft-versus-host disease following allogeneic bone marrow transplantation. Ann Hematol. 1997;74:1–6. doi: 10.1007/s002770050246. [DOI] [PubMed] [Google Scholar]
- 39.Zeis M, Uharek L, Glass B, Steinmann J, Dreger P, Gassmann W, et al. Allogeneic MHC-mismatched activated natural killer cells administered after bone marrow transplantation provide a strong graft-versus-leukaemia effect in mice. Br J Haematol. 1997;96:757–61. doi: 10.1046/j.1365-2141.1997.d01-2101.x. [DOI] [PubMed] [Google Scholar]
- 40.Habif G, Crinier A, Andre P, Vivier E, Narni-Mancinelli E. Targeting natural killer cells in solid tumors. Cell Mol Immunol. 2019;16:415–22. doi: 10.1038/s41423-019-0224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wijermans PW, Gerrits WBJ, Haak HL. Severe immunodeficiency in patients treated with fludarabine monophosphate. Eur J Haematol. 1993;50:292–6. doi: 10.1111/j.1600-0609.1993.tb00165.x. [DOI] [PubMed] [Google Scholar]
- 42.Mackall CL, Fleisher TA, Brown MR, Magrath IT, Shad AT, Horowitz ME, et al. Lymphocyte depletion during treatment with intensive chemotherapy for cancer. Blood. 1994;84:2221–8. [PubMed] [Google Scholar]
- 43.Kotsakis A, Sarra E, Peraki M, Koukourakis M, Apostolaki S, Souglakos J, et al. Docetaxel-induced lymphopenia in patients with solid tumors - a prospective phenotypic analysis. Cancer. 2000;89:1380–6. doi: 10.1002/1097-0142(20000915)89:6<1380::aid-cncr23>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 44.McGinnes K, Florence J, Penny R. The effect of radiotherapy on the natural-killer (NK)-cell activity of cancer-patients. J Clin Immunol. 1987;7:210–7. doi: 10.1007/BF00915726. [DOI] [PubMed] [Google Scholar]
- 45.Díaz-Basabe A, Strati F, Facciotti F. License to Kill: When iNKT Cells Are Granted the Use of Lethal Cytotoxicity. Int J Mol Sci. 2020;21:3909. doi: 10.3390/ijms21113909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 47.Shimizu K, Iyoda T, Yamasaki S, Kadowaki N, Tojo A, Fujii AS. NK and NKT Cell-Mediated Immune Surveillance against Hematological Malignancies. Cancers (Basel) 2020;12:817. doi: 10.3390/cancers12040817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li Y, Li G, Zhang J, Wu X, Chen X. The dual roles of human gammadelta T cells: anti-tumor or tumor-promoting. Front Immunol. 2020;11:619954. doi: 10.3389/fimmu.2020.619954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Niu C, Jin H, Li M, Xu J, Xu D, Hu J, et al. In vitro analysis of the proliferative capacity and cytotoxic effects of ex vivo induced natural killer cells, cytokine-induced killer cells, and gamma-delta T cells. BMC Immunol. 2015;16:61. doi: 10.1186/s12865-015-0124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haeryfar SMM, Shaler CR, Rudak PT. Mucosa-associated invariant T cells in malignancies: a faithful friend or formidable foe? Cancer Immunol Immunother. 2018;67:1885–96. doi: 10.1007/s00262-018-2132-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mempel TR, Henrickson SE, von Andrian UH. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature. 2004;427:154–9. doi: 10.1038/nature02238. [DOI] [PubMed] [Google Scholar]
- 52.Malhotra D, Burrack KS, Jenkins MK, Frosch AE. Antigen-specific CD4(+) T cells exhibit distinct kinetic and phenotypic patterns during primary and secondary responses to infection. Front Immunol. 2020;11:2125. doi: 10.3389/fimmu.2020.02125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rosenberg J, Huang J. CD8(+) T cells and NK cells: parallel and complementary soldiers of immunotherapy. Curr Opin Chem Eng. 2018;19:9–20. doi: 10.1016/j.coche.2017.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Irvine DJ, Purbhoo MA, Krogsgaard M, Davis MM. Direct observation of ligand recognition by T cells. Nature. 2002;419:845–9. doi: 10.1038/nature01076. [DOI] [PubMed] [Google Scholar]
- 55.Purbhoo MA, Irvine DJ, Huppa JB, Davis MM. T cell killing does not require the formation of a stable mature immunological synapse. Nat Immunol. 2004;5:524–30. doi: 10.1038/ni1058. [DOI] [PubMed] [Google Scholar]
- 56.June CH, O'Connor RS, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. science. 2018;359:1361–5. doi: 10.1126/science.aar6711. [DOI] [PubMed] [Google Scholar]
- 57.Abu-Shah E, Trendel N, Kruger P, Nguyen J, Pettmann J, Kutuzov M, et al. Human CD8(+) T cells exhibit a shared antigen threshold for different effector responses. J Immunol. 2020;205:1503–12. doi: 10.4049/jimmunol.2000525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, et al. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331:44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ljunggren HG, Malmberg KJ. Prospects for the use of NK cells in immunotherapy of human cancer. Nat Rev Immunol. 2007;7:329–39. doi: 10.1038/nri2073. [DOI] [PubMed] [Google Scholar]
- 60.Herrmann J, Berberich H, Hartmann J, Beyer S, Davies K, Koch J. Homo-oligomerization of the activating natural killer cell receptor NKp30 ectodomain increases its binding affinity for cellular ligands. J Biol Chem. 2014;289:765–77. doi: 10.1074/jbc.M113.514786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Henager SH, Hale MA, Maurice NJ, Dunnington EC, Swanson CJ, Peterson MJ, et al. Combining different design strategies for rational affinity maturation of the MICA-NKG2D interface. Protein Sci. 2012;21:1396–402. doi: 10.1002/pro.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang H, Qi J, Zhang S, Li Y, Tan S, Gao GF. Binding mode of the side-by-side two-IgV molecule CD226/DNAM-1 to its ligand CD155/Necl-5. Proc Natl Acad Sci USA. 2019;116:988–96. doi: 10.1073/pnas.1815716116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cui W, Kaech SM. Generation of effector CD8+ T cells and their conversion to memory T cells. Immunological Rev. 2010;236:151–66. doi: 10.1111/j.1600-065X.2010.00926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee SH, Bar-Haim E, Machlenkin A, Goldberger O, Volovitz I, Vadai E, et al. In vivo rejection of tumor cells dependent on CD8 cells that kill independently of perforin and FasL. Cancer Gene Ther. 2004;11:237–48. doi: 10.1038/sj.cgt.7700678. [DOI] [PubMed] [Google Scholar]
- 65.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–74. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 66.Strohl WR, Naso M. Bispecific T-Cell Redirection versus Chimeric Antigen Receptor (CAR)-T Cells as Approaches to Kill Cancer Cells. Antibodies (Basel) 2019;8:41. doi: 10.3390/antib8030041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van den Broek MF, Hengartner H. The role of perforin in infections and tumour surveillance. Exp Physiol. 2000;85:681–5. doi: 10.1017/s0958067000020972. [DOI] [PubMed] [Google Scholar]
- 68.Dixon KJ, Wu J, Walcheck B. Engineering Anti-Tumor Monoclonal Antibodies and Fc Receptors to Enhance ADCC by Human NK Cells. Cancers (Basel) 2021;13:312. doi: 10.3390/cancers13020312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ochoa MC, Minute L, Rodriguez I, Garasa S, Perez-Ruiz E, Inogés S, et al. Antibody-dependent cell cytotoxicity: immunotherapy strategies enhancing effector NK cells. Immunol Cell Biol. 2017;95:347–55. doi: 10.1038/icb.2017.6. [DOI] [PubMed] [Google Scholar]
- 70.Fauriat C, Long EO, Ljunggren HG, Bryceson YT. Regulation of human NK-cell cytokine and chemokine production by target cell recognition. Blood. 2010;115:2167–76. doi: 10.1182/blood-2009-08-238469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Frank K, Paust S. Dynamic natural killer cell and T cell responses to influenza infection. Front Cell Infect Microbiol. 2020;10:425. doi: 10.3389/fcimb.2020.00425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations. Annu Rev Immunol. 2010;28:445–89. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Macallan DC, Worth A, Asquith B, Ghattas H, Beverley PC. Measurement and modeling of human T cell kinetics. Eur J Immunol. 2003;33:2316–26. doi: 10.1002/eji.200323763. [DOI] [PubMed] [Google Scholar]
- 74.Macallan DC, Wallace DL, Irvine AJ, Asquith B, Worth A, Ghattas H, et al. Rapid turnover of T cells in acute infectious mononucleosis. Eur J Immunol. 2003;33:2655–65. doi: 10.1002/eji.200324295. [DOI] [PubMed] [Google Scholar]
- 75.Zhang Y, Wallace DL, de Lara CM, Ghattas H, Asquith B, Worth A, et al. In vivo kinetics of human natural killer cells: the effects of ageing and acute and chronic viral infection. Immunology. 2007;121:258–65. doi: 10.1111/j.1365-2567.2007.02573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jamieson AM, Isnard P, Dorfman JR, Coles MC, Raulet DH. Turnover and proliferation of NK cells in steady state and lymphopenic conditions. J Immunol. 2004;172:864–70. doi: 10.4049/jimmunol.172.2.864. [DOI] [PubMed] [Google Scholar]
- 77.Sun JC, Beilke JN, Lanier LL. Immune memory redefined: characterizing the longevity of natural killer cells. Immunological Rev. 2010;236:83–94. doi: 10.1111/j.1600-065X.2010.00900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.O’Leary JG, Goodarzi M, Drayton DL, von Andrian UH. T cell– and B cell–independent adaptive immunity mediated by natural killer cells. Nat Immunol. 2006;7:507–16. doi: 10.1038/ni1332. [DOI] [PubMed] [Google Scholar]
- 79.Konduri V, Oyewole-Said D, Vazquez-Perez J, Weldon SA, Halpert MM, Levitt JM, et al. CD8(+)CD161(+) T-cells: cytotoxic memory cells with high therapeutic potential. Front Immunol. 2020;11:613204. doi: 10.3389/fimmu.2020.613204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cerwenka A, Lanier LL. Natural killer cell memory in infection, inflammation and cancer. Nat Rev Immunol. 2016;16:112–23. doi: 10.1038/nri.2015.9. [DOI] [PubMed] [Google Scholar]
- 81.Myers JA, Miller JS. Exploring the NK cell platform for cancer immunotherapy. Nat Rev Clin Oncol. 2021;18:85–100. doi: 10.1038/s41571-020-0426-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.O’Sullivan TE, Sun JC, Lanier LL. Natural killer cell memory. Immunity. 2015;43:634–45. doi: 10.1016/j.immuni.2015.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Paust S, von Andrian UH. Natural killer cell memory. Nat Immunol. 2011;12:500–8. doi: 10.1038/ni.2032. [DOI] [PubMed] [Google Scholar]
- 84.Brudno JN, Kochenderfer JN. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood. 2016;127:3321–30. doi: 10.1182/blood-2016-04-703751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Brudno JN, Kochenderfer JN. Recent advances in CAR T-cell toxicity: Mechanisms, manifestations and management. Blood Rev. 2019;34:45–55. doi: 10.1016/j.blre.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pfefferle A, Huntington ND. You Have Got a Fast CAR: Chimeric Antigen Receptor NK Cells in Cancer Therapy. Cancers (Basel) 2020;12:706. doi: 10.3390/cancers12030706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rice J, Nagle S, Randall J, Hinson HE. Chimeric antigen receptor T cell-related neurotoxicity: mechanisms, clinical presentation, and approach to treatment. Curr Treat Options Neurol. 2019;21:40. doi: 10.1007/s11940-019-0580-3. [DOI] [PubMed] [Google Scholar]
- 88.Zeiser R, Longo DL, Blazar BR. Acute graft-versus-host disease—biologic process, prevention, and therapy. N Engl J Med. 2017;377:2167–79. doi: 10.1056/NEJMra1609337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lupo KB, Matosevic S. Natural Killer Cells as Allogeneic Effectors in Adoptive Cancer Immunotherapy. Cancers (Basel) 2019;11:769. doi: 10.3390/cancers11060769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shah NN, Baird K, Delbrook CP, Fleisher TA, Kohler ME, Rampertaap S, et al. Acute GVHD in patients receiving IL-15/4-1BBL activated NK cells following T-cell-depleted stem cell transplantation. Blood. 2015;125:784–92. doi: 10.1182/blood-2014-07-592881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hosokai R, Masuko M, Shibasaki Y, Saitoh A, Furukawa T, Imai C. Donor killer immunoglobulin-like receptor haplotype B/x induces severe acute graft-versus-host disease in the presence of human leukocyte antigen mismatch in T cell-replete hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2017;23:606–11. doi: 10.1016/j.bbmt.2016.12.638. [DOI] [PubMed] [Google Scholar]
- 92.Sterner RC, Sterner RM. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 2021;11:69. doi: 10.1038/s41408-021-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Morgan MA, Buning H, Sauer M, Schambach A. Use oF Cell And Genome Modification Technologies To Generate Improved “Off-the-Shelf” CAR T and CAR NK cells. Front Immunol. 2020;11:1965. doi: 10.3389/fimmu.2020.01965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wendel P, Reindl LM, Bexte T, Künnemeyer L, Särchen V, Albinger N, et al. Arming Immune Cells for Battle: A Brief Journey through the Advancements of T and NK Cell Immunotherapy. Cancers (Basel) 2021;13:1481. doi: 10.3390/cancers13061481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vacca P, Pietra G, Tumino N, Munari E, Mingari MC, Moretta L. Exploiting human NK cells in tumor therapy. Front Immunol. 2020;10:3013. doi: 10.3389/fimmu.2019.03013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang W, Jiang J, Wu C. CAR-NK for tumor immunotherapy: clinical transformation and future prospects. Cancer Lett. 2020;472:175–80. doi: 10.1016/j.canlet.2019.11.033. [DOI] [PubMed] [Google Scholar]
- 97.Demicheli R, Retsky MW, Hrushesky WJ, Baum M, Gukas ID. The effects of surgery on tumor growth: a century of investigations. Ann Oncol. 2008;19:1821–8. doi: 10.1093/annonc/mdn386. [DOI] [PubMed] [Google Scholar]
- 98.Dianat-Moghadam H, Mahari A, Heidarifard M, Parnianfard N, Pourmousavi-Kh L, Rahbarghazi R, et al. NK cells-directed therapies target circulating tumor cells and metastasis. Cancer Lett. 2021;497:41–53. doi: 10.1016/j.canlet.2020.09.021. [DOI] [PubMed] [Google Scholar]
- 99.Alhabbab RY. Targeting Cancer Stem Cells by Genetically Engineered Chimeric Antigen Receptor T Cells. Front Genet. 2020;11:312. doi: 10.3389/fgene.2020.00312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dianat-Moghadam H, Rokni M, Marofi F, Panahi Y, Yousefi M. Natural killer cell–based immunotherapy: From transplantation toward targeting cancer stem cells. J Cell Physiol. 2019;234:259–73. doi: 10.1002/jcp.26878. [DOI] [PubMed] [Google Scholar]
- 101.Ames E, Canter RJ, Grossenbacher SK, Mac S, Chen M, Smith RC, et al. NK cells preferentially target tumor cells with a cancer stem cell phenotype. J Immunol. 2015;195:4010–9. doi: 10.4049/jimmunol.1500447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kim GR, Ha GH, Bae JH, Oh SO, Kim SH, Kang CD. Metastatic colon cancer cell populations contain more cancer stem-like cells with a higher susceptibility to natural killer cell-mediated lysis compared with primary colon cancer cells. Oncol Lett. 2015;9:1641–6. doi: 10.3892/ol.2015.2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cheung AS, Zhang DKY, Koshy ST, Mooney DJ. Scaffolds that mimic antigen-presenting cells enable ex vivo expansion of primary T cells. Nat Biotechnol. 2018;36:160–9. doi: 10.1038/nbt.4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Levine BL, Miskin J, Wonnacott K, Keir C. Global manufacturing of CAR T cell therapy. Mol Ther -Methods Clin Dev. 2017;4:92–101. doi: 10.1016/j.omtm.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lock D, Mockel-Tenbrinck N, Drechsel K, Barth C, Mauer D, Schaser T, et al. Automated manufacturing of potent CD20-directed chimeric antigen receptor T cells for clinical use. Hum Gene Ther. 2017;28:914–25. doi: 10.1089/hum.2017.111. [DOI] [PubMed] [Google Scholar]
- 106.Lu TL, Pugach O, Somerville R, Rosenberg SA, Kochenderfer JN, Better M, et al. A rapid cell expansion process for production of engineered autologous CAR-T cell therapies. Hum Gene Ther Methods. 2016;27:209–18. doi: 10.1089/hgtb.2016.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chabannon C, Mfarrej B, Guia S, Ugolini S, Devillier R, Blaise D, et al. Manufacturing natural killer cells as medicinal products. Front Immunol. 2016;7:504. doi: 10.3389/fimmu.2016.00504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Oyer JL, Pandey V, Igarashi RY, Somanchi SS, Zakari A, Solh M, et al. Natural killer cells stimulated with PM21 particles expand and biodistribute in vivo: clinical implications for cancer treatment. Cytotherapy. 2016;18:653–63. doi: 10.1016/j.jcyt.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 109.Romanski A, Uherek C, Bug G, Seifried E, Klingemann H, Wels WS, et al. CD19-CAR engineered NK-92 cells are sufficient to overcome NK cell resistance in B-cell malignancies. J Cell Mol Med. 2016;20:1287–94. doi: 10.1111/jcmm.12810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wiesinger M, März J, Kummer M, Schuler G, Dörrie J, Schuler-Thurner B, et al. Clinical-scale production of CAR-T cells for the treatment of melanoma patients by mRNA transfection of a CSPG4-specific CAR under full GMP compliance. Cancers. 2019;11:18. doi: 10.3390/cancers11081198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pomeroy EJ, Hunzeker JT, Kluesner MG, Lahr WS, Smeester BA, Crosby MR, et al. A genetically engineered primary human natural killer cell platform for cancer immunotherapy. Mol Ther. 2020;28:52–63. doi: 10.1016/j.ymthe.2019.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Boissel L, Betancur M, Lu W, Wels WS, Marino T, Van Etten RA, et al. Comparison of mRNA and lentiviral based transfection of natural killer cells with chimeric antigen receptors recognizing lymphoid antigens. Leuk Lymphoma. 2012;53:958–65. doi: 10.3109/10428194.2011.634048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ueda T, Kumagai A, Iriguchi S, Yasui Y, Miyasaka T, Nakagoshi K, et al. Non-clinical efficacy, safety and stable clinical cell processing of induced pluripotent stem cell-derived anti-glypican-3 chimeric antigen receptor-expressing natural killer/innate lymphoid cells. Cancer Sci. 2020;111:1478–90. doi: 10.1111/cas.14374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lapteva N, Durett AG, Sun J, Rollins LA, Huye LL, Fang J, et al. Large-scale ex vivo expansion and characterization of natural killer cells for clinical applications. Cytotherapy. 2012;14:1131–43. doi: 10.3109/14653249.2012.700767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Min B, Choi H, Her JH, Jung MY, Kim HJ, Jung MY, et al. Optimization of large-scale expansion and cryopreservation of human natural killer cells for anti-tumor therapy. Immune Netw. 2018;18:e31. doi: 10.4110/in.2018.18.e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chong EA, Levine BL, Grupp SA, Davis M, Siegel DL, Maude SL, et al. CD19-Directed CAR T-cell (CTL019) product viability and clinical outcomes in Non-Hodgkin lymphomas and B-cell acute lymphoblastic leukemia. Blood. 2018;132:3–197. [Google Scholar]
- 117.Panch SR, Srivastava SK, Elavia N, McManus A, Liu S, Jin P, et al. Effect of cryopreservation on autologous chimeric antigen receptor T cell characteristics. Mol Ther. 2019;27:1275–85. doi: 10.1016/j.ymthe.2019.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–7. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 119.Geller MA, Cooley S, Judson PL, Ghebre R, Carson LF, Argenta PA, et al. A phase II study of allogeneic natural killer cell therapy to treat patients with recurrent ovarian and breast cancer. Cytotherapy. 2011;13:98–107. doi: 10.3109/14653249.2010.515582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Peled T, Brachya G, Persi N, Lador C, Olesinski E, Landau E, et al. Enhanced In Vivo Persistence and Proliferation of NK cells expanded in culture with the small molecule nicotinamide: development of a clinical-applicable method for NK expansion. Blood. 2017;130:4–657. [Google Scholar]
- 121.Porter DL, Hwang WT, Frey NV, Lacey SF, Shaw PA, Loren AW, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med. 2015;7:12. doi: 10.1126/scitranslmed.aac5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yee C, Thompson JA, Byrd D, Riddell SR, Roche P, Celis E, et al. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci USA. 2002;99:16168–73. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhu H, Blum RH, Bernareggi D, Ask EH, Wu Z, Hoel HJ, et al. Metabolic reprograming via deletion of CISH in human iPSC-derived NK cells promotes in vivo persistence and enhances anti-tumor activity. Cell Stem Cell. 2020;27:224–37.e226. doi: 10.1016/j.stem.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhou J, Shen X, Huang J, Hodes RJ, Rosenberg SA, Robbins PF. Telomere length of transferred lymphocytes correlates with in vivo persistence and tumor regression in melanoma patients receiving cell transfer therapy. J Immunol. 2005;175:7046–52. doi: 10.4049/jimmunol.175.10.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Leong JW, Chase JM, Romee R, Schneider SE, Sullivan RP, Cooper MA, et al. Preactivation with IL-12, IL-15, and IL-18 induces CD25 and a functional high-affinity IL-2 receptor on human cytokine-induced memory-like natural killer cells. Biol Blood Marrow Transplant. 2014;20:463–73. doi: 10.1016/j.bbmt.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sievers NM, Dorrie J, Schaft N. CARs: beyond T cells and T cell-derived signaling domains. Int J Mol Sci. 2020;21:30. doi: 10.3390/ijms21103525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lim WA, June CH. The principles of engineering immune cells to treat cancer. Cell. 2017;168:724–40. doi: 10.1016/j.cell.2017.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Xiao L, Cen D, Gan H, Sun Y, Huang N, Xiong H, et al. Adoptive Transfer of NKG2D CAR mRNA-Engineered Natural Killer Cells in Colorectal Cancer Patients. Mol Ther. 2019;27:1114–25. doi: 10.1016/j.ymthe.2019.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Li P, Morris DL, Willcox BE, Steinle A, Spies T, Strong RK. Complex structure of the activating immunoreceptor NKG2D and its MHC class I-like ligand MICA. Nat Immunol. 2001;2:443–51. doi: 10.1038/87757. [DOI] [PubMed] [Google Scholar]
- 130.Gudipati V, Rydzek J, Doel-Perez I, Gonçalves VDR, Scharf L, Königsberger S, et al. Inefficient CAR-proximal signaling blunts antigen sensitivity. Nat Immunol. 2020;21:848–56. doi: 10.1038/s41590-020-0719-0. [DOI] [PubMed] [Google Scholar]
- 131.Li Y, Hermanson DL, Moriarity BS, Kaufman DS. Human iPSC-derived natural killer cells engineered with chimeric antigen receptors enhance anti-tumor activity. Cell Stem Cell. 2018;23:181–92. doi: 10.1016/j.stem.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Davis ZB, Vallera DA, Miller JS, Felices M. Natural killer cells unleashed: checkpoint receptor blockade and BiKE/TriKE utilization in NK-mediated anti-tumor immunotherapy. Semin Immunol. 2017;31:64–75. doi: 10.1016/j.smim.2017.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sarhan D, Brandt L, Felices M, Guldevall K, Lenvik T, Hinderlie P, et al. 161533 TriKE stimulates NK-cell function to overcome myeloid-derived suppressor cells in MDS. Blood Adv. 2018;2:1459–69. doi: 10.1182/bloodadvances.2017012369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cheng Y, Zheng X, Wang X, Chen Y, Wei H, Sun R, et al. Trispecific killer engager 161519 enhances natural killer cell function and provides anti-tumor activity against CD19-positive cancers. Cancer Biol Med. 2020;17:1026–38. doi: 10.20892/j.issn.2095-3941.2020.0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Felices M, Kodal B, Hinderlie P, Kaminski MF, Cooley S, Weisdorf DJ, et al. Novel CD19-targeted TriKE restores NK cell function and proliferative capacity in CLL. Blood Adv. 2019;3:897–907. doi: 10.1182/bloodadvances.2018029371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Topp MS, Gökbuget N, Stein AS, Zugmaier G, O'Brien S, Bargou RC, et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol. 2015;16:57–66. doi: 10.1016/S1470-2045(14)71170-2. [DOI] [PubMed] [Google Scholar]
- 137.Herrmann M, Krupka C, Deiser K, Brauchle B, Marcinek A, Ogrinc Wagner A, et al. Bifunctional PD-1 × αCD3 × αCD33 fusion protein reverses adaptive immune escape in acute myeloid leukemia. Blood. 2018;132:2484–94. doi: 10.1182/blood-2018-05-849802. [DOI] [PubMed] [Google Scholar]
- 138.Topp MS, Duell J, Zugmaier G, Attal M, Moreau P, Langer C, et al. Anti-B-Cell Maturation Antigen BiTE Molecule AMG 420 Induces Responses in Multiple Myeloma. J Clin Oncol. 2020;38:775–83. doi: 10.1200/JCO.19.02657. [DOI] [PubMed] [Google Scholar]
- 139.Koehl U, Brehm C, Huenecke S, Zimmermann SY, Kloess S, Bremm M, et al. Clinical grade purification and expansion of NK cell products for an optimized manufacturing protocol. Front Oncol. 2013;3:118. doi: 10.3389/fonc.2013.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wagner J, Pfannenstiel V, Waldmann A, Bergs JWJ, Brill B, Huenecke S, et al. A Two-Phase Expansion Protocol Combining Interleukin (IL)-15 and IL-21 Improves Natural Killer Cell Proliferation and Cytotoxicity against Rhabdomyosarcoma. Front Immunol. 2017;8:676. doi: 10.3389/fimmu.2017.00676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Heinze A, Grebe B, Bremm M, Huenecke S, Munir TA. Graafen L, et al. The synergistic use of IL-15 and IL-21 for the generation of NK cells from CD3/CD19-depleted grafts improves their ex vivo expansion and cytotoxic potential against neuroblastoma: perspective for optimized immunotherapy post haploidentical stem cell transplantation. Front Immunol. 2019;10:2816. doi: 10.3389/fimmu.2019.02816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tanaka Y, Nakazawa T, Nakamura M, Nishimura F, Matsuda R, Omoto K, et al. Ex vivo-expanded highly purified natural killer cells in combination with temozolomide induce antitumor effects in human glioblastoma cells in vitro. PLoS ONE. 2019;14:e0212455. doi: 10.1371/journal.pone.0212455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Nhung HTM, Anh BV, Huyen TL, Hiep DT, Thao CT, Lam PN, et al. Ex vivo expansion of human peripheral blood natural killer cells and cytotoxic T lymphocytes from lung cancer patients. Oncol Lett. 2018;15:5730–8. doi: 10.3892/ol.2018.8029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Liem NT, Phong NV, Kien NT, Anh BV, Huyen TL, Thao CT, et al. Phase I clinical trial using autologous ex vivo expanded NK cells and cytotoxic T lymphocytes for cancer treatment in Vietnam. Int J Mol Sci. 2019;20:3166. doi: 10.3390/ijms20133166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Liu M, Meng Y, Zhang L, Han Z, Feng X. High-efficient generation of natural killer cells from peripheral blood with preferable cell vitality and enhanced cytotoxicity by combination of IL-2 IL-15 and IL-18. Biochem Biophys Res Commun. 2021;534:149–56. doi: 10.1016/j.bbrc.2020.12.012. [DOI] [PubMed] [Google Scholar]
- 146.Kweon S, Phan MT, Chun S, Yu H, Kim J, Kim S, et al. Expansion of Human NK Cells Using K562 Cells Expressing OX40 Ligand and Short Exposure to IL-21. Front Immunol. 2019;10:879. doi: 10.3389/fimmu.2019.00879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Michen S, Frosch J, Füssel M, Schackert G, Momburg F, Temme A. Artificial feeder cells expressing ligands for killer cell immunoglobulin-like receptors and CD94/NKG2A for expansion of functional primary natural killer cells with tolerance to self. Cytotherapy. 2020;22:354–68. doi: 10.1016/j.jcyt.2020.02.004. [DOI] [PubMed] [Google Scholar]
- 148.Lim SA, Kim TJ, Lee JE, Sonn CH, Kim K, Kim J, et al. Ex vivo expansion of highly cytotoxic human NK cells by cocultivation with irradiated tumor cells for adoptive immunotherapy. Cancer Res. 2013;73:2598–607. doi: 10.1158/0008-5472.CAN-12-2893. [DOI] [PubMed] [Google Scholar]
- 149.Sakamoto N, Ishikawa T, Kokura S, Okayama T, Oka K, Ideno M, et al. Phase I clinical trial of autologous NK cell therapy using novel expansion method in patients with advanced digestive cancer. J Transl Med. 2015;13:277. doi: 10.1186/s12967-015-0632-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Fernández A, Navarro-Zapata A, Escudero A, Matamala N, Ruz-Caracuel B, Mirones I, et al. Optimizing the Procedure to Manufacture Clinical-Grade NK Cells for Adoptive Immunotherapy. Cancers (Basel) 2021;13:577. doi: 10.3390/cancers13030577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Moseman JE, Foltz JA, Sorathia K, Heipertz EL, Lee DA. Evaluation of serum-free media formulations in feeder cell-stimulated expansion of natural killer cells. Cytotherapy. 2020;22:322–8. doi: 10.1016/j.jcyt.2020.02.002. [DOI] [PubMed] [Google Scholar]
- 152.Denman CJ, Senyukov VV, Somanchi SS, Phatarpekar PV, Kopp LM, Johnson JL, et al. Membrane-bound IL-21 promotes sustained ex vivo proliferation of human natural killer cells. PLoS ONE. 2012;7:e30264. doi: 10.1371/journal.pone.0030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Fujisaki H, Kakuda H, Imai C, Mullighan CG, Campana D. Replicative potential of human natural killer cells. Br J Haematol. 2009;145:606–13. doi: 10.1111/j.1365-2141.2009.07667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wang X, Jasinski DL, Medina JL, Spencer DM, Foster AE, Bayle JH. Inducible MyD88/CD40 synergizes with IL-15 to enhance antitumor efficacy of CAR-NK cells. Blood Adv. 2020;4:1950–64. doi: 10.1182/bloodadvances.2020001510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Imamura M, Shook D, Kamiya T, Shimasaki N, Chai SMH, Coustan-Smith E, et al. Autonomous growth and increased cytotoxicity of natural killer cells expressing membrane-bound interleukin-15. Blood. 2014;124:1081–8. doi: 10.1182/blood-2014-02-556837. [DOI] [PubMed] [Google Scholar]
- 156.Chanswangphuwana C, Allan DSJ, Chakraborty M, Reger RN, Childs RW. Augmentation of NK Cell Proliferation and Anti-tumor Immunity by Transgenic Expression of Receptors for EPO or TPO. Mol Ther. 2021;29:47–59. doi: 10.1016/j.ymthe.2020.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mehta RS, Shpall EJ, Rezvani K. Cord blood as a source of natural killer cells. Front Med. 2016;2. [DOI] [PMC free article] [PubMed]
- 158.Sarvaria A, Jawdat D, Madrigal JA, Saudemont A. Umbilical cord blood natural killer cells, their characteristics, and potential clinical applications. Front Immunol. 2017;8:329. doi: 10.3389/fimmu.2017.00329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Goldenson BH, Zhu H, Wang YZM, Heragu N, Bernareggi D, Ruiz-Cisneros A, et al. Umbilical cord blood and iPSC-derived natural killer cells demonstrate key differences in cytotoxic activity and KIR profiles. Front Immunol. 2020;11:561553. doi: 10.3389/fimmu.2020.561553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hosseini E, Ghasemzadeh M, Kamalizad M, Schwarer AP. Ex vivo expansion of CD3(depleted) cord blood-MNCs in the presence of bone marrow stromal cells; an appropriate strategy to provide functional NK cells applicable for cellular therapy. Stem Cell Res. 2017;19:148–55. doi: 10.1016/j.scr.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 161.Reina-Ortiz C, Constantinides M, Fayd-Herbe-de-Maudave A, Présumey J, Hernandez J, Cartron G, et al. Expanded NK cells from umbilical cord blood and adult peripheral blood combined with daratumumab are effective against tumor cells from multiple myeloma patients. Oncoimmunology. 2020;10:1853314. doi: 10.1080/2162402X.2020.1853314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Shah N, Martin-Antonio B, Yang H, Ku S, Lee DA, Cooper LJN, et al. Antigen presenting cell-mediated expansion of human umbilical cord blood yields log-scale expansion of natural killer cells with anti-myeloma activity. PLoS ONE. 2013;8:e76781. doi: 10.1371/journal.pone.0076781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Mu YX, Zhao YX, Li BY, Bao HJ, Jiang H, Qi XL, et al. A simple method for in vitro preparation of natural killer cells from cord blood. BMC Biotechnol. 2019;19:80. doi: 10.1186/s12896-019-0564-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhu H, Kaufman DS. An Improved Method to Produce Clinical-Scale Natural Killer Cells from Human Pluripotent Stem Cells. Methods Mol Biol. 2019;2048:107–19. doi: 10.1007/978-1-4939-9728-2_12. [DOI] [PubMed] [Google Scholar]
- 165.Domogala A, Blundell M, Thrasher A, Lowdell MW, Madrigal JA, Saudemont A. Natural killer cells differentiated in vitro from cord blood CD34+ cells are more advantageous for use as an immunotherapy than peripheral blood and cord blood natural killer cells. Cytotherapy. 2017;19:710–20. doi: 10.1016/j.jcyt.2017.03.068. [DOI] [PubMed] [Google Scholar]
- 166.Grzywacz B, Kataria N, Sikora M, Oostendorp RA, Dzierzak EA, Blazar BR, et al. Coordinated acquisition of inhibitory and activating receptors and functional properties by developing human natural killer cells. Blood. 2006;108:3824–33. doi: 10.1182/blood-2006-04-020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Spanholtz J, Tordoir M, Eissens D, Preijers F, van der Meer A, Joosten I, et al. High log-scale expansion of functional human natural killer cells from umbilical cord blood CD34-positive cells for adoptive cancer immunotherapy. PLoS ONE. 2010;5:e9221. doi: 10.1371/journal.pone.0009221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Spanholtz J, Preijers F, Tordoir M, Trilsbeek C, Paardekooper J, de Witte T, et al. Clinical-grade generation of active NK cells from cord blood hematopoietic progenitor cells for immunotherapy using a closed-system culture process. PLoS ONE. 2011;6:e20740. doi: 10.1371/journal.pone.0020740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Basar R, Daher M, Rezvani K. Next-generation cell therapies: the emerging role of CAR-NK cells. Blood Adv. 2020;4:5868–76. doi: 10.1182/bloodadvances.2020002547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Dolstra H, Roeven MWH, Spanholtz J, Hangalapura BN, Tordoir M, Maas F, et al. Successful transfer of umbilical cord blood CD34(+) hematopoietic stem and progenitor-derived NK cells in older acute myeloid leukemia patients. Clin Cancer Res. 2017;23:4107–18. doi: 10.1158/1078-0432.CCR-16-2981. [DOI] [PubMed] [Google Scholar]
- 171.Yvon ES, Burga R, Powell A, Cruz CR, Fernandes R, Barese C, et al. Cord blood natural killer cells expressing a dominant negative TGF-β receptor: Implications for adoptive immunotherapy for glioblastoma. Cytotherapy. 2017;19:408–18. doi: 10.1016/j.jcyt.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 172.Zhao X, Cai L, Hu Y, Wang H Cord-blood natural killer cell-based immunotherapy for cancer. Front Immunol. 2020;11. [DOI] [PMC free article] [PubMed]
- 173.Burga RA, Yvon E, Chorvinsky E, Fernandes R, Cruz CRY, Bollard CM. Engineering the TGFβ Receptor to Enhance the Therapeutic Potential of Natural Killer Cells as an Immunotherapy for Neuroblastoma. Clin Cancer Res. 2019;25:4400–12. doi: 10.1158/1078-0432.CCR-18-3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Liu E, Tong Y, Dotti G, Shaim H, Savoldo B, Mukherjee M, et al. Cord blood NK cells engineered to express IL-15 and a CD19-targeted CAR show long-term persistence and potent antitumor activity. Leukemia. 2018;32:520–31. doi: 10.1038/leu.2017.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Daher M, Basar R, Gokdemir E, Baran N, Uprety N, Nunez Cortes AK, et al. Targeting a cytokine checkpoint enhances the fitness of armored cord blood CAR-NK cells. Blood. 2021;137:624–36. doi: 10.1182/blood.2020007748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Shankar K, Capitini CM, Saha K. Genome engineering of induced pluripotent stem cells to manufacture natural killer cell therapies. Stem Cell Res Ther. 2020;11:234. doi: 10.1186/s13287-020-01741-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Klingemann H, Boissel L, Toneguzzo F. Natural Killer Cells for Immunotherapy - Advantages of the NK-92 Cell Line over Blood NK Cells. Front Immunol. 2016;7:91. doi: 10.3389/fimmu.2016.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Knorr DA, Ni Z, Hermanson D, Hexum MK, Bendzick L, Cooper LJ, et al. Clinical-scale derivation of natural killer cells from human pluripotent stem cells for cancer therapy. Stem Cells Transl Med. 2013;2:274–83. doi: 10.5966/sctm.2012-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zeng J, Tang SY, Toh LL, Wang S. Generation of “Off-the-Shelf” natural killer cells from peripheral blood cell-derived induced pluripotent stem cells. Stem Cell Rep. 2017;9:1796–812. doi: 10.1016/j.stemcr.2017.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Mesquitta WT, Wandsnider M, Kang H, Thomson J, Moskvin O, Suknuntha K, et al. UM171 expands distinct types of myeloid and NK progenitors from human pluripotent stem cells. Sci Rep. 2019;9:6622. doi: 10.1038/s41598-019-43054-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Woll PS, Grzywacz B, Tian X, Marcus RK, Knorr DA, Verneris MR, et al. Human embryonic stem cells differentiate into a homogeneous population of natural killer cells with potent in vivo antitumor activity. Blood. 2009;113:6094–101. doi: 10.1182/blood-2008-06-165225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ni Z, Knorr DA, Clouser CL, Hexum MK, Southern P, Mansky LM, et al. Human pluripotent stem cells produce natural killer cells that mediate anti-HIV-1 activity by utilizing diverse cellular mechanisms. J Virol. 2010;85:43–50. doi: 10.1128/JVI.01774-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ng ES, Davis R, Stanley EG, Elefanty AG. A protocol describing the use of a recombinant protein-based, animal product-free medium (APEL) for human embryonic stem cell differentiation as spin embryoid bodies. Nat Protoc. 2008;3:768–76. doi: 10.1038/nprot.2008.42. [DOI] [PubMed] [Google Scholar]
- 184.Hermanson DL, Bendzick L, Pribyl L, McCullar V, Vogel RI, Miller JS, et al. Induced pluripotent stem cell-derived natural killer cells for treatment of ovarian cancer. Stem Cells. 2016;34:93–101. doi: 10.1002/stem.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kim K, Abdal Dayem A, Gil M, Yang GM, Lee SB, Kwon OH, et al. 3,2'-Dihydroxyflavone Improves the Proliferation and Survival of Human Pluripotent Stem Cells and Their Differentiation into Hematopoietic Progenitor Cells. J Clin Med. 2020;9:669. doi: 10.3390/jcm9030669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ni Z, Knorr DA, Bendzick L, Allred J, Kaufman DS. Expression of chimeric receptor CD4zeta by natural killer cells derived from human pluripotent stem cells improves in vitro activity but does not enhance suppression of HIV infection in vivo. Stem Cells. 2014;32:1021–31. doi: 10.1002/stem.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Cichocki F, Bjordahl R, Gaidarova S, Mahmood S, Abujarour R, Wang H, et al. iPSC-derived NK cells maintain high cytotoxicity and enhance in vivo tumor control in concert with T cells and anti-PD-1 therapy. Sci Transl Med. 2020;12:eaaz5618. doi: 10.1126/scitranslmed.aaz5618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wilber A, Linehan JL, Tian X, Woll PS, Morris JK, Belur LR, et al. Efficient and stable transgene expression in human embryonic stem cells using transposon-mediated gene transfer. Stem Cells. 2007;25:2919–27. doi: 10.1634/stemcells.2007-0026. [DOI] [PubMed] [Google Scholar]
- 189.Snyder KM, Hullsiek R, Mishra HK, Mendez DC, Li Y, Rogich A, et al. Expression of a recombinant high affinity IgG Fc receptor by engineered NK cells as a docking platform for therapeutic mAbs to target cancer cells. Front Immunol. 2018;9:2873. doi: 10.3389/fimmu.2018.02873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Bjordahl R, Gaidarova S, Goodridge JP, Mahmood S, Bonello G, Robinson M, et al. FT576: a novel multiplexed engineered off-the-shelf natural killer cell immunotherapy for the dual-targeting of CD38 and Bcma for the treatment of multiple myeloma. Blood. 2019;134:3214.
- 191.Goodridge JP, Mahmood S, Zhu H, Gaidarova S, Blum R, Bjordahl R, et al. FT596: translation of first-of-kind multi-antigen targeted off-the-shelf CAR-NK cell with engineered persistence for the treatment of B Cell Malignancies. Blood. 2019;134:301.
- 192.Angelos MG, et al. Aryl hydrocarbon receptor inhibition promotes hematolymphoid development from human pluripotent stem cells. Blood. 2017;129:3428–39. doi: 10.1182/blood-2016-07-730440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Gong JH, Maki G, Klingemann HG. Characterization of a human cell line (NK-92) with phenotypical and functional characteristics of activated natural killer cells. Leukemia. 1994;8:652–8. [PubMed] [Google Scholar]
- 194.Maki G, Klingemann HG, Martinson JA, Tam YK. Factors regulating the cytotoxic activity of the human natural killer cell line, NK-92. J Hematother Stem Cell Res. 2001;10:369–83. doi: 10.1089/152581601750288975. [DOI] [PubMed] [Google Scholar]
- 195.Cheng M, Ma J, Chen Y, Zhang J, Zhao W, Zhang J, et al. Establishment, characterization, and successful adaptive therapy against human tumors of NKG cell, a new human NK cell line. Cell Transplant. 2011;20:1731–46. doi: 10.3727/096368911X580536. [DOI] [PubMed] [Google Scholar]
- 196.Yagita M, Huang CL, Umehara H, Matsuo Y, Tabata R, Miyake M, et al. A novel natural killer cell line (KHYG-1) from a patient with aggressive natural killer cell leukemia carrying a p53 point mutation. Leukemia. 2000;14:922–30. doi: 10.1038/sj.leu.2401769. [DOI] [PubMed] [Google Scholar]
- 197.Nagata H, Konno A, Kimura N, Zhang Y, Kimura M, Demachi A, et al. Characterization of novel natural killer (NK)-cell and gammadelta T-cell lines established from primary lesions of nasal T/NK-cell lymphomas associated with the Epstein-Barr virus. Blood. 2001;97:708–13. [DOI] [PubMed]
- 198.Tsuchiyama J, Yoshino T, Mori M, Kondoh E, Oka T, Akagi T, et al. Characterization of a novel human natural killer-cell line (NK-YS) established from natural killer cell lymphoma/leukemia associated with Epstein-Barr virus infection. Blood. 1998;92:1374–83. [PubMed] [Google Scholar]
- 199.Chen IM, Whalen M, Bankhurst A, Sever CE, Doshi R, Hardekopf D, et al. A new human natural killer leukemia cell line, IMC-1. A complex chromosomal rearrangement defined by spectral karyotyping: functional and cytogenetic characterization. Leuk Res. 2004;28:275–84. doi: 10.1016/s0145-2126(03)00254-6. [DOI] [PubMed] [Google Scholar]
- 200.Nowakowska P, Romanski A, Miller N, Odendahl M, Bonig H, Zhang C, et al. Clinical grade manufacturing of genetically modified, CAR-expressing NK-92 cells for the treatment of ErbB2-positive malignancies. Cancer Immunol Immunother. 2018;67:25–38. doi: 10.1007/s00262-017-2055-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Chrobok M, Dahlberg CIM, Sayitoglu EC, Beljanski V, Nahi H, Gilljam M, et al. Functional Assessment for Clinical Use of Serum-Free Adapted NK-92 Cells. Cancers (Basel) 2019;11:69. doi: 10.3390/cancers11010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Jiang W, Zhang J, Tian Z. Functional characterization of interleukin-15 gene transduction into the human natural killer cell line NKL. Cytotherapy. 2008;10:265–74. doi: 10.1080/14653240801965156. [DOI] [PubMed] [Google Scholar]
- 203.Zhang J, Sun R, Wei HM, Zhang JH, Tian ZG. Characterization of interleukin-15 gene-modified human natural killer cells: implications for adoptive cellular immunotherapy. Haematologica. 2004;89:338–47. [PubMed] [Google Scholar]
- 204.Zhang R, Sun R, Wei HM, Zhang JH, Tian ZG. Characterization of stem cell factor gene-modified human natural killer cell line, NK-92 cells: Implication in NK cell-based adoptive cellular immunotherapy. Oncol Rep. 2004;11:1097–106. [PubMed] [Google Scholar]
- 205.Xu Y, Liu Q, Zhong M, Wang Z, Chen Z, Zhang Y, et al. 2B4 costimulatory domain enhancing cytotoxic ability of anti-CD5 chimeric antigen receptor engineered natural killer cells against T cell malignancies. J Hematol Oncol. 2019;12:49. doi: 10.1186/s13045-019-0732-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Suck G, Odendahl M, Nowakowska P, Seidl C, Wels WS, Klingemann HG, et al. NK-92: an ‘off-the-shelf therapeutic’ for adoptive natural killer cell-based cancer immunotherapy. Cancer Immunol Immunother. 2016;65:485–92. doi: 10.1007/s00262-015-1761-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Nowakowska P, Odendahlet M, Bönig H, Zhang C, Naundorf S, Becker P, et al. Optimization and validation of processes related to the GMP compliant manufacture of genetically modified car expressing NK-92 cells. Cytotherapy. 2015;17:S25. [Google Scholar]
- 208.Bjordahl R, Zhu H, Rogers P, Gaidarova S, Ge M, Blum R, et al. FT516, an off-the-shelf engineered NK cell therapeutic product for universal anti-tumor targeting strategy in combination with monoclonal antibodies. Cancer Res. 2019;79.
- 209.Rezner B, Solchaga L, Reyes L, Lin A, Abujarour R, Lee T, et al. cGMP mass production of FT538, a first-of-kind, off-the-shelf, multiplexed engineered natural killer cell cancer immunotherapy derived from a clonal master induced pluripotent stem cell line. Blood. 2020;136:25. [Google Scholar]
- 210.Veluchamy J. An off the shelf, GMP compliant, fully closed and semi-automated large-scale production system for allogeneic NK cells. Cytotherapy. 2020;22:S161–2. [Google Scholar]
- 211.Williams SM, Sumstad D, Kadidlo D, Curtsinger J, Luo X, Miller JS, et al. Clinical-scale production of cGMP compliant CD3/CD19 cell-depleted NK cells in the evolution of NK cell immunotherapy at a single institution. Transfusion. 2018;58:1458–67. doi: 10.1111/trf.14564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Lim O, Lee Y, Chung H, Her JH, Kang SM, Jung MY, et al. GMP-compliant, large-scale expanded allogeneic natural killer cells have potent cytolytic activity against cancer cells in vitro and in vivo. PLoS ONE. 2013;8:e53611. doi: 10.1371/journal.pone.0053611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Oyer J, Copik A, Pileggi J, Streefland M, Lee D, Igarashi R. Bridging NK cell expansion methods towards a feeder-cell free scalable GMP production of hyperfunctional NK cells. Mol Ther. 2020;28:191–2. [Google Scholar]
- 214.Bröker K, Sinelnikov E, Gustavus D, Schumacher U, Pörtner R, Hoffmeister H, et al. Mass Production of Highly Active NK Cells for Cancer Immunotherapy in a GMP Conform Perfusion Bioreactor. Front Bioeng Biotechnol. 2019;7:194. doi: 10.3389/fbioe.2019.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Granzin M, Soltenborn S, Müller S, Kollet J, Berg M, Cerwenka A, et al. Fully automated expansion and activation of clinical-grade natural killer cells for adoptive immunotherapy. Cytotherapy. 2015;17:621–32. doi: 10.1016/j.jcyt.2015.03.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Thakkar A, Igarashi RY, Lee DA. Automated closed-system large-scale expansion of clinical-grade natural killer cells. Cytotherapy. 2019;21:S31–2. [Google Scholar]
- 217.Zhang C, Oberoi P, Oelsner S, Waldmann A, Lindner A, Tonn T, et al. Chimeric Antigen Receptor-Engineered NK-92 Cells: An Off-the-Shelf Cellular Therapeutic for Targeted Elimination of Cancer Cells and Induction of Protective Antitumor Immunity. Front Immunol. 2017;8:533. doi: 10.3389/fimmu.2017.00533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Siegler EL, Zhu YN, Wang P, Yang LL. Off-the-shelf CAR-NK cells for cancer immunotherapy. Cell Stem Cell. 2018;23:160–1. doi: 10.1016/j.stem.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 219.Felices M, Lenvik AJ, McElmurry R, Chu S, Hinderlie P, Bendzick L, et al. Continuous treatment with IL-15 exhausts human NK cells via a metabolic defect. JCI Insight. 2018;3:e96219. doi: 10.1172/jci.insight.96219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Saito S, Harada Y, Morodomi Y, Onimaru M, Yoshida K, Kyuragi R, et al. Ex vivo generation of highly purified and activated natural killer cells from human peripheral blood. Hum Gene Ther Methods. 2013;24:241–52. doi: 10.1089/hgtb.2012.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Nagai K, Harada Y, Harada H, Yanagihara K, Yonemitsu Y, Miyazaki Y. Highly activated ex vivo-expanded natural killer cells in patients with solid tumors in a Phase I/IIa clinical study. Anticancer Res. 2020;40:5687–700. doi: 10.21873/anticanres.14583. [DOI] [PubMed] [Google Scholar]
- 222.Li F, Sheng Y, Hou W, Sampath P, Byrd D, Thorne S, et al. CCL5-armed oncolytic virus augments CCR5-engineered NK cell infiltration and antitumor efficiency. J Immunother Cancer. 2020;8:e000131. doi: 10.1136/jitc-2019-000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Choi YH, Lim EJ, Kim SW, Moon YW, Park KS, An H-J. Correction to: IL-27 enhances IL-15/IL-18-mediated activation of human natural killer cells. J Immunother Cancer. 2019;7:211. doi: 10.1186/s40425-019-0688-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Choi J-W, Lee ES, Kim SY, Park SI, Oh S, Kang JH, et al. Cytotoxic effects of ex vivo-expanded natural killer cell-enriched lymphocytes (MYJ1633) against liver cancer. BMC Cancer. 2019;19:817. doi: 10.1186/s12885-019-6034-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Damodharan SN, Walker KL, Forsberg MH, McDowell KA, Bouchlaka MN, Drier DA, et al. Analysis of ex vivo expanded and activated clinical-grade human NK cells after cryopreservation. Cytotherapy. 2020;22:450–7. doi: 10.1016/j.jcyt.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Zhao X-Y, Jiang Q, Jiang H, Hu L-J, Zhao T, Yu X-X, et al. Expanded clinical‐grade membrane‐bound IL‐21/4‐1BBL NK cell products exhibit activity against acute myeloid leukemia in vivo. Eur J Immunol. 2020;50:1374–85. doi: 10.1002/eji.201948375. [DOI] [PubMed] [Google Scholar]
- 227.Jong AY, Wu C-H, Li J, Sun J, Fabbri M, Wayne AS, et al. Large-scale isolation and cytotoxicity of extracellular vesicles derived from activated human natural killer cells. J Extracell Vesicles. 2017;6:1294368. doi: 10.1080/20013078.2017.1294368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Ciurea SO, Schafer JR, Bassett R, Denman CJ, Cao K, Willis D, et al. Phase 1 clinical trial using mbIL21 ex vivo–expanded donor-derived NK cells after haploidentical transplantation. Blood. 2017;130:1857–68. doi: 10.1182/blood-2017-05-785659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Vela M, Corral D, Carrasco P, Fernández L, Valentín J, González B, et al. Haploidentical IL-15/41BBL activated and expanded natural killer cell infusion therapy after salvage chemotherapy in children with relapsed and refractory leukemia. Cancer Lett. 2018;422:107–17. doi: 10.1016/j.canlet.2018.02.033. [DOI] [PubMed] [Google Scholar]
- 230.Fernández L, Leivas A, Valentín J, Escudero A, Corral D, de Paz R, et al. How do we manufacture clinical‐grade interleukin‐15–stimulated natural killer cell products for cancer treatment? Transfusion. 2018;58:1340–7. doi: 10.1111/trf.14573. [DOI] [PubMed] [Google Scholar]
- 231.Muñoz Builes M, Vela Cuenca M, Fuster Soler JL, Astigarraga I, Pascual Martínez A, Vagace Valero JM, et al. Study protocol for a phase II multicentre prospective non-randomised clinical trial to assess the safety and efficacy of infusing allogeneic activated and expanded natural killer cells as consolidation therapy for paediatric acute myeloblastic leukaemia. BMJ Open. 2020;10:e029642. doi: 10.1136/bmjopen-2019-029642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Mani R, Rajgolikar G, Nunes J, Zapolnik K, Wasmuth R, Mo X, et al. Fc-engineered anti-CD33 monoclonal antibody potentiates cytotoxicity of membrane-bound interleukin-21 expanded natural killer cells in acute myeloid leukemia. Cytotherapy. 2020;22:369–76. doi: 10.1016/j.jcyt.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Khatua S, Cooper LJN, Sandberg DI, Ketonen L, Johnson JM, Rytting ME, et al. Phase I study of intraventricular infusions of autologous ex vivo expanded NK cells in children with recurrent medulloblastoma and ependymoma. Neuro Oncol. 2020;22:1214–25. doi: 10.1093/neuonc/noaa047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Ishikawa E, Tsuboi K, Saijo K, Harada H, Takano S, Nose T, et al. Autologous natural killer cell therapy for human recurrent malignant glioma. Anticancer Res. 2004;24:1861–71. [PubMed] [Google Scholar]
- 235.Peighambarzadeh F, Najafalizadeh A, Esmaeil N, Rezaei A, Ashrafi F, Ganjalikhani Hakemi M. Optimization of in vitro expansion and activation of human natural killer cells against breast cancer cell line. Avicenna J Med Biotechnol. 2020;12:17–23. [PMC free article] [PubMed] [Google Scholar]
- 236.Calvo T, Reina-Ortiz C, Giraldos D, Gascón M, Woods D, Asenjo J, et al. Expanded and activated allogeneic NK cells are cytotoxic against B-chronic lymphocytic leukemia (B-CLL) cells with sporadic cases of resistance. Sci Rep. 2020;10:19398. doi: 10.1038/s41598-020-76051-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Granzin M, Stojanovic A, Miller M, Childs R, Huppert V, Cerwenka A. Highly efficient IL-21 and feeder cell-driven ex vivo expansion of human NK cells with therapeutic activity in a xenograft mouse model of melanoma. Oncoimmunology. 2016;5:e1219007. doi: 10.1080/2162402X.2016.1219007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Pedroso JF, de Souza Valim V, Pezzi A, Furlan JM, Lenhart G, Sehn F, et al. An experimental study comparing the expansion of peripheral blood natural killer (NK) cells cultured with artificial antigen-presenting cells in the presence or absence of bone marrow mesenchymal stem cells (MSCs) Mol Biotechnol. 2020;62:306–15. doi: 10.1007/s12033-020-00250-2. [DOI] [PubMed] [Google Scholar]
- 239.Vodyanik MA, Bork JA, Thomson JA, Slukvin II. Human embryonic stem cell–derived CD34+ cells: efficient production in the coculture with OP9 stromal cells and analysis of lymphohematopoietic potential. Blood. 2005;105:617–26. doi: 10.1182/blood-2004-04-1649. [DOI] [PubMed] [Google Scholar]
- 240.Woll PS, Martin CH, Miller JS, Kaufman DS. Human embryonic stem cell-derived NK cells acquire functional receptors and cytolytic activity. J Immunol. 2005;175:5095–103. doi: 10.4049/jimmunol.175.8.5095. [DOI] [PubMed] [Google Scholar]
- 241.Larbi A, Gombert J-M, Auvray C, l'Homme B, Magniez A, Féraud O, et al. The HOXB4 homeoprotein promotes the ex vivo enrichment of functional human embryonic stem cell-derived NK cells. PLoS ONE. 2012;7:e39514. doi: 10.1371/journal.pone.0039514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Bock AM, Knorr D, Kaufman DS. Development expansion and in vivo monitoring of human NK cells from human embryonic stem cells (hESCs) and and induced pluripotent stem cells (iPSCs). J Vis Exp. 2013:e50337. 10.3791/50337. [DOI] [PMC free article] [PubMed]
- 243.Hermanson DL, Ni Z, Kaufman DS. Human pluripotent stem cells as a renewable source of natural killer cells. In: Cheng T, editor. Hematopoietic differentiation of human pluripotent stem cells. SpringerBriefs in Stem Cells, vol 6. Dordrecht: Springer; 2015. 10.1007/978-94-017-7312-6_5.
- 244.Knorr DA, Bock A, Brentjens RJ, Kaufman DS. Engineered human embryonic stem cell-derived lymphocytes to study in vivo trafficking and immunotherapy. Stem Cells Dev. 2013;22:1861–9. doi: 10.1089/scd.2012.0608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Zhu H, Blum RH, Bjordahl R, Gaidarova S, Rogers P, Lee TT, et al. Pluripotent stem cell–derived NK cells with high-affinity noncleavable CD16a mediate improved antitumor activity. Blood. 2020;135:399–410. doi: 10.1182/blood.2019000621. [DOI] [PMC free article] [PubMed] [Google Scholar]