Abstract
Retinal, vitreous humor, brain, and cerebrospinal fluid (CSF) foscarnet levels were measured by high-performance liquid chromatography after administration of an intravenous dose of 120 mg/kg of body weight to 32 pigmented rabbits. A pharmacokinetic analysis was done using a two-compartment model. The penetration ratios, defined as ratios of retinal, vitreous humor, brain, and CSF areas under the concentration-time curve from 0 to 2 h were 110% ± 1%, 12.3% ± 0.7%, 118% ± 1%, and 20.2% ± 2.2%, respectively. These results suggest a good penetration of foscarnet into the retinal and brain tissues, reaching higher concentrations than those estimated from vitreous humor and CSF levels.
Foscarnet is a drug used extensively in the treatment of retinitis and neurological conditions caused by cytomegalovirus (CMV) in patients with AIDS, and there are data supporting the use of foscarnet with aciclovir or ganciclovir in the treatment of retinitis caused by other herpesviruses (4, 15, 19). It has been suggested that intravenous ganciclovir or foscarnet maintenance therapy of CMV retinitis may result in subtherapeutic intraocular concentrations, a factor implicated in the progression of this disease (1). However, there are few data on concentrations of foscarnet in tissue, and the levels in retina or brain have not been described for either humans or experimental models. The intraocular penetration of foscarnet has been assessed only in vitreal samples from a few patients with CMV retinitis (1). Likewise, the penetration of foscarnet into the central nervous system has been evaluated only from single determinations in cerebrospinal fluid (CSF) and single ratios of concentrations in CSF to concentrations in plasma in samples obtained at variable and arbitrary intervals from patients with nonuniform dosages. Consequently, the results have been very variable, 13 to 340% of the simultaneous concentration in plasma (11, 21–23). Moreover, to assume that retinal and brain foscarnet levels are similar to those measured in vitreous humor and CSF may be erroneous. Due to the difficulties in obtaining tissue samples from humans, we used a rabbit model for evaluating the retinal and brain penetration of foscarnet after its intravenous administration. The ratios of the areas under the concentration-time curve (AUCs) for tissues and CSF to the AUCs for serum were used to obtain more accurate results (16). We have also determined whether the concentrations detected in retina and brain are similar to those in vitreous humor and CSF. In humans, no significant differences were observed in plasma concentrations between single- and multiple-dose administration (25); therefore, the study was designed to obtain samples after a single dose of the drug.
Study design, drug administration, and sampling.
Thirty-two healthy, pigmented rabbits with a mean weight of 3 kg were used. The study was conducted according to the Ministerio de Agricultura guidelines. After animals were anesthetized with an intramuscular injection of xylazine (12 mg/kg of body weight) and ketamine (60 mg/kg), a jugular vein was catheterized by a surgical procedure and maintained as permeable by a 0.9% saline solution-lock technique. The entire experiments were conducted under surgical anesthesia. Each rabbit received 120 mg of foscarnet (Astra Pharmaceutical, Södertälje, Sweden) per kg, as an intravenous dose over a 3-min period, since this is a usual dose in humans during the maintenance treatment of CMV retinitis in patients with AIDS. Blood (2 ml) was taken before and at 5, 15, 30, 45, 60, 75, 90, and 120 min after the end of infusion. Retina, vitreous humor, brain, and CSF samples were obtained at 30 and 90 min and 60 and 120 min, so that each animal yielded tissue data for two different time points, before being euthanatized with intravenous pentobarbital. Vitreous humor was obtained by cutting the eyes just behind the lens (mean volume obtained, 638 ± 184 μl); afterwards, the retina was carefully dissected (mean weight obtained, 49.3 ± 13.5 mg). Brain tissue was obtained through a small craniectomy (mean weight obtained, 596 ± 169 mg), and CSF was obtained by puncture of the cisterna magna (mean volume obtained, 528 ± 193 μl). After centrifugation, plasma and CSF samples were stored at −80°C until testing. Before being frozen, vitreous humor and retinal and brain tissues were sonicated at 0.5 cps for 40 s (50-W sonicator; Sonics & Materials Inc., Danbury, Conn.). For processing, each sample was transferred to a micropartition tube (Centricon 30; Amicon Inc., Beverly, Mass.) and centrifuged at 1,500 × g for 20 min.
Assay procedure.
Concentrations of foscarnet were determined according to the modified method of Pettersson and Nordgren (20), using a high-performance liquid chromatograph with an electrochemical detector (Gilson Medical Electronics, Inc., Middleton, Wis.). The analytical column (125 by 4 mm [inside diameter]) was a Lichospher 100 RP-18 with 5-μm particles (Merck, Darmstadt, Germany). The volume injected was 20 μl, and the flow rate was 0.7 ml/min. Quantification was based on measuring standard solutions of foscarnet (Astra Pharmaceutical) in 0.9% (wt/vol) NaCl solution and hydrochlorothiazide as the internal standard. The detection limit was 10 μg/ml. Calibration lines were linear (rxy > 0.9000) over a range of 10 to 1,200 μg/ml. An analysis of variance was carried out to determine interassay [F(1–27) = 0.84; P = 0.5343] and intra-assay [F(1–37) = 0.72; P = 0.6692] variability; no statistically significant differences were observed between them. The intra- and interassay coefficients of variation were 1 and 1.7%, respectively. Recovery of foscarnet from plasma, retina, vitreous humor, brain, and CSF, after adding known concentrations of foscarnet to them, was 86, 82.7, 79.8, 84, and 94.8%, respectively.
Pharmacokinetic analysis.
Compartmental pharmacokinetic parameters were calculated from the concentration-time curve data (12). The corresponding macroconstants were calculated from the biexponential equation Cst = A0e−αt + B0e−βt, where Cst is the concentration of the drug in serum at time t. A0 and B0, and α and β, are the intercepts and exponents, respectively, of the two exponential phases. Twenty data per time point were used to determine the serum pharmacokinetic parameters, which were calculated manually, and 8 to 10 data for each tissue per time point were used. The AUC from 0 h to infinity (AUC0–∞) was calculated up to the time of the last quantifiable serum concentration using the trapezoidal rule and then to infinity using the quotient of the last measurable concentration to the terminal-phase rate constant, which was calculated by the above-mentioned curve fitting. Results were expressed per milliliter, assuming tissue densities as 1. The penetration ratio was defined as the ratio of the AUC0–2 for tissues and CSF to the AUC0–2 for serum.
Results.
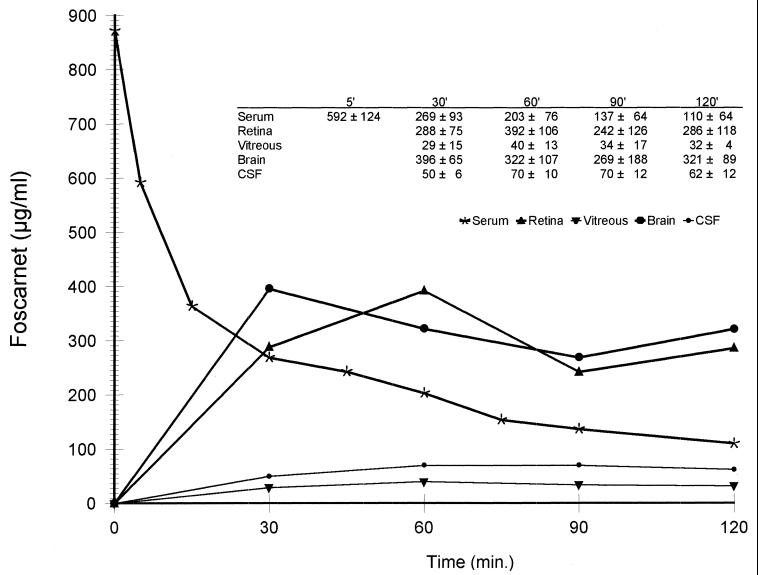
Figure 1 shows the mean serum and tissue concentration-time curves after the intravenous administration of foscarnet (120 mg/kg). The data fitted to a two-compartment model, the equation being Cst = 0.49e−10.02t + 0.382e−0.636t. The profile clearly shows the two distinct phases associated with a two-compartment model. The α phase is basically the rapid distribution of the drug (A/α < B/β), while the β phase is basically its elimination, after reaching the stationary equilibrium state. Foscarnet has a rapid disappearance from serum with a mean α-phase half-life of 0.065 ± 0.001 h and a β-phase half-life of 1.09 ± 0.26 h. Table 1 summarizes the values of the pharmacokinetic parameters for foscarnet obtained with this model.
FIG. 1.
Mean (± standard deviation; micrograms per milliliter) serum and tissue concentration-time curves after an intravenous dose of foscarnet (120 mg/kg).
TABLE 1.
Pharmacokinetic parameters of foscarnet after intravenous administration of 120 mg/kg to rabbits
| Parameter (unite)a | Value (mean ± SD) |
|---|---|
| Concn (μg/ml) | |
| 0 h | 872 ± 120 |
| Maximum in peripheral compartment | 343 ± 92 |
| A0 | 490 ± 45 |
| B0 | 382 ± 75 |
| Phase (h−1) | |
| α | 10.02 ± 0.24 |
| β | 0.636 ± 0.12 |
| t1/2 (h) | |
| α | 0.065 ± 0.001 |
| β | 1.09 ± 0.26 |
| tss (h) | 0.37 ± 0.078 |
| V (ml/kg) | |
| Vc | 137.6 ± 22 |
| Vp | 132.4 ± 14.2 |
| Vss | 270 ± 37 |
| CL (ml/kg/min) | 2.86 ± 0.91 |
| k (h−1) | |
| k1–2 | 4.57 ± 0.01 |
| k2–1 | 4.47 ± 0.12 |
| kel | 1.34 ± 0.2 |
| AUC (μg · h/ml) | |
| AUC0–2 | 484 ± 166 |
| AUC0–∞ | 649 ± 38 |
t1/2, half-life; tss, time in steady state; Vc, Vp, and Vss, volume of distribution in the central or peripheral compartment or at steady state, respectively; CL, clearance; k, rate constant; kel, elimination rate constant.
High levels of foscarnet were found in retina and brain, with AUC0–2 of 532 ± 183 and 574 ± 202 μg · h/ml, respectively. Penetration into both the retina and the brain was remarkably high, with estimated penetration ratios of 110% ± 1% and 118% ± 1%, respectively. Lower levels of foscarnet were found in vitreous humor and CSF, with AUC0–2 of 59.7 ± 23.0 and 93 ± 1.8 μg · h/ml and penetration ratios of 12.3% ± 0.7% and 20.2% ± 2.2%, respectively.
Discussion.
In this model, the estimated distribution volumes suggest that foscarnet is a drug widely distributed in different organs, as deduced from the equation Vss/Vc − 1 = 0.96 (12), where Vss is volume of distribution at steady state and Vc is volume of distribution in central compartment, in spite of a short biological half-life in both α and β phases. Our data show that foscarnet has a good penetration in both retinal and brain tissues, reaching levels far above the 50% inhibitory concentration (120 μg/ml; range, 7.5 to 240) for most strains of human CMV (7). Foscarnet is a very small and highly negatively charged molecule, with a protein binding level of 15% and with an organic/water partition coefficient of 0.426 (3). These physicochemical properties would facilitate its diffusion through the blood-brain and blood-retinal barriers (17). These high ratios may also be due to a slower elimination from retina and brain than from serum, so that the ratio of drug concentrations in these tissues and serum increases with time after infusion, as frequently occurs with other drugs (13, 16). However, the concentrations and AUC0–2 observed in the vitreous humor are much lower than those in the retina. Thus, the retinal concentrations reached after the intravenous administration of foscarnet cannot be estimated from those observed in the vitreous humor.
In the same way, levels of foscarnet in CSF are lower than those observed in brain. CSF drug concentrations are frequently lower than those of the cerebral extracellular fluid and the brain tissue, overall when CSF is obtained from ventricles or the cisterna magna (6, 18, 26). However, the presence of an AUC in the brain sixfold higher than that in the CSF suggests that, at least in this animal, there may be a high efflux clearance of foscarnet from CSF. This could be produced via the arachnoid villi and nonarachnoidal CSF drainage pathways (cribiform area, orbital area, and inner ear) present in the rabbit and other lower mammals (8, 15). In addition, as foscarnet is a weak acid (9), there could be an active efflux from the CSF, as occurs with other weak organic acid drugs (5).
Although the intravitreal levels of foscarnet observed in the patients studied by Arevalo et al. (1) were similar to those we have found in rabbits, it is difficult to know whether our data are entirely applicable to humans since the few reported pharmacokinetic studies of foscarnet in humans have been carried out with heterogeneous doses, rates of perfusions, and pharmacokinetic analyses. It would be expected that the concentrations of foscarnet in these tissues would be similar to or higher than those observed in rabbits, since in humans the Vss of foscarnet is higher and the half-life is longer than those in rabbits (2, 24, 26).
From our study, it cannot be ruled out that the current dosages of foscarnet during the maintenance phase of CMV retinitis (90 to 120 mg/day, in a single dose) give rise to subtherapeutic concentrations in the retina during part of the day and that this may be a factor in the progression of the disease. Nevertheless, our results show that foscarnet penetrates well into the retina and brain tissue, reaching higher concentrations than those estimated from vitreous humor and CSF.
Acknowledgments
This work was supported by grant SAF97-0012 from the Comisión Interministerial de Ciencia y Tecnología, by grant 64/96 from the Consejería de Salud, Junta de Andalucía, and by Astra S.A., Spain.
REFERENCES
- 1.Arevalo J F, Gonzalez C, Capparelli E V, Kirsch L S, Garcia R F, Quiceno J I, Connor J D, Gambertoglio J, Bergeron-Lynn G, Freman W R. Intravitreous and plasma concentrations of ganciclovir and foscarnet after intravenous therapy in patients with AIDS and cytomegalovirus retinitis. J Infect Dis. 1995;172:951–956. doi: 10.1093/infdis/172.4.951. [DOI] [PubMed] [Google Scholar]
- 2.Aweeka F, Gambertoglio J, Mills J, Jacobson M A. Pharmacokinetics of intermittently administered intravenous foscarnet in the treatment of acquired immunodeficiency syndrome patients with serious cytomegalovirus retinitis. Antimicrob Agents Chemother. 1989;33:742–745. doi: 10.1128/aac.33.5.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chrisp P, Clissold S. Foscarnet. A review of its antiviral activity, pharmacokinetic properties and therapeutic use in immunocompromised patients with cytomegalovirus retinitis. Drugs. 1991;41:104–129. doi: 10.2165/00003495-199141010-00009. [DOI] [PubMed] [Google Scholar]
- 4.Ciulla T A, Rutledge B K, Morley M G, Duker J S. The progressive outer retinal necrosis syndrome: successful treatment with combination antiviral therapy. Ophthalmic Surg Lasers. 1998;29:198–206. [PubMed] [Google Scholar]
- 5.Dacey R G, Sande M A. Effect of probenecid on cerebrospinal fluid concentrations of penicillin and cephalosporin derivatives. Antimicrob Agents Chemother. 1974;6:437–441. doi: 10.1128/aac.6.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davson H, Segal M B. Physiology of the CSF and blood-brain barriers. Boca Raton, Fla: CRC Press, Inc.; 1996. [Google Scholar]
- 7.Drew W L, Miner R C, Saleh E. Antiviral susceptibility of cytomegalovirus: criteria for detecting resistance to antivirals. Clin Diagn Virol. 1993;1:179–185. doi: 10.1016/0928-0197(93)90012-t. [DOI] [PubMed] [Google Scholar]
- 8.Erlich S S, McComb J G, Hyman S, Weiss M H. Ultrastructure of the orbital pathway for cerebrospinal fluid drainage in rabbits. J Neurosurg. 1989;70:926–931. doi: 10.3171/jns.1989.70.6.0926. [DOI] [PubMed] [Google Scholar]
- 9.García-Andreu J, Lucero M J, León M J, López-Cortés L F. Estudio del foscarnet como alternativa al tratamiento antiherpético. Cienc Pharm. 1997;7:209–218. [Google Scholar]
- 10.Hengge U R, Brockmeyer N H, Malessa R, Ravens U, Goos M. Foscarnet penetrates the blood-brain barrier. Rationale for therapy of cytomegalovirus encephalitis. Antimicrob Agents Chemother. 1993;37:1010–1014. doi: 10.1128/aac.37.5.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacobson M A, Causey D, Polsky B, Hardy D, Chown M, Davis R, et al. A dose-ranging study of daily maintenance intravenous foscarnet therapy for cytomegalovirus retinitis in AIDS. J Infect Dis. 1993;168:444–448. doi: 10.1093/infdis/168.2.444. [DOI] [PubMed] [Google Scholar]
- 12.Kagner J G. Pharmacokinetics for the pharmaceutical scientist. Lancaster, Pa: Technomic Publishing Co.; 1993. [Google Scholar]
- 13.Lupia R H, Ferencz N, Lertora J J, Aggarwal S K, George W J, Agrawal K C. Comparative pharmacokinetics of two prodrugs of zidovudine in rabbits: enhanced levels of zidovudine in brain tissue. Antimicrob Agents Chemother. 1993;37:818–824. doi: 10.1128/aac.37.4.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manzo R P, Gomez D G, Potts D G. Cerebrospinal fluid absorption in the rabbit. Inner ear pathways. Acta Otolaryngol. 1990;109:389–396. doi: 10.3109/00016489009125160. [DOI] [PubMed] [Google Scholar]
- 15.Moorthy R S, Weinberg D V, Teich S A, Berger B B, Minturn S K, Rao N A, et al. Management of varicella zoster virus retinitis in AIDS. Br J Ophthalmol. 1997;81:189–194. doi: 10.1136/bjo.81.3.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nau R, Zysk G, Thiel A, Prange H W. Pharmacokinetic quantification of the exchange of drugs between blood and cerebrospinal fluid in man. Eur J Clin Pharmacol. 1993;45:469–475. doi: 10.1007/BF00315520. [DOI] [PubMed] [Google Scholar]
- 17.Nau R, Sorgel F, Prange H W. Lipophilicity at pH 7.4 and molecular size govern the entry of the free serum fraction of drugs into the cerebrospinal fluid in humans with uninflamed meninges. J Neurol Sci. 1994;122:61–65. doi: 10.1016/0022-510x(94)90052-3. [DOI] [PubMed] [Google Scholar]
- 18.Nau R, Sörgel F, Prange H W. Pharmacokinetic optimisation of the treatment of bacterial central nervous system infections. Clin Pharmacokinet. 1998;35:223–246. doi: 10.2165/00003088-199835030-00005. [DOI] [PubMed] [Google Scholar]
- 19.Ormerod L D, Larkin J A, Margo C A, Pavan P R, Menosky M M, Haight D O, et al. Rapidly progressive herpetic retinal necrosis: a blinding disease characteristic of advanced AIDS. Clin Infect Dis. 1998;26:34–45. doi: 10.1086/516285. [DOI] [PubMed] [Google Scholar]
- 20.Pettersson K J, Nordgren T. Determination of phosphonoformate (foscarnet) in biological fluids by ion-pair reversed-phase liquid chromatography. J Chromatogr Biomed Appl. 1989;488:447–455. doi: 10.1016/s0378-4347(00)82968-0. [DOI] [PubMed] [Google Scholar]
- 21.Raffi F, Taburet A M, Ghaleh B, Huart A, Singlas E. Penetration of foscarnet into cerebrospinal fluid of AIDS patients. Antimicrob Agents Chemother. 1993;37:1777–1780. doi: 10.1128/aac.37.9.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seidel E A, Koening S, Polis M A. A dose escalation study to determine the toxicity and maximally tolerated dose of foscarnet. AIDS. 1993;7:941–945. [PubMed] [Google Scholar]
- 23.Sjovall J, Bergdahl S, Movin G, Ogenstad S, Saarimäki M. Pharmacokinetics of foscarnet and distribution to cerebrospinal fluid after intravenous infusion in patients with human immunodeficiency virus infection. Antimicrob Agents Chemother. 1989;33:1023–1031. doi: 10.1128/aac.33.7.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sjövall J, Karlsson A, Ogenstad S, Sandström E, Saarimäki M. Pharmacokinetics and absorption of foscarnet after intravenous and oral administration to patients with human immunodeficiency virus. Clin Pharmacol Ther. 1988;44:65–73. doi: 10.1038/clpt.1988.114. [DOI] [PubMed] [Google Scholar]
- 25.Taburet A M, Katlama C, Blanshard C, Zorza G, Gazzard D, Dohin E, et al. Pharmacokinetics of foscarnet after twice-daily administrations for treatment of cytomegalovirus disease in AIDS patients. Antimicrob Agents Chemother. 1992;36:1821–1824. doi: 10.1128/aac.36.9.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weisner B, Bernhardt W. Protein fractions of lumbar, cisternal and ventricular cerebrospinal fluid. J Neurol Sci. 1978;37:205–214. doi: 10.1016/0022-510x(78)90204-6. [DOI] [PubMed] [Google Scholar]